Abstract
Polydimethylsiloxane (PDMS) is a polymer material characterized by its flexibility, biocompatibility, non-toxicity, excellent stability, and high transparency. It is also easy to process and allows for control over its physical properties. However, its inherent hydrophobicity limits its application in certain fields. To address this limitation, research is being conducted to modify the surface properties of PDMS through polymer grafting. In this work, poly(ethylene glycol) methyl ether methacrylate (mPEG-MA) was grafted onto the PDMS surface to convert its hydrophobic characteristics to hydrophilicity. The tribological properties of the modified PDMS were then evaluated under conditions of hydrophilicity and water lubrication. Polymer grafting was performed by generating radicals on the surface of PDMS through ultraviolet (UV) irradiation using a photoinitiator, followed by grafting with mPEG-MA. The water contact angle, which serves as an indicator of hydrophilicity, was measured and revealed a decrease in the contact angle as the conditions for mPEG-MA grafting were intensified, signifying an increase in hydrophilicity. Additionally, the tribological properties under water lubrication improved with a higher degree of mPEG-MA grafting. Notably, PDMS grafted with a 20 wt.% mPEG-MA aqueous solution via UV irradiation for 12 h consistently maintained a coefficient of friction (COF) of less than 0.02 under water lubrication. Surface damage was observed locally in the dimples only under a load of 3 N.
1. Introduction
Polydimethylsiloxane (PDMS) has various properties such as non-toxicity [], transparency, flexibility, elasticity [], biocompatibility, hydrophobicity [], and insulation. Its ease of manufacturing and adaptability in terms of shape and physical characteristics further enhance its utility [,]. Consequently, PDMS is extensively utilized across various fields, including microfluidics, microneedles, biology, medicine, chemistry, and optics []. Especially, due to its flexibility, biocompatibility, non-toxicity, exceptional stability, and high transparency, PDMS is an ideal candidate for applications in biomedical devices, optical inspection, and wearable electronics. However, the hydrophobic surface of PDMS can exhibit cytotoxicity under certain conditions and has a high friction coefficient in a water-lubricated environment [,,,]. Therefore, it is essential to modify the surface to a hydrophilic one, depending on the specific environment in which PDMS is utilized [,,,,]. Changing the PDMS surface from hydrophobic to hydrophilic can significantly enhance its friction properties in water-based lubrication or in vivo environments [,,,]. Water-based lubricants are not only cost-effective but also more environmentally friendly compared to oil-based lubricants. Therefore, they offer advantages in various environments, such as in vivo settings and low-load systems [,,].
To modify the surface properties of PDMS, a technique involving the application of another polymer with the desired characteristics is used. Methods for coating one polymer onto the surface of another include using covalent bonding reactions or employing electrostatic self-assembly in a layer-by-layer approach [,,]. In the case of polymer coatings formed layer by layer through electrostatic self-assembly, ultraviolet (UV) light is used to induce covalent bonds between the polymers, thereby enhancing the stability of the coating [,,,]. One method to induce covalent bond reactions on polymer surfaces, thereby combining polymers with different properties, is polymer grafting [,,,]. Polymer grafting involves the creation of radicals on the surface of a polymer to facilitate the coating with a desired polymer. Techniques such as plasma treatment, ozone treatment, and UV irradiation are used to activate these radicals [,]. In the case of UV irradiation methods, radicals are generated on the polymer surface through a chemical reaction involving a photoinitiator [,,]. Polymer grafting can be categorized into two main types: ‘grafting-from’ and ‘grafting-to’. The process of reacting radicals generated on the polymer surface with a monomer that possesses the desired properties is referred to as the ‘grafting-from’ method. Conversely, the technique of coating by reacting these radicals with functional end groups is known as the ‘grafting-to’ method [,]. When hydrophilicity is required on polymer surfaces, poly(ethylene glycol) (PEG) can be utilized through grafting techniques. PEG has excellent biocompatibility due to its low toxicity, flexibility, and hydrophilic properties. In addition, PEG has the property of preventing opsonization, which allows polymers with low biocompatibility to continue to function in a biological environment through PEG grafting [,,,,]. Based on these properties, PEG is suitable for biomedical research, chemical analysis, cosmetics, and lubrication in moist environments. In PEG grafting, the molecular weight of PEG can be adjusted to control the properties of the resulting polymer. Low molecular weight PEG exhibits uniformity and smoothness, with film thickness equal to the molecular length [].
The motivation of this work is to develop a hydrophilic PDMS surface that demonstrates a low friction coefficient and high durability under water-based lubrication, particularly in the fields of biomedical engineering or polymer material mechanical systems. To change the hydrophobicity of the PDMS surface to hydrophilicity, poly(ethylene glycol) methyl ether methacrylate (mPEG-MA), a PEG-based polymer, was grafted onto the surface. Subsequently, the tribological properties of PDMS grafted with mPEG-MA were analyzed under water lubrication. A water contact angle test was conducted to evaluate hydrophilicity based on the conditions for grafting mPEG-MA onto the PDMS surface. The friction coefficient and surface damage were analyzed under water lubrication to examine the tribological properties in relation to the grafting conditions of mPEG-MA. Additionally, the tribological properties under water lubrication were evaluated according based on varying sliding speeds and loads. As a result, we identified mPEG-MA grafting conditions on PDMS that maintain stable tribological properties across various sliding speeds and loading conditions under water lubrication.
2. Materials and Methods
2.1. Fabrication of Specimen Plates and Hemispheres Using PDMS
In this work, PDMS was selected as the material for the water-lubricated friction test. PDMS specimens of appropriate size for a pin-on-disk friction tester along with PDMS hemispheres to serve as counter surfaces, were manufactured. First, the following procedure was followed to produce a flat PDMS plate. The PDMS prepolymer (Sylgard 184, Dow Corning, Midland, MI, USA) was mixed at its curing agent at a weight ratio of 10:1. After removing the bubbles from the mixture, the PDMS was poured into a petri dish and heated in a dry oven at 75 °C for 2 h. The thickness of the finished PDMS specimen was adjusted to 4–5 mm.
Next, the PDMS hemisphere was fabricated following the procedure outlined below. Figure 1 (left) shows a schematic diagram of the PDMS hemisphere fabrication process. In the first step, a hemisphere mold made of PDMS was fabricated to create a PDMS hemisphere with a diameter of 6 mm for use in a pin-on-disk friction tester. A 6 mm diameter stainless steel ball was placed on a PDMS mixture, prepared at a mass ratio of 10:1, and heated in a dry oven at 75 °C for 2 h. After heating, the steel ball was removed to complete the hemisphere mold. The finished mold was then coated with a 50 nm layer of gold, which served as an anti-sticking layer for demolding the PDMS hemisphere. The PDMS mixture, prepared at a mass ratio of 10:1, was poured into the gold-coated hemisphere mold and heated at 75 °C for 2 h. After heating, the PDMS hemisphere was removed from the mold. Figure 1 (right) shows an image of the completed PDMS plate and hemisphere, created following the procedure outlined above.
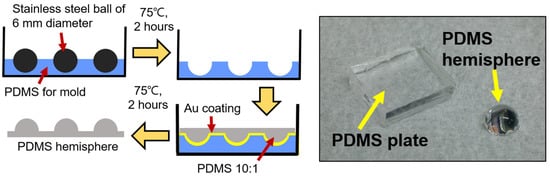
Figure 1.
(left) Schematic diagram of the process for fabricating a PDMS hemisphere and (right) images of the completed PDMS plate and hemisphere.
2.2. Grafting mPEG-MA onto PDMS
To make the PDMS surface hydrophilic, a polymer grafting technique utilizing a PEG-based polymer was employed. The ‘grafting-to’ method was used to easily control the molecular weight of grafted PEG. For this purpose, mPEG-MA, which contains a methacrylate structure that can react with surface radicals, was purchased from Sigma-Aldrich (Steinheim, Germany) and used as the grafting material. The molecular weight of mPEG-MA was determined to be 300 to ensure uniformity in the grafted coating. Figure 2 presents a schematic diagram of the polymer grafting process used in this work. mPEG-MA grafting was performed in two steps: surface activation and polymer grafting. In the surface activation step, UV irradiation was employed to form radicals on the PDMS surface, using Irgacure 2959 (BASF) as a photoinitiator. The PDMS plate and hemisphere were immersed in a 0.5% wt. aqueous solution of Irgacure 2959 and then exposed to a UV lamp with a wavelength of 254 nm for 2 h. Irgacure 2959 is activated by UV light reacts with the PDMS surface to generate radicals. Afterward, polymer grafting was conducted using mPEG-MA. After immersing the PDMS plate and hemisphere from the previous step in the mPEG-MA aqueous solution, they were exposed to a UV lamp with a wavelength of 254 nm to facilitate the reaction between the radicals on the PDMS surface and mPEG-MA. To assess the surface properties of PDMS under different grafting conditions of mPEG-MA, the grafting parameters were established as shown in the Table 1.
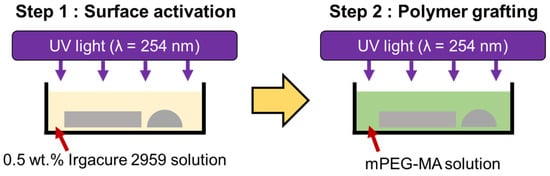
Figure 2.
Schematic diagram of the mPEG-MA grafting process.

Table 1.
Grafting conditions of mPEG-MA on PDMS.
3. Experimental Details
3.1. Hydrophilicity Measurement and Water-Lubricated Friction Experiment
To evaluate the surface hydrophilicity resulting from changes in the grafting conditions of mPEG-MA on the PDMS surface, water contact angle measurements were performed. This method is simple and convenient for quantifying the hydrophilicity of material surfaces. Generally, a water contact angle greater than 90 degrees indicates a hydrophobic surface, while an angle less than 90 degrees indicates a hydrophilic surface [,]. To measure the water contact angle, specimens of bare PDMS, 10% 12H, 10% 18H, 10% 24H, 20% 12H, 20% 18H, and 20% 24H. A water droplet of approximately 0.05 cc was placed on the surface of each specimen, and the angle formed between the specimen surface and the water droplet was measured using an optical device.
Subsequently, a friction test was performed using a pin-on-disk type tribometer (CSM Instruments, Peseux, Switzerland) to investigate the water lubrication characteristics based on the mPEG-MA grafting conditions. Figure 3 is an image of the pin-on-disk type tribometer and PDMS specimen used in this work. The maximum allowable vertical load and rotation speed of the pin-on-disk tribometer used in this work are 30 N and 550 rpm, respectively. The specimens used in the friction test included bare PDMS, 10% 12H, 10% 18H, 10% 24H, 20% 12H, 20% 18H, and 20% 24H, along with a 6-mm-diameter PDMS hemisphere manufactured under the same conditions as the specimens, which served as the counter surface. Deionized water was used as the lubricant in the water-lubricated environment. The friction test conditions were set to a load of 1 N, a sliding speed of 1 mm/s, and 100 cycles at room temperature. These normal load and sliding speed conditions were established with reference to previous studies that conducted water-lubricated friction tests on hydrophilic polymers [,,]. To ensure the repeatability of the experimental data, the friction test was conducted three times for each condition. After the friction tests were completed, damage to the specimen surface was assessed using a 3D laser confocal microscope (VK-X210, Keyence, Osaka, Japan).

Figure 3.
Pin-on-disk type tribometer and a mounted PDMS specimen.
3.2. Load-Dependent and Speed-Dependent Water Lubrication Experiments on mPEG-MA Grafted PDMS
Based on the results of the water lubrication tests of PDMS under the previously performed mPEG-MA grafting conditions, the grafting conditions that effectively exhibited the water lubrication effect were selected. The friction characteristics under water lubrication were evaluated by varying the load and sliding speed for both bare PDMS and PDMS grafted with a 20% mPEG-MA solution via UV irradiation for 12 h (20% 12H specimen). The friction test was conducted using the same pin-on-disk tribometer as in the previous test, with deionized water serving as the lubricant in a water-lubricated environment at room temperature. The test conditions were set to vertical loads of 1, 2, and 3 N, while the sliding speed was varied at 1, 5, 10, 50, and 100 mm/s for each load condition. A 100-cycle friction test was performed under these parameters. These normal load and sliding speed conditions were established with reference to previous studies that conducted water-lubricated friction tests on hydrophilic polymers [,,].
3.3. Durability Evaluation of mPEG-MA Grafted PDMS Under Water Lubrication
To evaluate the durability of the 20% 12H specimens under water lubrication, friction tests were conducted at load conditions of 1, 2, and 3 N, with a sliding speed of 10 mm/s and a total of 1000 cycles. Surface damage was assessed after the tests. Friction tests were conducted at room temperature using the pin-on-disk friction tester, with deionized water serving as the lubricant. To ensure the repeatability of the experimental data, each condition was tested three times. After completing the friction tests, the 3D laser confocal microscope was used to measure the damage on the specimen surface.
4. Results and Discussion
4.1. Hydrophilicity and Water Lubrication Properties
To evaluate the hydrophilicity of the PDMS surface grafted with mPEG-MA, the water contact angle was measured. Figure 4 shows the results of the water contact angle measurements for bare PDMS, 10% 12H, 10% 18H, 10% 24H, 20% 12H, 20% 18H, and 20% 24H specimens. The contact angles were measured as follows: 109.6 degrees for bare PDMS, 54.8 degrees for 10% 12H, 46 degrees for 10% 18H, 36.9 degrees for 10% 24H, and 24 degrees for 20% 12H, 20% 18H and 20% 24H. Generally, a water contact angle of less than 90 degrees is classified as hydrophilic [,]. Therefore, it was confirmed that the PDMS surface became hydrophilic through the grafting of mPEG-MA. Additionally, the results showed that the water contact angle decreased as the concentration of the mPEG-MA aqueous solution and the UV irradiation time increased. This indicates that hydrophilicity improved with stronger mPEG-MA grafting conditions.
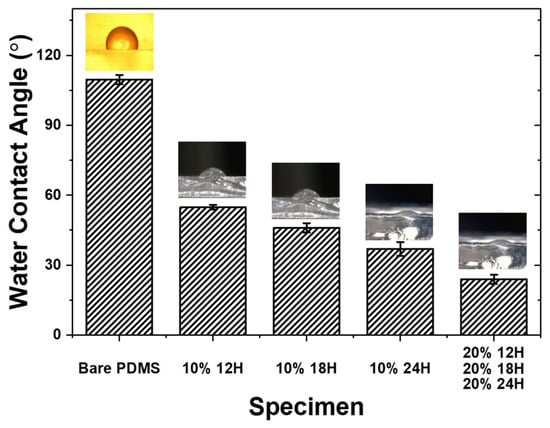
Figure 4.
Water contact angle of bare PDMS and PDMS grafted with mPEG-MA.
Next, the evaluation of water lubrication for PDMS according to the mPEG-MA grafting conditions was conducted. The friction tests were performed under the following conditions: a load of 1 N, a sliding speed of 1 mm/s, and 100 cycles at room temperature, using deionized water as a lubricant. Figure 5 presents the coefficient of friction (COF) graph for bare PDMS and PDMS grafted with mPEG-MA, measured under water lubrication. The average COF for the water-lubricated friction test was measured at 0.34 for the bare PDMS specimen. The highest COF was recorded at 0.5 for the 10% 12H specimen, followed by 0.3 for the 10% 18H specimen and 0.25 for the 10% 24H specimen, indicating a decrease in average COF with increasing UV exposure time. The 20% 12H, 20% 18H, and 20% 24H specimens exhibited low friction coefficients of 0.02, 0.03, and 0.02, respectively.
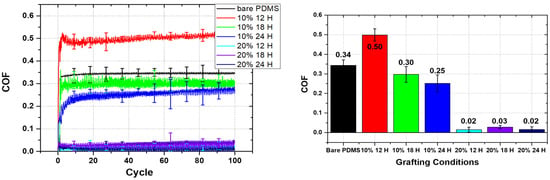
Figure 5.
COF of bare PDMS and PDMS grafted with mPEG-MA performed at 1 N and 1 mm/s under water lubrication (left), and average COF of each specimens (right).
Figure 6 shows the surface image of a PDMS specimen after a water-lubricated friction test, captured using a 3D laser confocal microscope. No surface damage was observed on the bare PDMS specimens. For the 10% 12H specimens, cracks and tearing occurred most frequently after 100 cycles of friction testing. The 10% 18H specimens showed cracks and slight tearing, while the 10% 24H specimens exhibited only cracks. In contrast, no surface damage, such as cracks or tearing, was observed on the 20% 12H, 20% 18H, and 20% 24H specimens. For the 10% 12H, 10% 18H, and 10% 24H specimens, where surface damage was observed, the COF increased significantly during the initial cycles of the water lubrication test, as shown in the COF graph in Figure 4. This increase is attributed to the insufficient durability of the grafted mPEG-MA layer, which led to cracks or tearing. These defects disrupted the water lubrication and increased surface roughness, resulting in higher friction coefficients. In addition, as the mPEG-MA grafting conditions were strengthened, the average COF decreased, and surface damage was reduced. Based on the water contact angle test results, it was determined that as the grafting conditions were strengthened, and the hydrophilicity of the specimen surface increased, allowing more water to act as a lubricant between the PDMS plate and the hemisphere during the water-lubricated friction test []. Additionally, grafted mPEG-MA absorbed water in a water-lubricated environment and swelled to form a convex texture and enhance the hydrodynamic lubrication effect []. For the 20% 12H, 20% 18H, and 20% 24H specimens, no surface damage was observed in the grafted mPEG-MA layer during the friction test. This ensured continuous water lubrication, allowing the initially low coefficient of friction to be consistently maintained.
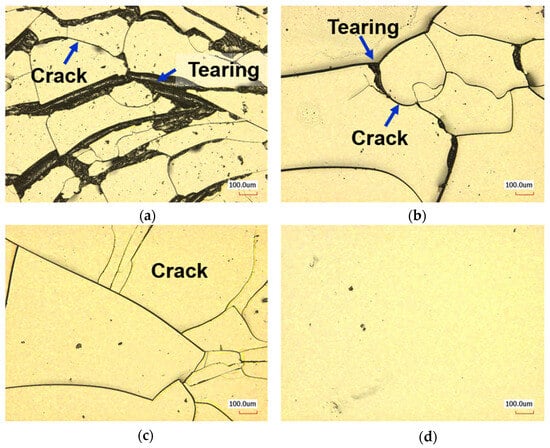
Figure 6.
Surface damage images of PDMS grafted mPEG-MA after friction test under water lubrication (a) 10% 12H, (b) 10% 18H, (c) 10% 24H, and (d) 20% 12H.
4.2. Tribological Properties at Varying Loads and Speeds Under Water Lubrication
In the above experiments, the average friction coefficients of the 20% 12H, 20% 18H, and 20% 24H specimens were similar at 0.02–0.03, and no surface damage occurred. Based on these results, the 20% 12H, 20% 18H, and 20% 24H specimens were analyzed to have similar water lubrication characteristics. Therefore, the tribological properties of the 20% 12H specimen, which took the shortest mPEG-MA grafting time, were analyzed under water lubrication. The friction tests were conducted using deionized water as a lubricant for 100 cycles at room temperature. The lubrication properties were examined by varying the vertical load and sliding speed. Figure 7 presents the load-dependent graphs of the COF of 20% 12H specimen measured under various sliding speed conditions during water lubrication. In all conditions, the COF of the 20% 12H specimen remained constant without any increase during the 100-cycle friction test, unlike the COF of the 10% 12H, 10% 18H, and 10% 24H specimens in Figure 5. Under the 1 N load condition, the average friction coefficient ranged from 0.009 to 0.016; under the 2 N load condition, it ranged from 0.009 to 0.013; and under the 3 N load condition, it ranged from 0.009 to 0.021. Additionally, no damage, such as cracks or tearing, was observed in the grafted mPEG-MA layer. This indicates that the water lubrication provided by mPEG-MA grafting was consistently maintained under vertical load conditions ranging from 1 to 3 N and sliding speeds from 1 to 100 mm/s.
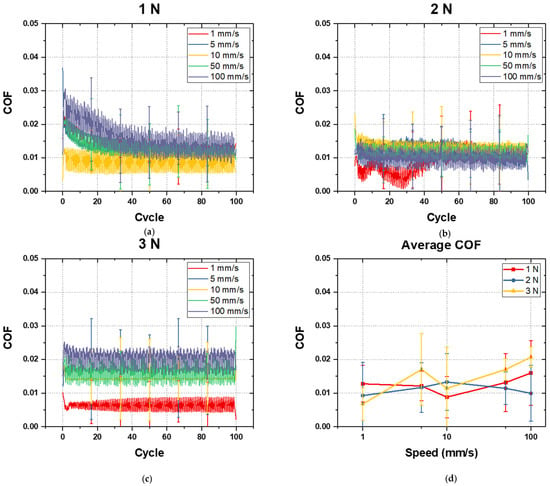
Figure 7.
COF of 20% 12H specimen performed under water lubrication (a) at 1 N and 1~100 mm/s, (b) at 2 N and 1~100 mm/s, (c) at 3 N and 1~100 mm/s, and (d) average COF with respect to normal load and sliding speed.
4.3. Durability Evaluation Under Water Lubrication
Additional friction tests were conducted to evaluate the durability of the mPEG-MA layer in the 20% 12H specimen under water lubrication. Deionized water was used as the lubricant, and the friction test was performed at a sliding speed of 10 mm/s for 1000 cycles in a room temperature environment. Figure 8 presents the COF graph for water-lubricated specimens under load conditions of 1, 2, and 3 N over 1000 cycles. The average coefficient of friction for the 20% 12H specimen under water lubrication was measured at 0.008 for 1 N, 0.011 for 2 N, and 0.015 for 3 N. After the friction test, surface damage was assessed using a 3D laser confocal microscope to evaluate the durability of the mPEG-MA layer. As shown in the left image of Figure 9, no surface damage was observed under the 1 N and 2 N load conditions. However, as can be seen in the right image of Figure 9, surface dimples were noted under the 3 N condition. Figure 10 shows an image measuring the size of the dimples that formed after a water-lubricated friction test under a 3 N load. The diameter of the dimples was measured to be between 130 and 200 μm. Despite the surface damage caused by the dimples, the COF remained stably maintained at 0.015 for 1000 cycles under the 3 N load condition. The Hertzian contact area that occurs between the PDMS plate and the hemisphere was calculated using the elastic modulus of PDMS of 1.2 MPa and the Poisson’s ratio of 0.46 when a 3 N load was applied during the friction test. The contact area diameter of PDMS was calculated to be approximately 400 μm. This is sufficiently larger than the diameter of the measured dimple, ensuring that adequate water lubrication was continuously maintained in the mPEG-MA layer throughout the friction test, preventing any increase in the COF. Furthermore, it was analyzed that the formed dimple acted as a reservoir for the lubricant, aiding in the maintenance of water lubrication.
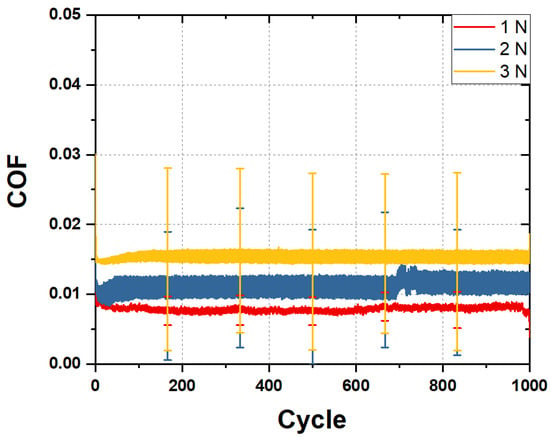
Figure 8.
COF of 20% 12H specimen performed at 1–3 N and 1000 cycles under water lubrication.

Figure 9.
Surface damage images of 20% 12H specimen at 1 N and 2 N load conditions (left) and at 3 N load conditions (right).
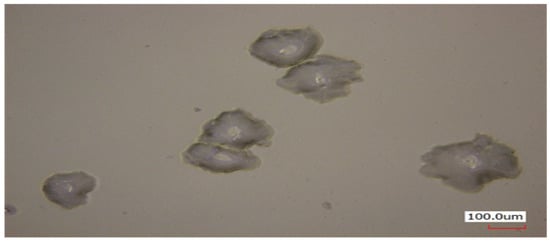
Figure 10.
Dimple images generated after performing water lubricated friction test for 1000 cycles under 3 N load conditions.
5. Conclusions
In this work, the hydrophobicity of the PDMS surface was converted to hydrophilicity to enhance water lubrication properties. To achieve this, mPEG-MA was grafted onto PDMS to increase the surface hydrophilicity. As the grafting conditions of mPEG-MA were intensified, the water contact angle of PDMS decreased from 109.6 degrees to 23.9 degrees, and the average friction coefficient under water lubrication decreased from 0.34 to 0.02. Furthermore, as the mPEG-MA grafting conditions were intensified, surface damage to the PDMS during the water-lubricated friction test was reduced. For PDMS grafted with a 20 wt.% mPEG-MA aqueous solution under UV irradiation for 12, 18, and 24 h, no surface damage was observed under a 1 N load and 100 test cycles. Based on the experimental results, the water-lubricated tribological properties of PDMS grafted with 20 wt.% mPEG-MA aqueous solution were evaluated after UV irradiation for 12 h. The average COF of the specimens remained consistent, ranging from 0.009 to 0.021 under vertical loads of 1~3 N and sliding speeds of 1~100 mm/s. Surface damage on mPEG-MA grafted PDMS was not detected under load tests of 1 N and 2 N. Although dimples appeared on mPEG-MA grafted PDMS under a 3 N load, the COF remained constant at 0.015 due to the lubrication storage effect of the dimples. The results of this work are expected to contribute to the application of mPEG-MA grafting technology, which exhibits excellent tribological properties under water lubrication, in environments where water-based lubrication of PDMS is required.
Author Contributions
Conceptualization, T.-H.K.; methodology, T.-H.K. and D.-E.K.; validation, T.-H.K.; investigation, T.-H.K.; writing—original draft, T.-H.K. and D.-E.K.; supervision and administration, D.-E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2020R1A2C2004714).
Data Availability Statement
Dataset available on request from the authors.
Acknowledgments
The authors would like to thank CSM Instruments, Co. BASF Korean branch for their cooperation and for the provision of materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hobbs, E.; Keplinger, M.; Calandra, J. Toxicity of polydimethylsiloxanes in certain environmental systems. Environ. Res. 1975, 10, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.-H.; Kim, D.-E. Development of highly durable and low friction micro-structured PDMS coating based on bio-inspired surface design. CIRP Ann. 2015, 64, 519–522. [Google Scholar] [CrossRef]
- Tong, L.; Zhou, W.; Zhao, Y.; Yu, X.; Wang, H.; Chu, P.K. Enhanced cytocompatibility and reduced genotoxicity of polydimethylsiloxane modified by plasma immersion ion implantation. Colloids Surf. B Biointerfaces 2016, 148, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chung, C.-K. PDMS microfabrication and design for microfluidics and sustainable energy application. Micromachines 2021, 12, 1350. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Ghosh, I.; Mukherjee, A. Poly(dimethylsiloxane) Induces Cytotoxicity and Genotoxicity in Human Lymphocytes. Proc. Zool. Soc. 2020, 73, 82–85. [Google Scholar] [CrossRef]
- Ertel, S.I.; Ratner, B.D.; Kaul, A.; Schway, M.B.; Horbett, T.A. In vitro study of the intrinsic toxicity of synthetic surfaces to cells. J. Biomed. Mater. Res. 1994, 28, 667–675. [Google Scholar] [CrossRef]
- Khadivi, P.; Salami-Kalajahi, M.; Roghani-Mamaqani, H. Evaluation of in vitro cytotoxicity and properties of polydimethylsiloxane-based polyurethane/crystalline nanocellulose bionanocomposites. J. Biomed. Mater. Res. Part A 2019, 107, 1771–1778. [Google Scholar] [CrossRef]
- Zhou, J.; Khodakov, D.A.; Ellis, A.V.; Voelcker, N.H. Surface modification for PDMS-based microfluidic devices. Electrophoresis 2012, 33, 89–104. [Google Scholar] [CrossRef]
- Hemmilä, S.; Cauich-Rodríguez, J.V.; Kreutzer, J.; Kallio, P. Rapid, simple, and cost-effective treatments to achieve long-term hydrophilic PDMS surfaces. Appl. Surf. Sci. 2012, 258, 9864–9875. [Google Scholar] [CrossRef]
- Trantidou, T.; Elani, Y.; Parsons, E.; Ces, O. Hydrophilic surface modification of PDMS for droplet microfluidics using a simple, quick, and robust method via PVA deposition. Microsyst. Nanoeng. 2017, 3, 16091. [Google Scholar] [CrossRef]
- Bodas, D.; Khan-Malek, C. Formation of more stable hydrophilic surfaces of PDMS by plasma and chemical treatments. Microelectron. Eng. 2006, 83, 1277–1279. [Google Scholar] [CrossRef]
- Vesterkvist, P.S.; Misiorek, J.O.; Spoof, L.E.; Toivola, D.M.; Meriluoto, J.A. Comparative cellular toxicity of hydrophilic and hydrophobic microcystins on Caco-2 cells. Toxins 2012, 4, 1008–1023. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Takahara, A. Tribological properties of hydrophilic polymer brushes under wet conditions. Chem. Rec. 2010, 10, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Hiratsuka, K.-I.; Sasada, T. Role of water in the lubrication of hydrogel. Wear 2006, 261, 500–504. [Google Scholar] [CrossRef]
- Hu, D.; Guo, Z.; Jun, T.; Yuan, C. A novel hydrophilic PVA fiber reinforced thermoplastic polyurethane materials for water-lubricated stern bearing. Fibers Polym. 2021, 22, 171–183. [Google Scholar] [CrossRef]
- Lee, S.; Spencer, N.D. Aqueous lubrication of polymers: Influence of surface modification. Tribol. Int. 2005, 38, 922–930. [Google Scholar] [CrossRef]
- Rahman, M.H.; Warneke, H.; Webbert, H.; Rodriguez, J.; Austin, E.; Tokunaga, K.; Rajak, D.K.; Menezes, P.L. Water-based lubricants: Development, properties, and performances. Lubricants 2021, 9, 73. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, D.-E. Water lubrication of stainless steel using reduced graphene oxide coating. Sci. Rep. 2015, 5, 17034. [Google Scholar] [CrossRef]
- Kim, H.J.; Shin, D.G.; Kim, D.-E. Frictional behavior between silicon and steel coated with graphene oxide in dry sliding and water lubrication conditions. Int. J. Precis. Eng. Manuf. -Green Technol. 2016, 3, 91–97. [Google Scholar] [CrossRef]
- Gokaltun, A.; Yarmush, M.L.; Asatekin, A.; Usta, O.B. Recent advances in nonbiofouling PDMS surface modification strategies applicable to microfluidic technology. Technology 2017, 5, 1–12. [Google Scholar] [CrossRef]
- Pinto, S.; Alves, P.; Matos, C.; Santos, A.; Rodrigues, L.; Teixeira, J.; Gil, M. Poly(dimethyl siloxane) surface modification by low pressure plasma to improve its characteristics towards biomedical applications. Colloids Surf. B Biointerfaces 2010, 81, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Chawla, K.; Lee, S.; Lee, B.P.; Dalsin, J.L.; Messersmith, P.B.; Spencer, N.D. A novel low-friction surface for biomedical applications: Modification of poly(dimethylsiloxane)(PDMS) with polyethylene glycol (PEG)-DOPA-lysine. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 90, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Olugebefola, S.C.; Kuhlman, W.A.; Rubner, M.F.; Mayes, A.M. Photopatterned nanoporosity in polyelectrolyte multilayer films. Langmuir 2008, 24, 5172–5178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, B.; Wang, R.; Zhang, G.; Hou, X.; Wu, L. Fabrication of a covalently attached self-assembly multilayer film based on CdTe nanoparticles. J. Colloid Interface Sci. 2002, 247, 361–365. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, J.; Wu, T.; Sun, Y.; Wang, Z.; Zhang, X.; Shen, J.; Cao, W. Fabrication of a covalently attached multilayer via photolysis of layer-by-layer self-assembled films containing diazo-resins. Chem. Commun. 1998, 17, 1853–1854. [Google Scholar] [CrossRef]
- Sun, J.; Wu, T.; Liu, F.; Wang, Z.; Zhang, X.; Shen, J. Covalently attached multilayer assemblies by sequential adsorption of polycationic diazo-resins and polyanionic poly(acrylic acid). Langmuir 2000, 16, 4620–4624. [Google Scholar] [CrossRef]
- Kato, K.; Uchida, E.; Kang, E.-T.; Uyama, Y.; Ikada, Y. Polymer surface with graft chains. Prog. Polym. Sci. 2003, 28, 209–259. [Google Scholar] [CrossRef]
- Ko, J.; Cho, K.; Han, S.W.; Sung, H.K.; Baek, S.W.; Koh, W.-G.; Yoon, J.S. Hydrophilic surface modification of poly(methyl methacrylate)-based ocular prostheses using poly(ethylene glycol) grafting. Colloids Surf. B Biointerfaces 2017, 158, 287–294. [Google Scholar] [CrossRef]
- He, Q.; Liu, Z.; Xiao, P.; Liang, R.; He, N.; Lu, Z. Preparation of hydrophilic poly(dimethylsiloxane) stamps by plasma-induced grafting. Langmuir 2003, 19, 6982–6986. [Google Scholar] [CrossRef]
- Alves, P.; Pinto, S.; Kaiser, J.-P.; Bruinink, A.; de Sousa, H.C.; Gil, M. Surface grafting of a thermoplastic polyurethane with methacrylic acid by previous plasma surface activation and by ultraviolet irradiation to reduce cell adhesion. Colloids Surf. B Biointerfaces 2011, 82, 371–377. [Google Scholar] [CrossRef]
- Eren, T.N.; Kariksiz, N.; Demirci, G.; Tuncel, D.; Okte, N.; Acar, H.Y.; Avci, D. Irgacure 2959-functionalized poly(ethyleneimine) s as improved photoinitiators: Enhanced water solubility, migration stability and visible-light operation. Polym. Chem. 2021, 12, 2772–2785. [Google Scholar] [CrossRef]
- Wilems, T.S.; Lu, X.; Kurosu, Y.E.; Khan, Z.; Lim, H.J.; Smith Callahan, L.A. Effects of free radical initiators on polyethylene glycol dimethacrylate hydrogel properties and biocompatibility. J. Biomed. Mater. Res. Part A 2017, 105, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yang, X.; Zhang, Y.; Cheng, X.; Xu, Y.; Bai, Y.; Shao, L. Segregation-induced in situ hydrophilic modification of poly(vinylidene fluoride) ultrafiltration membranes via sticky poly(ethylene glycol) blending. J. Membr. Sci. 2018, 563, 22–30. [Google Scholar] [CrossRef]
- Zhu, X.-Y.; Jun, Y.; Staarup, D.; Major, R.; Danielson, S.; Boiadjiev, V.; Gladfelter, W.; Bunker, B.; Guo, A. Grafting of high-density poly(ethylene glycol) monolayers on Si (111). Langmuir 2001, 17, 7798–7803. [Google Scholar] [CrossRef]
- Kim, C.-L.; Kim, D.-E.; Kim, H.-J. Control of Surface Energy using Bilayer Metallic Film Heterostructures. Tribol. Lubr. 2019, 35, 350–355. [Google Scholar] [CrossRef]
- Zhao, T.; Jiang, L. Contact angle measurement of natural materials. Colloids Surf. B Biointerfaces 2018, 161, 324–330. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).