Investigating Patients with Pulmonary Hypertension Under Detector-Based Spectral Computed Tomography
Abstract
1. Introduction
2. Materials and Methods
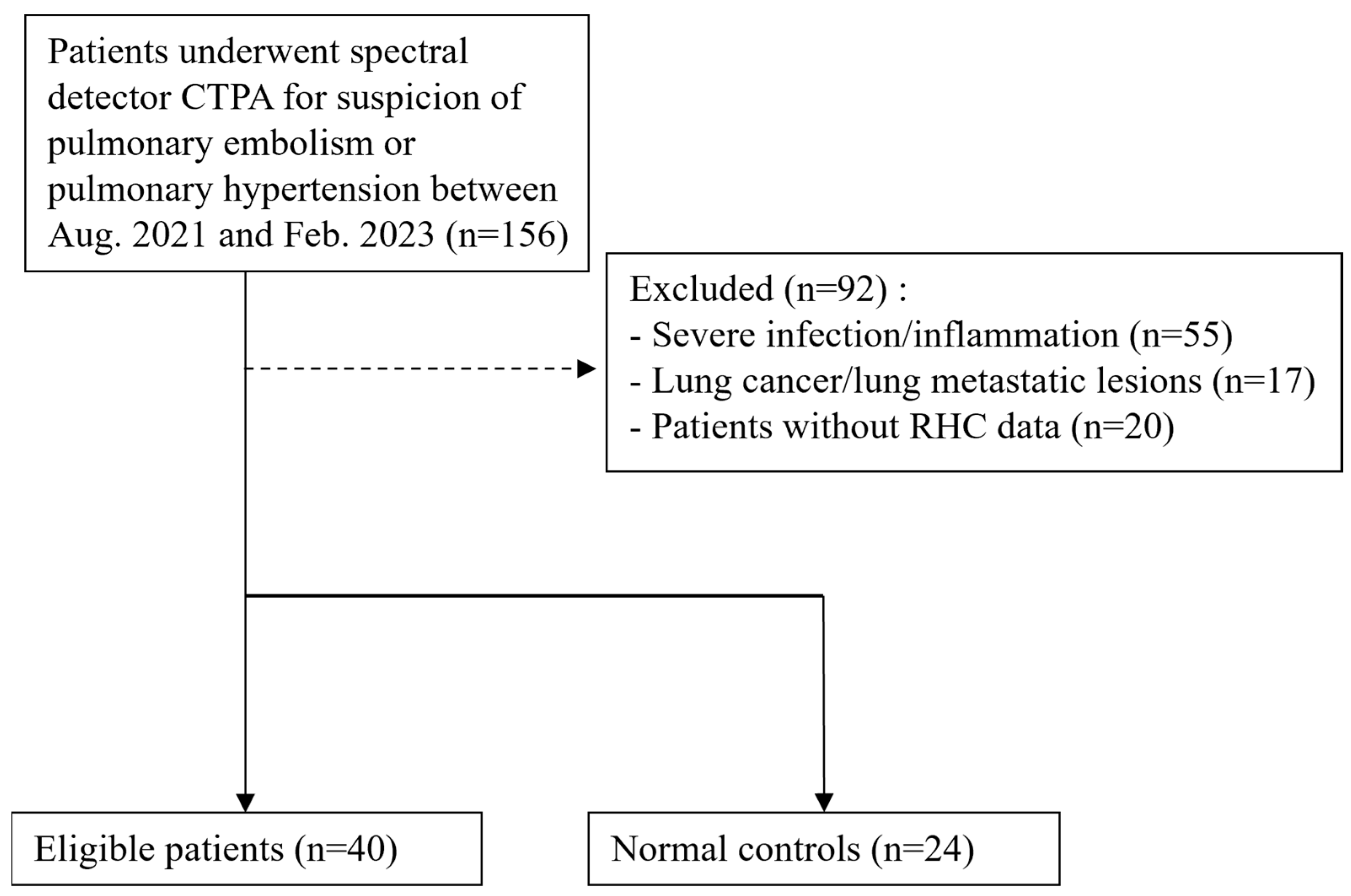
2.1. Patient Population
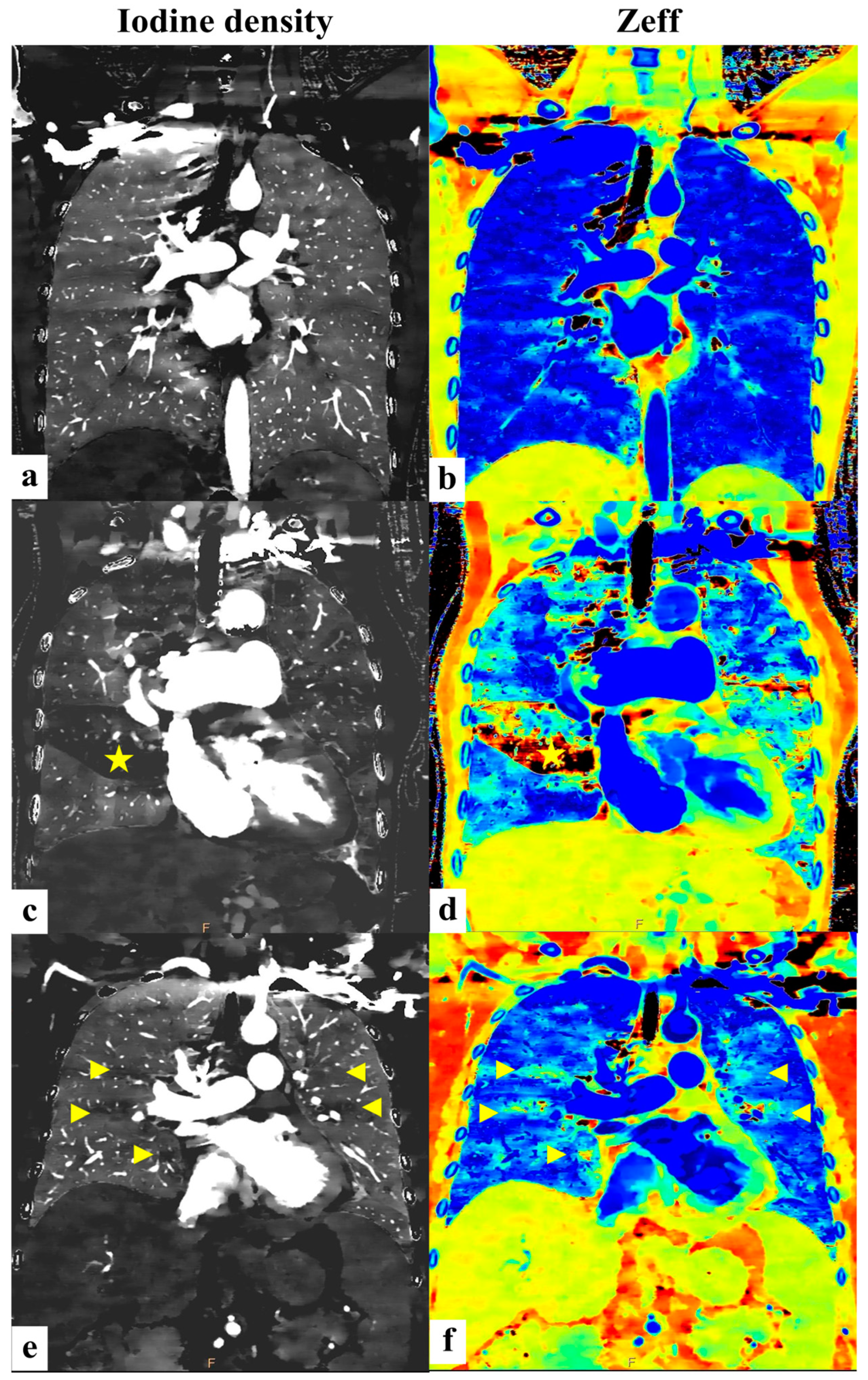
2.2. Dual-Energy CT Pulmonary Angiography Imaging and Analysis
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. Differentiation Between PH Patients and Controls
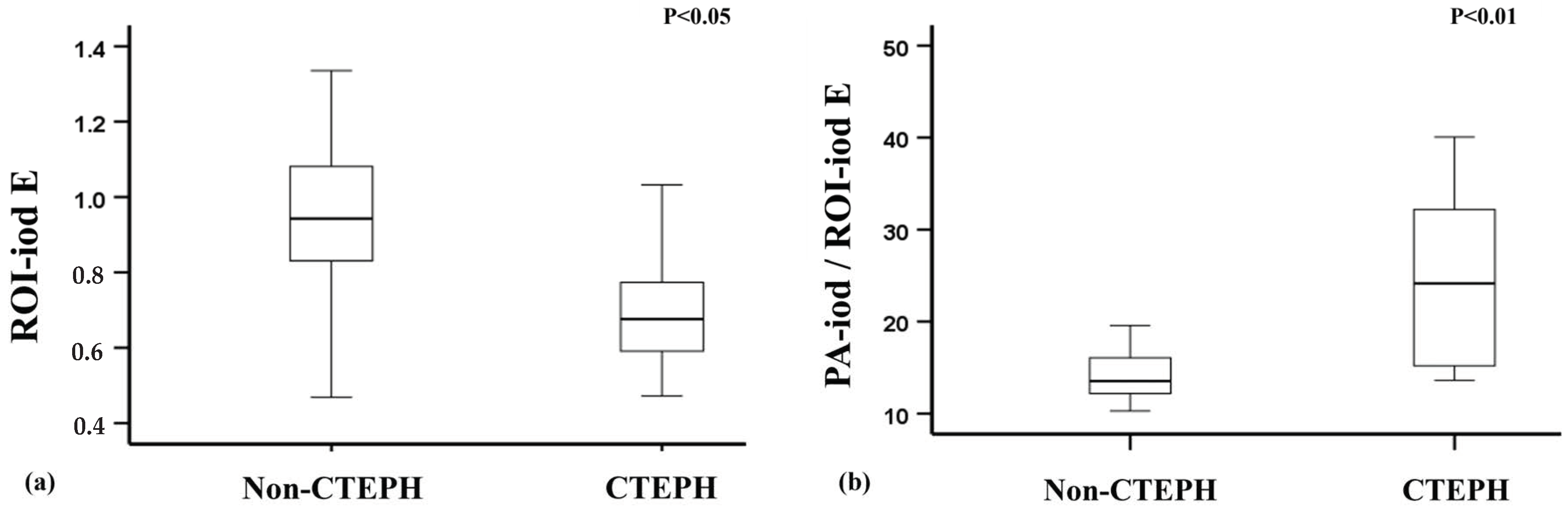
3.3. Differentiation Between CTEPH Patients and Non-CTEPH Patients
4. Discussion
- The ratio of iodine density in the main pulmonary artery to that in the lung parenchyma (PA-iod/ROI-iod) is significantly elevated in PH patients compared to healthy controls.
- Among PH subtypes, patients with chronic thromboembolic pulmonary hypertension (CTEPH) exhibit even higher PA-iod/ROI-iod ratios than those with other forms of PH.
- The effective atomic number (Zeff) is notably lower in PH patients relative to controls.
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PH | pulmonary hypertension |
| SDCT | detector-based spectral computed tomography |
| Iod | iodine density |
| Zeff | effective atomic number |
| ROI | region of interest |
| CTEPH | chronic thromboembolic pulmonary hypertension |
| PBV | pulmonary blood volume |
References
- Hoeper, M.M.; Humbert, M.; Souza, R.; Idrees, M.; Kawut, S.M.; Sliwa-Hahnle, K.; Jing, Z.-C.; Gibbs, J.S.R. A global view of pulmonary hypertension. Lancet Respir. Med. 2016, 4, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Noordegraaf, A.V.; Chin, K.M.; Haddad, F.; Hassoun, P.M.; Hemnes, A.R.; Hopkins, S.R.; Kawut, S.M.; Langleben, D.; Lumens, J.; Naeije, R. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: An update. Eur. Respir. J. 2019, 53, 1801900. [Google Scholar] [CrossRef] [PubMed]
- Gall, H.; Felix, J.F.; Schneck, F.K.; Milger, K.; Sommer, N.; Voswinckel, R.; Franco, O.H.; Hofman, A.; Schermuly, R.T.; Weissmann, N.; et al. The Giessen Pulmonary Hypertension Registry: Survival in pulmonary hypertension subgroups. J. Heart Lung Transplant. 2017, 36, 957–967. [Google Scholar] [CrossRef]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Lee, S.H.; Voswinckel, R.; Palazzini, M.; Jais, X.; Marinelli, A.; Barst, R.J.; Ghofrani, H.A.; Jing, Z.-C.; Opitz, C.; et al. Complications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centers. J. Am. Coll. Cardiol. 2006, 48, 2546–2552. [Google Scholar] [CrossRef]
- Janda, S.; Shahidi, N.; Gin, K.; Swiston, J. Diagnostic accuracy of echocardiography for pulmonary hypertension: A systematic review and meta-analysis. Heart 2011, 97, 612–622. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713, quiz 786–788. [Google Scholar]
- Swift, A.J.; Rajaram, S.; Marshall, H.; Condliffe, R.; Capener, D.; Hill, C.; Davies, C.; Hurdman, J.; Elliot, C.A.; Wild, J.M.; et al. Black blood MRI has diagnostic and prognostic value in the assessment of patients with pulmonary hypertension. Eur. Radiol. 2012, 22, 695–702. [Google Scholar] [CrossRef]
- Swift, A.J.; Lu, H.; Uthoff, J.; Garg, P.; Cogliano, M.; Taylor, J.; Metherall, P.; Zhou, S.; Johns, C.S.; Alabed, S.; et al. A machine learning cardiac magnetic resonance approach to extract disease features and automate pulmonary arterial hypertension diagnosis. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 236–245. [Google Scholar] [CrossRef]
- Shehata, M.L.; Harouni, A.A.; Skrok, J.; Basha, T.A.; Boyce, D.; Lechtzin, N.; Mathai, S.C.; Girgis, R.; Osman, N.F.; Lima, J.A.C.; et al. Regional and global biventricular function in pulmonary arterial hypertension: A cardiac MR imaging study. Radiology 2013, 266, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, A.; Hansell, D.M. Computed tomography signs of pulmonary hypertension: Old and new observations. Clin. Radiol. 2009, 64, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Zhou, M.; Liu, D.; Long, X.; Guo, T.; Kong, X. Diagnostic accuracy of computed tomography for chronic thromboembolic pulmonary hypertension: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0126985. [Google Scholar] [CrossRef]
- Ende-Verhaar, Y.M.; Meijboom, L.J.; Kroft, L.J.; Beenen, L.F.; Boon, G.J.; Middeldorp, S.; Nossent, E.J.; Symersky, P.; Huisman, M.V.; Bogaard, H.J.; et al. Usefulness of standard computed tomography pulmonary angiography performed for acute pulmonary embolism for identification of chronic thromboembolic pulmonary hypertension: Results of the InShape III study. J. Heart Lung Transplant. 2019, 38, 731–738. [Google Scholar] [CrossRef]
- Aycock, R.D.; Westafer, L.M.; Boxen, J.L.; Majlesi, N.; Schoenfeld, E.M.; Bannuru, R.R. Acute Kidney Injury After Computed Tomography: A Meta-Analysis. Ann. Emerg. Med. 2018, 71, 44–53.e4. [Google Scholar] [CrossRef]
- Gleeson, T.G.; Bulugahapitiya, S. Contrast-induced nephropathy. AJR Am. J. Roentgenol. 2004, 183, 1673–1689. [Google Scholar] [CrossRef]
- Ellis, J.H.; Cohan, R.H. Reducing the risk of contrast-induced nephropathy: A perspective on the controversies. AJR Am. J. Roentgenol. 2009, 192, 1544–1549. [Google Scholar] [CrossRef]
- Kim, N.H.; Delcroix, M.; Jais, X.; Madani, M.M.; Matsubara, H.; Mayer, E.; Ogo, T.; Tapson, V.F.; Ghofrani, H.-A.; Jenkins, D.P. Chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801915. [Google Scholar] [CrossRef]
- He, J.; Fang, W.; Lv, B.; He, J.-G.; Xiong, C.-M.; Liu, Z.-H.; He, Z.-X. Diagnosis of chronic thromboembolic pulmonary hypertension: Comparison of ventilation/perfusion scanning and multidetector computed tomography pulmonary angiography with pulmonary angiography. Nucl. Med. Commun. 2012, 33, 459–463. [Google Scholar] [CrossRef]
- Tunariu, N.; Gibbs, S.J.; Win, Z.; Gin-Sing, W.; Graham, A.; Gishen, P.; Al-Nahhas, A. Ventilation-Perfusion scintigraphy is more sensitive than multidetector CTPA in detecting chronic thromboembolic pulmonary disease as a treatable cause of pulmonary hypertension. J. Nucl. Med. 2007, 48, 680–684. [Google Scholar] [CrossRef]
- Lisbona, R.; Kreisman, H.; Novales-Diaz, J.; Derbekyan, V.; Lisbona, H.K.R.; Barbosa, E.J.M.; Gupta, N.K.; Torigian, D.A.; Gefter, W.B.; Soin, J.S.; et al. Perfusion lung scanning: Differentiation of primary from thromboembolic pulmonary hypertension. AJR Am. J. Roentgenol. 1985, 144, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Fishman, A.J.; Moser, K.M.; Fedullo, P.F. Perfusion lung scans vs pulmonary angiography in evaluation of suspected primary pulmonary hypertension. Chest 1983, 84, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Rich, S.; Pietra, G.G.; Kieras, K.; Hart, K.; Brundage, B.H. Primary pulmonary hypertension: Radiographic and scintigraphic patterns of histologic subtypes. Ann. Intern. Med. 1986, 105, 499–502. [Google Scholar] [CrossRef]
- Wang, M.; Ma, R.; Wu, D.; Xiong, C.; He, J.; Wang, L.; Sun, X.; Fang, W. Value of lung perfusion scintigraphy in patients with idiopathic pulmonary arterial hypertension: A patchy pattern to consider. Pulm. Circ. 2019, 9, 2045894018816968. [Google Scholar] [CrossRef]
- Johnson, T.R.C. Dual-Energy CT: General Principles. AJR Am. J. Roentgenol. 2012, 199 (Suppl. S5), S3–S8. [Google Scholar] [CrossRef]
- Johnson, T.R.C.; Nikolaou, K.; Wintersperger, B.J.; Leber, A.W.; von Ziegler, F.; Rist, C.; Buhmann, S.; Knez, A.; Reiser, M.F.; Becker, C.R. Dual-source CT cardiac imaging: Initial experience. Eur. Radiol. 2006, 16, 1409–1415. [Google Scholar] [CrossRef]
- Rajiah, P.; Abbara, S.; Halliburton, S.S. Spectral detector CT for cardiovascular applications. Diagn. Interv. Radiol. 2017, 23, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Ameli-Renani, S.; Ramsay, L.; Bacon, J.L.; Rahman, F.; Nair, A.; Smith, V.; Baskerville, K.; Devaraj, A.; Madden, B.; Vlahos, I. Dual-energy computed tomography in the assessment of vascular and parenchymal enhancement in suspected pulmonary hypertension. J. Thorac. Imaging 2014, 29, 98–106. [Google Scholar] [CrossRef]
- Wolfram, S.; Stephan, S.; Franziska, F.; Miriam, K.; Jens, H.; Gregor, P.; Lars, G.; Hans-Ulrich, K. Correlation of quantitative dual-energy computed tomography iodine maps and abdominal computed tomography perfusion measurements: Are single-acquisition dual-energy computed tomography iodine maps more than a reduced-dose surrogate of conventional computed tomography perfusion? Investig. Radiol. 2015, 50, 703–708. [Google Scholar]
- De Santis, D.; Eid, M.; De Cecco, C.N.; Jacobs, B.E.; Albrecht, M.H.; Varga-Szemes, A.; Tesche, C.; Caruso, D.; Laghi, A.; Schoepf, U.J. Dual-Energy Computed Tomography in Cardiothoracic Vascular Imaging. Radiol. Clin. N. Am. 2018, 56, 521–534. [Google Scholar] [CrossRef]
- Zhang, L.J.; Zhou, C.S.; Schoepf, U.J.; Sheng, H.X.; Wu, S.Y.; Krazinski, A.W.; Silverman, J.R.; Meinel, F.G.; Zhao, Y.E.; Zhang, Z.J.; et al. Dual-energy CT lung ventilation/perfusion imaging for diagnosing pulmonary embolism. Eur. Radiol. 2013, 23, 2666–2675. [Google Scholar]
- Masy, M.; Giordano, J.; Petyt, G.; Hossein-Foucher, C.; Duhamel, A.; Kyheng, M.; De Groote, P.; Fertin, M.; Lamblin, N.; Bervar, J.-F.; et al. Dual-energy CT (DECT) lung perfusion in pulmonary hypertension: Concordance rate with V/Q scintigraphy in diagnosing chronic thromboembolic pulmonary hypertension (CTEPH). Eur. Radiol. 2018, 28, 5100–5110. [Google Scholar] [CrossRef] [PubMed]
- Hoey, E.T.D.; Agrawal, S.K.B.; Ganesh, V.; Gopalan, D.; Screaton, N.J. Dual energy CT pulmonary angiography: Findings in a patient with chronic thromboembolic pulmonary hypertension. Thorax 2009, 64, 1012. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoey, E.T.D.; Mirsadraee, S.; Pepke-Zaba, J.; Jenkins, D.P.; Gopalan, D.; Screaton, N.J. Dual-energy CT angiography for assessment of regional pulmonary perfusion in patients with chronic thromboembolic pulmonary hypertension: Initial experience. AJR Am. J. Roentgenol. 2011, 196, 524–532. [Google Scholar] [CrossRef]
- Renard, B.; Remy-Jardin, M.; Santangelo, T.; Faivre, J.-B.; Tacelli, N.; Remy, J.; Duhamel, A. Dual-energy CT angiography of chronic thromboembolic disease: Can it help recognize links between the severity of pulmonary arterial obstruction and perfusion defects? Eur. J. Radiol. 2011, 79, 467–472. [Google Scholar] [CrossRef]
- Verbelen, T.; Godinas, L.; Dorfmüller, P.; Gopalan, D.; Condliffe, R.; Delcroix, M. Clinical-radiological-pathological correlation in chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2023, 32, 230149. [Google Scholar] [CrossRef]
- Abozeed, M.; Conic, S.; Bullen, J.; Rizk, A.; Bin Saeedan, M.; Karim, W.; Heresi, G.A.; Renapurkar, R.D. Dual energy CT based scoring in chronic thromboembolic pulmonary hypertension and correlation with clinical and hemodynamic parameters: A retrospective cross-sectional study. Cardiovasc. Diagn. Ther. 2022, 12, 305–313. [Google Scholar] [CrossRef]
- Hahn, L.D.; Papamatheakis, D.G.; Fernandes, T.M.; Poch, D.S.; Yang, J.; Shen, J.; Hoh, C.K.; Hsiao, A.; Kerr, K.M.; Pretorius, V.; et al. Multidisciplinary Approach to Chronic Thromboembolic Pulmonary Hypertension: Role of Radiologists. Radiographics 2023, 43, e220078. [Google Scholar] [CrossRef]
- Murty, R.C. Effective atomic numbers of heterogeneous materials. Nature 1965, 207, 398–399. [Google Scholar] [CrossRef]
- Levet, A.; Özdemir, Y. Determination of effective atomic numbers, effective electrons numbers, total atomic cross-sections and buildup factor of some compounds for different radiation sources. Radiat. Phys. Chem. 2017, 130, 171–176. [Google Scholar] [CrossRef]
- Landry, G.; Seco, J.; Gaudreault, M.; Verhaegen, F. Deriving effective atomic numbers from DECT based on a parameterization of the ratio of high and low linear attenuation coefficients. Phys. Med. Biol. 2013, 58, 6851–6866. [Google Scholar] [CrossRef]
- Li, K.; Li, Y.; Qi, Z.; Garrett, J.W.; Grist, T.M.; Chen, G.-H. Quantitative lung perfusion blood volume using dual energy CT-based effective atomic number (Zeff) imaging. Med. Phys. 2021, 48, 6658–6672. [Google Scholar] [CrossRef]


| PH Case (n = 33) | Control (n = 24) | |
|---|---|---|
| Age | 62 [44.0–80.5] | 60 [39.3–71.5] |
| Sex | ||
| Female | 23 [69.7%] | 12 [50%] |
| Male | 10 [30.3%] | 12 [50%] |
| Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|
| Num. of patients | 8 (24.2%) | 7 (21.2%) | 9 (27.3%) | 9 (27.3%) |
| Normal Control (n = 24) | PH Case (n = 33) | p-Value | |
|---|---|---|---|
| Sex | 0.132 | ||
| Female | 12 (50.0%) | 23 (69.7%) | |
| Male | 12 (50.0%) | 10 (30.3%) | |
| ROI-iod A | 1.03 (0.88–1.19) | 0.94 (0.76–1.21) | 0.207 |
| ROI-iod B | 1.02 (0.79–1.10) | 0.91 (0.67–1.03) | 0.091 |
| ROI-iod C | 0.99 (0.83–1.16) | 0.81 (0.68–1.07) | 0.037 * |
| ROI-iod D | 0.87 (0.72–1.01) | 0.83 (0.66–1.04) | 0.884 |
| ROI-iod E | 0.98 (0.82–1.13) | 0.91 (0.69–1.06) | 0.139 |
| Zeff A | 10.46 (10.37–10.82) | 9.94 (9.68–10.30) | <0.001 ** |
| Zeff B | 10.30 (10.07–10.55) | 9.95 (9.59–10.26) | 0.003 ** |
| Zeff C | 10.38 (10.15–10.60) | 9.83 (9.42–10.14) | <0.001 ** |
| Zeff D | 10.14 (9.92–10.51) | 9.87 (9.14–10.06) | 0.001 ** |
| Zeff E | 10.28 (10.20–10.49) | 9.80 (9.44–10.15) | <0.001 ** |
| PA-iod/ROI-iod A | 11.92 (8.48–13.34) | 13.55 (11.18–16.96) | 0.011 * |
| PA-iod/ROI-iod B | 12.20 (10.80–14.73) | 15.01 (13.39–19.85) | 0.003 ** |
| PA-iod/ROI-iod C | 11.89 (9.54–13.82) | 14.22 (12.59–21.58) | 0.001 ** |
| PA-iod/ROI-iod D | 12.69 (10.29–17.39) | 15.57 (12.78–19.75) | 0.047 * |
| PA-iod/ROI-iod E | 12.34 (9.64–14.04) | 14.70 (12.61–17.49) | 0.005 ** |
| Aa-iod/ROI-iod A | 9.82 (7.34–12.37) | 10.13 (6.42–12.96) | 0.808 |
| Aa-iod/ROI-iod B | 10.52 (8.52–12.88) | 11.45 (7.49–13.02) | 0.923 |
| Aa-iod/ROI-iod C | 9.99 (7.88–12.47) | 10.47 (7.57–13.20) | 0.722 |
| Aa-iod/ROI-iod D | 10.82 (9.61–13.95) | 10.99 (7.74–12.53) | 0.316 |
| Aa-iod/ROI-iod E | 10.09 (8.14–12.53) | 10.88 (7.26–12.95) | 0.987 |
| Non-CTEPH (n = 24) | CTEPH (n = 9) | p-Value | |
|---|---|---|---|
| Sex | 1.000 | ||
| Female | 17 (70.8%) | 6 (66.7%) | |
| Male | 7 (29.2%) | 3 (33.3%) | |
| ROI-iod A | 1.04 (0.83–1.26) | 0.76 (0.67–0.87) | 0.006 ** |
| ROI-iod B | 0.96 (0.73–1.10) | 0.72 (0.63–0.92) | 0.043 * |
| ROI-iod C | 0.84 (0.74–1.07) | 0.63 (0.49–0.85) | 0.035 * |
| ROI-iod D | 0.95 (0.75–1.13) | 0.65 (0.46–0.86) | 0.026 * |
| ROI-iod E | 0.94 (0.82–1.09) | 0.68 (0.58–0.87) | 0.015 * |
| Zeff A | 9.94 (9.66–10.27) | 10.03 (9.66–10.45) | 0.872 |
| Zeff B | 9.92 (9.52–10.29) | 10.00 (9.63–10.40) | 0.657 |
| Zeff C | 9.69 (9.40–10.04) | 9.96 (9.52–10.36) | 0.210 |
| Zeff D | 9.79 (9.35–10.00) | 9.87 (8.90–10.22) | 0.903 |
| Zeff E | 9.79 (9.43–10.12) | 9.94 (9.46–10.30) | 0.686 |
| PA-iod/ROI-iod A | 13.16 (10.90–14.64) | 20.32 (14.0131.74) | 0.005 ** |
| PA-iod/ROI-iod B | 13.83 (12.33–16.74) | 21.33 (15.88–29.70) | 0.005 ** |
| PA-iod/ROI-iod C | 13.43 (12.26–17.59) | 24.25 (15.23–42.08) | 0.012 * |
| PA-iod/ROI-iod D | 13.81 (12.05–17.66) | 27.52 (15.13–40.81) | 0.010 * |
| PA-iod/ROI-iod E | 13.54 (12.17–16.21) | 24.16 (14.56–33.63) | 0.006 ** |
| Aa-iod/ROI-iod A | 9.41 (6.18–12.65) | 11.31 (9.33–13.79) | 0.196 |
| Aa-iod/ROI-iod B | 9.45 (7.48–13.48) | 12.00 (9.30–13.01) | 0.544 |
| Aa-iod/ROI-iod C | 9.82 (6.87–12.78) | 13.14 (9.42–16.33) | 0.124 |
| Aa-iod/ROI-iod D | 9.59 (7.11–12.23) | 11.88 (9.65–6.29) | 0.124 |
| Aa-iod/ROI-iod E | 9.62 (6.55–12.56) | 12.71 (9.57–13.15) | 0.196 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.-F.; Chang, Y.-P.; Chai, J.-W. Investigating Patients with Pulmonary Hypertension Under Detector-Based Spectral Computed Tomography. Diagnostics 2025, 15, 1069. https://doi.org/10.3390/diagnostics15091069
Cheng H-F, Chang Y-P, Chai J-W. Investigating Patients with Pulmonary Hypertension Under Detector-Based Spectral Computed Tomography. Diagnostics. 2025; 15(9):1069. https://doi.org/10.3390/diagnostics15091069
Chicago/Turabian StyleCheng, Hsien-Fu, Yu-Pin Chang, and Jyh-Wen Chai. 2025. "Investigating Patients with Pulmonary Hypertension Under Detector-Based Spectral Computed Tomography" Diagnostics 15, no. 9: 1069. https://doi.org/10.3390/diagnostics15091069
APA StyleCheng, H.-F., Chang, Y.-P., & Chai, J.-W. (2025). Investigating Patients with Pulmonary Hypertension Under Detector-Based Spectral Computed Tomography. Diagnostics, 15(9), 1069. https://doi.org/10.3390/diagnostics15091069






