Optical Coherence Tomography Angiography, Elastography, and Attenuation Imaging for Evaluation of Liver Regeneration
Abstract
1. Introduction
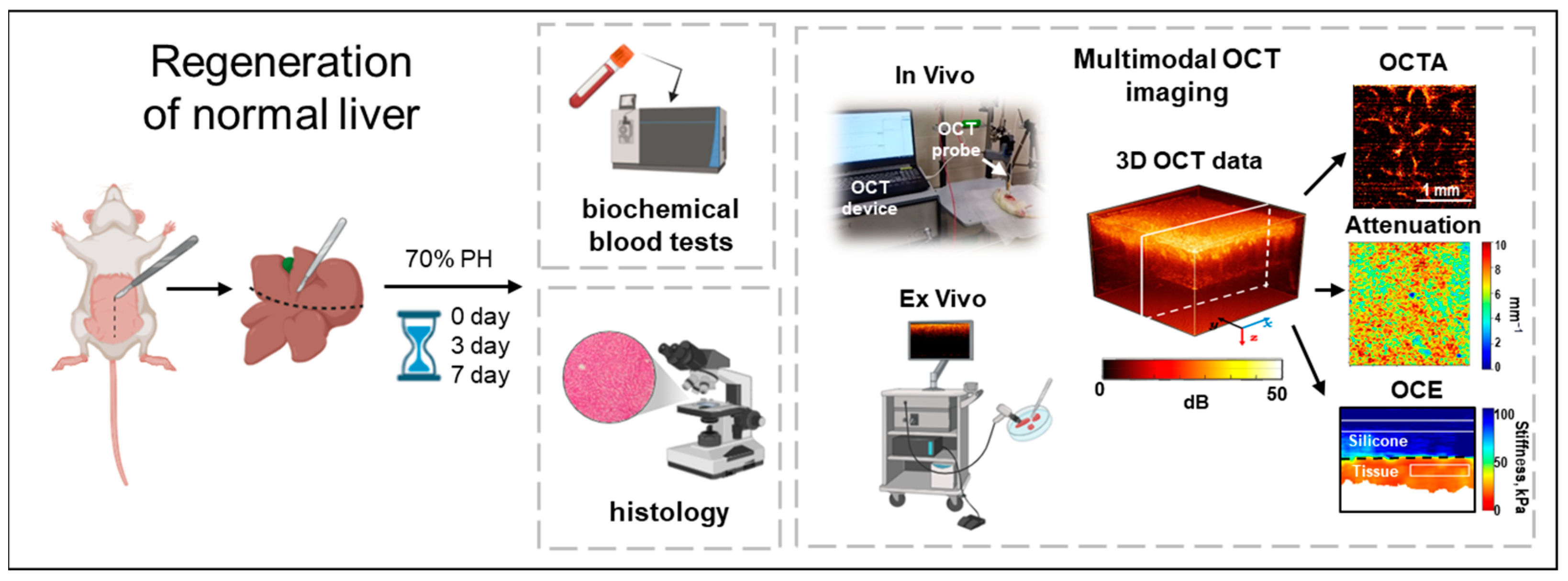
2. Materials and Methods
2.1. Animal Model
2.2. Evaluation of Liver Weight Recovery
2.3. Biochemical Blood Tests
2.4. Multimodal Optical Coherence Tomography (MM OCT) System and Data Acquisition
2.5. OCT Attenuation Coefficient Mapping
2.6. OCT-Based Angiography (OCTA) Imaging
2.7. OCT-Based Elastography (OCE) Imaging
2.8. Histological and Morphometric Analysis
2.9. Statistical Analysis
3. Results
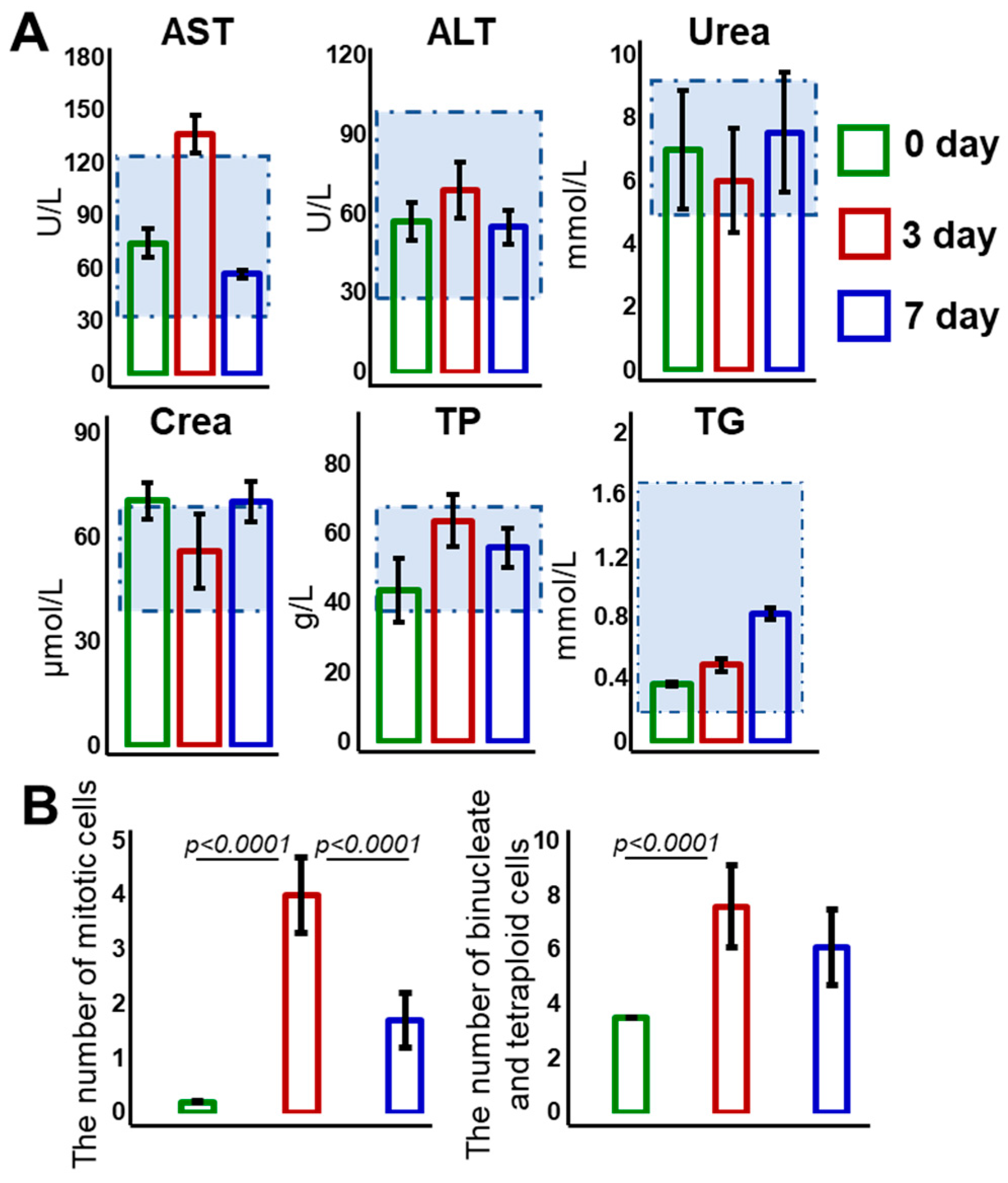
3.1. Weight Dynamics and Biochemical and Histological Evaluation of Liver Regeneration
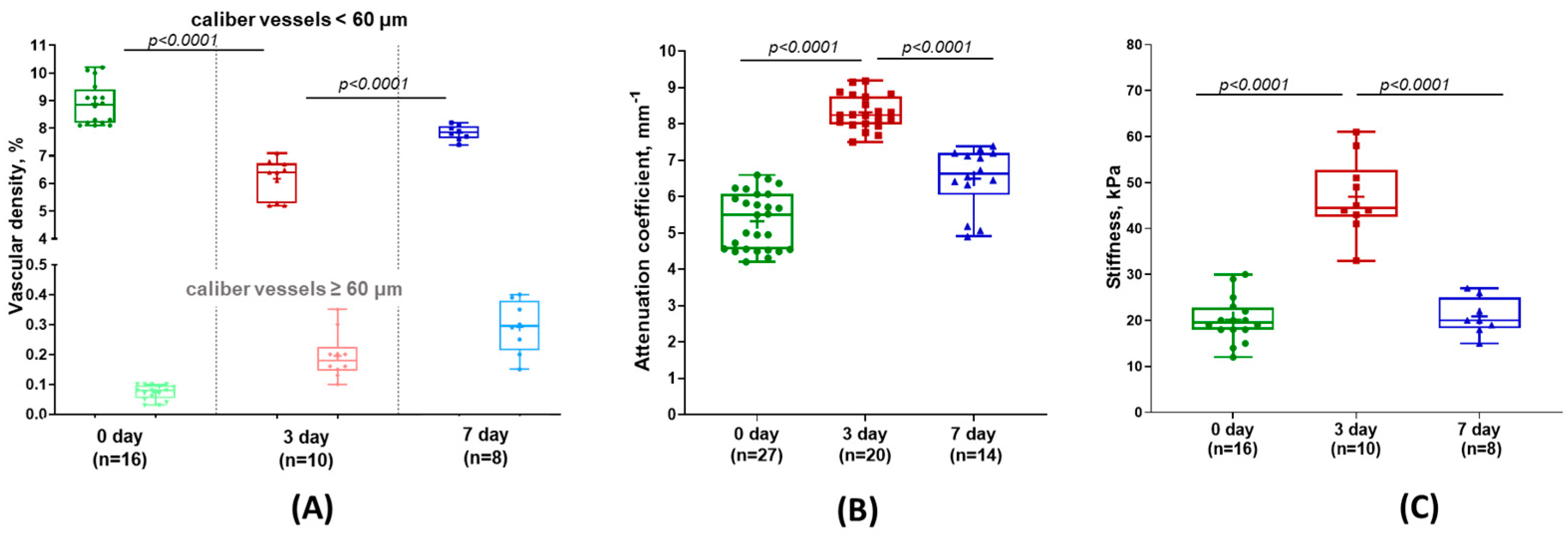
3.2. Evaluation of Liver Microcirculation During Its Regeneration
3.3. Attenuation Coefficient for Assessing Liver Tissue Structure
3.4. Evaluation of Changes in Tissue Stiffness During Liver Regeneration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MM OCT | Multimodal Optical Coherence Tomography |
| OCT | Optical Coherence Tomography |
| OCTA | Optical Coherence Tomography Angiography |
| OCE | Optical Coherence Tomography Elastography |
| PH | Partial Hepatectomy |
| ECM | Extracellular Matrix |
| AST | Aspartate Aminotransferase |
| ALT | Alanine Aminotransferase |
| TP | Total Protein |
| TGs | Triglycerides |
References
- Petrowsky, H.; Fritsch, R.; Guckenberger, M.; De Oliveira, M.L.; Dutkowski, P.; Clavien, P.A. Modern therapeutic approaches for the treatment of malignant liver tumours. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 755–772. [Google Scholar] [CrossRef] [PubMed]
- Bekheit, M.; Grundy, L.; Salih, A.K.; Bucur, P.; Vibert, E.; Ghazanfar, M. Post-hepatectomy liver failure: A timeline centered review. Hepatobiliary Pancreat. Dis. Int. 2023, 22, 554–569. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.K.; Barbas, A.S.; Marroquin, C.E.; Morse, M.A.; Kuo, P.C.; Clary, B.M. Resection of noncolorectal nonneuroendocrine liver metastases: A comparative analysis. J. Am. Coll. Surg. 2007, 204, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Sposito, C.; Zhou, J.; Pinna, A.D.; De Carlis, L.; Fan, J.; Cescon, M.; Sandro, S.D.; Yi-feng, H.; Lauterio, A.; et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology 2018, 154, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Angelico, R.; Siragusa, L.; Serenari, M.; Scalera, I.; Kauffman, E.; Lai, Q.; Vitale, A. Rescue liver transplantation after post-hepatectomy acute liver failure: A systematic review and pooled analysis. Transplant. Rev. 2023, 37, 100773. [Google Scholar] [CrossRef]
- Mahmud, N.; Panchal, S.; Turrentine, F.E.; Kaplan, D.E.; Zaydfudim, V.M. Performance of risk prediction models for post-operative mortality in patients undergoing liver resection. Am. J. Surg. 2023, 225, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulos, G.K.; Bhushan, B. Liver regeneration: Biological and pathological mechanisms and implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 40–55. [Google Scholar] [CrossRef]
- Rodimova, S.A.; Kuznetsova, D.S.; Bobrov, N.V.; Gulin, A.A.; Reunov, D.G.; Karabut, M.M.; Shcheslavskiy, V.I.; Vdovina, N.V.; Zagainov, V.E. Interrogation of the liver during regeneration by fluorescence lifetime imaging and mass spectrometry. IEEE J. Sel. Top. Quantum Electron. 2021, 27, 1–11. [Google Scholar] [CrossRef]
- Rodimova, S.; Bobrov, N.; Mozherov, A.; Elagin, V.; Karabut, M.; Shchechkin, I.; Kozlov, D.; Krylov, D.; Gavrina, A.; Zagainov, V. Optical biomedical imaging reveals criteria for violated liver regenerative potential. Cells 2023, 12, 479. [Google Scholar] [CrossRef]
- Rodimova, S.; Kozlova, V.; Bobrov, N.; Kozlov, D.; Mozherov, A.; Elagin, V.; Shchechkin, I.; Kuzmin, D.; Gavrina, A.; Zagainov, V. Novel Optical Criteria and Mechanisms of Critical Decline in Liver Regenerative Potential. Cells 2024, 13, 2015. [Google Scholar] [CrossRef]
- Chowdhury, A.B.; Mehta, K.J. Liver biopsy for assessment of chronic liver diseases: A synopsis. Clin. Exp. Med. 2023, 23, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Moiseev, A.; Sherstnev, E.; Kiseleva, E.; Achkasova, K.; Potapov, A.; Yashin, K.; Sirotkina, M.; Gelikonov, G.; Matkivsky, V.; Shilyagin, P. Depth-resolved method for attenuation coefficient calculation from optical coherence tomography data for improved biological structure visualization. J. Biophotonics 2023, 16, e202100392. [Google Scholar] [CrossRef]
- Vermeer, K.A.; Mo, J.; Weda, J.J.A.; Lemij, H.G.; Boer, J.F.D. Depth-resolved model-based reconstruction of attenuation coef-fcients in optical coherence tomography. Biomed. Opt. Express 2014, 5, 322–337. [Google Scholar] [CrossRef]
- Foo, K.Y.; Chin, L.; Zilkens, R.; Lakhiani, D.D.; Fang, Q.; Sanderson, R.; Dessauvagie, B.F.; Latham, B.; McLaren, S.; Saunders, C.M.; et al. Three-dimensional Mapping of the Attenuation Coefficient in Optical Coherence Tomography to Enhance Breast Tissue Microarchitecture Contrast. J. Biophotonics 2020, 13, e201960201. [Google Scholar] [CrossRef]
- Boer, J.F.D.; Hitzenberger, C.K.; Yasuno, Y. Polarization sensitive optical coherence tomography: A review. Biomed. Opt. Express 2017, 8, 1838–1873. [Google Scholar] [CrossRef] [PubMed]
- South, F.A.; Chaney, E.J.; Marjanovic, M.; Adie, S.G.; Boppart, S.A. Differentiation of Ex Vivo Human Breast Tissue Using Polarization-Sensitive Optical Coherence Tomography. Biomed. Opt. Express 2014, 5, 3417. [Google Scholar] [CrossRef]
- Matveev, L.A.; Zaitsev, V.Y.; Gelikonov, G.V.; Matveyev, A.L.; Moiseev, A.A.; Ksenofontov, S.Y.; Gelikonov, V.M.; Sirotkina, M.A.; Gladkova, N.D.; Demidov, V.; et al. Hybrid M-mode-like OCT imaging of three-dimensional microvasculature in vivo using reference-free processing of complex valued B-scans. Opt. Lett. 2015, 40, 1472–1475. [Google Scholar] [CrossRef] [PubMed]
- Larin, K.V.; Sampson, D.D. Optical coherence elastography—OCT at work in tissue biomechanics. Biomed. Opt. Express 2017, 8, 1172–1202. [Google Scholar] [CrossRef]
- Zaitsev, V.Y.; Matveyev, A.L.; Matveev, L.A.; Sovetsky, A.A.; Hepburn, M.S.; Mowla, A.; Kennedy, B.F. Strain and elasticity imaging in compression optical coherence elastography: The two-decade perspective and recent advances. J. Biophotonics 2021, 14, e202000257. [Google Scholar] [CrossRef]
- Mukherjee, P.; Fukuda, S.; Lukmanto, D.; Yamashita, T.; Okada, K.; Makita, S.; El-Sadek, I.A.; Miyazawa, A.; Zhu, L.; Morishita, R. Label-free metabolic imaging of non-alcoholic-fatty-liver-disease (NAFLD) liver by volumetric dynamic optical coherence tomography. Biomed. Opt. Express 2022, 13, 4071–4086. [Google Scholar] [CrossRef]
- Higgins, G.M. Experimental pathology of the liver. Restoration of the liver of the white rat following partial surgical removal. Arch. Pathol. Lab. Med. 1931, 12, 1186–1202. [Google Scholar]
- Amygdalos, I.; Hachgenei, E.; Burkl, L.; Vargas, D.; Goßmann, P.; Wolff, L.I.; Druzenko, M.; Frye, M.; König, N.; Schmitt, R.H. Optical coherence tomography and convolutional neural networks can differentiate colorectal liver metastases from liver parenchyma ex vivo. J. Cancer Res. Clin. Oncol. 2023, 149, 3575–3586. [Google Scholar] [CrossRef] [PubMed]
- Gelikonov, V.M.; Gelikonov, G.V.; Terpelov, D.A.; Shilyagin, P.A. Electronic interface systems for goals of spectral domain optical coherence tomography. Instrum. Exp. Tech. 2012, 55, 392–398. [Google Scholar] [CrossRef]
- Gong, P.; Almasian, M.; van Soest, G.; de Bruin, D.; van Leeuwen, T.; Sampson, D.; Faber, D. Parametric imaging of attenuation by optical coherence tomography: Review of models, methods, and clinical translation. J. Biomed. Opt. 2020, 25, 040901. [Google Scholar] [CrossRef] [PubMed]
- Moiseev, A.; Ksenofontov, S.; Sirotkina, M.; Kiseleva, E.; Gorozhantseva, M.; Shakhova, N.; Matveev, L.; Zaitsev, V.; Matveyev, A.; Zagaynova, E.; et al. Optical Coherence Tomogra-phy-Based Angiography Device with Real-Time Angiography B-Scans Visualization and Hand-Held Probe for Everyday Clinical Use. J. Biophotonics 2018, 11, e201700292. [Google Scholar] [CrossRef]
- Reif, R.; Qin, J.; An, L.; Zhi, Z.; Dziennis, S.; Wang, R. Quantifying optical microangiography images obtained from a spectral domain optical coherence tomography system. Int. J. Biomed. Imaging 2012, 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Kan, Z.; Madoff, D.C. Liver anatomy: Microcirculation of the liver. Semin. Interv. Radiol. 2008, 25, 77–85. [Google Scholar] [CrossRef]
- Maslennikova, A.V.; Sirotkina, M.A.; Moiseev, A.A.; Finagina, E.S.; Ksenofontov, S.Y.; Gelikonov, G.V.; Matveev, L.A.; Kiseleva, E.B.; Zaitsev, V.Y.; Zagaynova, E.V. In-vivo longitudinal imaging of microvascular changes in irradiated oral mucosa of radiotherapy cancer patients using optical coherence tomography. Sci. Rep. 2017, 7, 16505. [Google Scholar] [CrossRef]
- Kennedy, B.F.; Koh, S.H.; McLaughlin, R.A.; Kennedy, K.M.; Munro, P.R.T.; Sampson, D.D. Strain estimation in phase-sensitive optical coherence elastography. Biomed. Opt. Express 2012, 3, 1865–1879. [Google Scholar] [CrossRef]
- Matveyev, A.L.; Matveev, L.A.; Sovetsky, A.A.; Gelikonov, G.V.; Moiseev, A.A.; Zaitsev, V.Y. Vector Method for Strain Estimation in Phase-Sensitive Optical Coherence Elastography. Laser Phys. Lett. 2018, 15, 065603. [Google Scholar] [CrossRef]
- Zykov, A.A.; Matveyev, A.L.; Sovetsky, A.A.; Matveev, L.A.; Zaitsev, V.Y. Vector method of strain estimation in OCT-elastography with adaptive choice of scale for estimating interframe phase-variation gradients. Laser Phys. Lett. 2023, 20, 95601. [Google Scholar] [CrossRef]
- Zykov, A.A.; Matveyev, A.L.; Sovetsky, A.A.; Zaitsev, V.Y. Optimization of Preliminary Vector Averaging for Improving Strain-Estimation Accuracy in Phase-Sensitive Optical Coherence Elastography. J. Biomed. Photonics Eng. 2023, 9, 040311. [Google Scholar] [CrossRef]
- Sovetsky, A.A.; Matveyev, A.L.; Matveev, L.A.; Gubarkova, E.V.; Plekhanov, A.A.; Sirotkina, M.A.; Gladkova, N.D.; Zaitsev, V.Y. Full-Optical Method of Local Stress Standardization to Exclude Nonlinearity-Related Ambiguity of Elasticity Estimation in Compressional Optical Coherence Elastography. Laser Phys. Lett. 2020, 17, 065601. [Google Scholar] [CrossRef]
- Cardiff, R.D.; Miller, C.H.; Munn, R.J. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb. Protoc. 2014, 2014, 411. [Google Scholar] [CrossRef]
- Popescu, R.; Filimon, M.N.; Dumitrescu, G.; Ciochina, L.P.; Dumitrascu, V.; Vlad, D.; Verdes, D. Histological and morphometrical studies in liver regeneration in mice. Sci. Pap. Anim. Sci. Biotechnol. 2012, 45, 203–207. [Google Scholar]
- Mukherjee, P.; Miyazawa, A.; Fukuda, S.; Yamashita, T.; Lukmanto, D.; Okada, K.; El-Sadek, I.A.; Zhu, L.; Makita, S.; Oshika, T.; et al. Label-free functional and structural imaging of liver microvascular complex in mice by Jones matrix optical coherence tomography. Sci. Rep. 2021, 11, 20054. [Google Scholar] [CrossRef]
- Morsiani, E.; Aleotti, A. Haemodynamic and ultrastructural observations on the rat liver after two-thirds partial hepatectomy. J. Anat. 1998, 192, 507–515. [Google Scholar] [CrossRef]
- Campana, L.; Esser, H.; Huch, M.; Forbes, S. Liver regeneration and inflammation: From fundamental science to clinical applications. Nat. Rev. Mol. Cell Biol. 2021, 22, 608–624. [Google Scholar] [CrossRef]
- Vollmar, B.; Menger, M.D. The hepatic microcirculation: Mechanistic contributions and therapeutic targets in liver injury and repair. Physiol. Rev. 2009, 89, 1269–1339. [Google Scholar] [CrossRef]
- Drexler, W.; Liu, M.; Kumar, A.; Kamali, T.; Unterhuber, A.; Leitgeb, R.A. Optical Coherence Tomography Today: Speed, Contrast, and Multimodality. J. Biomed. Opt. 2014, 19, 071412. [Google Scholar] [CrossRef]
- Leitgeb, R.; Placzek, F.; Rank, E.; Krainz, L.; Haindl, R.; Li, Q.; Liu, M.; Andreana, M.; Unterhuber, A.; Schmoll, T.; et al. Enhanced Medical Diagnosis for DOCTors: A Perspective of Optical Coherence Tomography. J. Biomed. Opt. 2021, 26, 100601. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wei, H.; Ye, X.; Hu, K.; Wu, G.; Yang, H.; He, Y.; Xie, S.; Guo, Z. Influence of nanoparticles accumulation on optical properties of human normal and cancerous liver tissue in vitro estimated by OCT. Phys. Med. Biol. 2015, 60, 1385–1397. [Google Scholar] [CrossRef] [PubMed]
- Parsa, P.; Jacques, S.L.; Nishioka, N.S. Optical properties of rat liver between 350 and 2200 nm. Appl. Opt. 1989, 28, 2325–2330. [Google Scholar] [CrossRef]
- Gilgenkrantz, H.; de l’Hortet, A.C. Understanding liver regeneration: From mechanisms to regenerative medicine. Am. J. Pathol. 2018, 188, 1316–1327. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, H.; Li, Y.; Gong, L.; Zheng, G.; Wang, X.; Luan, W.; Li, S.; Ma, F.; Ni, L. NAFLD induction delays postoperative liver regeneration of ALPPS in rats. Dig. Dis. Sci. 2019, 64, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Eipel, C.; Abshagen, K.; Vollmar, B. Regulation of hepatic blood flow: The hepatic arterial buffer response revisited. World J. Gastroenterol. 2010, 16, 6046. [Google Scholar] [CrossRef]
- Vowinkel, T.; Granger, D.N. Gastrointestinal Blood Flow. In Yamada’ s Textbook of Gastroenterology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 161–182. [Google Scholar]
- Lorenz, L.; Axnick, J.; Buschmann, T.; Henning, C.; Urner, S.; Fang, S.; Nurmi, H.; Eichhorst, N.; Holtmeier, R.; Bódis, K. Mechanosensing by β1 integrin induces angiocrine signals for liver growth and survival. Nature 2018, 562, 128–132. [Google Scholar] [CrossRef]
- Dold, S.; Richter, S.; Kollmar, O.; von Heesen, M.; Scheuer, C.; Laschke, M.W.; Vollmar, B.; Schilling, M.K.; Menger, M.D. Portal hyperperfusion after extended hepatectomy does not induce a hepatic arterial buffer response (HABR) but impairs mitochondrial redox state and hepatocellular oxygenation. PLoS ONE 2015, 10, e0141877. [Google Scholar] [CrossRef]
- Große-Segerath, L.; Lammert, E. Role of vasodilation in liver regeneration and health. Biol. Chem. 2021, 402, 1009–1019. [Google Scholar] [CrossRef]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Gupta, K.; Ng, I.C.; Xing, J.; Yang, Y.A.; Yu, H. Mechanosensing in liver regeneration. Semin. Cell Dev. Biol. 2017, 71, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Bomo, J.; Ezan, F.; Tiaho, F.; Bellamri, M.; Langouet, S.; Theret, N.; Baffet, G. Increasing 3D Matrix Rigidity Strengthens Proliferation and Spheroid Development of Human Liver Cells in a Constant Growth Factor Environment. J. Cell. Biochem. 2016, 117, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.K.; Wilhelm, J.; Fassett, J.T. Regulation of hepatocyte cell cycle progression and differentiation by type I collagen structure. Curr. Top. Dev. Biol. 2006, 72, 205–236. [Google Scholar] [CrossRef]
- Li, W.; Wu, Y.; Hu, W.; Zhou, J.; Shu, X.; Zhang, X.; Zhang, Z.; Wu, H.; Du, Y.; Lü, D.; et al. Direct mechanical exposure initiates hepatocyte proliferation. JHEP Rep. 2023, 5, 100905. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodimova, S.; Gubarkova, E.; Bobrov, N.; Shchechkin, I.; Kozlova, V.; Zolotova, N.; Potapov, A.; Kiseleva, E.; Gelikonov, G.; Gladkova, N.; et al. Optical Coherence Tomography Angiography, Elastography, and Attenuation Imaging for Evaluation of Liver Regeneration. Diagnostics 2025, 15, 977. https://doi.org/10.3390/diagnostics15080977
Rodimova S, Gubarkova E, Bobrov N, Shchechkin I, Kozlova V, Zolotova N, Potapov A, Kiseleva E, Gelikonov G, Gladkova N, et al. Optical Coherence Tomography Angiography, Elastography, and Attenuation Imaging for Evaluation of Liver Regeneration. Diagnostics. 2025; 15(8):977. https://doi.org/10.3390/diagnostics15080977
Chicago/Turabian StyleRodimova, Svetlana, Ekaterina Gubarkova, Nikolai Bobrov, Ilya Shchechkin, Vera Kozlova, Natalia Zolotova, Arseniy Potapov, Elena Kiseleva, Grigory Gelikonov, Natalia Gladkova, and et al. 2025. "Optical Coherence Tomography Angiography, Elastography, and Attenuation Imaging for Evaluation of Liver Regeneration" Diagnostics 15, no. 8: 977. https://doi.org/10.3390/diagnostics15080977
APA StyleRodimova, S., Gubarkova, E., Bobrov, N., Shchechkin, I., Kozlova, V., Zolotova, N., Potapov, A., Kiseleva, E., Gelikonov, G., Gladkova, N., Zagainov, V., Zagaynova, E., & Kuznetsova, D. (2025). Optical Coherence Tomography Angiography, Elastography, and Attenuation Imaging for Evaluation of Liver Regeneration. Diagnostics, 15(8), 977. https://doi.org/10.3390/diagnostics15080977








