Molecular Genetic Analysis of Bone Marrow Core Biopsy as an Alternative or Adjunct to Bone Marrow Aspirate and/or Peripheral Blood in Hematologic Myeloid Neoplasms
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Collection and Specimens
2.2. DNA and RNA Isolation, Next-Generation Sequencing (NGS)
2.3. Statistical Analysis
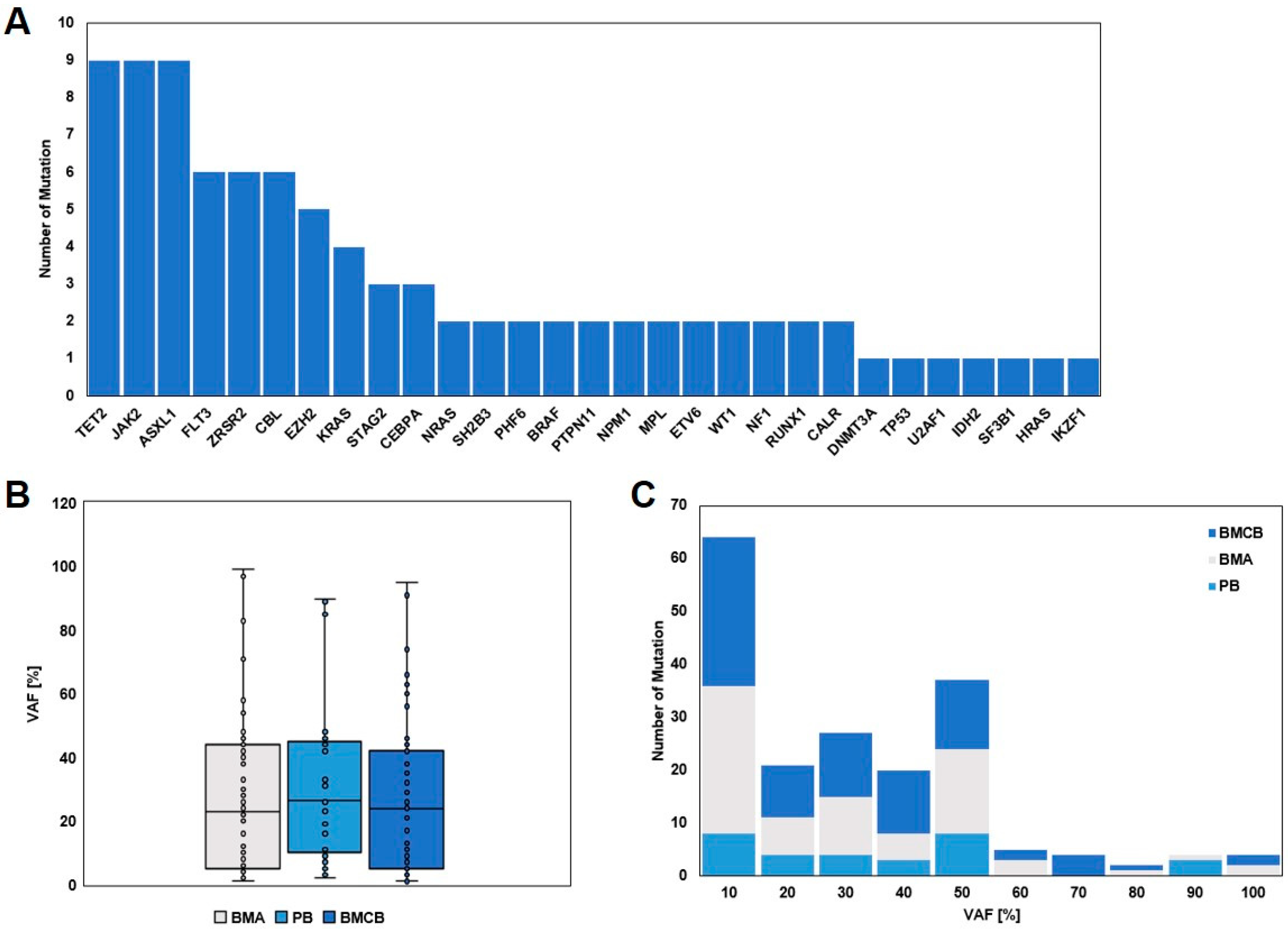
3. Results
3.1. Description of the Collection and Specimens
3.2. DNA and RNA Quality Assessment and AmpliSeq Performance
3.3. Molecular Genetic Analysis and Detection of Mutated Genes
3.4. Comparison of the Three Specimens, BMA, BMCB, and PB, and per Case
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International consensus classification of myeloid neoplasms and acute leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the world health organization classification of haematolymphoid tumours: Myeloid and histiocytic/dendritic neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the world health organization classification of haematolymphoid tumours: Lymphoid neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Erber, W.N.; Porwit, A.; Tomonaga, M.; Peterson, L.C. Icsh guidelines for the standardization of bone marrow specimens and reports. Int. J. Lab. Hematol. 2008, 30, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Rack, K.A.; van den Berg, E.; Haferlach, C.; Beverloo, H.B.; Costa, D.; Espinet, B.; Foot, N.; Jeffries, S.; Martin, K.; O’Connor, S.; et al. European recommendations and quality assurance for cytogenomic analysis of haematological neoplasms. Leukemia 2019, 33, 1851–1867. [Google Scholar] [CrossRef]
- Cherry, A.M.; Slovak, M.L.; Campbell, L.J.; Chun, K.; Eclache, V.; Haase, D.; Haferlach, C.; Hildebrandt, B.; Iqbal, A.M.; Jhanwar, S.C.; et al. Will a peripheral blood (pb) sample yield the same diagnostic and prognostic cytogenetic data as the concomitant bone marrow (bm) in myelodysplasia? Leuk. Res. 2012, 36, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Jansko-Gadermeir, B.; Leisch, M.; Gassner, F.J.; Zaborsky, N.; Dillinger, T.; Hutter, S.; Risch, A.; Melchardt, T.; Egle, A.; Drost, M.; et al. Myeloid ngs analyses of paired samples from bone marrow and peripheral blood yield concordant results: A prospective cohort analysis of the agmt study group. Cancers 2023, 15, 2305. [Google Scholar] [CrossRef]
- Jumniensuk, C.; Nobori, A.; Lee, T.; Senaratne, T.N.; Rao, D.; Pullarkat, S. Concordance of peripheral blood and bone marrow next-generation sequencing in hematologic neoplasms. Adv. Hematol. 2022, 2022, 8091746. [Google Scholar] [CrossRef]
- Bartels, S.; Schipper, E.; Hasemeier, B.; Kreipe, H.; Lehmann, U. Routine clinical mutation profiling using next generation sequencing and a customized gene panel improves diagnostic precision in myeloid neoplasms. Oncotarget 2016, 7, 30084–30093. [Google Scholar] [CrossRef]
- Dintner, S.; Schmutz, M.; Sommer, S.; Langer, A.; Hirschbühl, K.; Claus, R.; Schmid, C.; Trepel, M.; Märkl, B. NGS-based molecular genetics of leukemia—A powerful and decentralized approach. Die Pathol. 2023, 44, 155–159. [Google Scholar] [CrossRef]
- Haferlach, T.; Nagata, Y.; Grossmann, V.; Okuno, Y.; Bacher, U.; Nagae, G.; Schnittger, S.; Sanada, M.; Kon, A.; Alpermann, T.; et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia 2014, 28, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.J.; Shen, D.; Ding, L.; Shao, J.; Koboldt, D.C.; Chen, K.; Larson, D.E.; McLellan, M.D.; Dooling, D.; Abbott, R.; et al. Clonal architecture of secondary acute myeloid leukemia. N. Engl. J. Med. 2012, 366, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic classification and prognosis in acute myeloid leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Papaemmanuil, E.; Gerstung, M.; Malcovati, L.; Tauro, S.; Gundem, G.; Van Loo, P.; Yoon, C.J.; Ellis, P.; Wedge, D.C.; Pellagatti, A.; et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood 2013, 122, 3616–3627, quiz 3699. [Google Scholar] [CrossRef]
- Sadigh, S.; Kim, A.S. Molecular pathology of myeloid neoplasms: Molecular pattern recognition. Surg. Pathol. Clin. 2021, 14, 517–528. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanaka, T. Clonal evolution and hierarchy in myeloid malignancies. Trends Cancer 2023, 9, 707–715. [Google Scholar] [CrossRef]
- Palomo, L.; Meggendorfer, M.; Hutter, S.; Twardziok, S.; Ademà, V.; Fuhrmann, I.; Fuster-Tormo, F.; Xicoy, B.; Zamora, L.; Acha, P.; et al. Molecular landscape and clonal architecture of adult myelodysplastic/myeloproliferative neoplasms. Blood 2020, 136, 1851–1862. [Google Scholar] [CrossRef]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of aml in adults: 2022 recommendations from an international expert panel on behalf of the eln. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Orazi, A. Histopathology in the diagnosis and classification of acute myeloid leukemia, myelodysplastic syndromes, and myelodysplastic/myeloproliferative diseases. Pathobiology 2007, 74, 97–114. [Google Scholar] [CrossRef]
- Desalphine, M.; Bagga, P.K.; Gupta, P.K.; Kataria, A.S. To evaluate the role of bone marrow aspiration and bone marrow biopsy in pancytopenia. J. Clin. Diagn. Res. 2014, 8, Fc11-15. [Google Scholar] [CrossRef]
- Tefferi, A.; Guglielmelli, P.; Lasho, T.L.; Gangat, N.; Ketterling, R.P.; Pardanani, A.; Vannucchi, A.M. Mipss70+ version 2.0: Mutation and karyotype-enhanced international prognostic scoring system for primary myelofibrosis. J. Clin. Oncol. 2018, 36, 1769–1770. [Google Scholar] [CrossRef] [PubMed]
- Behrmann, L.; Wellbrock, J.; Fiedler, W. Acute myeloid leukemia and the bone marrow niche-take a closer look. Front. Oncol. 2018, 8, 444. [Google Scholar] [CrossRef] [PubMed]
- Comazzetto, S.; Shen, B.; Morrison, S.J. Niches that regulate stem cells and hematopoiesis in adult bone marrow. Dev. Cell 2021, 56, 1848–1860. [Google Scholar] [CrossRef] [PubMed]
- Medyouf, H. The microenvironment in human myeloid malignancies: Emerging concepts and therapeutic implications. Blood 2017, 129, 1617–1626. [Google Scholar] [CrossRef]
- Pimenta, D.B.; Varela, V.A.; Datoguia, T.S.; Caraciolo, V.B.; Lopes, G.H.; Pereira, W.O. The bone marrow microenvironment mechanisms in acute myeloid leukemia. Front. Cell Dev. Biol. 2021, 9, 764698. [Google Scholar] [CrossRef]


 = concordance,
= concordance,  = gain of information,
= gain of information,  = loss of information. Abbreviations: (s)AML: (secondary) acute myeloid leukemia, PMF: primary myelofibrosis, MDS: myelodysplastic syndrome, PV: polycythemia vera, CCUS: clonal cytopenia of undetermined significance, CMML: chronic myelomonocytic leukemia, ET: essential thrombocytosis, MDS/MPN: myelodysplastic syndrome/myeloproliferative neoplasia—not otherwise specified, BMA: bone marrow aspiration, BMCB: bone marrow core biopsy, PB: peripheral blood, bloody tap: no bone marrow fragments in the aspirate, only blood.
= loss of information. Abbreviations: (s)AML: (secondary) acute myeloid leukemia, PMF: primary myelofibrosis, MDS: myelodysplastic syndrome, PV: polycythemia vera, CCUS: clonal cytopenia of undetermined significance, CMML: chronic myelomonocytic leukemia, ET: essential thrombocytosis, MDS/MPN: myelodysplastic syndrome/myeloproliferative neoplasia—not otherwise specified, BMA: bone marrow aspiration, BMCB: bone marrow core biopsy, PB: peripheral blood, bloody tap: no bone marrow fragments in the aspirate, only blood.
 = concordance,
= concordance,  = gain of information,
= gain of information,  = loss of information. Abbreviations: (s)AML: (secondary) acute myeloid leukemia, PMF: primary myelofibrosis, MDS: myelodysplastic syndrome, PV: polycythemia vera, CCUS: clonal cytopenia of undetermined significance, CMML: chronic myelomonocytic leukemia, ET: essential thrombocytosis, MDS/MPN: myelodysplastic syndrome/myeloproliferative neoplasia—not otherwise specified, BMA: bone marrow aspiration, BMCB: bone marrow core biopsy, PB: peripheral blood, bloody tap: no bone marrow fragments in the aspirate, only blood.
= loss of information. Abbreviations: (s)AML: (secondary) acute myeloid leukemia, PMF: primary myelofibrosis, MDS: myelodysplastic syndrome, PV: polycythemia vera, CCUS: clonal cytopenia of undetermined significance, CMML: chronic myelomonocytic leukemia, ET: essential thrombocytosis, MDS/MPN: myelodysplastic syndrome/myeloproliferative neoplasia—not otherwise specified, BMA: bone marrow aspiration, BMCB: bone marrow core biopsy, PB: peripheral blood, bloody tap: no bone marrow fragments in the aspirate, only blood.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirschbühl, K.; Märkl, B.; Müller, G.; Schaller, T.; Claus, R.; Sommer, S.; Schmutz, M.; Trepel, M.; Schmid, C.; Dintner, S. Molecular Genetic Analysis of Bone Marrow Core Biopsy as an Alternative or Adjunct to Bone Marrow Aspirate and/or Peripheral Blood in Hematologic Myeloid Neoplasms. Diagnostics 2025, 15, 991. https://doi.org/10.3390/diagnostics15080991
Hirschbühl K, Märkl B, Müller G, Schaller T, Claus R, Sommer S, Schmutz M, Trepel M, Schmid C, Dintner S. Molecular Genetic Analysis of Bone Marrow Core Biopsy as an Alternative or Adjunct to Bone Marrow Aspirate and/or Peripheral Blood in Hematologic Myeloid Neoplasms. Diagnostics. 2025; 15(8):991. https://doi.org/10.3390/diagnostics15080991
Chicago/Turabian StyleHirschbühl, Klaus, Bruno Märkl, Gernot Müller, Tina Schaller, Rainer Claus, Sebastian Sommer, Maximilian Schmutz, Martin Trepel, Christoph Schmid, and Sebastian Dintner. 2025. "Molecular Genetic Analysis of Bone Marrow Core Biopsy as an Alternative or Adjunct to Bone Marrow Aspirate and/or Peripheral Blood in Hematologic Myeloid Neoplasms" Diagnostics 15, no. 8: 991. https://doi.org/10.3390/diagnostics15080991
APA StyleHirschbühl, K., Märkl, B., Müller, G., Schaller, T., Claus, R., Sommer, S., Schmutz, M., Trepel, M., Schmid, C., & Dintner, S. (2025). Molecular Genetic Analysis of Bone Marrow Core Biopsy as an Alternative or Adjunct to Bone Marrow Aspirate and/or Peripheral Blood in Hematologic Myeloid Neoplasms. Diagnostics, 15(8), 991. https://doi.org/10.3390/diagnostics15080991





