We read with interest the excellent review manuscript from Huang, S.-W. and Liu, Y.-K. [1], which describes that pediatric chest pain is a common chief complaint in the emergency department. Not surprisingly, children with chest pain are usually brought to the emergency department by their parents out of fear of heart disease. However, chest pain in the pediatric population is generally a benign disease. In this review, we have identified musculoskeletal pain as the most prevalent etiology of chest pain in the pediatric population, accounting for 38.7–86.3% of cases, followed by pulmonary (1.8–12.8%), gastrointestinal (0.3–9.3%), psychogenic (5.1–83.6%), and cardiac chest pain (0.3–8.0%). Various diagnostic procedures for cardiac chest pain are commonly used in the emergency department, including electrocardiogram (ECG), chest radiography, cardiac troponin examination, and echocardiography. However, these examinations demonstrate limited sensitivity in identifying cardiac etiologies, with sensitivities ranging from 0 to 17.8% for ECG and 11.0 to 17.2% for chest radiography. To avoid the overuse of these diagnostic tools, a well-designed standardized algorithm for pediatric chest pain could decrease unnecessary examination without missing severe diseases [2,3,4]. Our primary concern is that no attention has been given to exercise-induced intraventricular gradients, which are easily detectable using exercise stress echocardiography and have been associated with chest pain and other symptoms [5,6,7,8,9,10,11,12,13], including in children. We present the case of a 15-year-old boy, a rugby player, who experienced severe chest pain followed by syncope during a match. Upon evaluation at the emergency department, he showed a significant increase in troponin levels. Coronary angiography (Figure 1) and CT angiography (Figure 2) revealed normal results. However, an exercise stress echocardiogram identified a significant intraventricular gradient (Figure 3).
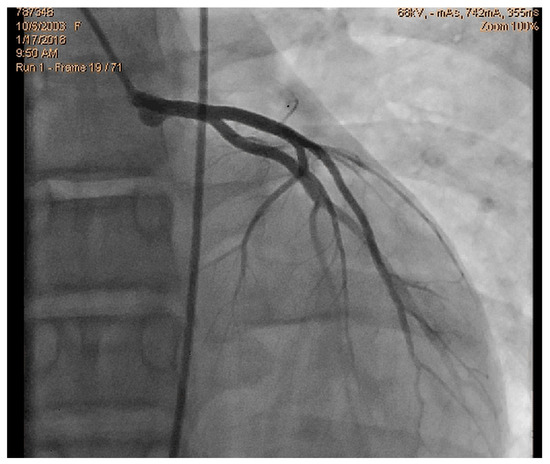
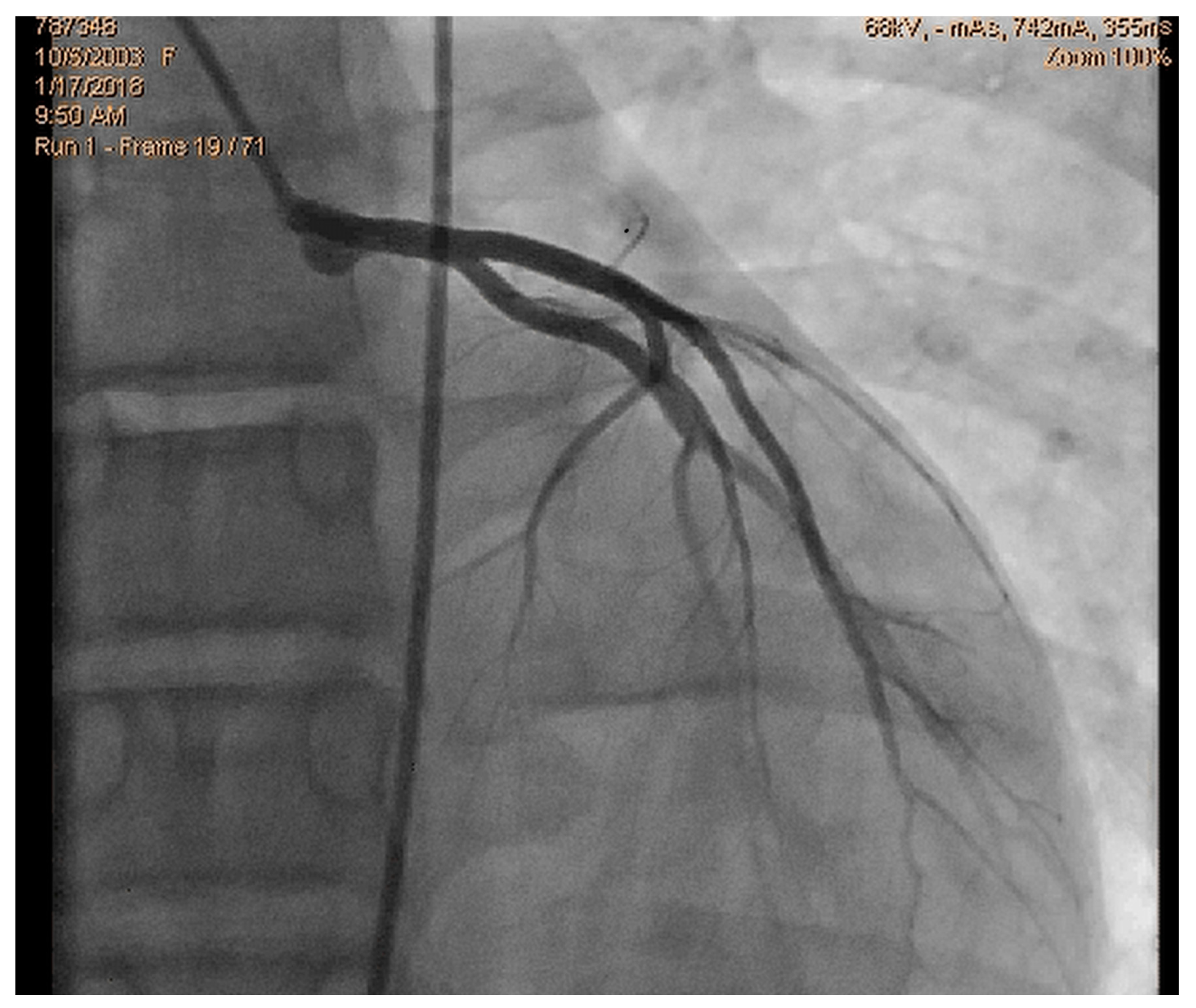
Figure 1.
Normal coronary angiography.
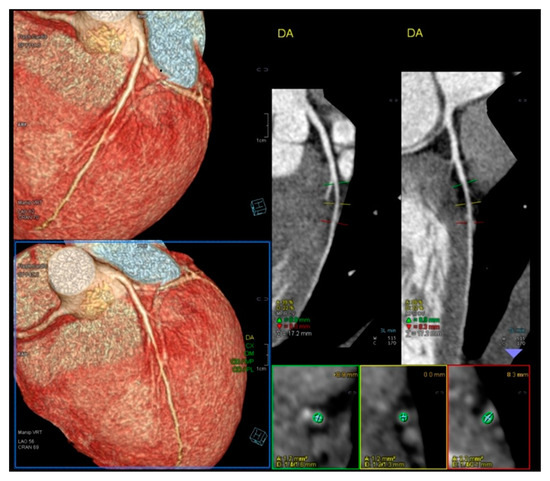
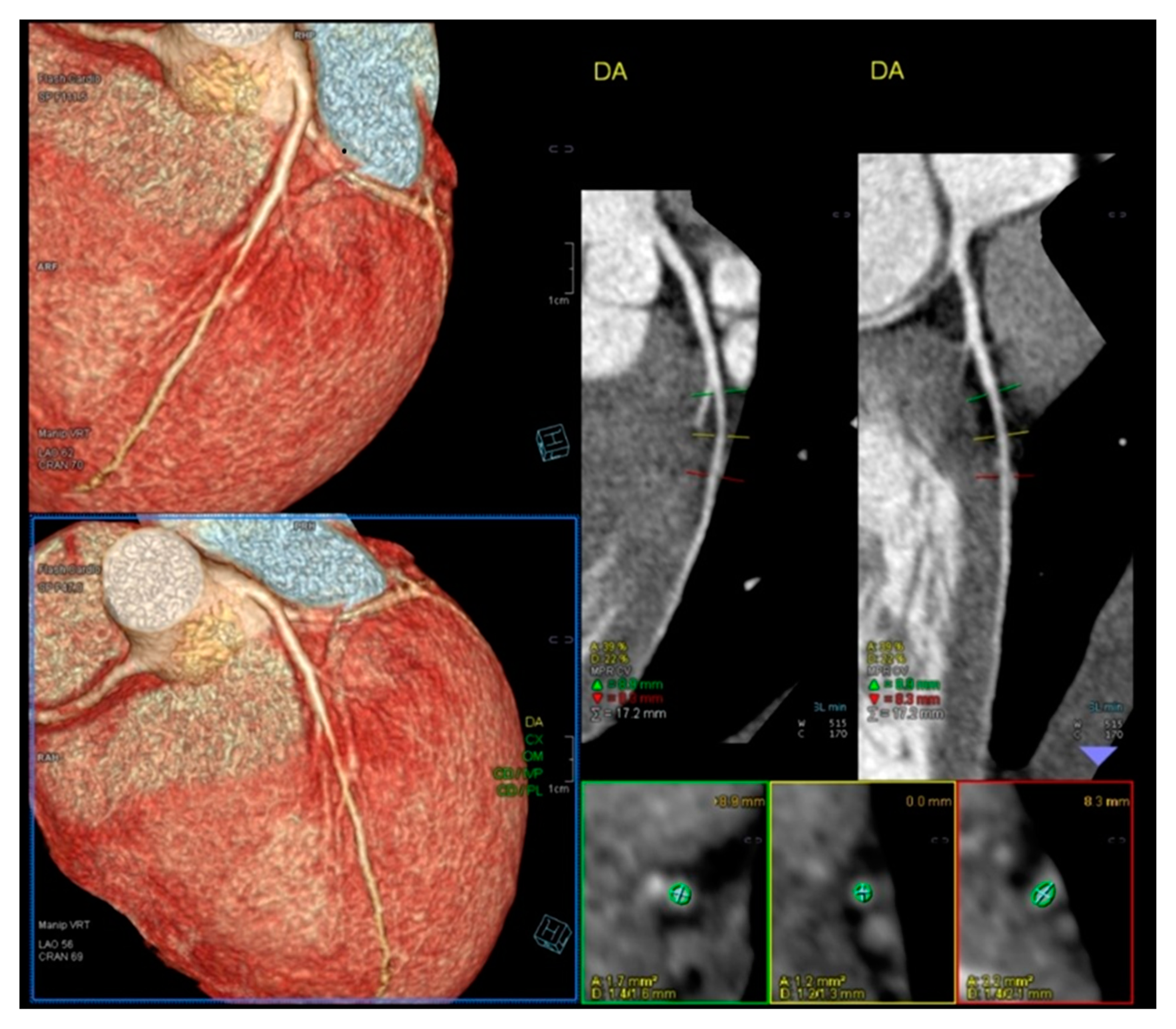
Figure 2.
Normal Angio TC of coronary arteries.
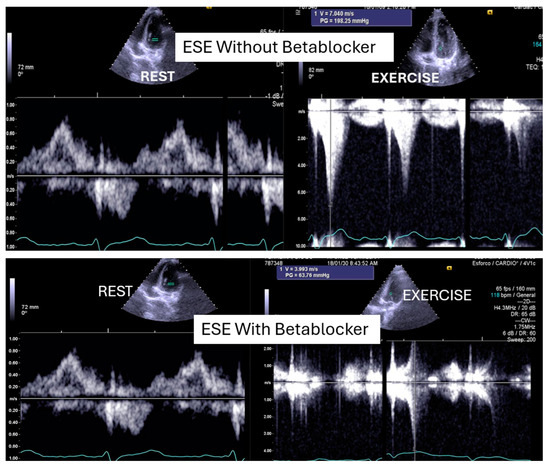
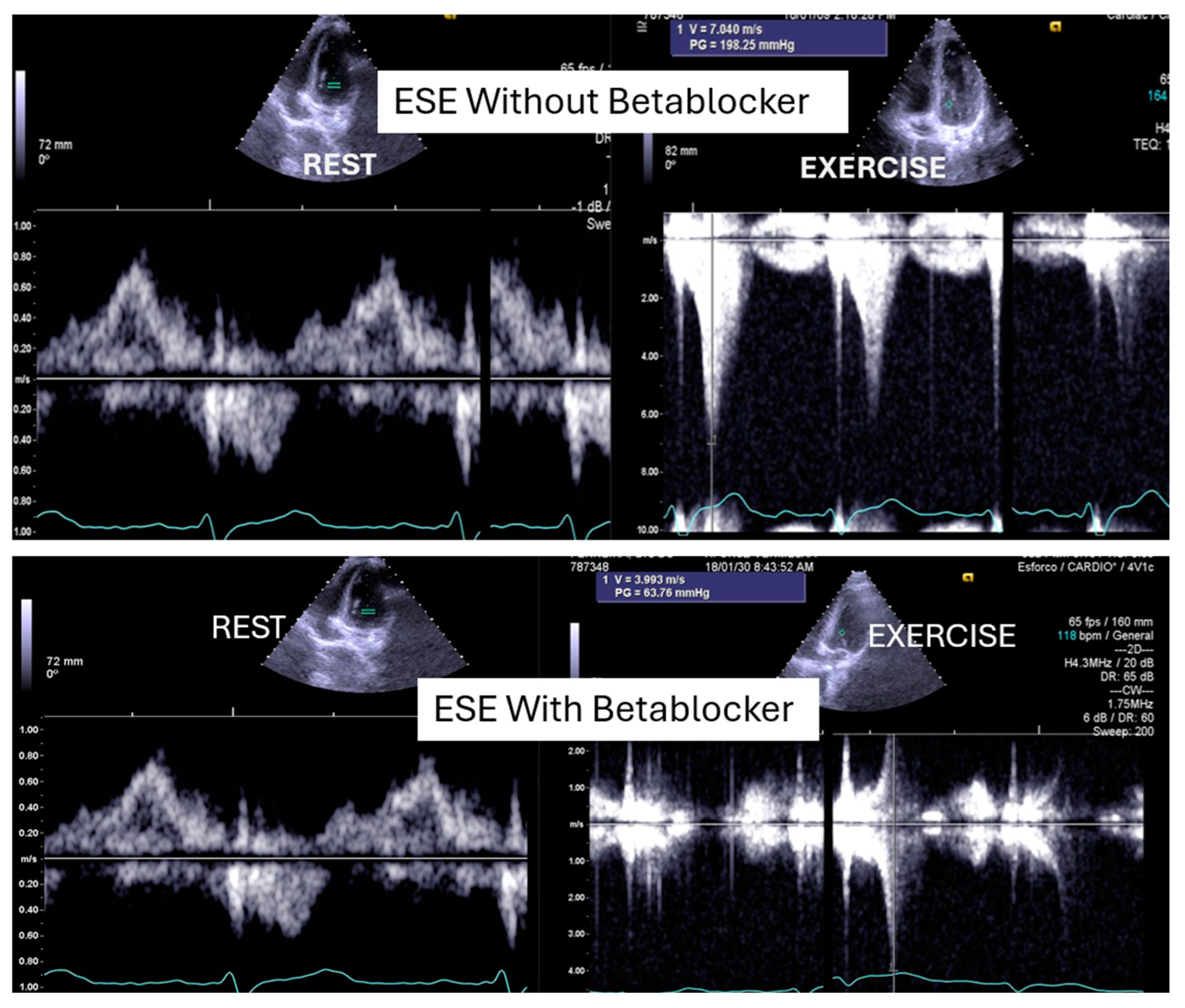
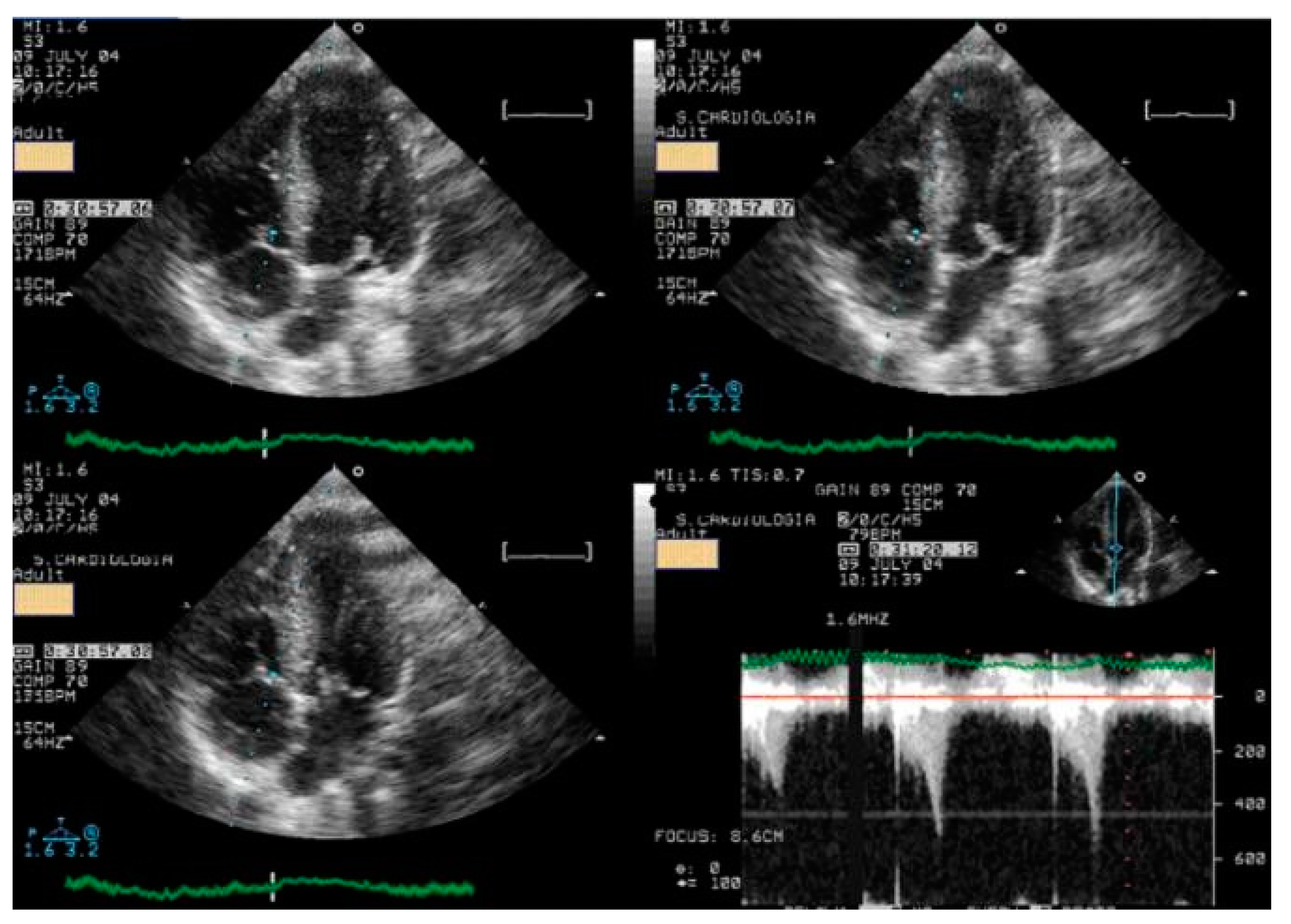
Figure 3.
An intraventricular-induced gradient in one child with chest pain followed by syncope during a rugby match. In the upper image, there is a huge intraventricular gradient and in the lower image, there is a small intraventricular gradient under treatment with bisoprolol.
This was considered the most likely cause of the clinical event. This test was repeated under bisoprolol therapy. In our experience with 139 athletes [11], 58 (41%) were under 18 years old—46 of whom were evaluated for exercise-related symptoms—and 20 (34%) developed an intraventricular gradient during exercise. We strongly advocate for exercise stress echocardiography to be considered for children presenting with exercise-related symptoms in the emergency department at the appropriate time. According to our experience [8], approximately 40% of children with clear exercise-related symptoms, like angina, dizziness, syncope, ST alterations in ECG, or ST alterations in exercise stress ECG (Figure 4), develop mid-ventricular obstruction (MVO) (Figure 5), which appears to be a relatively high prevalence; we recognize this warrants further explanation regarding the mechanisms of development and relationship to chest pain.
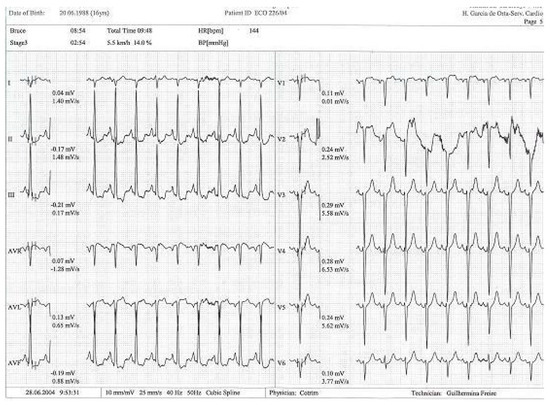
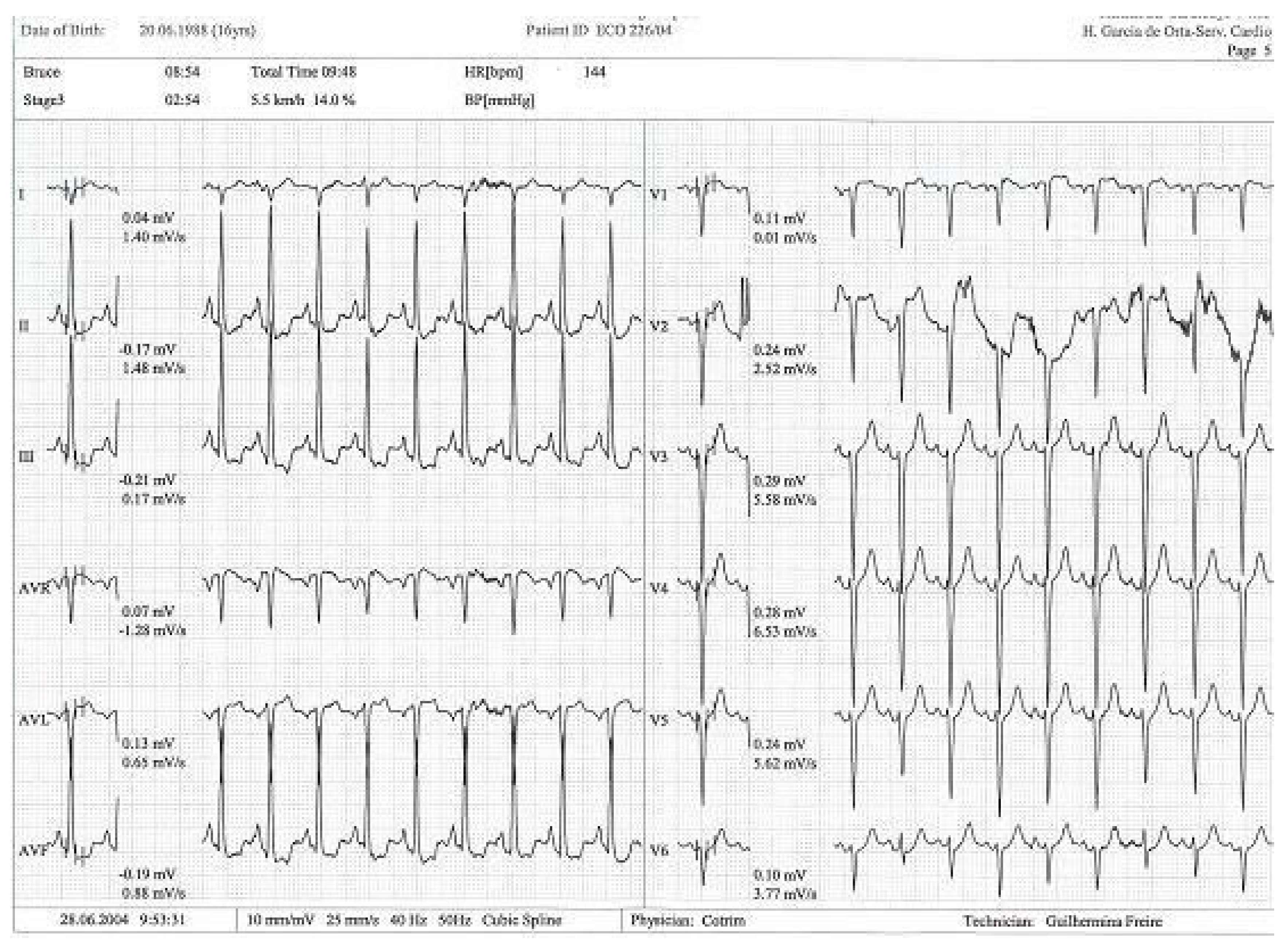
Figure 4.
Exercise test with alteration in ST segment in DII, DIII, and avF [14].
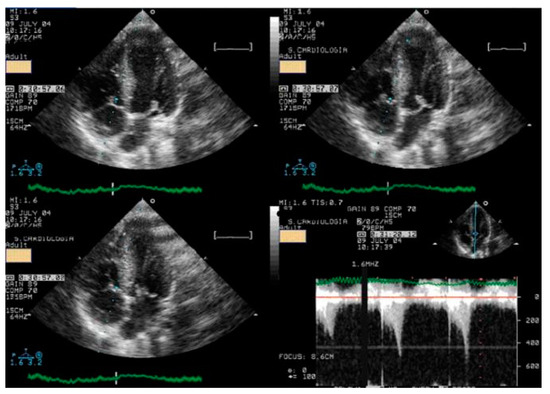
Figure 5.
At peak exercise, systolic anterior movement of mitral valve and significant intraventricular gradient was detected [14].
In our experience and in the literature [5,6,7,8,9,10,11,12,13,14,15,16], chest pain (exercise angina) has been related to an anatomically small LV chamber, small LVOT, and to an increased relative wall thickness. Additionally, a certain level of hypohydration—characterized by a reduction in left ventricular volumes and commonly linked to intense exercise—may be a potential contributing factor to MVO.As most of the children were referred by other centers, these children were not systematically followed up longitudinally. However, it is our knowledge that four have participated in the genetic study for myocardiopathy and one developed HCM [10]. The increase in intraventricular pressure causing perturbation in subendocardial perfusion is the potential mechanism for ischemia, chest pain, and ST alterations [15,16]. Furthermore, using beta-blockers in children without structural cardiac abnormalities remains a controversial approach. The use of beta-blockers [17,18] is recommended and suitable for pediatric arrhythmias, hypertension, heart failure, hypertrophic cardiomyopathy, migraine prophylaxis, hyperthyroidism, and infantile hemangiomas. Beta-adrenergic receptor antagonists, commonly known as beta-blockers, are divided into three generations based on their receptor selectivity. First-generation beta-blockers (e.g., propranolol) are non-selective and block both β1 and β2 receptors. Second-generation beta-blockers (e.g., metoprolol) are relatively selective for the β1 receptor, while third-generation beta blockers (e.g., carvedilol) block β1, β2, and α1 receptors. Beta-blockers are frequently used to treat adult cardiac conditions, such as hypertension, atrial arrhythmias, and chronic heart failure. Similarly, they are considered a first-line treatment for many pediatric tachyarrhythmias, both in non-operative and peri-operative settings [19]. However, despite their widespread use in children, there is a significant lack of pediatric-specific data to determine precise dosing and personalized treatment. As a result, most pediatric treatment decisions are based on data extrapolated from adult studies. The most commonly prescribed oral beta-blockers for children include atenolol, carvedilol, metoprolol, propranolol, and bisoprolol [19]. The use of beta-blockers is recommended for adult patients with exercise-induced IVPG, whether or not they have hypertrophic cardiomyopathy [20,21,22,23,24]. Based on both our findings and the existing literature, we suggest that children would also benefit from the same treatment (Figure 3).
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huang, S.-W.; Liu, Y.-K. Pediatric Chest Pain: A Review of Diagnostic Tools in the Pediatric Emergency Department. Diagnostics 2024, 14, 526. [Google Scholar] [CrossRef]
- Verghese, G.R.; Friedman, K.G.; Rathod, R.H.; Meiri, A.; Saleeb, S.F.; Graham, D.A.; Geggel, R.L.; Fulton, D.R. Resource Utilization Reduction for Evaluation of Chest Pain in Pediatrics Using a Novel Standardized Clinical Assessment and Management Plan (SCAMP). J. Am. Heart Assoc. 2012, 1, jah3-e000349. [Google Scholar] [CrossRef]
- Saleeb, S.F.; McLaughlin, S.R.; Graham, D.A.; Friedman, K.G.; Fulton, D.R. Resource reduction in pediatric chest pain: Standardized clinical assessment and management plan. Congenit. Heart Dis. 2018, 13, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Kane, D.A.; Friedman, K.G.; Fulton, D.R.; Geggel, R.L.; Saleeb, S.F. Needles in Hay II: Detecting Cardiac Pathology by the Pediatric Chest Pain Standardized Clinical Assessment and Management Plan. Congenit. Heart Dis. 2016, 11, 396–402. [Google Scholar] [CrossRef]
- Cotrim, C.; Palinkas, E.D.; Cotrim, N. The Importance of Left Ventricular Outflow Tract and Mid-Ventricular Gradients in Stress Echocardiography: A Narrative Review. J. Clin. Med. 2023, 12, 5292. [Google Scholar] [CrossRef] [PubMed]
- Cotrim, C.; Picano, E. Step G for Gradients in Stress Echocardiography. In Stress Echocardiography; Picano, E., Ed.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Lopes, A.; Cotrim, C.; Martins, J.D.; Pinto, F. Exercise-induced intraventricular obstruction in a child with near syncope and chest pain during exercise. Pediatr. Cardiol. 2011, 32, 1032–1035. [Google Scholar] [CrossRef]
- Cotrim, N.; Café, H.M.; Guardado, J.; Cordeiro, P.; Cotrim, H.; Martins, R.; Baquero, L.; Cotrim, C. Clinical Application of Exercise Stress Echocardiography in an Outpatient Pediatric Population. J. Clin. Med. 2024, 13, 2191. [Google Scholar] [CrossRef] [PubMed]
- Cotrim, C.; Almeida, A.G.; Carrageta, M. Exercise-induced intra-ventricular gradients as a frequent potential cause of myocardial ischemia in cardiac syndrome X patients. Cardiovasc. Ultrasound. 2008, 6, 3. [Google Scholar] [CrossRef]
- Cotrim, N.; Castilho, B.; Cotrim, C.; Guardado, J.; Baquero, L. An Unexpected Finding in an Adolescent Rowing Athlete With Angina Pectoris—A Case Report. Clin. J. Sport Med. 2024, 35, 242–245. [Google Scholar] [CrossRef]
- Lopes, L.R.; Cotrim, C.; Cruz, I.; Picano, E.; Pinto, F.; Pereira, H. Left ventricular outflow tract obstruction as a primary phenotypic expression of hypertrophic cardiomyopathy in mutation carriers without hypertrophy. Int. J. Cardiol. 2014, 176, 1264–1267. [Google Scholar] [CrossRef]
- Cotrim, C.; Almeida, A.R.; Miranda, R.; Almeida, A.G.; Cotrim, H.; Picano, E.; Carrageta, M. Stress-induced intraventricular gradients in symptomatic athletes during upright exercise continuous wave Doppler echocardiography. Am. J. Cardiol. 2010, 106, 1808–1812. [Google Scholar] [CrossRef] [PubMed]
- Cotrim, C.; Lopes, L.R.; Almeida, A.R.; Miranda, R.; Ana, A.G.; Cotrim, H.; Andrade, J.P.; Carrageta, M. Efficacy of beta-blocker therapy in symptomatic athletes with exercise-induced intra-ventricular gradients. Cardiovasc. Ultrasound. 2010, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Cotrim, C.; Almeida, A.G.; Carrageta, M. Clinical significance of intraventricular gradient during effort in an adolescent karate player. Cardiovasc. Ultrasound. 2007, 5, 39. [Google Scholar] [CrossRef]
- Cabrera-Bueno, F.; Gómez-Doblas, J.J.; Muñoz-García, A.; García-Pinilla, J.M.; Navarro, M.J.; de Teresa-Galván, E. Effort angina, normal coronary angiogram, and dynamic left ventricular obstruction. J. Am. Soc. Echocardiogr. 2007, 20, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.A.; Ashkir, Z.; Raman, B.; Bueno-Orovio, A. Mechanisms and prognostic impact of myocardial ischemia in hypertrophic cardiomyopathy. Int. J. Cardiovasc. Imaging. 2023, 39, 1979–1996. [Google Scholar] [CrossRef]
- Walton, M.; Wagner, J.B. Pediatric Beta Blocker Therapy: A Comprehensive Review of Development and Genetic Variation to Guide Precision-Based Therapy in Children, Adolescents, and Young Adults. Genes 2024, 15, 379. [Google Scholar] [CrossRef]
- Schranz, D. Can Pediatric Heart Failure Therapy Be Improved? Yes, It Can, But…. Paediatr. Drugs. 2022, 24, 567–571. [Google Scholar] [CrossRef]
- Cabrera-Bueno, F.; García-Pinilla, J.M.; Gómez-Doblas, J.J.; Montiel-Trujillo, A.; Rodríguez-Bailón, I.; de Teresa-Galván, E. Beta-blocker therapy for dynamic left ventricular outflow tract obstruction induced by exercise. Int. J. Cardiol. 2007, 117, 222–226. [Google Scholar] [CrossRef]
- Lau, T.K.; Navarijo, J.; Stainback, R.F. Pseudo-False-Positive exercise treadmill testing. Tex Heart Inst. J. 2001, 28, 308–311. [Google Scholar]
- Al-Nasser, F.; Duncan, A.; Sharma, R.; O’Sullivan, C.; Coats, A.J.S.; Anker, S.D.; Henein, M.Y. Beta-blocker therapy for dynamic left-ventricular outflow tract obstruction. Int. J. Cardiol. 2002, 86, 199–205. [Google Scholar] [CrossRef]
- Östman-Smith, I.; Wettrell Göran Riesenfelf, T. A cohort study of childhood hypertrophic cardiomyopathy. Improved survival following high-dose beta-adrenoceptor antagonist treatment. J. Am. Coll. Cardiol. 1999, 34, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Ommen, S.R.; Ho, C.Y.; Asif, I.M.; Balaji, S.; Burke, M.A.; Day, S.M.; Dearani, J.A.; Epps, K.C.; Evanovich, L.; Ferrari, V.A.; et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR Guideline for the Management of Hypertrophic Cardiomyopathy: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1239–e1311, Erratum in Circulation 2024, 150, e198. https://doi.org/10.1161/CIR.0000000000001277. [Google Scholar] [CrossRef] [PubMed]
- Nanni, U.; Ferroni, P.; Riondino, S.; Spila, A.; Valente, M.G.; Del Monte, G.; Roselli, M.; Guadagni, F. Convention for the Protection of Human Rights and Dignity of the Human Being with Regard to the Application of Biology and Medicine: Convention on Human Rights and Biomedicine. J. Med. Philos. 2000, 25, 259–266. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).