Myocardial Infarction with Nonobstructive Coronary Arteries (MINOCA): Current Insights into Pathophysiology, Diagnosis, and Management
Abstract
1. Introduction
2. Epidemiology and Prognosis
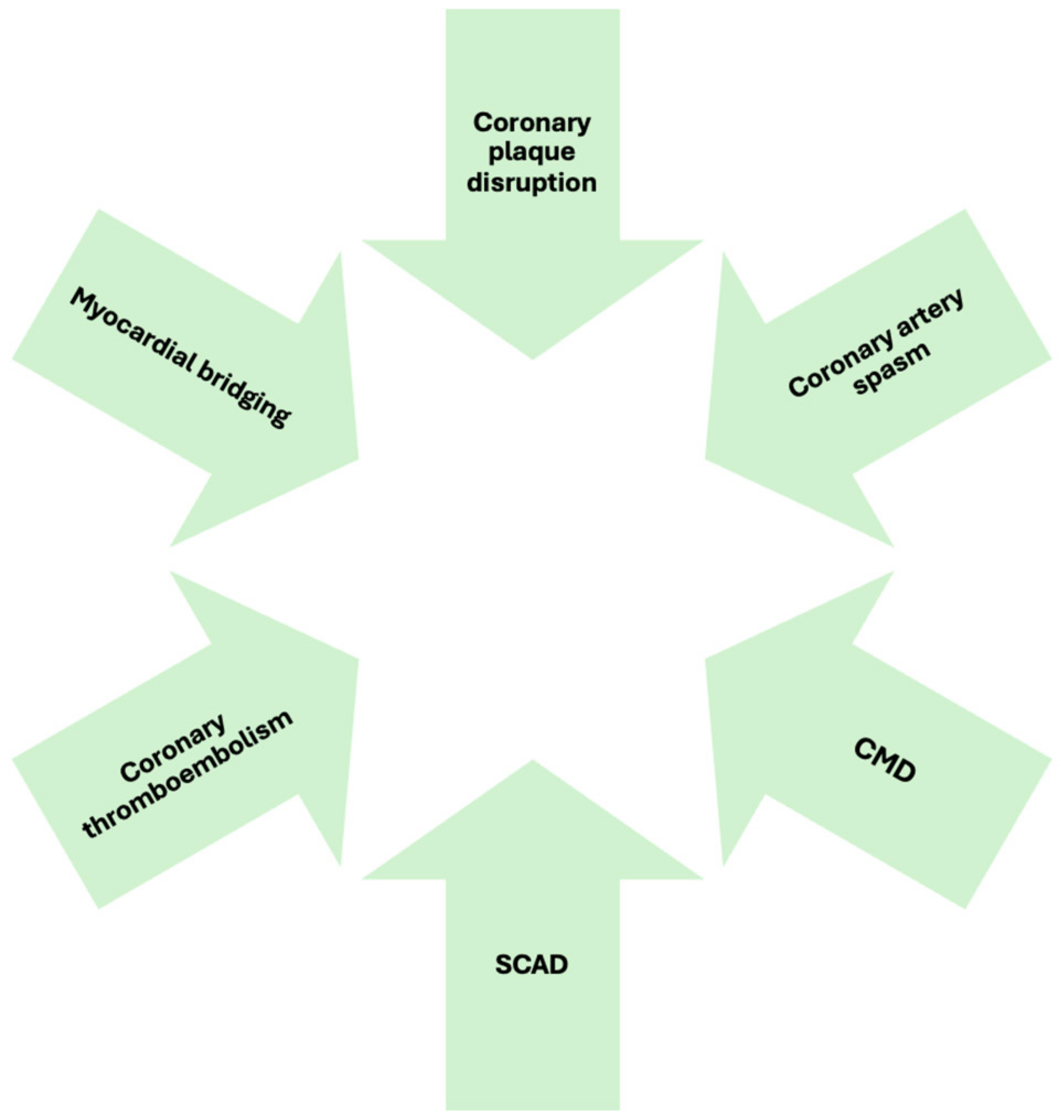
3. Pathophysiology
3.1. Atherosclerotic Causes
- Coronary plaque disruption
3.2. Non-Atherosclerotic Causes
- Coronary Artery Spasm
- Coronary Microvascular Dysfunction
- Coronary embolism and thrombosis
- Spontaneous Coronary Artery Dissection
- Myocardial bridging
3.3. Supply–Demand Mismatch
3.4. MINOCA Mimics
4. Clinical Presentation
5. Diagnosis
- Intravascular imaging (OCT and IVUS)
- Intracoronary functional test (Ach/ergonovine)
- Coronary physiology assessments (FFR, CMR, and IMR)
6. Management
7. Sex-Related Considerations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agewall, S.; Beltrame, J.F.; Reynolds, H.R.; Niessner, A.; Rosano, G.; Caforio, A.L.; De Caterina, R.; Zimarino, M.; Roffi, M.; Kjeldsen, K.; et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur. Heart J. 2017, 38, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Tamis-Holland, J.E.; Jneid, H.; Reynolds, H.R.; Agewall, S.; Brilakis, E.S.; Brown, T.M.; Lerman, A.; Cushman, M.; Kumbhani, D.J.; Arslanian-Engoren, C.; et al. Contemporary Diagnosis and Management of Patients With Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease: A Scientific Statement From the American Heart Association. Circulation 2019, 139, e891–e908. [Google Scholar] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction. Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, B.; Baron, T.; Albertucci, M.; Prati, F. Myocardial Infarction with Non-Obstructive Coronary Artery Disease’. Available online: https://eurointervention.pcronline.com/article/myocardial-infarction-with-non-obstructive-coronary-artery-disease (accessed on 5 February 2025).
- Boivin-Proulx, L.-A.; Haddad, K.; Lombardi, M.; Chong, A.Y.; Escaned, J.; Mukherjee, S.; Forcillo, J.; Potter, B.J.; Coutinho, T.; Pacheco, C. Pathophysiology of Myocardial Infarction with Nonobstructive Coronary Artery Disease: A Contemporary Systematic Review. CJC Open 2024, 6, 380–390. [Google Scholar] [CrossRef]
- Pasupathy, S.; Air, T.; Dreyer, R.; Tavella, R.; Beltrame, J.F. Systematic Review of Patients Presenting With Suspected Myocardial Infarction and Nonobstructive Coronary Arteries. Circulation 2015, 131, 861–870. [Google Scholar] [CrossRef]
- Eggers, K.M.; Hjort, M.; Baron, T.; Jernberg, T.; Nordenskjöld, A.M.; Tornvall, P.; Lindahl, B. Morbidity and cause-specific mortality in first-time myocardial infarction with nonobstructive coronary arteries. J. Intern. Med. 2019, 285, 419–428. [Google Scholar] [CrossRef]
- Pelliccia, F.; Pasceri, V.; Niccoli, G.; Tanzilli, G.; Speciale, G.; Gaudio, C.; Crea, F.; Camici, P.G. Predictors of Mortality in Myocardial Infarction and Nonobstructed Coronary Arteries: A Systematic Review and Meta-Regression. Am. J. Med. 2020, 133, 73–83.e4. [Google Scholar] [CrossRef]
- Nordenskjöld, A.M.; Lagerqvist, B.; Baron, T.; Jernberg, T.; Hadziosmanovic, N.; Reynolds, H.R.; Tornvall, P.; Lindahl, B. Reinfarction in Patients with Myocardial Infarction with Nonobstructive Coronary Arteries (MINOCA): Coronary Findings and Prognosis. Am. J. Med. 2019, 132, 335–346. [Google Scholar]
- Hjort, M.; Lindahl, B.; Baron, T.; Jernberg, T.; Tornvall, P.; Eggers, K.M. Prognosis in relation to high-sensitivity cardiac troponin T levels in patients with myocardial infarction and non-obstructive coronary arteries. Am. Heart J. 2018, 200, 60–66. [Google Scholar] [CrossRef]
- Pasupathy, S.; Lindahl, B.; Litwin, P.; Tavella, R.; Williams, M.J.; Air, T.; Zeitz, C.; Smilowitz, N.R.; Reynolds, H.R.; Eggers, K.M.; et al. Survival in Patients With Suspected Myocardial Infarction With Nonobstructive Coronary Arteries: A Comprehensive Systematic Review and Meta-Analysis From the MINOCA Global Collaboration. Circ. Cardiovasc. Qual. Outcomes 2021, 14, e007880. [Google Scholar] [CrossRef]
- Canton, L.; Fedele, D.; Bergamaschi, L.; Foà, A.; Di Iuorio, O.; Tattilo, F.P.; Rinaldi, A.; Angeli, F.; Armillotta, M.; Sansonetti, A.; et al. Sex- and age-related differences in outcomes of patients with acute myocardial infarction: MINOCA vs. MIOCA. Eur. Heart J. Acute Cardiovasc. Care 2023, 12, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. The pathophysiology of acute coronary syndromes. Heart 2000, 83, 361–366. [Google Scholar] [CrossRef] [PubMed]
- White, S.J.; Newby, A.C.; Johnson, T.W. Endothelial erosion of plaques as a substrate for coronary thrombosis. Thromb. Haemost. 2018, 115, 509–519. [Google Scholar] [CrossRef]
- Virmani, R.; Burke, A.; Farb, A.; Kolodgie, F.D. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 2006, 47, C13–C18. [Google Scholar] [CrossRef]
- Reynolds, H.R.; Maehara, A.; Kwong, R.Y.; Sedlak, T.; Saw, J.; Smilowitz, N.R.; Mahmud, E.; Wei, J.; Marzo, K.; Matsumura, M.; et al. Coronary Optical Coherence Tomography and Cardiac Magnetic Resonance Imaging to Determine Underlying Causes of Myocardial Infarction With Nonobstructive Coronary Arteries in Women. Circulation 2021, 143, 624–640. [Google Scholar] [CrossRef]
- Opolski, M.; Spiewak, M.; Marczak, M.; Debski, A.; Knaapen, P.; Schumacher, S.P.; Staruch, A.D.; Grodecki, K.; Chmielak, Z.; Lazarczyk, H.; et al. Mechanisms of Myocardial Infarction in Patients With Nonobstructive Coronary Artery Disease: Results From the Optical Coherence Tomography Study. JACC Cardiovasc. Imaging 2019, 12, 2210–2221. [Google Scholar] [CrossRef]
- Gerbaud, E.; Arabucki, F.; Nivet, H.; Barbey, C.; Cetran, L.; Chassaing, S.; Seguy, B.; Lesimple, A.; Cochet, H.; Montaudon, M.; et al. OCT and CMR for the Diagnosis of Patients Presenting With MINOCA and Suspected Epicardial Causes. JACC Cardiovasc. Imaging 2020, 13, 2619–2631. [Google Scholar] [CrossRef]
- Beltrame, J.F.; Crea, F.; Kaski, J.C.; Ogawa, H.; Ong, P.; Sechtem, U.; Shimokawa, H.; Bairey Merz, C.N.; Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for vasospastic angina. Eur. Heart J. 2017, 38, 2565–2568. [Google Scholar] [CrossRef]
- Scalone, G.; Niccoli, G.; Crea, F.; Pathophysiology, E.C. Diagnosis and management of MINOCA: An update. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 54–62. [Google Scholar] [CrossRef]
- Montone, R.A.; Niccoli, G.; Fracassi, F.; Russo, M.; Gurgoglione, F.; Camma, G.; Lanza, G.A.; Crea, F. Patients with acute myocardial infarction and non-obstructive coronary arteries: Safety and prognostic relevance of invasive coronary provocative tests. Eur. Heart J. 2018, 39, 91–98. [Google Scholar] [CrossRef]
- Ong, P.; Camici, P.G.; Beltrame, J.F.; Crea, F.; Shimokawa, H.; Sechtem, U.; Kaski, J.C.; Merz, C.N.; Coronary Vasomotion Disorders International Study Group. International standardization of diagnostic criteria for microvascular angina. Int. J. Cardiol. 2018, 250, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; Conejo, T. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 144, e368–e454. [Google Scholar] [CrossRef] [PubMed]
- Del Buono, M.G.; Montone, R.A.; Camilli, M.; Carbone, S.; Narula, J.; Lavie, C.J.; Niccoli, G.; Crea, F. Coronary Microvascular Dysfunction Across the Spectrum of Cardiovascular Diseases: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1352–1371. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; Camici, G.; Merz, C.N.B. Coronary microvascular dysfunction: An update. Eur. Heart J. 2014, 35, 1101–1111. [Google Scholar] [CrossRef]
- Godo, S.; Takahashi, J.; Yasuda, S.; Shimokawa, H. Role of Inflammation in Coronary Epicardial and Microvascular Dysfunction. Eur. Cardiol. 2021, 16, e13. [Google Scholar] [CrossRef]
- Odaka, Y.; Takahashi, J.; Tsuburaya, R.; Nishimiya, K.; Hao, K.; Matsumoto, Y.; Ito, K.; Sakata, Y.; Miyata, S.; Manita, D.; et al. Plasma concentration of serotonin is a novel biomarker for coronary microvascular dysfunction in patients with suspected angina and unobstructive coronary arteries. Eur. Heart J. 2017, 38, 489–496. [Google Scholar] [CrossRef]
- Takahashi, J.; Suda, A.; Nishimiya, K.; Godo, S.; Yasuda, S.; Shimokawa, H. Pathophysiology and Diagnosis of Coronary Functional Abnormalities. Eur. Cardiol. 2021, 16, e30. [Google Scholar] [CrossRef]
- Raphael, C.E.; Heit, J.A.; Reeder, G.S.; Bois, M.C.; Maleszewski, J.J.; Tilbury, R.T.; Holmes, D.R. Coronary Embolus: An Underappreciated Cause of Acute Coronary Syndromes. JACC Cardiovasc. Interv. 2018, 11, 172–180. [Google Scholar] [CrossRef]
- El Sabbagh, A.; Al-Hijji, M.A.; Thaden, J.J.; Pislaru, S.V.; Pislaru, C.; Pellikka, P.A.; Arruda-Olson, A.M.; Grogan, M.; Greason, K.L.; Maleszewski, J.J.; et al. Cardiac Myxoma: The Great Mimicker. JACC Cardiovasc. Imaging 2017, 10, 203–206. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, J.; Chen, W.; Yang, X.; Liu, Y.; Cong, X.; Huang, Z.; Li, N. Acute myocardial infarction as the first sign of infective endocarditis: A case report. J. Int. Med. Res. 2020, 48, 300060520980598. [Google Scholar] [CrossRef]
- Karameh, M.; Golomb, M.; Arad, A.; Kalmnovich, G.; Herzog, E. Multi-Valvular Non-bacterial Thrombotic Endocarditis Causing Sequential Pulmonary Embolism, Myocardial Infarction, and Stroke: A Case Report and Literature Review. Cureus 2022, 14, e32261. [Google Scholar] [CrossRef] [PubMed]
- Talebi, S.; Jadhav, P.; Tamis-Holland, J.E. Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease (MINOCA): A Review of the Present and Preview of the Future. Curr. Atheroscler. Rep. 2021, 23, 49. [Google Scholar] [CrossRef] [PubMed]
- Pasupathy, S.; Rodgers, S.; Tavella, R.; McRae, S.; Beltrame, J.F. Risk of Thrombosis in Patients Presenting with Myocardial Infarction with Nonobstructive Coronary Arteries (MINOCA). TH Open Companion J. Thromb. Haemost. 2018, 2, e167–e172. [Google Scholar] [CrossRef]
- Shen, Y.-M.; Nagalla, S. Hypercoagulable Workup in Thrombotic Cardiovascular Diseases. Circulation 2018, 138, 229–231. [Google Scholar] [CrossRef]
- Stepien, K.; Nowak, K.; Wypasek, E.; Zalewski, J.; Undas, A. High prevalence of inherited thrombophilia and antiphospholipid syndrome in myocardial infarction with non-obstructive coronary arteries: Comparison with cryptogenic stroke. Int. J. Cardiol. 2019, 290, 1–6. [Google Scholar] [CrossRef]
- Saw, J.; Starovoytov, A.; Aymong, E.; Inohara, T.; Alfadhel, M.; McAlister, C.; Samuel, R.; Grewal, T.; Parolis, J.A.; Sheth, T.; et al. Canadian Spontaneous Coronary Artery Dissection Cohort Study: 3-Year Outcomes. J. Am. Coll. Cardiol. 2022, 80, 1585–1597. [Google Scholar] [CrossRef]
- García-Guimaraes, M.; Bastante, T.; Macaya, F.; Roura, G.; Sanz, R.; Alvarado, J.C.; Tizon, H.; Flores-Ríos, X.; Moreu, J.; Ojeda, S.; et al. Spontaneous coronary artery dissection in Spain: Clinical and angiographic characteristics, management, and in-hospital events. Rev. Espanola Cardiol. Engl. Ed. 2021, 74, 15–23. [Google Scholar] [CrossRef]
- Waterbury, T.M.; Tweet, M.S.; Hayes, S.N.; Eleid, M.F.; Bell, M.R.; Lerman, A.; Singh, M.; Best, P.J.; Lewis, B.R.; Rihal, C.S.; et al. Early Natural History of Spontaneous Coronary Artery Dissection. Circ. Cardiovasc. Interv. 2018, 11, e006772. [Google Scholar] [CrossRef]
- Hayes, S.N.; Tweet, M.S.; Adlam, D.; Kim, E.S.; Gulati, R.; Price, J.E.; Rose, C.H. Spontaneous Coronary Artery Dissection: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 961–984. [Google Scholar] [CrossRef]
- Alfonso, F.; Paulo, M.; Gonzalo, N.; Dutary, J.; Jimenez-Quevedo, P.; Lennie, V.; Escaned, J.; Bañuelos, C.; Hernandez, R.; Macaya, C. Diagnosis of spontaneous coronary artery dissection by optical coherence tomography. J. Am. Coll. Cardiol. 2012, 59, 1073–1079. [Google Scholar] [CrossRef]
- Guerra, E.; Bergamaschi, L.; Tuttolomondo, D.; Pizzi, C.; Sartorio, D.; Gaibazzi, N. Contrast Stress Echocardiography Findings in Myocardial Bridging Compared to Normal Coronary Course, With and Without Coronary Artery Disease. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2023, 36, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- La, S.; Beltrame, J.; Tavella, R. Sex-specific and ethnicity-specific differences in MINOCA. Nat. Rev. Cardiol. 2024, 21, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Buller, P.; Kern, A.; Tyczyński, M.; Rosiak, W.; Figatowski, W.; Gil, R.J.; Bil, J. The Comparison of Predicting Factors and Outcomes of MINOCA and STEMI Patients in the 5-Year Follow-Up. J. Pers. Med. 2023, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Duchnowski, P.; Śmigielski, W. Usefulness of myocardial damage biomarkers in predicting cardiogenic shock in patients undergoing heart valve surgery. Kardiol. Pol. 2024, 82, 423–426. [Google Scholar] [CrossRef]
- Mileva, N.; Paolisso, P.; Gallinoro, E.; Fabbricatore, D.; Munhoz, D.; Bergamaschi, L.; Belmonte, M.; Panayotov, P.; Pizzi, C.; Barbato, E.; et al. Diagnostic and Prognostic Role of Cardiac Magnetic Resonance in MINOCA: Systematic Review and Meta-Analysis. JACC Cardiovasc. Imaging 2023, 16, 376–389. [Google Scholar] [CrossRef]
- Collste, O.; Sörensson, P.; Frick, M.; Agewall, S.; Daniel, M.; Henareh, L.; Ekenbäck, C.; Eurenius, L.; Guiron, C.; Jernberg, T.; et al. Myocardial infarction with normal coronary arteries is common and associated with normal findings on cardiovascular magnetic resonance imaging: Results from the Stockholm Myocardial Infarction with Normal Coronaries study. J. Intern. Med. 2013, 273, 189–196. [Google Scholar] [CrossRef]
- Dastidar, A.G.; Baritussio, A.; De Garate, E.; Drobni, Z.; Biglino, G.; Singhal, P.; Milano, E.G.; Angelini, G.D.; Dorman, S.; Strange, J.; et al. Prognostic Role of CMR and Conventional Risk Factors in Myocardial Infarction With Nonobstructed Coronary Arteries. JACC Cardiovasc. Imaging 2019, 12, 1973–1982. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Harden, S.; Abid, N.; Peebles, C.; Nicholas, Z.; Jones, T.; Mckenzie, D.; Curzen, N. Troponin-positive chest pain with unobstructed coronary arteries: Definitive differential diagnosis using cardiac MRI. Br. J. Radiol. 2012, 85, e461–e466. [Google Scholar] [CrossRef]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef]
- Cundari, G.; Galea, N.; De Rubeis, G.; Frustaci, A.; Cilia, F.; Mancuso, G.; Marchitelli, L.; Catapano, F.; Carbone, I.; Catalano, C.; et al. Use of the new Lake Louise Criteria improves CMR detection of atypical forms of acute myocarditis. Int. J. Cardiovasc. Imaging 2021, 37, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Lintingre, P.-F.; Nivet, H.; Clément-Guinaudeau, S.; Camaioni, C.; Sridi, S.; Corneloup, O.; Gerbaud, E.; Coste, P.; Dournes, G.; Latrabe, V.; et al. High-Resolution Late Gadolinium Enhancement Magnetic Resonance for the Diagnosis of Myocardial Infarction With Nonobstructed Coronary Arteries. JACC Cardiovasc. Imaging 2020, 13, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Dastidar, A.G.; Rodrigues, J.C.; Johnson, T.W.; De Garate, E.; Singhal, P.; Baritussio, A.; Scatteia, A.; Strange, J.; Nightingale, A.K.; Angelini, G.D.; et al. Myocardial Infarction With Nonobstructed Coronary Arteries: Impact of CMR Early After Presentation. JACC Cardiovasc. Imaging 2017, 10, 1204–1206. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, L.; Foà, A.; Paolisso, P.; Renzulli, M.; Angeli, F.; Fabrizio, M.; Bartoli, L.; Armillotta, M.; Sansonetti, A.; Amicone, S.; et al. Prognostic Role of Early Cardiac Magnetic Resonance in Myocardial Infarction With Nonobstructive Coronary Arteries. JACC Cardiovasc. Imaging 2024, 17, 149–161. [Google Scholar] [CrossRef]
- Yamamoto, M.H.; Maehara, A.; Song, L.; Matsumura, M.; Chin, C.Y.; Losquadro, M.; Sosa, F.A.; Mintz, G.S.; Shlofmitz, R.A. Optical Coherence Tomography Assessment of Morphological Characteristics in Suspected Coronary Artery Disease, but Angiographically Nonobstructive Lesions. Cardiovasc. Revascularization Med. Mol. Interv. 2019, 20, 475–479. [Google Scholar] [CrossRef]
- Taruya, A.; Tanaka, A.; Nishiguchi, T.; Ozaki, Y.; Kashiwagi, M.; Yamano, T.; Matsuo, Y.; Ino, Y.; Kitabata, H.; Takemoto, K.; et al. Lesion characteristics and prognosis of acute coronary syndrome without angiographically significant coronary artery stenosis. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 202–209. [Google Scholar] [CrossRef]
- Mas-Lladó, C.; Maristany, J.; Gomez-Lara, J.; Pascual, M.; Alameda, M.D.; Gomez-Jaume, A.; Peral-Disdier, V. Optical Coherence Tomography for the Diagnosis of Exercise-Related Acute Cardiovascular Events and Inconclusive Coronary Angiography. J. Intervent. Cardiol. 2020, 2020, 8263923. [Google Scholar] [CrossRef]
- Zeng, M.; Zhao, C.; Bao, X.; Liu, M.; He, L.; Xu, Y.; Meng, W.; Qin, Y.; Weng, Z.; Yi, B.; et al. Clinical Characteristics and Prognosis of MINOCA Caused by Atherosclerotic and Nonatherosclerotic Mechanisms Assessed by OCT. JACC Cardiovasc. Imaging 2023, 16, 521–532. [Google Scholar] [CrossRef]
- Barbieri, L.; D’Errico, A.; Avallone, C.; Gentile, D.; Provenzale, G.; Guagliumi, G.; Tumminello, G.; Carugo, S. Optical Coherence Tomography and Coronary Dissection: Precious Tool or Useless Surplus? Front. Cardiovasc. Med. 2022, 9, 822998. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar]
- Takagi, Y.; Yasuda, S.; Takahashi, J.; Tsunoda, R.; Ogata, Y.; Seki, A.; Sumiyoshi, T.; Matsui, M.; Goto, T.; Tanabe, Y.; et al. Clinical implications of provocation tests for coronary artery spasm: Safety, arrhythmic complications, and prognostic impact: Multicentre Registry Study of the Japanese Coronary Spasm Association. Eur. Heart J. 2013, 34, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Ong, P.; Athanasiadis, A.; Borgulya, G.; Vokshi, I.; Bastiaenen, R.; Kubik, S.; Hill, S.; Schäufele, T.; Mahrholdt, H.; Kaski, J.C.; et al. Clinical usefulness, angiographic characteristics, and safety evaluation of intracoronary acetylcholine provocation testing among 921 consecutive white patients with unobstructed coronary arteries. Circulation 2014, 129, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Curzen, N.; Rana, O.; Nicholas, Z.; Golledge, P.; Zaman, A.; Oldroyd, K.; Hanratty, C.; Banning, A.; Wheatcroft, S.; Hobson, A.; et al. Does routine pressure wire assessment influence management strategy at coronary angiography for diagnosis of chest pain? The RIPCORD study. Circ. Cardiovasc. Interv. 2014, 7, 248–255. [Google Scholar] [CrossRef]
- Usui, E.; Murai, T.; Kanaji, Y.; Hoshino, M.; Yamaguchi, M.; Hada, M.; Hamaya, R.; Kanno, Y.; Lee, T.; Yonetsu, T.; et al. Clinical significance of concordance or discordance between fractional flow reserve and coronary flow reserve for coronary physiological indices, microvascular resistance, and prognosis after elective percutaneous coronary intervention. EuroIntervention J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2018, 14, 798–805. [Google Scholar] [CrossRef]
- AlBadri, A.; Bairey Merz, C.N.; Johnson, B.D.; Wei, J.; Mehta, P.K.; Cook-Wiens, G.; Reis, S.E.; Kelsey, S.F.; Bittner, V.; Sopko, G.; et al. Impact of Abnormal Coronary Reactivity on Long-Term Clinical Outcomes in Women. J. Am. Coll. Cardiol. 2019, 73, 684–693. [Google Scholar] [CrossRef]
- Lindahl, B.; Baron, T.; Erlinge, D.; Hadziosmanovic, N.; Nordenskjöld, A.; Gard, A.; Jernberg, T. Medical Therapy for Secondary Prevention and Long-Term Outcome in Patients With Myocardial Infarction With Nonobstructive Coronary Artery Disease. Circulation 2017, 135, 1481–1489. [Google Scholar] [CrossRef]
- Kovach, C.; Hebbe, A.; O’Donnell, C.I.; Plomondon, M.E.; Hess, P.L.; Rahman, A.; Mulukutla, S.; Waldo, S.W.; Valle, J.A. Comparison of Patients With Nonobstructive Coronary Artery Disease With Versus Without Myocardial Infarction (from the VA Clinical Assessment Reporting and Tracking [CART] Program). Am. J. Cardiol. 2021, 146, 1–7. [Google Scholar] [CrossRef]
- Abdu, F.A.; Liu, L.; Mohammed, A.Q.; Xu, B.; Yin, G.; Xu, S.; Xu, Y.; Che, W. Effect of Secondary Prevention Medication on the Prognosis in Patients With Myocardial Infarction With Nonobstructive Coronary Artery Disease. J. Cardiovasc. Pharmacol. 2020, 76, 678. [Google Scholar] [CrossRef]
- Ciliberti, G.; Verdoia, M.; Merlo, M.; Zilio, F.; Vatrano, M.; Bianco, F.; Mancone, M.; Zaffalon, D.; Bonci, A.; Boscutti, A.; et al. Pharmacological therapy for the prevention of cardiovascular events in patients with myocardial infarction with non-obstructed coronary arteries (MINOCA): Insights from a multicentre national registry. Int. J. Cardiol. 2021, 327, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Paolisso, P.; Bergamaschi, L.; Saturi, G.; D'Angelo, E.C.; Magnani, I.; Toniolo, S.; Stefanizzi, A.; Rinaldi, A.; Bartoli, L.; Angeli, F.; et al. Secondary Prevention Medical Therapy and Outcomes in Patients With Myocardial Infarction With Non-Obstructive Coronary Artery Disease. Front. Pharmacol. 2020, 10, 1606. [Google Scholar] [CrossRef]
- Eggers, K.M.; Hadziosmanovic, N.; Baron, T.; Hambraeus, K.; Jernberg, T.; Nordenskjöld, A.; Tornvall, P.; Lindahl, B. Myocardial Infarction with Nonobstructive Coronary Arteries: The Importance of Achieving Secondary Prevention Targets. Am. J. Med. 2018, 131, 524–531.e6. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.M.; Jiang, L.; Chen, Y.; Xie, J.; Pan, H.; Peto, R.; Collins, R.; Liu, L. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: Randomised placebo-controlled trial. Lancet 2005, 366, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Bijsterveld, N.R.; Moons, A.H. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N. Engl. J. Med. 2001, 345, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Prati, F.; Uemura, S.; Souteyrand, G.; Virmani, R.; Motreff, P.; Di Vito, L.; Biondi-Zoccai, G.; Halperin, J.; Fuster, V.; Ozaki, Y.; et al. OCT-Based Diagnosis and Management of STEMI Associated With Intact Fibrous Cap. JACC Cardiovasc. Imaging 2013, 6, 283–287. [Google Scholar] [CrossRef]
- Xing, L.; Yamamoto, E.; Sugiyama, T.; Jia, H.; Ma, L.; Hu, S.; Wang, C.; Zhu, Y.; Li, L.; Xu, M.; et al. EROSION Study (Effective Anti-Thrombotic Therapy Without Stenting: Intravascular Optical Coherence Tomography–Based Management in Plaque Erosion). Circ. Cardiovasc. Interv. 2017, 10, e005860. [Google Scholar] [CrossRef]
- Beltrame, J.F.; Crea, F.; Kaski, J.C.; Ogawa, H.; Ong, P.; Sechtem, U.; Shimokawa, H.; Merz, C.N.; Coronary Vasomotion Disorders International Study Group. The Who, What, Why, When, How and Where of Vasospastic Angina. Circ. J. Off. J. Jpn. Circ. Soc. 2016, 80, 289–298. [Google Scholar] [CrossRef]
- Beltrame, J.F.; Crea, F.; Camici, P. Advances in coronary microvascular dysfunction. Heart Lung Circ. 2009, 18, 19–27. [Google Scholar] [CrossRef]
- Lerman, A.; Burnett, J.C.; Higano, S.T.; McKinley, L.J.; Holmes, D.R. Long-term L-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation 1998, 97, 2123–2128. [Google Scholar] [CrossRef]
- Kurtoglu, N.; Akcay, A.; Dindar, I. Usefulness of oral dipyridamole therapy for angiographic slow coronary artery flow. Am. J. Cardiol. 2001, 87, 777–779. [Google Scholar] [CrossRef]
- Saha, S.; Ete, T.; Kapoor, M.; Jha, P.K.; Megeji, R.D.; Kavi, G.; Warjri, S.B.; Mishra, A. Effect of Ranolazine in Patients with Chest Pain and Normal Coronaries—A Hospital Based Study. J. Clin. Diagn. Res. JCDR 2017, 11, OC14–OC16. [Google Scholar] [CrossRef]
- Suhrs, H.E.; Michelsen, M.M.; Prescott, E. Treatment strategies in coronary microvascular dysfunction: A systematic review of interventional studies. Microcirc. N.Y.N. 1994 2019, 26, e12430. [Google Scholar] [CrossRef] [PubMed]
- Tweet, M.S.; Hayes, S.N.; Codsi, E.; Gulati, R.; Rose, C.H.; Best, J.M. Spontaneous Coronary Artery Dissection Associated With Pregnancy. J. Am. Coll. Cardiol. 2017, 70, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Saw, J.; Aymong, E.; Sedlak, T.; Buller, C.E.; Starovoytov, A.; Ricci, D.; Robinson, S.; Vuurmans, T.; Gao, M.; Humphries, K.; et al. Spontaneous Coronary Artery Dissection. Circ. Cardiovasc. Interv. 2014, 7, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Noguchi, T.; Haruta, S.; Yamamoto, Y.; Oshima, S.; Nakao, K.; Taniguchi, Y.; Yamaguchi, J.; Tsuchihashi, K.; Seki, A.; et al. Prognostic impact of spontaneous coronary artery dissection in young female patients with acute myocardial infarction: A report from the Angina Pectoris–Myocardial Infarction Multicenter Investigators in Japan. Int. J. Cardiol. 2016, 207, 341–348. [Google Scholar] [CrossRef]
- Features, C. Management, and Prognosis of Spontaneous Coronary Artery Dissection. Circulation 2012, 126, 579–588. [Google Scholar]
- Adlam, D.; Alfonso, F.; Maas, A.; Vrints, C.; Committee, W. European Society of Cardiology, acute cardiovascular care association, SCAD study group: A position paper on spontaneous coronary artery dissection. Eur. Heart J. 2018, 39, 3353–3368. [Google Scholar] [CrossRef]
- Saw, J.; Mancini, G.B.J.; Humphries, K.H. Contemporary Review on Spontaneous Coronary Artery Dissection. J. Am. Coll. Cardiol. 2016, 68, 297–312. [Google Scholar] [CrossRef]
- Saha, M.; McDaniel, J.K.; Zheng, X.L. Thrombotic thrombocytopenic purpura: Pathogenesis, diagnosis and potential novel therapeutics. J. Thromb. Haemost. JTH 2017, 15, 1889–1900. [Google Scholar] [CrossRef]
- Corban, M.T.; Hung, O.Y.; Eshtehardi, P.; Rasoul-Arzrumly, E.; McDaniel, M.; Mekonnen, G.; Timmins, L.H.; Lutz, J.; Guyton, R.A.; Samady, H. Myocardial bridging: Contemporary understanding of pathophysiology with implications for diagnostic and therapeutic strategies. J. Am. Coll. Cardiol. 2014, 63, 2346–2355. [Google Scholar] [CrossRef]
- Ide, T.; Ohtani, K.; Higo, T.; Tanaka, M.; Kawasaki, Y.; Tsutsui, H. Ivabradine for the Treatment of Cardiovascular Diseases. Circ. J. Off. J. Jpn. Circ. Soc. 2019, 83, 252–260. [Google Scholar] [CrossRef]
- Klues, H.G.; Schwarz, E.R.; vom Dahl, J.; Reffelmann, T.; Reul, H.; Potthast, K.; Schmitz, C.; Minartz, J.; Krebs, W.; Hanrath, I. Disturbed intracoronary hemodynamics in myocardial bridging: Early normalization by intracoronary stent placement. Circulation 1997, 96, 2905–2913. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Shen, J.; Xia, L.; Ding, W.; Wang, C. Surgical treatment of symptomatic left anterior descending myocardial bridges: Myotomy vs. bypass surgery. Surg. Today 2020, 50, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Nordenskjöld, A.M.; Agewall, S.; Atar, D.; Baron, T.; Beltrame, J.; Bergström, O.; Erlinge, D.; Gale, C.P.; López-Pais, J.; Jernberg, T.; et al. Randomized evaluation of beta blocker and ACE-inhibitor/angiotensin receptor blocker treatment in patients with myocardial infarction with non-obstructive coronary arteries (MINOCA-BAT): Rationale and design. Am. Heart J. 2021, 231, 96–104. [Google Scholar] [CrossRef]
- Ang, S.; Chia, J.E.; Krittanawong, C.; Lee, K.; Iglesias, J.; Misra, K.; Mukherjee, D. Sex Differences and Clinical Outcomes in Patients With Myocardial Infarction With Nonobstructive Coronary Arteries: A Meta-Analysis. J. Am. Heart Assoc. 2024, 13, e035329. [Google Scholar] [CrossRef]
- Pacheco, C.; Luu, J.; Mehta, K.; Wei, J.; Gulati, M.; Merz, C.N.B. INOCA and MINOCA: Are Women’s Heart Centres the Answer to Understanding and Management of These Increasing Populations of Women (and Men)? Can. J. Cardiol. 2022, 38, 1611–1614. [Google Scholar] [CrossRef]
- Cano-Castellote, M.; Afanador-Restrepo, D.F.; González-Santamaría, J.; Rodríguez-López, C.; Castellote-Caballero, Y.; Hita-Contreras, F.; Carcelén-Fraile, M.D.; Aibar-Almazán, A. Pathophysiology, Diagnosis and Treatment of Spontaneous Coronary Artery Dissection in Peripartum Women. J. Clin. Med. 2022, 11, 6657. [Google Scholar] [CrossRef]
- Mauricio, R.; Srichai, M.B.; Axel, L.; Hochman, J.S.; Reynolds, H.R. Stress Cardiac MRI in Women With Myocardial Infarction and Nonobstructive Coronary Artery Disease. Clin. Cardiol. 2016, 39, 596–602. [Google Scholar] [CrossRef]
- Wei, J.; Bakir, M.; Darounian, N.; Li, Q.; Landes, S.; Mehta, P.K.; Shufelt, C.L.; Handberg, E.M.; Kelsey, S.F.; Sopko, G.; et al. Myocardial Scar Is Prevalent and Associated With Subclinical Myocardial Dysfunction in Women With Suspected Ischemia But No Obstructive Coronary Artery Disease: From the Women’s Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction Study. Circulation 2018, 137, 874–876. [Google Scholar] [CrossRef]
- Lewey, J.; El Hajj, S.C.; Hayes, S.N. Spontaneous Coronary Artery Dissection: New Insights into This Not-So-Rare Condition. Annu. Rev. Med. 2022, 73, 339–354. [Google Scholar] [CrossRef]
- Caralis, D.G.; Deligonul, U.; Kern, M.J.; Cohen, J.D. Smoking is a risk factor for coronary spasm in young women. Circulation 1992, 85, 905–909. [Google Scholar] [CrossRef]
- Handberg, E.M.; Merz, C.N.; Cooper-Dehoff, R.M.; Wei, J.; Conlon, M.; Lo, M.C.; Boden, W.; Frayne, S.M.; Villines, T.; Spertus, J.A.; et al. Rationale and design of the Women’s Ischemia Trial to Reduce Events in Nonobstructive CAD (WARRIOR) trial. Am. Heart J. 2021, 237, 90–103. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tognola, C.; Maloberti, A.; Varrenti, M.; Mazzone, P.; Giannattasio, C.; Guarracini, F. Myocardial Infarction with Nonobstructive Coronary Arteries (MINOCA): Current Insights into Pathophysiology, Diagnosis, and Management. Diagnostics 2025, 15, 942. https://doi.org/10.3390/diagnostics15070942
Tognola C, Maloberti A, Varrenti M, Mazzone P, Giannattasio C, Guarracini F. Myocardial Infarction with Nonobstructive Coronary Arteries (MINOCA): Current Insights into Pathophysiology, Diagnosis, and Management. Diagnostics. 2025; 15(7):942. https://doi.org/10.3390/diagnostics15070942
Chicago/Turabian StyleTognola, Chiara, Alessandro Maloberti, Marisa Varrenti, Patrizio Mazzone, Cristina Giannattasio, and Fabrizio Guarracini. 2025. "Myocardial Infarction with Nonobstructive Coronary Arteries (MINOCA): Current Insights into Pathophysiology, Diagnosis, and Management" Diagnostics 15, no. 7: 942. https://doi.org/10.3390/diagnostics15070942
APA StyleTognola, C., Maloberti, A., Varrenti, M., Mazzone, P., Giannattasio, C., & Guarracini, F. (2025). Myocardial Infarction with Nonobstructive Coronary Arteries (MINOCA): Current Insights into Pathophysiology, Diagnosis, and Management. Diagnostics, 15(7), 942. https://doi.org/10.3390/diagnostics15070942





