Chance or Necessity—The Fungi Co−Occurring with Formica polyctena Ants
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Mutualistic Fungus–Ant Interaction in the Holarctic Region Is Understudied
1.2. Red Wood Ants’ Mounds Are Very Specific Microenvironments, Actively Shaped by Their Biocenosis
1.3. The Insight into F. polyctena Mycobiota Could Shed a Light on the Complex Interactions between Individuals within and between Nests
2. Materials and Methods
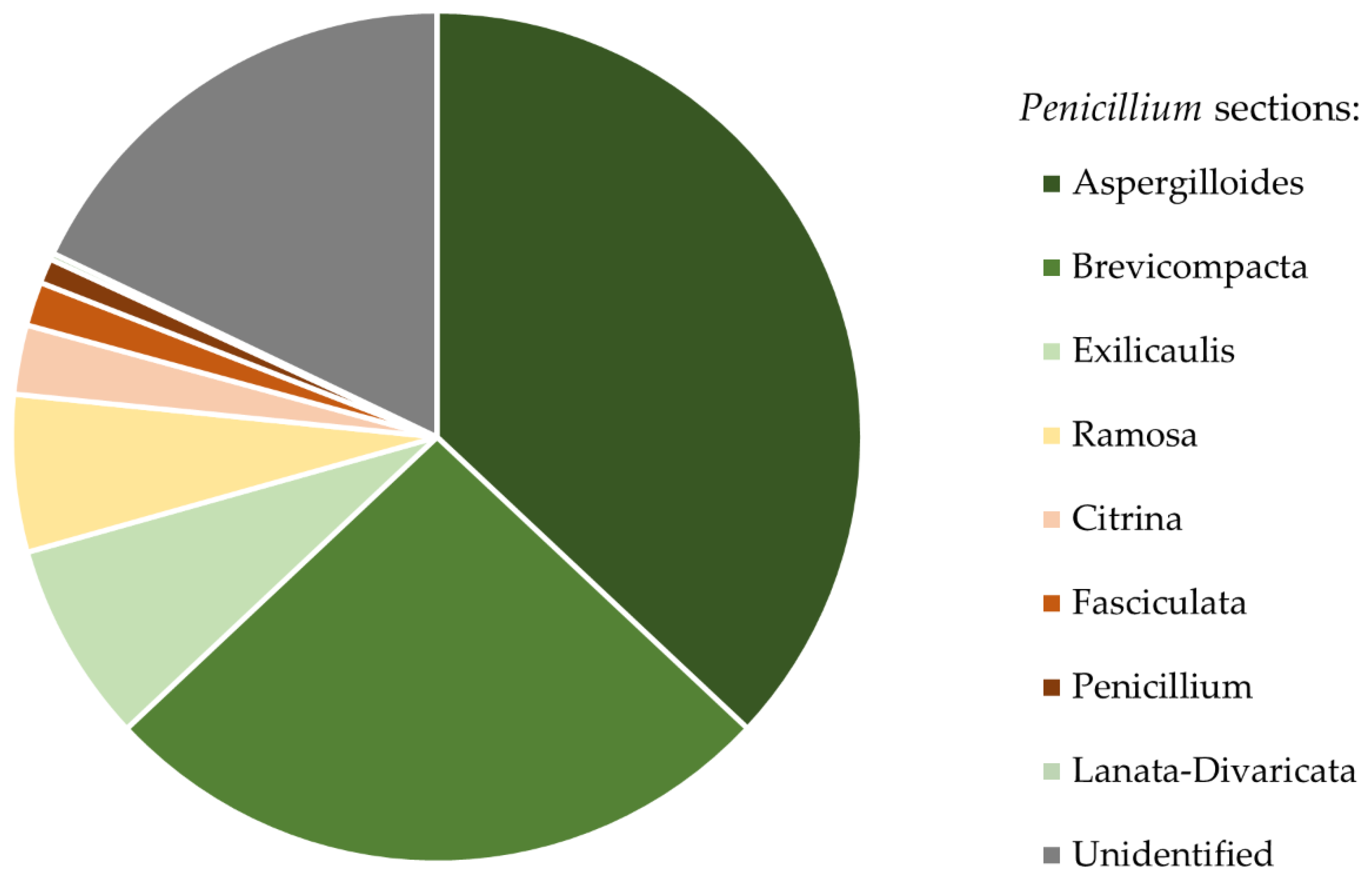
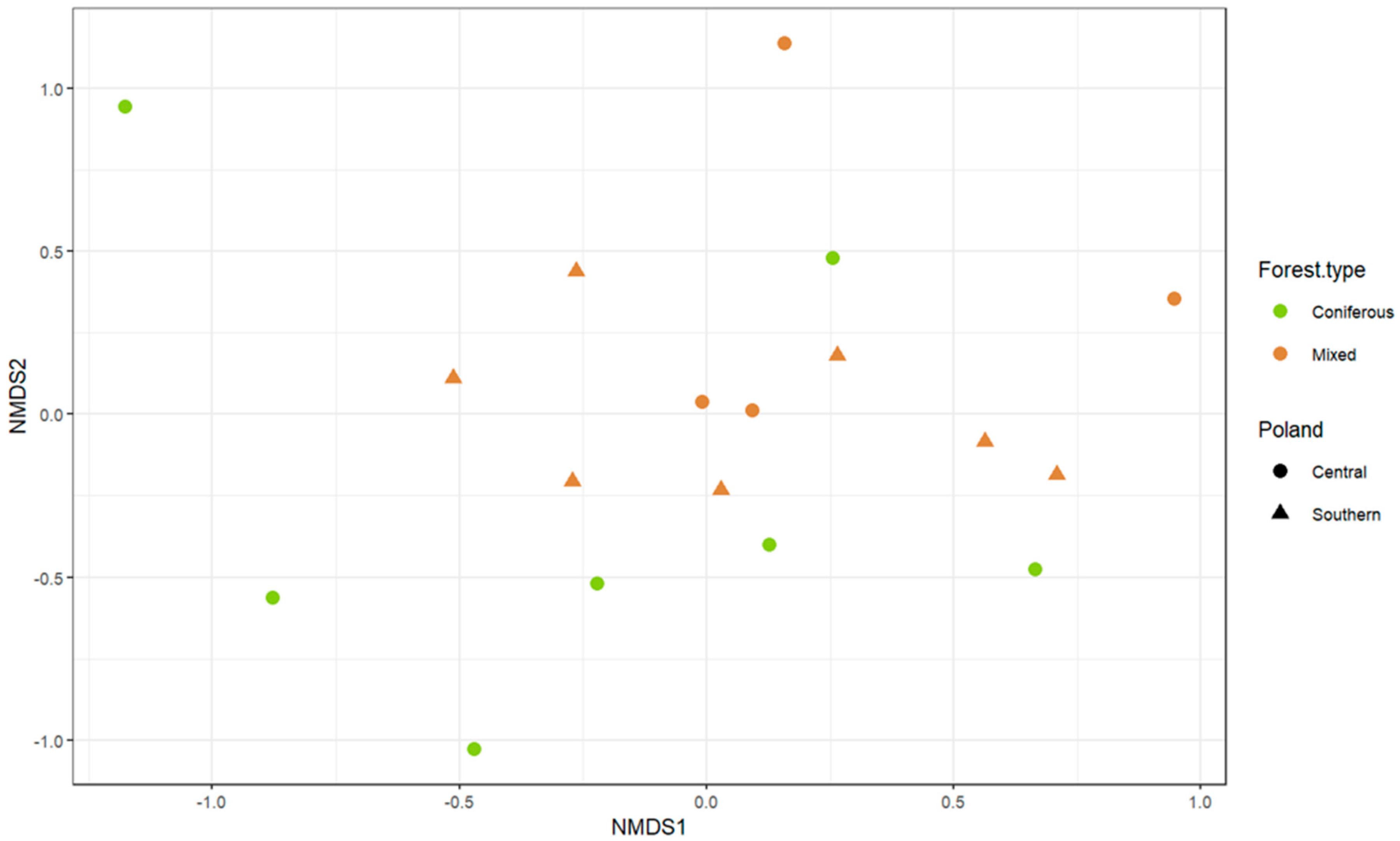
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mueller, U.G.; Rehner, S.A.; Schultz, T.R. The Evolution of Agriculture in Ants. Science 1998, 281, 2034–2038. [Google Scholar] [CrossRef]
- Mueller, U.G.; Schultz, T.R.; Currie, C.R.; Malloch, D. The Origin of the Attine Ant-Fungus Mutualism. Q. Rev. Biol. 2001, 76, 169–197. [Google Scholar] [CrossRef]
- Vega, F.E.; Blackwell, M. Insect-Fungal Associations: Ecology and Evolution; Oxford University Press: Oxford, UK, 2005; ISBN 0-19-803727-9. [Google Scholar]
- Haeder, S.; Wirth, R.; Herz, H.; Spiteller, D. Candicidin-Producing Streptomyces Support Leaf-Cutting Ants to Protect Their Fungus Garden against the Pathogenic Fungus Escovopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 4742–4746. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.L.; Mueller, U.G.; Mikheyev, A.S. Free-Living Fungal Symbionts (Lepiotaceae) of Fungus-Growing Ants (Attini: Formicidae). Mycologia 2009, 101, 206–210. [Google Scholar] [CrossRef]
- Voglmayr, H.; Mayer, V.; Maschwitz, U.; Moog, J.; Djieto-Lordon, C.; Blatrix, R. The Diversity of Ant-Associated Black Yeasts: Insights into a Newly Discovered World of Symbiotic Interactions. Fungal Biol. 2011, 115, 1077–1091. [Google Scholar] [CrossRef] [PubMed]
- Nepel, M.; Voglmayr, H.; Schönenberger, J.; Mayer, V.E. High Diversity and Low Specificity of Chaetothyrialean Fungi in Carton Galleries in a Neotropical Ant–Plant Association. PLoS ONE 2014, 9, e112756. [Google Scholar] [CrossRef] [PubMed]
- Nepel, M.; Voglmayr, H.; Blatrix, R.; Longino, J.T.; Fiedler, K.; Schönenberger, J.; Mayer, V.E. Ant-Cultivated Chaetothyriales in Hollow Stems of Myrmecophytic Cecropia Sp. Trees–Diversity and Patterns. Fungal Ecol. 2016, 23, 131–140. [Google Scholar] [CrossRef]
- Vasse, M.; Voglmayr, H.; Mayer, V.; Gueidan, C.; Nepel, M.; Moreno, L.; de Hoog, S.; Selosse, M.-A.; McKey, D.; Blatrix, R. A Phylogenetic Perspective on the Association between Ants (Hymenoptera: Formicidae) and Black Yeasts (Ascomycota: Chaetothyriales). Proc. R. Soc. B Biol. Sci. 2017, 284, 20162519. [Google Scholar] [CrossRef]
- Mayer, V.E.; Nepel, M.; Blatrix, R.; Oberhauser, F.B.; Fiedler, K.; Schönenberger, J.; Voglmayr, H. Transmission of Fungal Partners to Incipient Cecropia-Tree Ant Colonies. PLoS ONE 2018, 13, e0192207. [Google Scholar] [CrossRef]
- Moreno, L.F.; Mayer, V.; Voglmayr, H.; Blatrix, R.; Benjamin Stielow, J.; Teixeira, M.M.; Vicente, V.A.; de Hoog, S. Genomic Analysis of Ant Domatia-Associated Melanized Fungi (Chaetothyriales, Ascomycota). Mycol. Prog. 2019, 18, 541–552. [Google Scholar] [CrossRef]
- Espadaler, X.; Santamaria, S. Ecto-and Endoparasitic Fungi on Ants from the Holarctic Region. Psyche J. Entomol. 2012, 2012. [Google Scholar] [CrossRef]
- Vega, F.E.; Kaya, H.K. Insect Pathology; Academic Press: San Diego, CA, USA, 2012; ISBN 0-12-384984-5. [Google Scholar]
- Csata, E.; Erős, K.; Markó, B. Effects of the Ectoparasitic Fungus Rickia wasmannii on Its Ant Host Myrmica scabrinodis: Changes in Host Mortality and Behavior. Insectes Sociaux 2014, 61, 247–252. [Google Scholar] [CrossRef]
- Markó, B.; Csata, E.; Erős, K.; Német, E.; Czekes, Z.; Rózsa, L. Distribution of the Myrmecoparasitic Fungus Rickia wasmannii (Ascomycota: Laboulbeniales) across Colonies, Individuals, and Body Parts of Myrmica scabrinodis. J. Invertebr. Pathol. 2016, 136, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Wrzosek, M.; Dubiel, G.; Gorczak, M.; Pawłowska, J.; Tischer, M.; Bałazy, S. New Insights on the Phylogeny and Biology of the Fungal Ant Pathogen Aegeritella. J. Invertebr. Pathol. 2016, 133, 1–7. [Google Scholar] [CrossRef]
- Małagocka, J.; Jensen, A.B.; Eilenberg, J. Pandora formicae, a Specialist Ant Pathogenic Fungus: New Insights into Biology and Taxonomy. J. Invertebr. Pathol. 2017, 143, 108–114. [Google Scholar] [CrossRef] [PubMed]
- De Bekker, C.; Will, I.; Das, B.; Adams, R.M. The Ants (Hymenoptera: Formicidae) and Their Parasites: Effects of Parasitic Manipulations and Host Responses on Ant Behavioral Ecology. Myrmecol. News 2018, 28, 1–2. [Google Scholar]
- Wheeler, W.M.; Bailey, I.W. The Feeding Habits of Pseudomyrmine and Other Ants. Trans. Am. Philos. Soc. 1920, 22, 235–279. [Google Scholar] [CrossRef]
- Clark, D.W. Fungi Associated with the Infrabuccal Pockets of Camponotus Pennsylvanicus and Other Formicine Ants. Master’s Thesis, University of Toronto, Department of Botany, Toronto, ON, Canada, 2002. [Google Scholar]
- Mankowski, M.E.; Morrell, J.J. Yeasts Associated with the Infrabuccal Pocket and Colonies of the Carpenter Ant Camponotus vicinus. Mycologia 2004, 96, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Visagie, C.M.; Renaud, J.B.; Burgess, K.M.N.; Malloch, D.W.; Clark, D.; Ketch, L.; Urb, M.; Louis-Seize, G.; Assabgui, R.; Sumarah, M.W. Fifteen New Species of Penicillium. Pers. Mol. Phylogeny Evol. Fungi 2016, 36, 247. [Google Scholar] [CrossRef]
- Boots, B.; Clipson, N. Linking Ecosystem Modification by the Yellow Meadow Ant (Lasius flavus) to Microbial Assemblages in Different Soil Environments. Eur. J. Soil Biol. 2013, 55, 100–106. [Google Scholar] [CrossRef]
- Lindström, S.; Timonen, S.; Sundström, L.; Johansson, H. Ants Reign over a Distinct Microbiome in Forest Soil. Soil Biol. Biochem. 2019, 139, 107529. [Google Scholar] [CrossRef]
- Fernández-Marín, H.; Zimmerman, J.K.; Rehner, S.A.; Wcislo, W.T. Active Use of the Metapleural Glands by Ants in Controlling Fungal Infection. Proc. R. Soc. B Biol. Sci. 2006, 273, 1689–1695. [Google Scholar] [CrossRef]
- Brütsch, T.; Jaffuel, G.; Vallat, A.; Turlings, T.C.; Chapuisat, M. Wood Ants Produce a Potent Antimicrobial Agent by Applying Formic Acid on Tree-collected Resin. Ecol. Evol. 2017, 7, 2249–2254. [Google Scholar] [CrossRef]
- Cremer, S.; Armitage, S.A.; Schmid-Hempel, P. Social Immunity. Curr. Biol. 2007, 17, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Reber, A.; Purcell, J.; Buechel, S.D.; Buri, P.; Chapuisat, M. The Expression and Impact of Antifungal Grooming in Ants. J. Evol. Biol. 2011, 24, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Tragust, S.; Mitteregger, B.; Barone, V.; Konrad, M.; Ugelvig, L.V.; Cremer, S. Ants Disinfect Fungus-Exposed Brood by Oral Uptake and Spread of Their Poison. Curr. Biol. 2013, 23, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Castella, G.; Chapuisat, M.; Christe, P. Prophylaxis with Resin in Wood Ants. Anim. Behav. 2008, 75, 1591–1596. [Google Scholar] [CrossRef]
- Wilson-Rich, N.; Spivak, M.; Fefferman, N.H.; Starks, P.T. Genetic, Individual, and Group Facilitation of Disease Resistance in Insect Societies. Annu. Rev. Entomol. 2009, 54, 405–423. [Google Scholar] [CrossRef]
- Batey, S.F.; Greco, C.; Hutchings, M.I.; Wilkinson, B. Chemical Warfare between Fungus-Growing Ants and Their Pathogens. Curr. Opin. Chem. Biol. 2020, 59, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Currie, C.R. A Community of Ants, Fungi, and Bacteria: A Multilateral Approach to Studying Symbiosis. Annu. Rev. Microbiol. 2001, 55, 357–380. [Google Scholar] [CrossRef] [PubMed]
- Czechowski, W.; Radchenko, A.; Czechowska, W.; Vepsäläinen, K. The Ants of Poland with Reference to the Myrmecofauna of Europe; Natura Optima Dux Foundation: Warsaw, Poland, 2012; ISBN 8393077346. [Google Scholar]
- Frouz, J.; Jilková, V. The Effect of Ants on Soil Properties and Processes (Hymenoptera: Formicidae). Myrmecol. News 2008, 11, 191–199. [Google Scholar]
- Jílková, V.; Šebek, O.; Frouz, J. Mechanisms of PH Change in Wood Ant (Formica Polyctena) Nests. Pedobiologia 2012, 55, 247–251. [Google Scholar] [CrossRef]
- Romiguier, J.; Rolland, J.; Morandin, C.; Keller, L. Phylogenomics of Palearctic Formica Species Suggests a Single Origin of Temporary Parasitism and Gives Insights to the Evolutionary Pathway toward Slave-Making Behaviour. BMC Evol. Biol. 2018, 18, 1–8. [Google Scholar] [CrossRef]
- Domisch, T.; Finer, L.; Neuvonen, S.; Niemelä, P.; Risch, A.C.; Kilpeläinen, J.; Ohashi, M.; Jurgensen, M.F. Foraging Activity and Dietary Spectrum of Wood Ants (Formica rufa Group) and Their Role in Nutrient Fluxes in Boreal Forests. Ecol. Entomol. 2009, 34, 369–377. [Google Scholar] [CrossRef]
- Stockan, J.A.; Robinson, E.J. Wood Ant Ecology and Conservation; Cambridge University Press: Cambridge, UK, 2016; ISBN 1107048338. [Google Scholar]
- Krzysztofiak, L.; Krzysztofiak, A. Mrówki Środowisk Leśnych Polski; Stowarzyszenie Człowiek i Przyroda: Suwałki, Poland, 2006; ISBN 836011515X. [Google Scholar]
- Scherba, G. Reproduction, Nest Orientation and Population Structure of an Aggregation of Mound Nests Of Formica ulkei Emery («Formicidae»). Insectes Sociaux 1958, 5, 201–213. [Google Scholar] [CrossRef]
- Malozemova, L.A.; Koruma, N.P. Influence of Ants on Soil. Sov. J. Ecol. 1973, 450–452. [Google Scholar]
- Lockaby, B.G.; Adams, J.C. Pedoturbation of a Forest Soil by Fire Ants. Soil Sci. Soc. Am. J. 1985, 49, 220–223. [Google Scholar] [CrossRef]
- Frouz, J.; Holec, M.; Kalčík, J. The Effect of Lasius niger (Hymenoptera, Formicidae) Ant Nest on Selected Soil Chemical Properties. Pedobiologia 2003, 47, 205–212. [Google Scholar] [CrossRef]
- Kilpeläinen, J.; Finér, L.; Niemelä, P.; Domisch, T.; Neuvonen, S.; Ohashi, M.; Risch, A.C.; Sundström, L. Carbon, Nitrogen and Phosphorus Dynamics of Ant Mounds (Formica rufa Group) in Managed Boreal Forests of Different Successional Stages. Appl. Soil Ecol. 2007, 36, 156–163. [Google Scholar] [CrossRef]
- Chapuisat, M.; Oppliger, A.; Magliano, P.; Christe, P. Wood Ants Use Resin to Protect Themselves against Pathogens. Proc. R. Soc. B Biol. Sci. 2007, 274, 2013–2017. [Google Scholar] [CrossRef]
- Grishkan, I.B. Anthills at the Upper Kolyma River as Substrate for Micromycetes. Mikol. Fitopatol. 1989, 23, 105–109. [Google Scholar]
- Maksimova, I.A.; Glushakova, A.M.; Kachalkin, A.V.; Chernov, I.Y.; Panteleeva, S.N.; Reznikova, Z.I. Yeast Communities of Formica aquilonia Colonies. Microbiology 2016, 85, 124–129. [Google Scholar] [CrossRef]
- Golubev, V.I.; Bab’eva, I.P. Debaryomyces formicarius Sp. n. and Debaryomyces cantarellii Associated with the Ants of the Group Formica rufa L. J. Gen. Appl. Microbiol. 1972, 18, 249–254. [Google Scholar]
- Duff, L.B.; Urichuk, T.M.; Hodgins, L.N.; Young, J.R.; Untereiner, W.A. Diversity of Fungi from the Mound Nests of Formica ulkei and Adjacent Non-Nest Soils. Can. J. Microbiol. 2016, 62, 562–571. [Google Scholar] [CrossRef]
- Frouz, J. The Effect of Nest Moisture on Daily Temperature Regime in the Nests of Formica polyctena Wood Ants. Insectes Sociaux 2000, 47, 229–235. [Google Scholar] [CrossRef]
- Webber, J.F.; Gibbs, J.N. Insect Dissemination of Fungal Pathogens of Trees. Insect-Fungus Interact. 1989, 161–175. [Google Scholar]
- Abbott, S.P. Insects and Other Arthropods as Agents of Vector-Dispersal in Fungi. 2002. Available online: http://www.Com/Pdf/Abbottinsectdispersal.Pdf (accessed on 4 January 2021).
- Wyatt, T.T.; Wösten, H.A.; Dijksterhuis, J. Fungal spores for dispersion in space and time. In Advances in Applied Microbiology; Elsevier Academic Press: San Diego, CA, USA, 2013; Volume 85, pp. 43–91. ISBN 00652164. [Google Scholar]
- De Zarzuela, M.F.M.; Campos-Farinha, A.E.D.C.; Russomanno, O.M.; Kruppa, P.C.; Goncalez, E. Evaluation of Urban Ants (Hymenoptera: Formicidae) as Vectors of Microorganisms in Residential and Industrial Environments: II. Fungi. Sociobiology 2007, 50, 653–658. [Google Scholar]
- Duarte, A.P.M.; Attili-Angelis, D.; Baron, N.C.; Groenewald, J.Z.; Crous, P.W.; Pagnocca, F.C. Riding with the Ants. Pers. Mol. Phylogeny Evol. Fungi 2017, 38, 81. [Google Scholar] [CrossRef] [PubMed]
- Bird, A.E.; Hesketh, H.; Cross, J.V.; Copland, M. The Common Black Ant, Lasius niger (Hymenoptera: Formicidae), as a Vector of the Entomopathogen Lecanicillium longisporum to Rosy Apple Aphid, Dysaphis plantaginea (Homoptera: Aphididae). Biocontrol Sci. Technol. 2004, 14, 757–767. [Google Scholar] [CrossRef]
- Kurze, C.; Jenkins, N.E.; Hughes, D.P. Evaluation of Direct and Indirect Transmission of Fungal Spores in Ants. J. Invertebr. Pathol. 2020, 172, 107351. [Google Scholar] [CrossRef]
- Eisner, T.; Happ, G.M. The Infrabuccal Pocket of a Formicine Ant: A Social Filtration Device. Psyche 1962, 69, 107–116. [Google Scholar] [CrossRef]
- Regulation of the Minister of Environment dated December 16, 2016 on the protected species of animals. J. Laws 2016. Available online: https://dziennikustaw.gov.pl/DU/rok/2016/pozycja/2183 (accessed on 4 January 2021).
- Gardes, M.; Bruns, T.D. ITS Primers with Enhanced Specificity for Basidiomycetes-application to the Identification of Mycorrhizae and Rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- White, T.J. Analysis of Phylogenetic Relationships by Amplification and Direct Sequencing of Ribosomal Genes. In PCR Protocols: A Guide to Methods and Applications; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020. [Google Scholar]
- Oksanen, J.F.; Blanchet, G.; Friendly, M.; KIndt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package; 2019; Available online: https://github.com/vegandevs/vegan (accessed on 4 January 2021).
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Hadley, W. Ggplot2: Elegrant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 3319242776. [Google Scholar]
- Vandepol, N.; Liber, J.; Desirò, A.; Na, H.; Kennedy, M.; Barry, K.; Grigoriev, I.V.; Miller, A.N.; O’Donnell, K.; Stajich, J.E. Resolving the Mortierellaceae Phylogeny through Synthesis of Multi-Gene Phylogenetics and Phylogenomics. Fungal Divers. 2020, 104, 1–23. [Google Scholar] [CrossRef]
- Visagie, C.; Houbraken, J.; Frisvad, J.C.; Hong, S.-B.; Klaassen, C.; Perrone, G.; Seifert, K.; Varga, J.; Yaguchi, T.; Samson, R. Identification and Nomenclature of the Genus Penicillium. Stud. Mycol. 2014, 78, 343–371. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Kopchinskiy, A.G.; Kubicek, C.P. The First 100 Trichoderma Species Characterized by Molecular Data. Mycoscience 2006, 47, 55. [Google Scholar] [CrossRef]
- Efimenko, T.A.; Glukhova, A.A.; Demiankova, M.V.; Boykova, Y.V.; Malkina, N.D.; Sumarukova, I.G.; Vasilieva, B.F.; Rogozhin, E.A.; Ivanov, I.A.; Krassilnikov, V.A. Antimicrobial Activity of Microorganisms Isolated from Ant Nests of Lasius niger. Life 2020, 10, 91. [Google Scholar] [CrossRef]
- Kauserud, H.; Lie, M.; Stensrud, Ø.; Ohlson, M. Molecular Characterization of Airborne Fungal Spores in Boreal Forests of Contrasting Human Disturbance. Mycologia 2005, 97, 1215–1224. [Google Scholar] [CrossRef]
- Pereira, R.M. Akanthomyces Sp.: A Newly Discovered Fungal Pathogen Affecting Solenopsis invicta and Pogonomyrmex badius in Florida. Mycologia 1950, 42, 566–589. [Google Scholar]
- Aini, A.N.; Mongkolsamrit, S.; Wijanarka, W.; Thanakitpipattana, D.; Luangsa-Ard, J.J.; Budiharjo, A. Diversity of Akanthomyces on Moths (Lepidoptera) in Thailand. MycoKeys 2020, 71, 1. [Google Scholar] [CrossRef]
- Hsieh, L.; Tzean, S.; Wu, W. The Genus Akanthomyces on Spiders from Taiwan. Mycologia 1997, 89, 319–324. [Google Scholar] [CrossRef]
- Espadaler, X.; Monteserín, S. Aegeritella (Deuteromycetes) on Formica (Hymenoptera, Formicidae) in Spain. Orsis Org. Sist. 2003, 13–17. [Google Scholar]
- Balazy, S.; Wisniewski, J. Aegeritella superficialis Gen. et Sp. Nov., Epifityczny Grzyb Na Mrówkach z Rodzaju Formica L. Pr. Kom. Nauk Rol. Kom. Nauk Lesn. Pozn. Tow. Przyj. Nauk Wydz. Nauk Rol. Lesn. 1974, 38, 3–15. [Google Scholar]
- GBIF Home Page. 2021. Available online: GBIF.org (accessed on 4 January 2021).
- Wu, C.; Vellend, M.; Yuan, W.; Jiang, B.; Liu, J.; Shen, A.; Liu, J.; Zhu, J.; Yu, M. Patterns and Determinants of Plant Biodiversity in Non-Commercial Forests of Eastern China. PLoS ONE 2017, 12, e0188409. [Google Scholar] [CrossRef] [PubMed]
- Urbanová, M.; Šnajdr, J.; Baldrian, P. Composition of Fungal and Bacterial Communities in Forest Litter and Soil Is Largely Determined by Dominant Trees. Soil Biol. Biochem. 2015, 84, 53–64. [Google Scholar] [CrossRef]
- Park, M.S.; Oh, S.-Y.; Fong, J.J.; Houbraken, J.; Lim, Y.W. The Diversity and Ecological Roles of Penicillium in Intertidal Zones. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Gupta, V.K. New and Future Developments in Microbial Biotechnology and Bioengineering: Aspergillus System Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 0444635130. [Google Scholar]
- Rodrigues, A.; Mueller, U.G.; Ishak, H.D.; Bacci, M., Jr.; Pagnocca, F.C. Ecology of Microfungal Communities in Gardens of Fungus-Growing Ants (Hymenoptera: Formicidae): A Year-Long Survey of Three Species of Attine Ants in Central Texas. FEMS Microbiol. Ecol. 2011, 78, 244–255. [Google Scholar] [CrossRef]
- Davydenko, K.; Vasaitis, R.; Menkis, A. Fungi Associated with Ips acuminatus (Coleoptera: Curculionidae) in Ukraine with a Special Emphasis on Pathogenicity of Ophiostomatoid Species. Eur. J. Entomol. 2017, 114, 77–85. [Google Scholar] [CrossRef]
- Li, X.; Wheeler, G.S.; Ding, J. A Leaf-Rolling Weevil Benefits from General Saprophytic Fungi in Polysaccharide Degradation. Arthropod-Plant Interact. 2012, 6, 417–424. [Google Scholar] [CrossRef]
- Shruthi, K.; Yadav, P.S.; Prasad, B.S.; Chandra, M.S. Cellulase Production by Aspergillus unguis in Solid State Fermentation. J. For. Res. 2019, 30, 205–212. [Google Scholar] [CrossRef]
- Gharaei-Fathabad, E.; Tajick-Ghanbary, M.; Shahrokhi, N. Antimicrobial Properties of Penicillium Species Isolated from Agricultural Soils of Northern Iran. Res. J. Toxins 2009, 1, 1–7. [Google Scholar]
- Khokhar, I.; Haider, M.S.; Mukhtar, I.; Mushtaq, S. Biological Control of Aspergillus niger, the Cause of Black-Rot Disease of Allium cepa L. (Onion), by Penicillium Species. J. Agrobiol. 2012, 29, 23–28. [Google Scholar] [CrossRef]
- Leger, R.J.S.; Screen, S.E.; Shams-Pirzadeh, B. Lack of Host Specialization in Aspergillus flavus. Appl. Environ. Microbiol. 2000, 66, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Strakowska, J.; Błaszczyk, L.; Chełkowski, J. The Significance of Cellulolytic Enzymes Produced by Trichoderma in Opportunistic Lifestyle of This Fungus. J. Basic Microbiol. 2014, 54, S2–S13. [Google Scholar] [CrossRef]
- Adnan, M.; Islam, W.; Shabbir, A.; Khan, K.A.; Ghramh, H.A.; Huang, Z.; Chen, H.Y.; Lu, G. Plant Defense against Fungal Pathogens by Antagonistic Fungi with Trichoderma in Focus. Microb. Pathog. 2019, 129, 7–18. [Google Scholar] [CrossRef]
- De Santo, A.V.; Rutigliano, F.A.; Berg, B.; Fioretto, A.; Puppi, G.; Alfani, A. Fungal Mycelium and Decomposition of Needle Litter in Three Contrasting Coniferous Forests. Acta Oecologica 2002, 23, 247–259. [Google Scholar] [CrossRef]
- Gusteleva, L. Biosynthesis of Group B Vitamins by Yeasts--Symbionts of Xylophagous Insects. Mikrobiologiia 1975, 44, 45–47. [Google Scholar]
- Álvaro-Benito, M.; Polo, A.; González, B.; Fernández-Lobato, M.; Sanz-Aparicio, J. Structural and Kinetic Analysis of Schwanniomyces occidentalis Invertase Reveals a New Oligomerization Pattern and the Role of Its Supplementary Domain in Substrate Binding. J. Biol. Chem. 2010, 285, 13930–13941. [Google Scholar] [CrossRef]
- Wagner, L.; Stielow, B.; Hoffmann, K.; Petkovits, T.; Papp, T.; Vágvölgyi, C.; De Hoog, G.; Verkley, G.; Voigt, K. A Comprehensive Molecular Phylogeny of the Mortierellales (Mortierellomycotina) Based on Nuclear Ribosomal DNA. Pers. Mol. Phylogeny Evol. Fungi 2013, 30, 77. [Google Scholar] [CrossRef]
- Wrzosek, M.; Siedlecki, I.; Kopczyński, M. Whether ants choose between different species of Mortierella s.l.? In Behavioral Evidence for Taxonomic Distinctiveness of Entomortierella and Mortierella; in preparation.



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siedlecki, I.; Gorczak, M.; Okrasińska, A.; Wrzosek, M. Chance or Necessity—The Fungi Co−Occurring with Formica polyctena Ants. Insects 2021, 12, 204. https://doi.org/10.3390/insects12030204
Siedlecki I, Gorczak M, Okrasińska A, Wrzosek M. Chance or Necessity—The Fungi Co−Occurring with Formica polyctena Ants. Insects. 2021; 12(3):204. https://doi.org/10.3390/insects12030204
Chicago/Turabian StyleSiedlecki, Igor, Michał Gorczak, Alicja Okrasińska, and Marta Wrzosek. 2021. "Chance or Necessity—The Fungi Co−Occurring with Formica polyctena Ants" Insects 12, no. 3: 204. https://doi.org/10.3390/insects12030204
APA StyleSiedlecki, I., Gorczak, M., Okrasińska, A., & Wrzosek, M. (2021). Chance or Necessity—The Fungi Co−Occurring with Formica polyctena Ants. Insects, 12(3), 204. https://doi.org/10.3390/insects12030204





