Tough and Low Friction Polyvinyl Alcohol Hydrogels Loaded with Anti-inflammatories for Cartilage Replacement
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydrogels Preparation
- Annealing at 100 °C or 150 °C for 30 min (samples A100 and A150, respectively).
- High hydrostatic pressure at 70 °C and 600 MPa for 10 min (samples HHP). Samples were previously packed and sealed in polyamide/polyethylene vacuum bags (90 μ, Penta Ibérica - Soc. Ibérica de Embalagens, Lda, Torres Vedras, Portugal) filled with 200 mL of DD water and pre-heated at 70 °C for 10 min. Then, they were placed inside a polytetrafluoroethylene insulation vessel pre-filled with water at the same temperature and pressurized in a Hiperbaric 55 equipment (Burgos, Spain). The rate of pressurization till the plateau of 600 MPa was 250 MPa·min−1, while depressurization was instantaneous.
- Gamma radiation with a dose of 75 kGy (samples GR). The irradiation was done at room temperature using ⁶⁰Co as a source of γ-rays.
2.2. Hydrogels Characterization
2.2.1. Microstructural Analysis
2.2.2. Water Uptake
2.2.3. Wettability
2.2.4. Mechanical Properties
2.2.5. Tribological Behavior
2.2.6. Drug Loading and Release
2.2.7. HET-CAM Test
2.2.8. Cytotoxicity Evaluation
2.2.9. Statistical Analysis
3. Results and Discussion
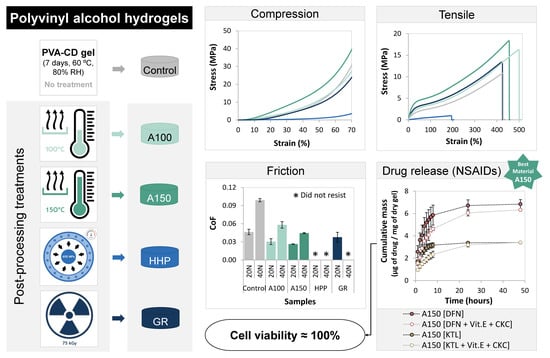
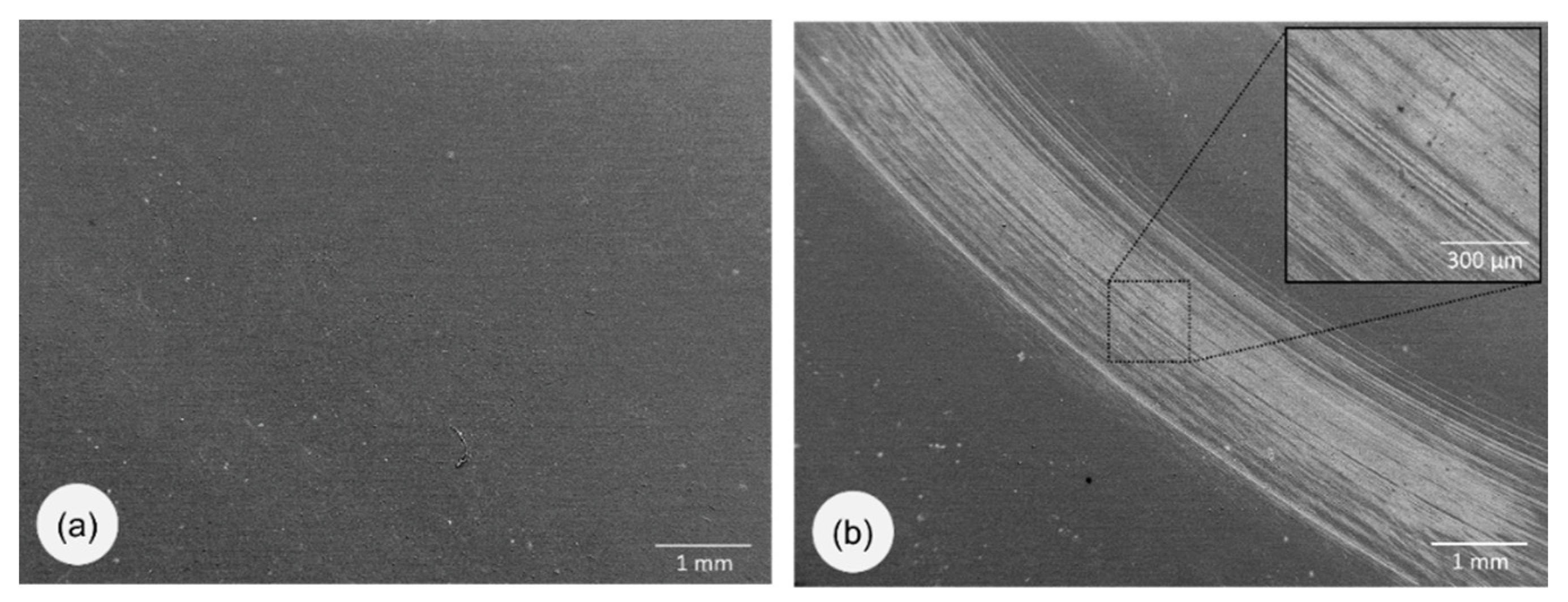
3.1. Morphology
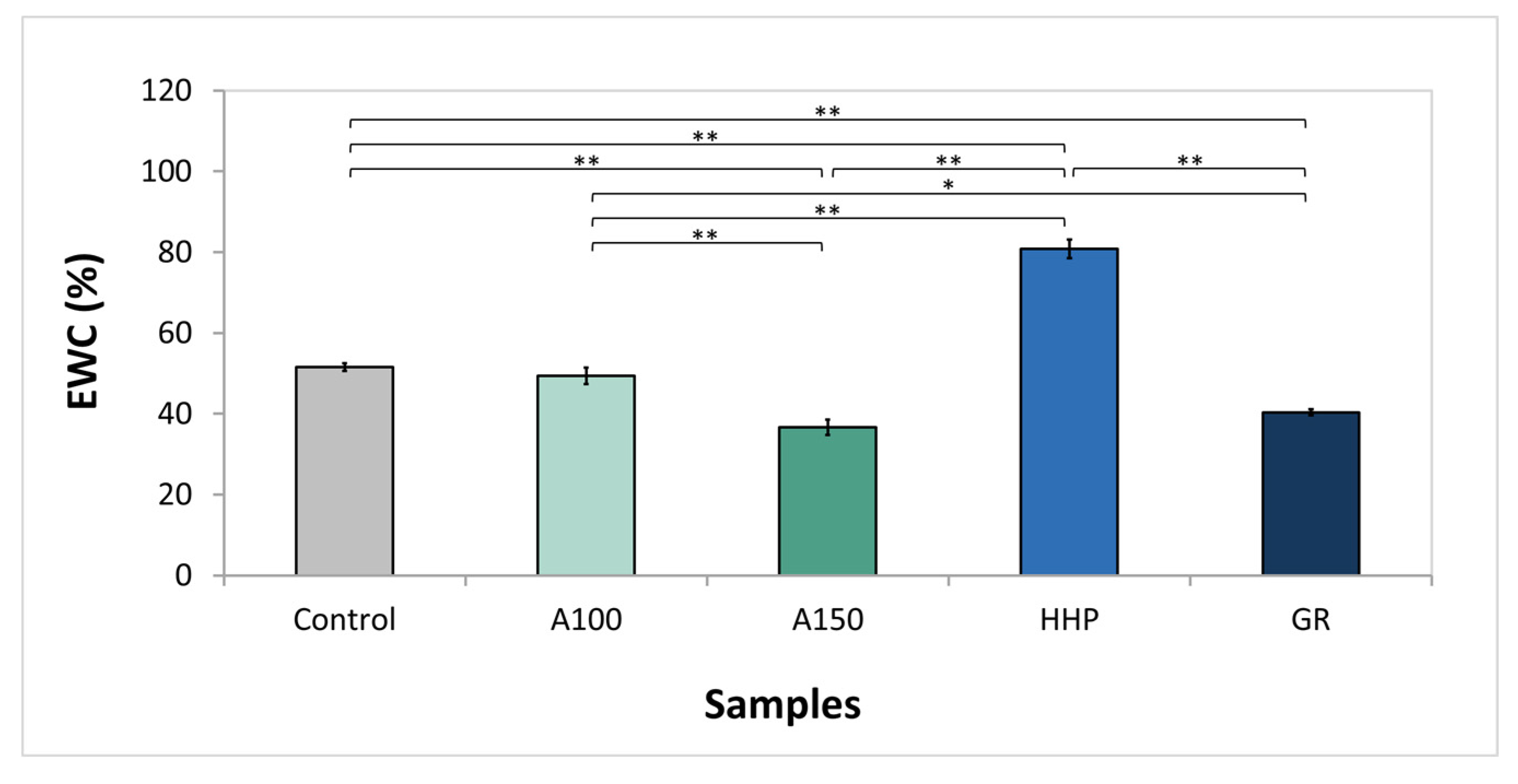
3.2. Water Uptake
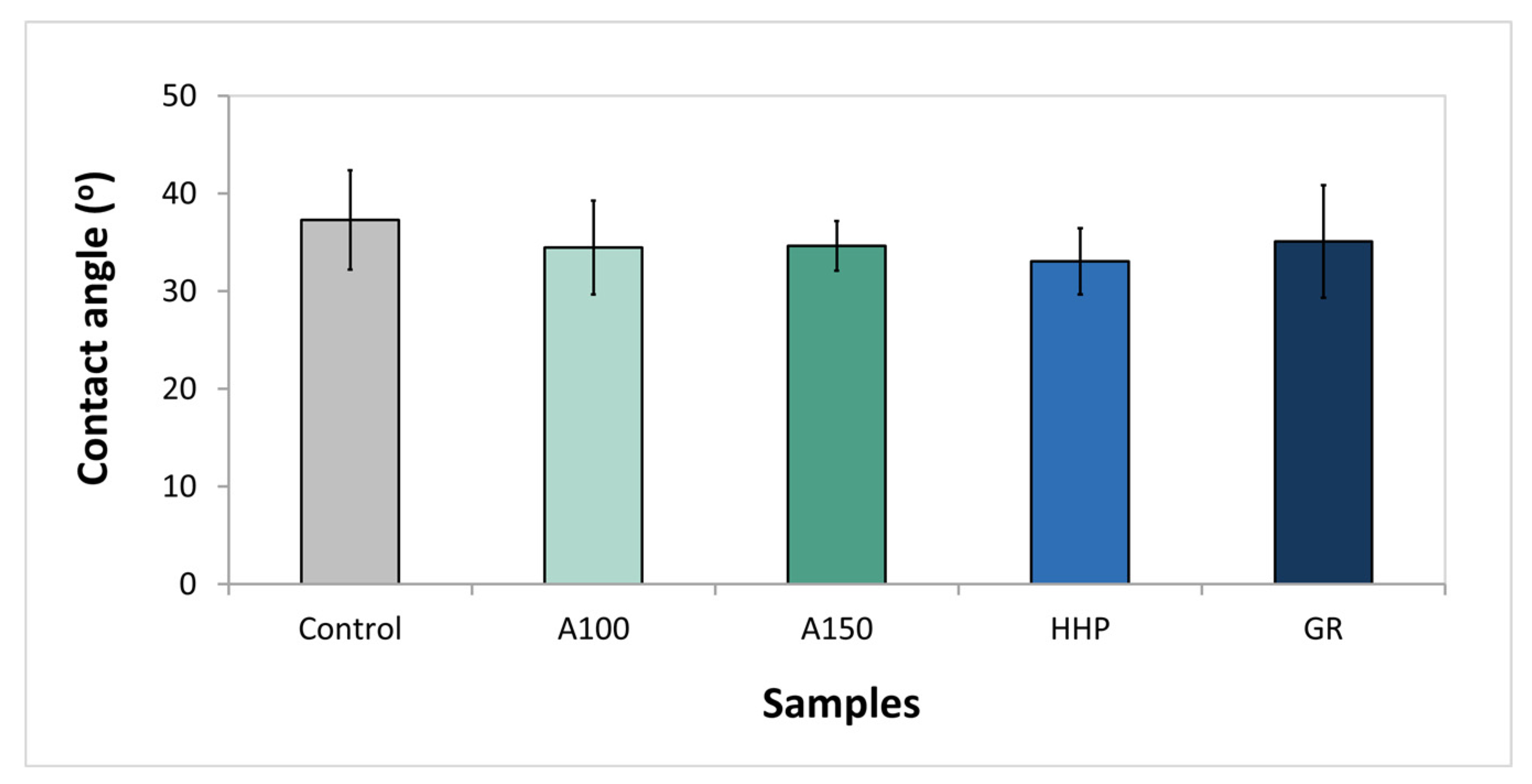
3.3. Wettability
3.4. Mechanical Properties
3.5. Tribological Behavior
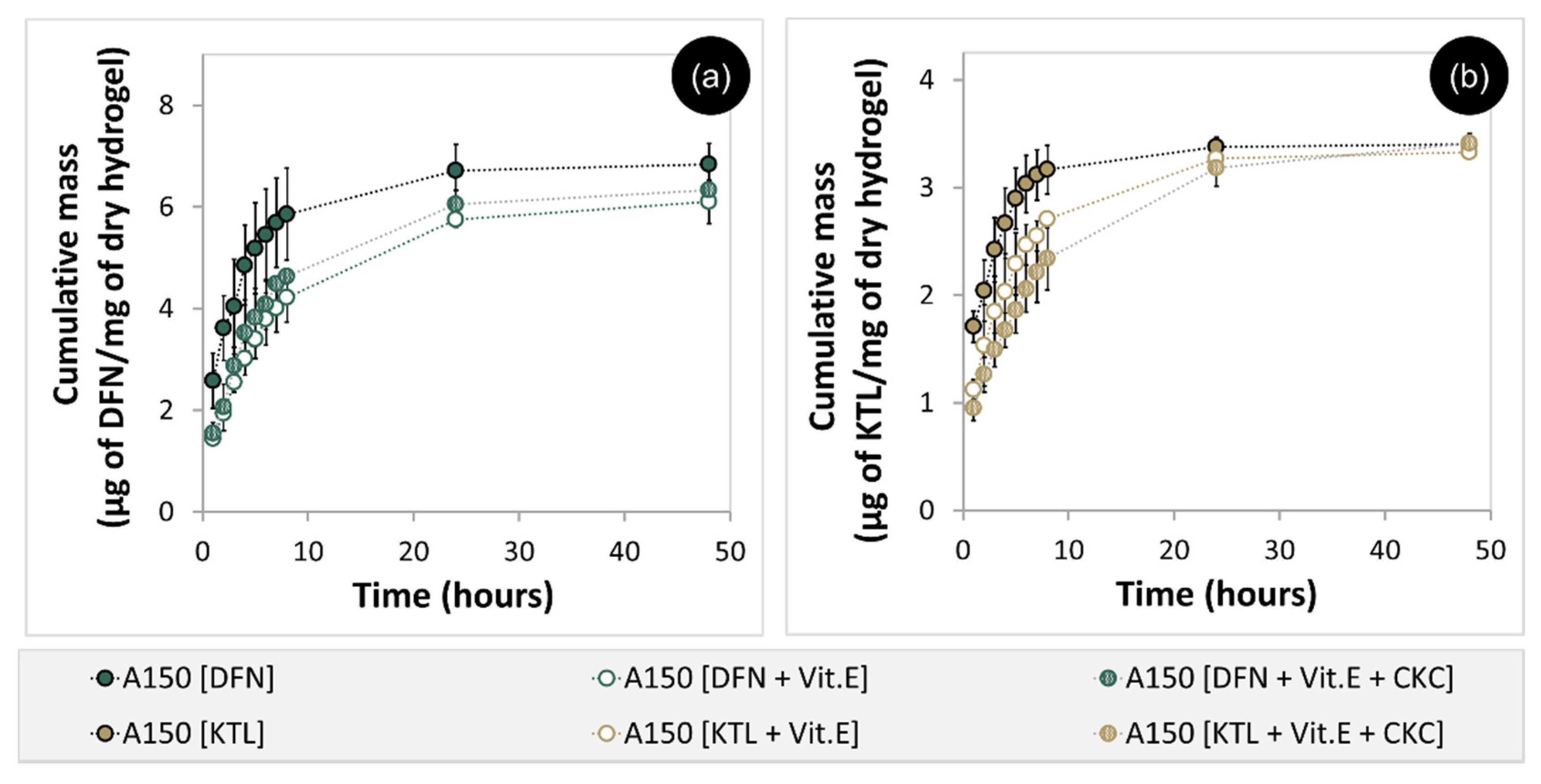
3.6. Drug Loading and Release
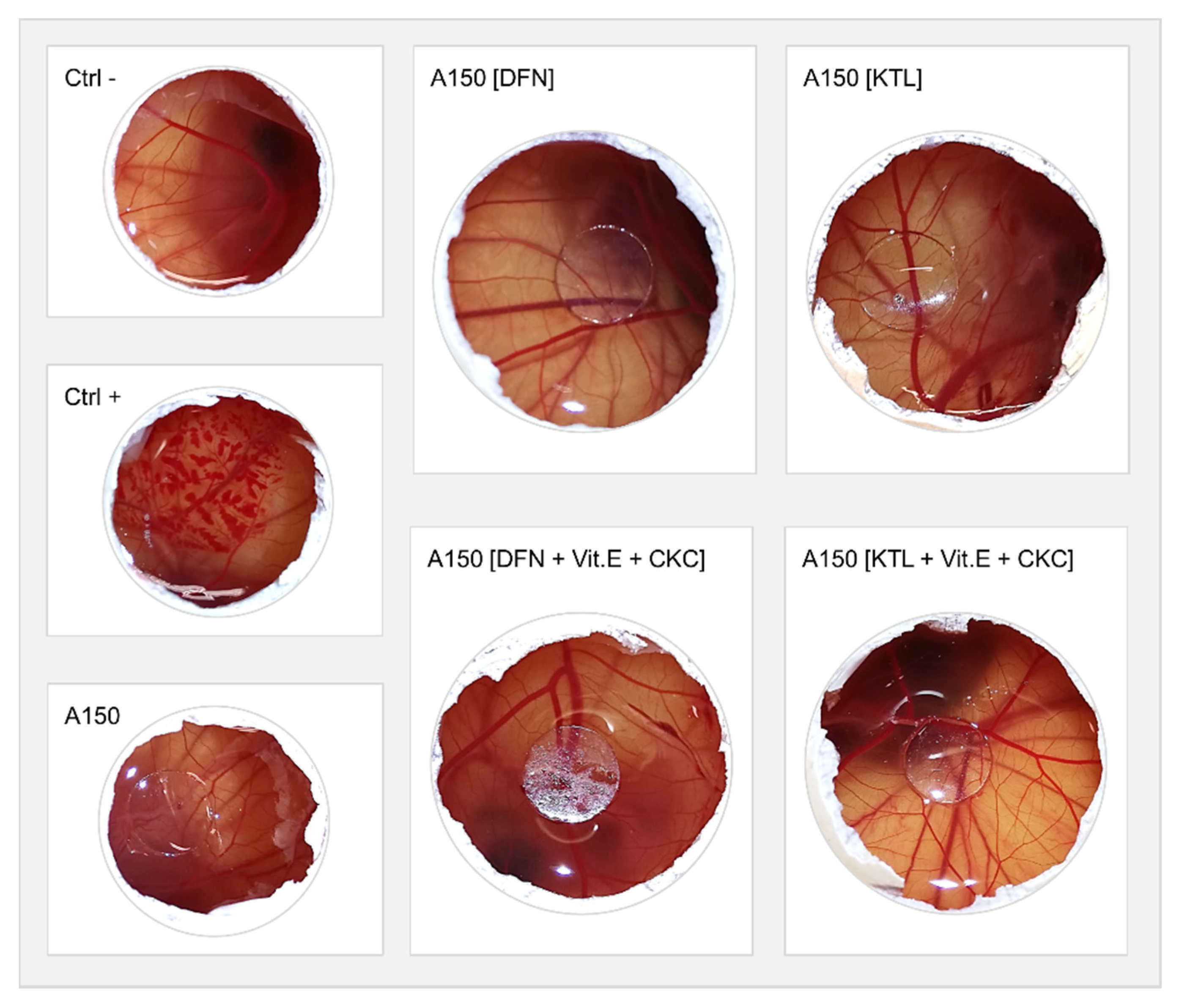
3.7. HET-CAM Test
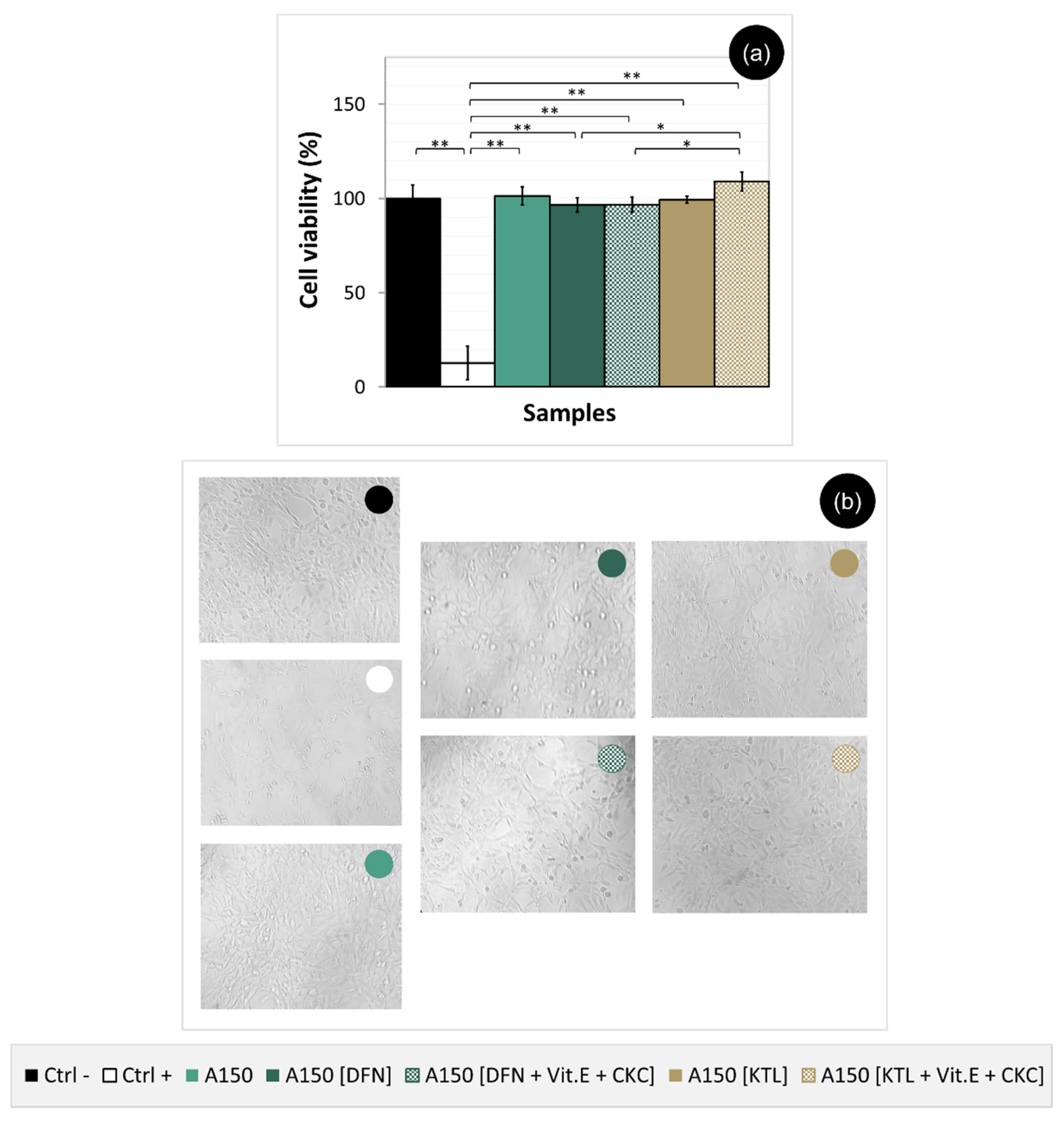
3.8. Cytotoxicity Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fox, A.J.S.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sport Heal. A Multidiscip. Approach 2009, 1, 461–468. [Google Scholar]
- Beddoes, C.; Whitehouse, M.; Briscoe, W.; Su, B. Hydrogels as a replacement material for damaged articular hyaline cartilage. Materials 2016, 9, 443. [Google Scholar] [CrossRef] [PubMed]
- Rawal, B.R.; Ribeiro, R.; Chouksey, M.; Tripathi, K. Biomaterials for cartilage repair: A review. J. Med. Sci. 2013, 13, 615–620. [Google Scholar] [CrossRef][Green Version]
- Bicho, D.; Pina, S.; Reis, R.L.; Oliveira, J.M. Commercial products for osteochondral tissue repair and regeneration. In Osteochondral Tissue Engineering; Oliveira, J., Pina, S., Reis, R., San Roman, J., Eds.; Springer: Cham, Switzerland, 2018; Volume 1058, pp. 415–428. [Google Scholar]
- Chuang, E.-Y.; Chiang, C.-W.; Wong, P.-C.; Chen, C.-H. Hydrogels for the application of articular cartilage tissue engineering: A review of hydrogels. Adv. Mater. Sci. Eng. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Jagur-Grodzinski, J. Polymers for tissue engineering, medical devices, and regenerative medicine. Concise general review of recent studies. Polym. Adv. Technol. 2006, 17, 395–418. [Google Scholar] [CrossRef]
- Święszkowski, W.; Ku, D.N.; Bersee, H.E.N.; Kurzydlowski, K.J. An elastic material for cartilage replacement in an arthritic shoulder joint. Biomaterials 2006, 27, 1534–1541. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Seidi, O.; Ribeiro, N.; Colaço, R.; Serro, A.P. Tribomechanical comparison between pva hydrogels obtained using different processing conditions and human cartilage. Materials 2019, 12, 3413. [Google Scholar] [CrossRef]
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1451–1457. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and applications of poly (vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. In Biopolymers PVA Hydrogels, Anionic Polymerisation Nanocomposites; Springer: Heidelberg/Berlin, Germany, 2000; Volume 153, pp. 37–65. [Google Scholar]
- Luo, X.; Akram, M.Y.; Yuan, Y.; Nie, J.; Zhu, X. Silicon dioxide/poly(vinyl alcohol) composite hydrogels with high mechanical properties and low swellability. J. Appl. Polym. Sci. 2019, 136, 46895. [Google Scholar] [CrossRef]
- Zhou, D.; Dong, Q.; Liang, K.; Xu, W.; Zhou, Y.; Xiao, P. Photocrosslinked methacrylated poly (vinyl alcohol)/hydroxyapatite nanocomposite hydrogels with enhanced mechanical strength and cell adhesion. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1882–1889. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z.; Fei, G.; Xia, H. Polydopamine particles reinforced poly (vinyl alcohol) hydrogel with nir light triggered shape memory and self-healing capability. Macromol. Rapid Commun. 2017, 38, 1700421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, P.C.; Edgren, D. Crosslinking reaction of poly (vinyl alcohol) with glyoxal. J. Polym. Res. 2010, 17, 725–730. [Google Scholar] [CrossRef]
- Park, J.-Y.; Hwang, K.-J.; Yoon, S.-D.; Lee, J.-H.; Lee, I.-H. Influence of glyoxal on preparation of poly (vinyl alcohol)/poly (acrylic acid) blend film. J. Nanosci. Nanotechnol. 2015, 15, 5955–5958. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Liu, T.; Jiao, C.; Peng, X.; Chen, Y.-N.; Chen, Y.; He, C.; Liu, R.; Wang, H. Super-strong and tough poly (vinyl alcohol)/poly (acrylic acid) hydrogels reinforced by hydrogen bonding. J. Mater. Chem. B 2018, 6, 8105–8114. [Google Scholar] [CrossRef]
- Chen, K.; Chen, G.; Wei, S.; Yang, X.; Zhang, D.; Xu, L. Preparation and property of high strength and low friction PVA-HA/PAA composite hydrogel using annealing treatment. Mater. Sci. Eng. C 2018, 91, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Holloway, J.L.; Lowman, A.M.; Vanlandingham, M.R.; Palmese, G.R. Chemical grafting for improved interfacial shear strength in UHMWPE/PVA-hydrogel fiber-based composites used as soft fibrous tissue replacements. Compos. Sci. Technol. 2013, 85, 118–125. [Google Scholar] [CrossRef]
- Jing, L.; Li, H.; Tay, R.Y.; Sun, B.; Tsang, S.H.; Cometto, O.; Lin, J.; Teo, E.H.T.; Tok, A.I.Y. Biocompatible hydroxylated boron nitride nanosheets/poly (vinyl alcohol) interpenetrating hydrogels with enhanced mechanical and thermal responses. ACS Nano 2017, 11, 3742–3751. [Google Scholar] [CrossRef]
- Kobayashi, M.; Hyu, H.S. Development and evaluation of polyvinyl alcohol-hydrogels as an artificial articular cartilage for orthopedic implants. Materials 2010, 3, 2753–2771. [Google Scholar] [CrossRef]
- Rejmontová, P.; Kovalcik, A.; Humpolíček, P.; Capáková, Z.; Wrzecionko, E.; Sáha, P. The use of fractionated Kraft lignin to improve the mechanical and biological properties of PVA-based scaffolds. RSC Adv. 2019, 9, 12346–12353. [Google Scholar] [CrossRef]
- Suzuki, A.; Sasaki, S. Swelling and mechanical properties of physically crosslinked poly (vinyl alcohol) hydrogels. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2015, 229, 828–844. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Suzuki, A. Factors influencing the swelling and elution properties of poly (vinyl alcohol) cast gels. Polym. Adv. Technol. 2016, 27, 318–324. [Google Scholar] [CrossRef]
- Negishi, J.; Nam, K.; Kimura, T.; Fujisato, T.; Kishida, A. High-hydrostatic pressure technique is an effective method for the preparation of PVA–heparin hybrid gel. Eur. J. Pharm. Sci. 2010, 41, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Lian, Z.; Zhang, Y.; Zhao, Y. Nano-TiO2 particles and high hydrostatic pressure treatment for improving functionality of polyvinyl alcohol and chitosan composite films and nano-TiO2 migration from film matrix in food simulants. Innov. Food Sci. Emerg. Technol. 2016, 33, 145–153. [Google Scholar] [CrossRef]
- Fukumori, T.; Nakaoki, T. Significant improvement of mechanical properties for polyvinyl alcohol film prepared from freeze/thaw cycled gel. Open J. Org. Polym. Mater. 2013, 3, 110–116. [Google Scholar] [CrossRef]
- Gonzalez, J.S.; Alvarez, V.A. The effect of the annealing on the poly (vinyl alcohol) obtained by freezing–thawing. Thermochim. Acta 2011, 521, 184–190. [Google Scholar] [CrossRef]
- Mondino, A.; González, M.; Romero, G.; Smolko, E. Physical properties of gamma irradiated poly (vinyl alcohol) hydrogel preparations. Radiat. Phys. Chem. 1999, 55, 723–726. [Google Scholar] [CrossRef]
- Sasaki, S.; Omata, S.; Murakami, T.; Nagasawa, N.; Taguchi, M.; Suzuki, A. Effect of gamma ray irradiation on friction property of poly(vinyl alcohol) cast-drying on freeze-thawed hybrid gel. Gels 2018, 4, 30. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Kou, L.; Xiao, S.; Sun, R.; Bao, S.; Yao, Q.; Chen, R. Biomaterial-engineered intra-articular drug delivery systems for osteoarthritis therapy. Drug Deliv. 2019, 26, 870–885. [Google Scholar] [CrossRef]
- Rey-Rico, A.; Madry, H.; Cucchiarini, M. Hydrogel-based controlled delivery systems for articular cartilage repair. Biomed. Res. Int. 2016, 2016, 1215263. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, F.; Mohammadi, S.S.; Tanideh, N.; Ahmadi, F. Hybrid scaffolds of hyaluronic acid and collagen loaded with prednisolone: An interesting system for osteoarthritis. Adv. Pharm. Bull. 2018, 8, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Bédouet, L.; Moine, L.; Pascale, F.; Nguyen, V.-N.; Labarre, D.; Laurent, A. Synthesis of hydrophilic intra-articular microspheres conjugated to ibuprofen and evaluation of anti-inflammatory activity on articular explants. Int. J. Pharm. 2014, 459, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Bi, X.; Li, H.; Huang, G. Enhanced targeting efficiency of PLGA microspheres loaded with Lornoxicam for intra-articular administration. Drug Deliv. 2011, 18, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Kawadkar, J.; Chauhan, M.K. Intra-articular delivery of genipin cross-linked chitosan microspheres of flurbiprofen: Preparation, characterization, in vitro and in vivo studies. Eur. J. Pharm. Biopharm. 2012, 81, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, C.; Bilge, S.S. Effects of nonsteroidal anti-inflammatory drugs at the molecular level. Eurasian J. Med. 2018, 50, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, P.; Galante, R.; Santos, L.; Alves de Matos, A.P.; Colaço, R.; Serro, A.P.; Saramago, B. Comparison of two hydrogel formulations for drug release in ophthalmic lenses. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1170–1180. [Google Scholar] [CrossRef]
- Filipe, H.P.; Henriques, J.; Reis, P.; Silva, P.C.; Quadrado, M.J.; Serro, A.P. Contact lenses as drug controlled release systems: A narrative review. Rev. Bras Oftalmol. 2016, 75, 241–247. [Google Scholar] [CrossRef][Green Version]
- Paradiso, P.; Serro, A.P.; Saramago, B.; Colaço, R.; Chauhan, A. Controlled release of antibiotics from vitamin e–loaded silicone-hydrogel contact lenses. J. Pharm. Sci. 2016, 105, 1164–1172. [Google Scholar] [CrossRef]
- Topete, A. Effects of Sterilization on Drug Loaded Soft Contact Lenses. Ph.D. Thesis, Instituto Superior Técnico—University of Lisbon, Lisbon, Portugal, 2015. [Google Scholar]
- Torres-Luna, C.; Hu, N.; Tammareddy, T.; Domszy, R.; Yang, J.; Wang, N.S.; Yang, A. Extended delivery of non-steroidal anti-inflammatory drugs through contact lenses loaded with Vitamin E and cationic surfactants. Contact Lens Anterior Eye 2019, 42, 546–552. [Google Scholar] [CrossRef]
- ICCVAM. The hen’s egg Test—Chorioallantoic membrane test method. In ICCVAM Test Method Evaluation Report: Current Validation Status of In Vitro Test Methods Proposed for Identifying Eye Injury Hazard Potential of Chemicals and Products; National Institute of Environmental Health Sciences: Research Triangle Park, NC, USA, 2010; pp. 19–25, NIH Publication No. 10-7553. [Google Scholar]
- Oliveira, A. Effects of Sterilization on Drug Loaded Ophthalmic Lenses Materials. Ph.D. Thesis, Instituto Superior Técnico—University of Lisbon, Lisbon, Portugal, 2016. [Google Scholar]
- Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity (ISO 10993-5); International Organization for Standardization: Geneva, Switzerland, 2009.
- Bandarra, S.; Mascarenhas, P.; Luís, A.R.; Catrau, M.; Bekman, E.; Ribeiro, A.C.; Félix, S.; Caldeira, J.; Barahona, I. In vitro and in silico evaluations of resin-based dental restorative material toxicity. Clin. Oral Investig. 2019. [Google Scholar] [CrossRef] [PubMed]
- Gulenoor, F.; Poddar, P. γ-irradiated polyvinyl alcohol (pva) and citric acid blend hydrogels: Swelling and absorption properties. Chem. Sci. J. 2016, 7, 1000125. [Google Scholar] [CrossRef]
- Hassan, M.R.; Chowdhury, A.B.M.A.; Islam, M.T.; Poddar, P.; Dafader, N.C.; Chowdhury, A.M.S. Swelling and absorption properties of polyvinyl alcohol (pva) and acrylic acid blend hydrogels: Effect of of γ-irradiation. En Chem. Technol. Ind. J. 2016, 11, 107. [Google Scholar]
- Zink, J.; Wyrobnik, T.; Prinz, T.; Schmid, M. Physical, chemical and biochemical modifications of protein-based films and coatings: An extensive review. Int. J. Mol. Sci. 2016, 17, 1376. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Wang, C.; Huang, G. A facile method to fabricate hybrid hydrogels with mechanical toughness using a novel multifunctional cross-linker. RSC Adv. 2017, 7, 35311–35319. [Google Scholar] [CrossRef]
- Shi, Y.; Xiong, D.; Zhang, J. Effect of irradiation dose on mechanical and biotribological properties of PVA/PVP hydrogels as articular cartilage. Tribol. Int. 2014, 78, 60–67. [Google Scholar] [CrossRef]
- Gaharwar, A.; Schexnailder, P.; Schmidt, G. Nanocomposite polymer biomaterials for tissue repair of bone and cartilage. In Nanobiomaterials Handbook; CRC Press: Boca Raton, EL, USA, 2011; pp. 1–28. [Google Scholar]
- Cao, Z.; Wang, Y.; Wang, H.; Ma, C.; Li, H.; Zheng, J.; Wu, J.; Huang, G. Tough, ultrastretchable and tear-resistant hydrogels enabled by linear macro-cross-linker. Polym. Chem. 2019, 10, 3503–3513. [Google Scholar] [CrossRef]
- Shi, F.-K.; Wang, X.-P.; Guo, R.-H.; Zhong, M.; Xie, X.-M. Highly stretchable and super tough nanocomposite physical hydrogels facilitated by the coupling of intermolecular hydrogen bonds and analogous chemical crosslinking of nanoparticles. J. Mater. Chem. B 2015, 3, 1187–1192. [Google Scholar] [CrossRef]
- Li, W.; Wang, D.; Yang, W.; Song, Y. Compressive mechanical properties and microstructure of PVA–HA hydrogels for cartilage repair. RSC Adv. 2016, 6, 20166–20172. [Google Scholar] [CrossRef]
- Pan, Y.-S.; Xiong, D.-S.; Ma, R.-Y. A study on the friction properties of poly (vinyl alcohol) hydrogel as articular cartilage against titanium alloy. Wear 2007, 262, 1021–1025. [Google Scholar] [CrossRef]
- Pan, Y.S.; Xiong, D.S.; Chen, X.L. Friction characteristics of poly (vinyl alcohol) hydrogel as an articular cartilage biomaterial. Key Eng. Mater. 2007, 330–332, 1297–1300. [Google Scholar] [CrossRef]
- Mow, V.C. The Role of Lubrication in Biomechanical Joints. J. Lubr. Technol. 1969, 91, 320–326. [Google Scholar] [CrossRef]
- Neville, A.; Morina, A.; Liskiewicz, T.; Yan, Y. Synovial joint lubrication—Does nature teach more effective engineering lubrication strategies? Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2007, 221, 1223–1230. [Google Scholar] [CrossRef]
- Li, F.; Su, Y.; Wang, J.; Wu, G.; Wang, C. Influence of dynamic load on friction behavior of human articular cartilage, stainless steel and polyvinyl alcohol hydrogel as artificial cartilage. J. Mater. Sci. Mater. Med. 2010, 21, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Forster, H.; Fisher, J. The influence of continuous sliding and subsequent surface wear on the friction of articular cartilage. Proc. Inst. Mech. Eng. H 1999, 213, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Brand, R.A. Joint contact stress: A reasonable surrogate for biological processes? Iowa Orthop. J. 2005, 25, 82–94. [Google Scholar] [PubMed]
- Serro, A.P.; Colaço, R.; Saramago, B. Effect of albumin adsorption on biotribological properties of artificial joint materials. In Proteins at Interfaces III State of the Art; Horbett, T., Brash, J.L., Norde, W., Eds.; ACS Symposium Series: Washington, DC, USA, 2012; Volume 1120, pp. 497–523. [Google Scholar]
- Ghalei, S.; Asadi, H.; Ghalei, B. Zein nanoparticle-embedded electrospun PVA nanofibers as wound dressing for topical delivery of anti-inflammatory diclofenac. J. Appl. Polym. Sci. 2018, 135, 46643. [Google Scholar] [CrossRef]
- Peng, C.-C.; Kim, J.; Chauhan, A. Extended delivery of hydrophilic drugs from silicone-hydrogel contact lenses containing vitamin E diffusion barriers. Biomaterials 2010, 31, 4032–4047. [Google Scholar] [CrossRef]
- Bengani, L.C.; Chauhan, A. Extended delivery of an anionic drug by contact lens loaded with a cationic surfactant. Biomaterials 2013, 34, 2814–2821. [Google Scholar] [CrossRef]
- Barney, R.; Carroll, J.; Delaet, D. Surfactant studies of quaternary ammonium compounds: Critical surfactant concentration. J. Surfactants Deterg. 2006, 9, 137–140. [Google Scholar] [CrossRef]
- Sinha, V.R.; Kumar, R.V.; Singh, G. Ketorolac tromethamine formulations: An overview. Expert Opin. Drug Deliv. 2009, 6, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Adeyeye, C.M.; Li, P.-K. Diclofenac sodium. In Analytical Profiles of Drug Substances; Academic Press: Cambridge, MA, USA, 1990; Volume 19, pp. 123–144. [Google Scholar]
- Kalweit, S.; Gerner, I.; Spielmann, H. Validation project of alternatives for the draize eye test. Mol. Toxicol. 1987, 1, 597–603. [Google Scholar] [PubMed]
- Spielmann, H. HET-CAM test. In In Vitro Toxicity Testing Protocols. Methods in Molecular BiologyTM; O’Hare, S., Atterwill, C.K., Eds.; Humana Press: Totowa, NJ, USA, 1995; pp. 199–204. [Google Scholar]
- Eder, C.; Falkner, E.; Nehrer, S.; Losert, U.M.; Schoeffl, H. Introducing the concept of the 3Rs into tissue engineering research. ALTEX 2006, 23, 17–23. [Google Scholar] [PubMed]
- Moreno-Jiménez, I.; Kanczler, J.M.; Hulsart-Billstrom, G.; Stefanie, I.; Oreffo, R.O.C. The chorioallantoic membrane assay for biomaterial testing in tissue engineering: A short-term in vivo preclinical model. Tissue Eng. Part C Methods 2017, 23, 938–952. [Google Scholar] [CrossRef]
- Falkner, E.; Eder, C.; Kapeller, B.; Fröschl, W.; Schmatz, C.; Macfelda, K.; Losert, U.M. The mandatory cam testing of cells and scaffolds for tissue engineering: Benefits for the three RS of cooperation with the vaccine industry. Altern. Lab. Anim. 2004, 32, 573–580. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, A.S.; Schweizer, S.; Nolasco, P.; Barahona, I.; Saraiva, J.; Colaço, R.; Serro, A.P. Tough and Low Friction Polyvinyl Alcohol Hydrogels Loaded with Anti-inflammatories for Cartilage Replacement. Lubricants 2020, 8, 36. https://doi.org/10.3390/lubricants8030036
Oliveira AS, Schweizer S, Nolasco P, Barahona I, Saraiva J, Colaço R, Serro AP. Tough and Low Friction Polyvinyl Alcohol Hydrogels Loaded with Anti-inflammatories for Cartilage Replacement. Lubricants. 2020; 8(3):36. https://doi.org/10.3390/lubricants8030036
Chicago/Turabian StyleOliveira, Andreia Sofia, Sara Schweizer, Pedro Nolasco, Isabel Barahona, Jorge Saraiva, Rogério Colaço, and Ana Paula Serro. 2020. "Tough and Low Friction Polyvinyl Alcohol Hydrogels Loaded with Anti-inflammatories for Cartilage Replacement" Lubricants 8, no. 3: 36. https://doi.org/10.3390/lubricants8030036
APA StyleOliveira, A. S., Schweizer, S., Nolasco, P., Barahona, I., Saraiva, J., Colaço, R., & Serro, A. P. (2020). Tough and Low Friction Polyvinyl Alcohol Hydrogels Loaded with Anti-inflammatories for Cartilage Replacement. Lubricants, 8(3), 36. https://doi.org/10.3390/lubricants8030036