Integrated Diagnostics for Atrial Fibrillation Recurrence: Exploratory Results from the PLACEBO Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
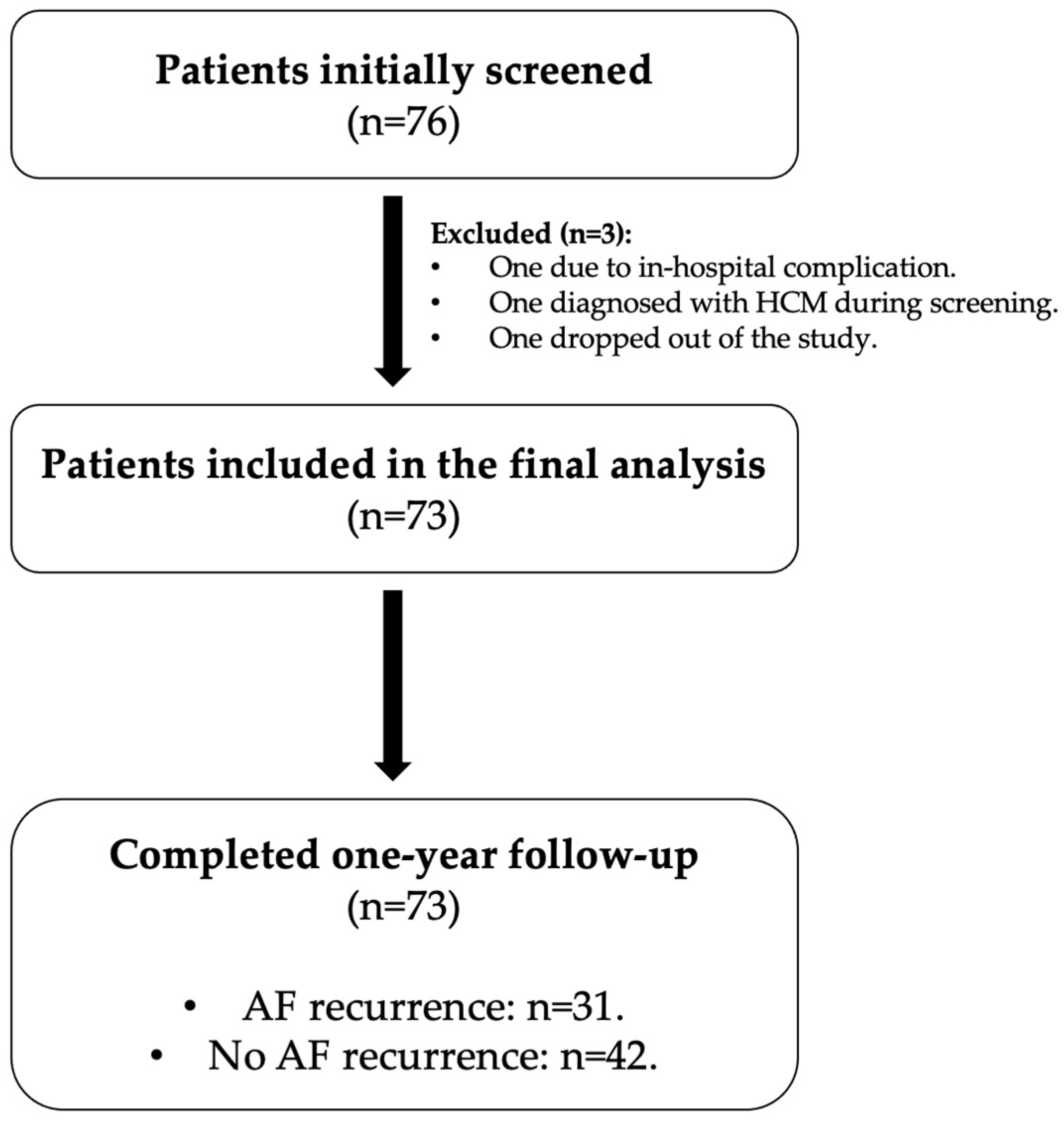
2.2. Study Population
2.3. Data Collection
2.3.1. Baseline
- Demographics and medical history: Demographic data [e.g., age, sex, body mass index (BMI)] and detailed clinical history, including cardiovascular risk factors, comorbidities, prior PAF episodes, and their characteristics, were recorded.
- CPET: CPET was performed on a cycle ergometer with continuous 12-lead ECG monitoring and respiratory gas analysis. The key parameters obtained included peak oxygen uptake (peak VO2), minute ventilation/carbon dioxide production (VE/VCO2), and other exercise indices with established prognostic value in cardiovascular conditions.
- Transthoracic echocardiography: A comprehensive echocardiographic assessment was conducted under stable hemodynamic conditions. The parameters included left ventricular (LV) ejection fraction (LVEF), global longitudinal strain (GLS), LV diastolic indices, and LA dimensions, volume, and strain. Right ventricular parameters, such as RV FAC and free wall strain, were also evaluated. All echocardiographic examinations were performed by a single experienced cardiologist to ensure consistency. For the echocardiographic measurements, the GE Vivid E95 ultrasound system (GE Healthcare, Chicago, IL, USA) was used. The 2D measurements were performed offline using the commercially available EchoPAC GE software package, version PC (GE Healthcare, Chicago, IL, USA). All measurements adhered to the European Association of Cardiovascular Imaging (EACVI) guidelines [19,20].
- Electrocardiographic (ECG) Holter monitoring (24 h): Continuous ECG data were recorded to assess AF episodes, premature atrial and ventricular contractions, HRV indices, and other rhythm-related metrics. For ambulatory ECG Holter monitoring, we utilized the GE Seer 1000 and GE CardioMem 4000 Holter monitors (GE Healthcare, USA). HRV analysis was performed using GE CardioDay ECG Holter software, v2.6 (GE Healthcare, Chicago, IL, USA).
- Plasma biomarker analysis: Blood samples were collected upon study enrollment, after at least 12 h of overnight fasting. Basic biochemical measurements, including brain natriuretic peptide (BNP) and high-sensitivity cardiac troponin I (hs-cTnI), were performed immediately after collection. Additional blood samples were obtained in vacuum collection tubes without heparin, EDTA, or gel (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and transferred to the laboratory within 1 h of vein puncture while maintained on ice. The samples were preserved on ice until centrifugation to minimize biochemical changes, as certain biomarkers, including Hcy, can continue to be produced by red blood cells post-collection. After a minimum of 45 min to allow clot formation, the blood samples were centrifuged at 4000 rpm for 10 min in a refrigerated centrifuge. The serum samples that were not analyzed immediately were stored at −80 °C. Lipemic or hemolyzed samples were excluded.
2.3.2. Follow-Up
2.3.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Demographic and Lifestyle Characteristics
3.2. Atrial Fibrillation Characteristics
3.3. Comorbidities
3.4. Echocardiographic Characteristics
3.5. CPET Results
3.6. Twenty-Four-Hour ECG Holter Monitoring
3.7. Regression
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | atrial fibrillation |
| PAF | paroxysmal atrial fibrillation |
| CPET | cardiopulmonary exercise testing |
| TTE | transthoracic echocardiography |
| RV FAC | right ventricular fractional area change |
| LA | left atrial |
| HCM | hypertrophic cardiomyopathy |
| GAL3 | galectin-3 |
| Hcy | homocysteine |
| HRV | heart rate variability |
| BMI | body mass index |
| Peak VO2 | peak oxygen uptake |
| VE/VCO2 | minute ventilation/carbon dioxide production ratio |
| LV | left ventricular |
| LVEF | left ventricular ejection fraction |
| GLS | global longitudinal strain |
| EACVI | European Association of Cardiovascular Imaging |
| ECG | electrocardiogram |
| BNP | brain natriuretic peptide |
| Hs-cTnI | high-sensitivity cardiac troponin I |
| CMIA | chemiluminescence |
| ROC | receiver operating characteristics |
| SD | standard deviation |
| OSA | obstructive sleep apnea |
| LVMI | left ventricular mass index |
| LAVI | left atrial volume index |
| VO2 AT | anaerobic threshold |
| PETCO2 | partial pressure of end-tidal carbon dioxide |
| OR | odds ratio |
| SDNN | standard deviation of normal to normal R-R intervals |
| SDRR | standard deviation of R-R intervals |
References
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int. J. Stroke 2021, 16, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Thrall, G.; Lane, D.; Carroll, D.; Lip, G.Y. Quality of life in patients with atrial fibrillation: A systematic review. Am. J. Med. 2006, 119, 448.e1–448.e19. [Google Scholar] [CrossRef] [PubMed]
- Peigh, G.; Zhou, J.; Rosemas, S.C.; Roberts, A.I.; Longacre, C.; Nayak, T.; Schwab, G.; Soderlund, D.; Passman, R.S. Impact of Atrial Fibrillation Burden on Health Care Costs and Utilization. JACC Clin. Electrophysiol. 2024, 10, 718–730. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar]
- Ruigómez, A.; Johansson, S.; Wallander, M.A.; García Rodríguez, L.A. Predictors and prognosis of paroxysmal atrial fibrillation in general practice in the UK. BMC Cardiovasc. Disord. 2005, 5, 20. [Google Scholar] [CrossRef]
- de Vos, C.B.; Pisters, R.; Nieuwlaat, R.; Prins, M.H.; Tieleman, R.G.; Coelen, R.J.; van den Heijkant, A.C.; Allessie, M.A.; Crijns, H.J. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J. Am. Coll. Cardiol. 2010, 55, 725–731. [Google Scholar] [CrossRef]
- Glaab, T.; Taube, C. Practical guide to cardiopulmonary exercise testing in adults. Respir. Res. 2022, 23, 9. [Google Scholar] [CrossRef]
- Boulmpou, A.; Teperikidis, E.; Papadopoulos, C.; Patoulias, D.I.; Charalampidis, P.; Mouselimis, D.; Tsarouchas, A.; Boutou, A.; Giannakoulas, G.; Vassilikos, V. The role of cardiopulmonary exercise testing in risk stratification and prognosis of atrial fibrillation: A scoping review of the literature. Acta Cardiol. 2023, 78, 274–287. [Google Scholar] [CrossRef]
- Troughton, R.W.; Asher, C.R.; Klein, A.L. The role of echocardiography in atrial fibrillation and cardioversion. Heart 2003, 89, 1447–1454. [Google Scholar] [CrossRef]
- Cameli, M.; Mandoli, G.E.; Loiacono, F.; Sparla, S.; Iardino, E.; Mondillo, S. Left atrial strain: A useful index in atrial fibrillation. Int. J. Cardiol. 2016, 220, 208–213. [Google Scholar] [CrossRef]
- Chou, S.H.; Kuo, C.T.; Hsu, L.A.; Ho, W.J.; Wang, C.L. Single-beat determination of right ventricular function in patients with atrial fibrillation. Echocardiograph 2010, 27, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Li, L.C.; Li, J.; Gao, J. Functions of galectin-3 and its role in fibrotic diseases. J. Pharmacol. Exp. Ther. 2014, 351, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Charalampidis, P.; Teperikidis, E.; Boulmpou, A.; Papadopoulos, C.E.; Potoupni, V.; Tsioni, K.; Rakitzi, P.; Karamitsos, T.; Vassilikos, V. Homocysteine as a Predictor of Paroxysmal Atrial Fibrillation-Related Events: A Scoping Review of the Literature. Diagnostics 2022, 12, 2192. [Google Scholar] [CrossRef] [PubMed]
- Geurts, S.; Tilly, M.J.; Arshi, B.; Stricker, B.H.C.; Kors, J.A.; Deckers, J.W.; de Groot, N.M.S.; Ikram, M.A.; Kavousi, M. Heart rate variability and atrial fibrillation in the general population: A longitudinal and Mendelian randomization study. Clin. Res. Cardiol. 2023, 112, 747–758. [Google Scholar] [CrossRef]
- Kim, S.H.; Lim, K.R.; Seo, J.H.; Ryu, D.R.; Lee, B.K.; Cho, B.R.; Chun, K.J. Higher heart rate variability as a predictor of atrial fibrillation in patients with hypertension. Sci. Rep. 2022, 12, 3702. [Google Scholar] [CrossRef]
- Mazaris, S.; Siasos, G.; Oikonomou, E.; Tsigkou, V.; Vavuranakis, M.; Kokkou, E.; Zaromitidou, M.; Papamikroulis, G.A.; Papavassiliou, A.G.; Papaioannou, S.; et al. Atrial Fibrillation: Biomarkers Determining Prognosis. Curr. Med. Chem. 2019, 26, 909–915. [Google Scholar] [CrossRef]
- Sánchez, F.J.; Pueyo, E.; Diez, E.R. Strain Echocardiography to Predict Postoperative Atrial Fibrillation. Int. J. Mol. Sci. 2022, 23, 1355. [Google Scholar] [CrossRef]
- Boulmpou, A.; Moysiadis, T.; Zormpas, G.; Teperikidis, E.; Vassilikos, V.; Giannakoulas, G.; Papadopoulos, C. Exploring the Feasibility of Integrating Cardiopulmonary Exercise Testing, Echocardiography, and Biomarkers for Predicting Atrial Fibrillation Recurrence: Rationale, Design and Protocol for a Prospective Cohort Study (The PLACEBO Trial). J. Clin. Med. 2025, 14, 1690. [Google Scholar] [CrossRef]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.R.; Roach, D.E.; Koshman, M.L.; Sheldon, R.S. Standard Deviation of Sequential Five-Minute R-R Interval Means (SDANN) is a prognostic marker, but not necessarily an autonomic marker. J. Am. Coll. Cardiol. 2006, 48, 1285–1286, author reply 6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kleiger, R.E.; Stein, P.K.; Bigger, J.T., Jr. Heart rate variability: Measurement and clinical utility. Ann. Noninvasive Electrocardiol. 2005, 10, 88–101. [Google Scholar] [CrossRef]
- Zhang, E.; Liang, S.; Sun, T.; Xu, J.; Lu, F.; Wu, D.; Zhang, J.; He, L.; Zhang, F.; Fan, S.; et al. Prognostic value of heart rate variability in atrial fibrillation recurrence following catheter ablation: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2022, 9, 1048398. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (Review). Int. J. Mol. Med. 2018, 41, 599–614. [Google Scholar] [CrossRef]
- Slack, R.J.; Mills, R.; Mackinnon, A.C. The therapeutic potential of galectin-3 inhibition in fibrotic disease. Int. J. Biochem. Cell Biol. 2021, 130, 105881. [Google Scholar] [CrossRef]
- Martínez-Martínez, E.; Brugnolaro, C.; Ibarrola, J.; Ravassa, S.; Buonafine, M.; López, B.; Fernández-Celis, A.; Querejeta, R.; Santamaria, E.; Fernández-Irigoyen, J.; et al. CT-1 (Cardiotrophin-1)-Gal-3 (Galectin-3) Axis in Cardiac Fibrosis and Inflammation. Hypertension 2019, 73, 602–611. [Google Scholar] [CrossRef]
- Procyk, G.; Czapla, A.; Jałocha, K.; Tymińska, A.; Grabowski, M.; Gąsecka, A. The role of galectin-3 in atrial fibrillation. J. Mol. Med. 2023, 101, 1481–1492. [Google Scholar] [CrossRef]
- Gong, M.; Cheung, A.; Wang, Q.S.; Li, G.; Goudis, C.A.; Bazoukis, G.; Lip, G.Y.H.; Baranchuk, A.; Korantzopoulos, P.; Letsas, K.P.; et al. Galectin-3 and risk of atrial fibrillation: A systematic review and meta-analysis. J. Clin. Lab. Anal. 2020, 34, e23104. [Google Scholar] [CrossRef]
- Linz, D.; McEvoy, R.D.; Cowie, M.R.; Somers, V.K.; Nattel, S.; Lévy, P.; Kalman, J.M.; Sanders, P. Associations of Obstructive Sleep Apnea With Atrial Fibrillation and Continuous Positive Airway Pressure Treatment: A Review. JAMA Cardiol. 2018, 3, 532–540. [Google Scholar] [CrossRef]
- Trzepizur, W.; Blanchard, M.; Ganem, T.; Balusson, F.; Feuilloy, M.; Girault, J.M.; Meslier, N.; Oger, E.; Paris, A.; Pigeanne, T.; et al. Sleep Apnea-Specific Hypoxic Burden, Symptom Subtypes, and Risk of Cardiovascular Events and All-Cause Mortality. Am. J. Respir. Crit. Care Med. 2022, 205, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Narkiewicz, K.; Somers, V.K. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol. Scand. 2003, 177, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Moula, A.I.; Parrini, I.; Tetta, C.; Lucà, F.; Parise, G.; Rao, C.M.; Mauro, E.; Parise, O.; Matteucci, F.; Gulizia, M.M.; et al. Obstructive Sleep Apnea and Atrial Fibrillation. J. Clin. Med. 2022, 11, 1242. [Google Scholar] [CrossRef] [PubMed]
- Nalliah, C.J.; Wong, G.R.; Lee, G.; Voskoboinik, A.; Kee, K.; Goldin, J.; Watts, T.; Linz, D.; Parameswaran, R.; Sugumar, H.; et al. Impact of CPAP on the Atrial Fibrillation Substrate in Obstructive Sleep Apnea: The SLEEP-AF Study. Clin. Electrophysiol. 2022, 8, 869–877. [Google Scholar] [CrossRef]
- Konecny, T.; Miles, W.M. Treating Obstructive Sleep Apnea and Atrial Fibrillation: Focus on Substrate, Triggers, and Those Evasive Outcomes. Clin. Electrophysiol. 2022, 8, 878–881. [Google Scholar] [CrossRef]
- Cao, Z.; Jia, Y.; Zhu, B. BNP and NT-proBNP as Diagnostic Biomarkers for Cardiac Dysfunction in Both Clinical and Forensic Medicine. Int. J. Mol. Sci. 2019, 20, 1820. [Google Scholar] [CrossRef]
- Hijazi, Z.; Siegbahn, A.; Andersson, U.; Granger, C.B.; Alexander, J.H.; Atar, D.; Gersh, B.J.; Mohan, P.; Harjola, V.P.; Horowitz, J.; et al. High-sensitivity troponin I for risk assessment in patients with atrial fibrillation: Insights from the Apixaban for Reduction in Stroke and other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Circulation 2014, 129, 625–634. [Google Scholar] [CrossRef]
- Ivanov, V.; Smereka, Y.; Rasputin, V.; Dmytriiev, K. Homocysteine and atrial fibrillation: Novel evidences and insights. Monaldi Arch. Chest Dis. 2023, 93. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, S.J.; Kim, J.; Chung, T.W.; Park, S.J.; Park, K.M.; Kim, J.S.; On, Y.K. Exercise capacity and risk of incident atrial fibrillation in healthy adults. Korean J. Intern. Med. 2023, 38, 872–878. [Google Scholar] [CrossRef]
- Tsuneoka, H.; Koike, A.; Nagayama, O.; Sakurada, K.; Kato, J.; Sato, A.; Yamashita, T.; Aonuma, K. Prognostic value of cardiopulmonary exercise testing in cardiac patients with atrial fibrillation. Int. Heart J. 2012, 53, 102–107. [Google Scholar] [CrossRef]
- Terada, T.; Keir, D.A.; Murias, J.M.; Vidal-Almela, S.; Buckley, J.; Reed, J.L. Variability of cardiopulmonary exercise testing in patients with atrial fibrillation and determination of exercise responders to high-intensity interval training and moderate-to-vigorous intensity continuous training. Appl. Physiol. Nutr. Metab. 2024, 49, 1636–1645. [Google Scholar] [CrossRef] [PubMed]
- Obokata, M.; Reddy, Y.N.V.; Melenovsky, V.; Pislaru, S.; Borlaug, B.A. Deterioration in right ventricular structure and function over time in patients with heart failure and preserved ejection fraction. Eur. Heart J. 2019, 40, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Aziz, E.F.; Kukin, M.; Javed, F.; Musat, D.; Nader, A.; Pratap, B.; Shah, A.; Enciso, J.S.; Chaudhry, F.A.; Herzog, E. Right ventricular dysfunction is a strong predictor of developing atrial fibrillation in acutely decompensated heart failure patients, ACAP-HF data analysis. J. Card. Fail. 2010, 16, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.E.; Yin, X.; Levy, D.; Vasan, R.S.; Magnani, J.W.; Ellinor, P.T.; McManus, D.D.; Lubitz, S.A.; Larson, M.G.; Benjamin, E.J. Galectin 3 and incident atrial fibrillation in the community. Am. Heart J. 2014, 167, 729–734. [Google Scholar] [CrossRef]
| Variables | Total Cases (n = 73) | AF Recurrence During 1 y Follow-Up NO (n = 42) | AF Recurrence During 1 y Follow-Up YES (n = 31) | p-Values |
|---|---|---|---|---|
| Age (years) | 60.0 (11.6) Range: 18.3–80.1 | 59.1 (13.1) Range: 18.3–75.9 | 61.3 (9.2) Range: 34.8–80.1 | 0.424 a |
| Male sex (n, %) | 35 (47.9%) | 18 (42.9%) | 17 (54.8%) | 0.311 b |
| Female sex (n, %) | 38 (52.1%) | 24 (57.1%) | 14 (45.2%) | 0.311 b |
| BMI (kg/m2) | 27.9 (4.4) | 28.2 (4.6) | 27.4 (4.1) | 0.414 a |
| BSA (m2) | 2.0 (0.2) | 1.9 (0.2) | 1.9 (0.2) | 0.695 a |
| Smoking (n, %) | 23 (31.5%) | 14 (33.3%) | 9 (29.0%) | 0.695 b |
| Alcohol (n, %) | 37 (50.7%) | 20 (47.6%) | 17 (54.8%) | 0.542 b |
| Physical activity (n, %) | 50 (68.5%) | 27 (64.3%) | 23 (74.2%) | 0.368 b |
| AF characteristics | ||||
| Total disease duration (months) | 40.0 (49.4) | 38.6 (54.3) | 41.9 (42.5) | 0.781 a |
| PAF episode in the last 6 months (n, %) | 58 (79.5%) | 34 (81%) | 24 (77.4%) | 0.712 b |
| PAF episode in the last 1 month (n, %) | 28 (38.4%) | 16 (38.1%) | 12 (38.7%) | 0.957 b |
| Presence of symptoms (n, %) | 68 (93.2%) | 39 (92.9%) | 29 (93.5%) | 0.908 b |
| EHRA score | 0.540 b | |||
| 1 (n, %) | 17 (23.3%) | 10 (23.8%) | 7 (22.6%) | |
| 2 (n, %) | 48 (65.8%) | 26 (61.9%) | 22 (71.0%) | |
| 3 (n, %) | 8 (11.0%) | 6 (14.3%) | 2 (6.5%) | |
| Comorbidities | ||||
| Arterial hypertension (n, %) | 33 (45.2%) | 18 (42.9%) | 15 (48.4%) | 0.639 b |
| Diabetes mellitus (n, %) | 6 (8.2%) | 3 (7.1%) | 3 (9.7%) | 0.697 b |
| Dyslipidemia (n, %) | 36 (49.3%) | 20 (47.6%) | 16 (51.6%) | 0.736 b |
| OSA (n, %) | 8 (11.0%) | 2 (4.8%) | 6 (19.4%) | 0.049 b |
| Coronary artery disease (n, %) | 3 (4.1%) | 2 (4.8%) | 1 (3.2%) | 0.744 b |
| Stroke (n, %) | 2 (2.7%) | 0 (0.0%) | 2 (6.5%) | 0.095 |
| Valvular disease (n, %) | 3 (4.1%) | 3 (7.1%) | 0 (0.0%) | 0.129 b |
| Thyroid disease (n, %) | 17 (23.3%) | 11 (26.2%) | 6 (19.4%) | 0.495 b |
| Variables | Total Cases (n = 73) | AF Recurrence Within 1 y Follow-Up NO (n = 42) | AF Recurrence Within 1 y Follow-Up YES (n = 31) | p-Values |
|---|---|---|---|---|
| Echocardiography | ||||
| LVEF (%) | 58.8 (13.6) | 57.0 (17.1) | 61.4 (5.4) | 0.126 a |
| LVMI (g/m2) | 92.4 (24.6) | 90.4 (19.3) | 95.1 (30.4) | 0.431 a |
| GLS (%) | −18.3 (2.7) | −18.6 (2.4) | −17.9 (3.1) | 0.339 a |
| E/E’ | 8.4 (4.1) | 8.7 (4.9) | 7.9 (2.9) | 0.441 a |
| LAVI (mL/m2) | 30.2 (9.9) | 31.1 (9.7) | 29.0 (10.1) | 0.374 a |
| LAVpreA (mL) | 43.6 (17.2) | 44.8 (17.7) | 42.0 (16.8) | 0.509 a |
| LA strain reservoir (%) | 24.8 (7.6) | 24.0 (7.4) | 26.0 (8.0) | 0.275 a |
| LA strain conduit (%) | −14.3 (5.0) | −14.4 (5.0) | −14.2 (5.0) | 0.833 a |
| LA strain contraction (%) | −11.5 (4.4) | −11.0 (4.5) | −12.1 (4.2) | 0.266 a |
| RV FAC (%) | 42.7 (7.9) | 44.6 (8.5) | 40.1 (6.3) | 0.014 a |
| RV FWS (%) | −23.0 (6.0) | −24.5 (6.1) | −21.3 (5.4) | 0.053 a |
| RV GS (%) | −20.6 (4.4) | −21.7 (4.5) | −19.3 (4.1) | 0.051 a |
| TAPSE (cm) | 2.6 (0.4) | 2.6 (0.5) | 2.5 (0.4) | 0.401 a |
| CPET | ||||
| Peak VO2 (mL/kg/min) | 18.6 (5.2) | 18.3 (5.6) | 19.0 (4.8) | 0.585 a |
| VO2 AT (mL/kg/min) | 12.7 (3.5) | 12.7 (3.8) | 12.8 (3.2) | 0.863 a |
| Work at VO2 max (Watts) | 108.2 (55.4) | 102.4 (59.0) | 116.2 (50.0) | 0.296 a |
| VO2/HR at VO2 max (mL/beat) | 11.8 (3.4) | 11.8 (3.0) | 12.0 (3.9) | 0.804 a |
| VE/VCO2 at VO2 max | 30.4 (4.7) | 30.4 (5.4) | 30.5 (3.7) | 0.967 a |
| PETCO2 at VO2 max (mmHg) | 36.0 (12.0) | 35.4 (13.7) | 36.9 (9.4) | 0.606 a |
| 24 h ECG Holter monitoring | ||||
| Total PVCs in 24 h | 46.3 (124.1) | 41.5 (127.5) | 53.0 (121.0) | 0.707 a |
| Total PACs in 24 h | 804.5 (1866.5) | 620.4 (1583.8) | 1067.7 (2213.4) | 0.334 a |
| Atrial fibrillation (n) | 7 (10.1%) | 0 (0.0%) | 7 (24.1%) | 0.001 b |
| Atrial flutter (n) | 1 (1.4%) | 0 (0.0%) | 1 (3.4%) | 0.237 b |
| Minimum heart rate (bpm) | 51.1 (6.4) | 51.0 (4.8) | 51.4 (8.1) | 0.758 a |
| Maximum heart rate (bpm) | 112.2 (20.1) | 107.8 (16.6) | 118.3 (23.0) | 0.031 a |
| QTc (ms) | 443.8 (23.1) | 444.0 (21.0) | 443.4 (26.0) | 0.920 a |
| SDNN (ms) | 144.6 (38.8) | 137.6 (37.6) | 154.2 (39.1) | 0.078 a |
| SDANN (ms) | 122 (37.1) | 117.7 (34.4) | 127.9 (40.4) | 0.264 a |
| SDRR (ms) | 39.4 (14.9) | 35.4 (10.5) | 45.0 (18.2) | 0.015 a |
| HRV-TI | 30.6 (8.9) | 30.0 (8.6) | 32.1 (9.3) | 0.258 a |
| PNN50 (%) | 12.8 (14.2) | 11.3 (13.9) | 15.0 (15.0) | 0.301 a |
| RMSSD (ms) | 70.1 (55.1) | 62.2 (45.6) | 81.0 (65.4) | 0.190 a |
| SDSD (ms) | 60.1 (48.4) | 53.0 (40.0) | 70.1 (57.2) | 0.143 a |
| Deceleration capacity (ms) | 5.8 (2.8) | 5.7 (2.3) | 5.9 (3.4) | 0.733 a |
| Acceleration capacity (ms) | −7.9 (3.5) | −7.4 (2.7) | −8.6 (4.3) | 0.140 a |
| Biomarkers | ||||
| hs-cTnI (pg/mL) | 6.6 (18.4) | 8.1 (24.0) | 4.5 (4.2) | 0.413 a |
| BNP (pg/mL) | 89.1 (75.6) | 95.7 (81.1) | 79.8 (67.5) | 0.381 a |
| Hcy (μmol/L) | 12.3 (3.9) | 12.5 (4.2) | 12.1 (3.5) | 0.692 a |
| GAL3 (ng/mL) | 12.2 (3.6) | 11.4 (3.7) | 13.2 (3.4) | 0.045 a |
| 95% Conf. Interval—OR | ||||
|---|---|---|---|---|
| Variable | Odds Ratio (OR) | p-Value | Lower | Upper |
| Sex—male | 0.618 | 0.312 | 0.243 | 1.573 |
| Age (years) | 1.017 | 0.420 | 0.976 | 1.061 |
| Weight (kg) | 1.000 | 0.983 | 0.968 | 1.032 |
| Height (cm) | 1.031 | 0.270 | 0.977 | 1.087 |
| BMI (kg/m2) | 0.955 | 0.410 | 0.857 | 1.065 |
| BSA (m2) | 1.623 | 0.690 | 0.150 | 17.516 |
| Total disease duration (months) | 1.001 | 0.778 | 0.992 | 1.011 |
| Total PAF episodes (n) | 1.120 | 0.330 | 0.892 | 1.406 |
| PAF episodes in the last 6 months—Yes | 1.040 | 0.865 | 0.660 | 1.640 |
| PAF episodes in the last 1 month—Yes | 1.267 | 0.585 | 0.542 | 2.963 |
| Presence of symptoms—Yes | 1.115 | 0.908 | 0.175 | 7.112 |
| EHRA Score 2 vs. 1 3 vs. 1 | 1.209 0.476 | 0.740 0.437 | 0.394 0.073 | 3.706 3.087 |
| Thyroid disease—Yes | 0.676 | 0.496 | 0.219 | 2.085 |
| OSA—Yes | 4.800 | 0.067 | 0.898 | 25.665 |
| Smoking—Yes | 0.818 | 0.696 | 0.299 | 2.239 |
| Diabetes mellitus—Yes | 1.393 | 0.698 | 0.262 | 7.416 |
| Arterial hypertension—Yes | 1.250 | 0.639 | 0.492 | 3.176 |
| Dyslipidemia—Yes | 1.173 | 0.736 | 0.463 | 2.971 |
| Alcohol—Yes | 1.336 | 0.542 | 0.526 | 3.389 |
| Physical activity—Yes | 1.597 | 0.369 | 0.574 | 4.441 |
| Use of beta-blocker—Yes | 0.692 | 0.453 | 0.265 | 1.807 |
| Use of antiarrhythmic drugs—Yes | 1.422 | 0.459 | 0.560 | 3.614 |
| Use of beta-blocker + antiarrhythmic drugs—Yes | 1.174 | 0.740 | 0.455 | 3.025 |
| WBC (cells/mL) | 1.000 | 0.188 | 1.000 | 1.001 |
| Hb (g/dL) | 0.964 | 0.820 | 0.705 | 1.318 |
| Ht (%) | 1.016 | 0.840 | 0.872 | 1.184 |
| RDW (%) | 1.036 | 0.878 | 0.656 | 1.637 |
| PLT (platelets/mL) | 1.004 | 0.401 | 0.995 | 1.012 |
| Mg (mg/dL) | 0.333 | 0.361 | 0.031 | 3.525 |
| Fe (μg/dL) | 0.989 | 0.196 | 0.972 | 1.006 |
| UIBC (μg/dL) | 0.998 | 0.751 | 0.987 | 1.010 |
| CRP (mg/L) | 0.997 | 0.969 | 0.847 | 1.173 |
| Ferritine (ng/mL) | 0.998 | 0.565 | 0.992 | 1.005 |
| hs-cTnI (ng/mL) | 0.984 | 0.468 | 0.944 | 1.027 |
| BNP (pg/mL) | 0.997 | 0.378 | 0.991 | 1.004 |
| PCT × 1000 (ng/mL × 1000) | 1.011 | 0.658 | 0.962 | 1.063 |
| Hcy (μmol/L) | 0.975 | 0.687 | 0.862 | 1.103 |
| GAL3 (ng/mL) | 1.149 | 0.051 | 0.999 | 1.322 |
| GAL3 > 10.95 ng/mL | 6.127 | 0.001 | 2.074 | 18.103 |
| Work at AT (Watts) | 1.004 | 0.590 | 0.989 | 1.019 |
| Work at VO2 max (Watts) | 1.005 | 0.292 | 0.996 | 1.013 |
| VT at VO2 max (L) | 1.830 | 0.144 | 0.814 | 4.115 |
| VO2 AT (mL/kg/min) | 1.012 | 0.861 | 0.887 | 1.155 |
| VO2 max (mL/kg/min) | 1.026 | 0.580 | 0.938 | 1.121 |
| VO2/HR at AT (mL/beat) | 1.002 | 0.984 | 0.842 | 1.191 |
| VO2/HR at VO2 max (mL/beat) | 1.019 | 0.800 | 0.880 | 1.181 |
| VE/VCO2 at AT * | 1.017 | 0.770 | 0.906 | 1.142 |
| VE/VCO2 at VO2 max * | 1.002 | 0.966 | 0.908 | 1.106 |
| PETCO2 at AT (mmHg) | 1.008 | 0.685 | 0.971 | 1.046 |
| PETCO2 at VO2 max (mmHg) | 1.010 | 0.621 | 0.971 | 1.051 |
| GLS (%) | 1.105 | 0.334 | 0.903 | 1.352 |
| LV mass (gr) | 1.004 | 0.406 | 0.995 | 1.012 |
| LVMI (g/m2) | 1.008 | 0.430 | 0.988 | 1.028 |
| LA diameter (cm) | 1.042 | 0.919 | 0.470 | 2.310 |
| LA volume max (mL) | 0.991 | 0.467 | 0.967 | 1.015 |
| LA volume min (mL) | 0.985 | 0.397 | 0.950 | 1.021 |
| LAVI (mL/m2) | 0.978 | 0.370 | 0.931 | 1.027 |
| LA ejection fraction (%) | 1.024 | 0.352 | 0.974 | 1.077 |
| LAVpreA (mL) | 0.990 | 0.505 | 0.963 | 1.019 |
| LA strain reservoir (%) | 1.036 | 0.273 | 0.973 | 1.103 |
| LA strain conduit (%) | 1.010 | 0.830 | 0.920 | 1.110 |
| LA strain contraction (%) | 0.940 | 0.263 | 0.843 | 1.048 |
| RV FAC (%) | 0.917 | 0.020 | 0.853 | 0.986 |
| RV FWS (%) | 1.108 | 0.060 | 0.996 | 1.232 |
| RV GS (%) | 1.150 | 0.059 | 0.995 | 1.330 |
| Total PVCs in 24 h (n) | 1.001 | 0.704 | 0.997 | 1.005 |
| Total SVE in 24 h (n) | 1.000 | 0.346 | 1.000 | 1.000 |
| QTc (msec) | 0.999 | 0.918 | 0.978 | 1.020 |
| SDNN (ms) | 1.012 | 0.082 | 0.999 | 1.025 |
| SDANN (ms) | 1.008 | 0.262 | 0.994 | 1.021 |
| SDRR (ms) | 1.053 | 0.015 | 1.010 | 1.098 |
| HRVTI * | 1.033 | 0.255 | 0.977 | 1.091 |
| PNN50 (%) | 1.018 | 0.303 | 0.984 | 1.054 |
| RMSSD (ms) | 1.006 | 0.171 | 0.997 | 1.015 |
| SDNN index | 1.014 | 0.131 | 0.996 | 1.033 |
| SDSD (ms) | 1.008 | 0.152 | 0.997 | 1.018 |
| Deceleration capacity (ms) | 1.032 | 0.729 | 0.865 | 1.231 |
| Acceleration capacity (ms) | 0.888 | 0.177 | 0.748 | 1.055 |
| 95% Conf. Interval—OR | ||||
|---|---|---|---|---|
| Variable | Odds Ratio (OR) | p-Value | Lower | Upper |
| GAL3 > 10.95 ng/mL | 5.206 | 0.006 | 1.618 | 16.748 |
| RV FAC (%) | 0.927 | 0.062 | 0.856 | 1.004 |
| SDRR (ms) | 1.061 | 0.021 | 1.009 | 1.116 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boulmpou, A.; Moysiadis, T.; Zormpas, G.; Teperikidis, E.; Tsioni, K.; Toumpourleka, M.; Zidrou, M.; Giannakoulas, G.; Vassilikos, V.; Papadopoulos, C. Integrated Diagnostics for Atrial Fibrillation Recurrence: Exploratory Results from the PLACEBO Trial. Diagnostics 2025, 15, 1105. https://doi.org/10.3390/diagnostics15091105
Boulmpou A, Moysiadis T, Zormpas G, Teperikidis E, Tsioni K, Toumpourleka M, Zidrou M, Giannakoulas G, Vassilikos V, Papadopoulos C. Integrated Diagnostics for Atrial Fibrillation Recurrence: Exploratory Results from the PLACEBO Trial. Diagnostics. 2025; 15(9):1105. https://doi.org/10.3390/diagnostics15091105
Chicago/Turabian StyleBoulmpou, Aristi, Theodoros Moysiadis, Georgios Zormpas, Eleftherios Teperikidis, Konstantina Tsioni, Maria Toumpourleka, Maria Zidrou, Georgios Giannakoulas, Vassilios Vassilikos, and Christodoulos Papadopoulos. 2025. "Integrated Diagnostics for Atrial Fibrillation Recurrence: Exploratory Results from the PLACEBO Trial" Diagnostics 15, no. 9: 1105. https://doi.org/10.3390/diagnostics15091105
APA StyleBoulmpou, A., Moysiadis, T., Zormpas, G., Teperikidis, E., Tsioni, K., Toumpourleka, M., Zidrou, M., Giannakoulas, G., Vassilikos, V., & Papadopoulos, C. (2025). Integrated Diagnostics for Atrial Fibrillation Recurrence: Exploratory Results from the PLACEBO Trial. Diagnostics, 15(9), 1105. https://doi.org/10.3390/diagnostics15091105









