Comparative Analysis of Volatiles Emitted from Tomato and Pepper Plants in Response to Infection by Two Whitefly-Transmitted Persistent Viruses
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Rearing and Maintenance of Non-Viruliferous and Viruliferous (TYLCV/PeWBVYV) Bemisia tabaci
2.2. Inoculation of Plants with Viruliferous (TYLCV/PeWBVYV) Whiteflies and Viral Detection
2.3. Volatile Extraction and Analysis Using Headspace Solid Phase Micro-Extraction (HS-SPME) Coupled with Gas Chromatography–Mass Spectrometry (GC-MS)
2.4. Statistics
3. Results
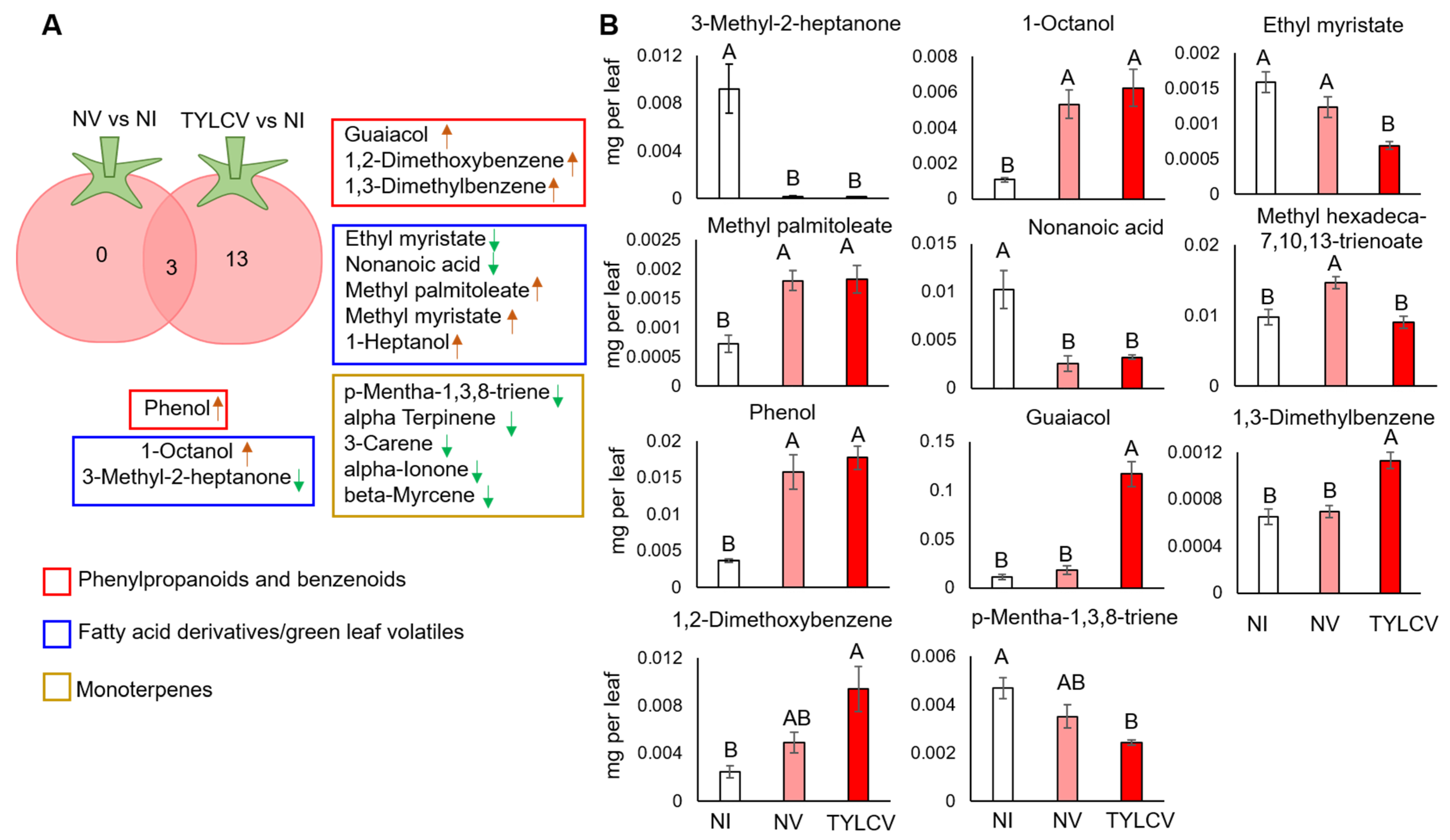
3.1. VOC Profiles of Tomato Plants Uninfected or Infected with TYLCV
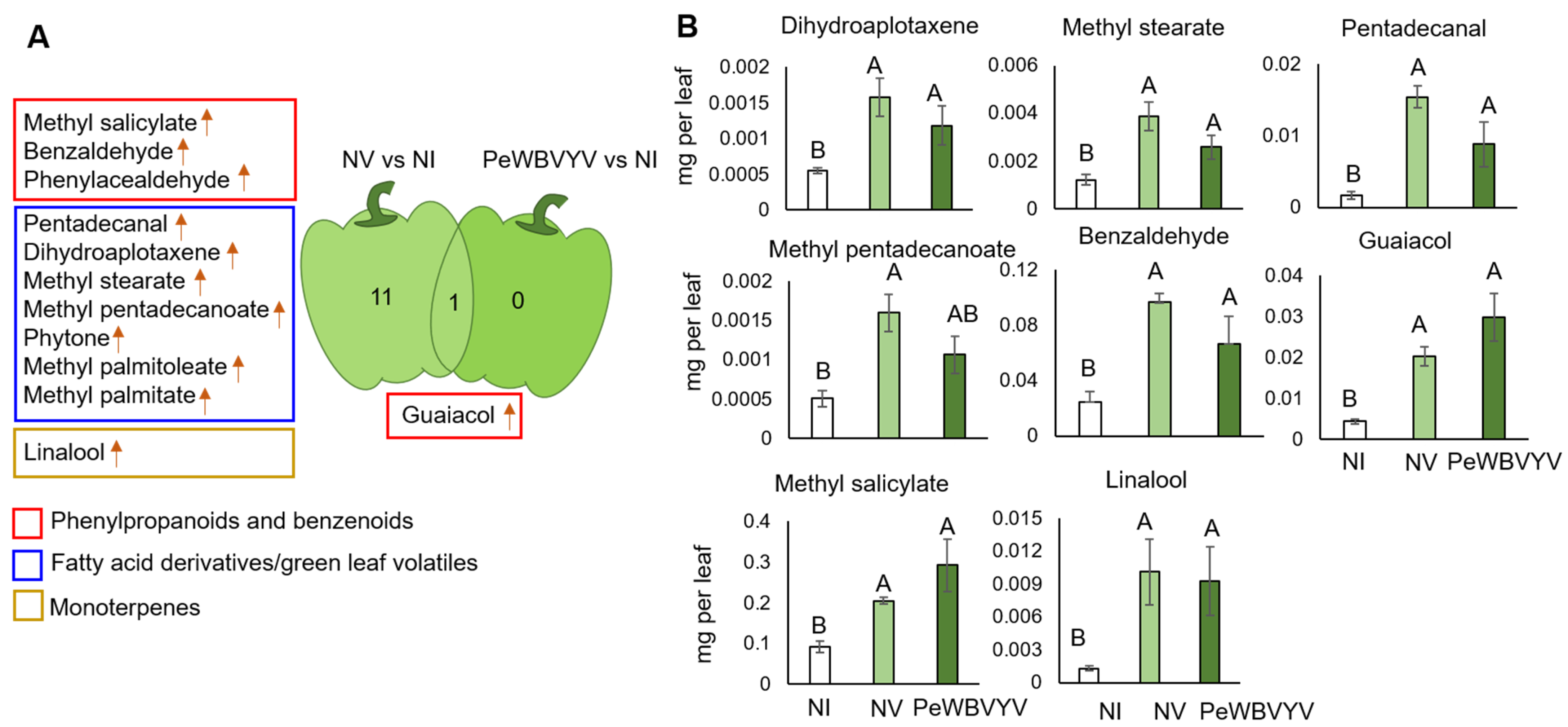
3.2. VOC Profiles of Pepper Leaves Uninfected or Infected with PeWBVYV
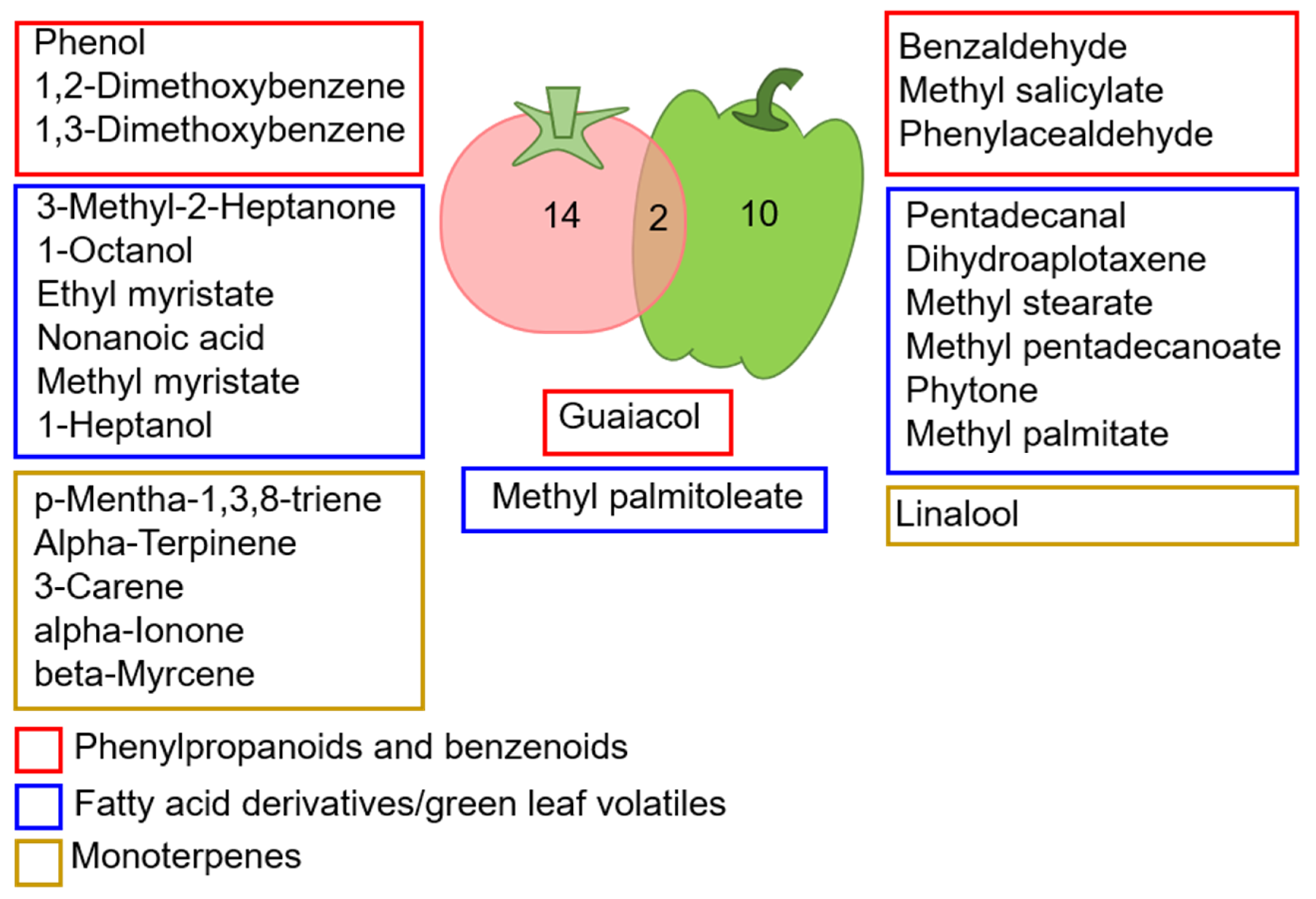
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statements
Acknowledgments
Conflicts of Interest
References
- Ghosh, S.; Ghanim, M. Factors determining transmission of persistent viruses by Bemisia tabaci and emergence of new virus–vector relationships. Viruses 2021, 13, 1808. [Google Scholar] [CrossRef]
- Byrne, D.N.; Bellows, T.S. Whitefly biology. Annu. Rev. Entomol. 1991, 36, 431–457. [Google Scholar] [CrossRef]
- Scholthof, K.G.; Adkins, S.; Czosnek, H.; Palukaitis, P.; Jacquot, E.; Hohn, T.; Hohn, B.; Saunders, K.; Candresse, T.; Ahlquist, P. Top 10 plant viruses in molecular plant pathology. Mol. Plant Pathol. 2011, 12, 938–954. [Google Scholar] [CrossRef]
- Moriones, E.; Navas-Castillo, J. Tomato yellow leaf curl virus, an emerging virus complex causing epidemics worldwide. Virus Res. 2000, 71, 123–134. [Google Scholar] [CrossRef]
- Gilbertson, R.L.; Batuman, O.; Webster, C.G.; Adkins, S. Role of the insect supervectors Bemisia tabaci and Frankliniella occidentalis in the emergence and global spread of plant viruses. Annu. Rev. Virol. 2015, 2, 67–93. [Google Scholar] [CrossRef]
- Navas-Castillo, J.; Fiallo-Olivé, E.; Sánchez-Campos, S. Emerging virus diseases transmitted by whiteflies. Annu. Rev. Phytopathol. 2011, 49, 219–248. [Google Scholar] [CrossRef]
- Ghosh, S.; Kanakala, S.; Lebedev, G.; Kontsedalov, S.; Silverman, D.; Alon, T.; Mor, N.; Sela, N.; Luria, N.; Dombrovsky, A.; et al. Transmission of a new polerovirus infecting pepper by the whitefly Bemisia tabaci. J. Virol. 2019, 93, e00488-19. [Google Scholar] [CrossRef]
- Costa, T.M.; Inoue-Nagata, A.K.; Vidal, A.H.; Ribeiro, S.G.; Nagata, T. The recombinant isolate of cucurbit aphid-borne yellows virus from Brazil is a polerovirus transmitted by whiteflies. Plant Pathol. 2020, 69, 1042–1050. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Gershenzon, J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 2010, 15, 176–184. [Google Scholar] [CrossRef]
- Vickers, C.E.; Gershenzon, J.; Lerdau, M.T.; Loreto, F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat. Chem. Biol. 2009, 5, 283–291. [Google Scholar] [CrossRef]
- De Moraes, C.M.; Mescher, M.C.; Tumlinson, J.H. Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 2001, 410, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Baldwin, I.T. Defensive function of herbivore-induced plant volatile emissions in nature. Science 2001, 291, 2141–2144. [Google Scholar] [CrossRef] [PubMed]
- Kreuzwieser, J.; Scheerer, U.; Kruse, J.; Burzlaff, T.; Honsel, A.; Alfarraj, S.; Georgiev, P.; Schnitzler, J.P.; Ghirardo, A.; Kreuzer, I.; et al. The Venus flytrap attracts insects by the release of volatile organic compounds. J. Exp. Bot. 2014, 65, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Zarate, S.I.; Kempema, L.A.; Walling, L.L. Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol. 2007, 143, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.-J.; Wei, J.-N.; Zhao, C.; Zhang, Y.-F.; Li, C.-Y.; Liu, S.-S.; Dicke, M.; Yu, X.-P.; Turlings, T.C.J. Airborne host–plant manipulation by whiteflies via an inducible blend of plant volatiles. Proc. Natl. Acad. Sci. USA 2019, 116, 7387–7396. [Google Scholar] [CrossRef]
- Ament, K.; Kant, M.R.; Sabelis, M.W.; Haring, M.A.; Schuurink, R.C. Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiol. 2004, 135, 2025–2037. [Google Scholar] [CrossRef]
- Hardie, J.; Isaacs, R.; Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M. Methyl salicylate and (-)-(1 R,5 S)-myrtenal are plant-derived repellents for black bean aphid, Aphis fabae Scop. (Homoptera: Aphididae). J. Chem. Ecol. 1994, 20, 2847–2855. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef]
- Dorokhov, Y.L.; Komarova, T.V.; Sheshukova, E.V. Volatile organic compounds and plant virus-host interaction. In Plant Virus-Host Interaction: Molecular Approaches and Viral Evolution; Academic Press: Cambridge, MA, USA, 2014; pp. 241–262. ISBN 9780124115842. [Google Scholar]
- Mann, R.S.; Ali, J.G.; Hermann, S.L.; Tiwari, S.; Pelz-Stelinski, K.S.; Alborn, H.T.; Stelinski, L.L. Induced release of a plant-defense volatile “deceptively” attracts insect vectors to plants infected with a bacterial pathogen. PLoS Pathog. 2012, 8, e1002610. [Google Scholar] [CrossRef]
- Eigenbrode, S.D.; Ding, H.; Shiel, P.; Berger, P.H. Volatiles from potato plants infected with potato leafroll virus attract and arrest the virus vector, Myzus persicae (Homoptera: Aphididae). Proc. R. Soc. B Biol. Sci. 2002, 269, 455–460. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Zhang, Z.; Zhang, C.; Zhou, X.; Zhang, D.; Liu, Y. The molecular mechanism of efficient transmission of plant viruses in variable virus–vector–plant interactions. Hortic. Plant J. 2021, 7, 501–508. [Google Scholar] [CrossRef]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proc. Natl. Acad. Sci. USA 2010, 107, 3600–3605. [Google Scholar] [CrossRef] [PubMed]
- Tungadi, T.; Groen, S.C.; Murphy, A.M.; Pate, A.E.; Iqbal, J.; Bruce, T.J.A.; Cunniffe, N.J.; Carr, J.P. Cucumber mosaic virus and its 2b protein alter emission of host volatile organic compounds but not aphid vector settling in tobacco. Virol. J. 2017, 14, 91. [Google Scholar] [CrossRef]
- Bleeker, P.M.; Diergaarde, P.J.; Ament, K.; Guerra, J.; Weidner, M.; Schütz, S.; de Both, M.T.J.; Haring, M.A.; Schuurink, R.C. The role of specific tomato volatiles in tomato-whitefly interaction. Plant Physiol. 2009, 151, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Yao, D.; Zhang, T.; Walling, L.L.; Yang, M.; Wang, Y.; Liu, S. Suppression of terpenoid synthesis in plants by a virus promotes its mutualism with vectors. Ecol. Lett. 2013, 16, 390–398. [Google Scholar] [CrossRef]
- Fereres, A.; Peñaflor, M.F.G.V.; Favaro, C.F.; Azevedo, K.E.X.; Landi, C.H.; Maluta, N.K.P.; Bento, J.M.S.; Lopes, J.R.S. Tomato infection by whitefly-transmitted circulative and non-circulative viruses induce contrasting changes in plant volatiles and vector behaviour. Viruses 2016, 8, 225. [Google Scholar] [CrossRef]
- Werner, B.J.; Mowry, T.M.; Bosque-Pérez, N.A.; Ding, H.; Eigenbrode, S.D. Changes in green peach aphid responses to potato leafroll virusinduced volatiles emitted during disease progression. Environ. Entomol. 2009, 38, 1429–1438. [Google Scholar] [CrossRef]
- Ngumbi, E.; Eigenbrode, S.D.; Bosque-Pérez, N.A.; Ding, H.; Rodriguez, A. Myzus persicae is arrested more by blends than by individual compounds elevated in headspace of plrv-infected potato. J. Chem. Ecol. 2007, 33, 1733–1747. [Google Scholar] [CrossRef]
- Jiménez-Martínez, E.S.; Bosque-Pérez, N.A.; Berger, P.H.; Zemetra, R.S.; Ding, H.; Eigenbrode, S.D. Volatile cues influence the response of Rhopalosiphum padi (Homoptera: Aphididae) to barley yellow dwarf virus–infected transgenic and untransformed wheat. Environ. Entomol. 2004, 33, 1207–1216. [Google Scholar] [CrossRef]
- Claudel, P.; Chesnais, Q.; Fouché, Q.; Krieger, C.; Halter, D.; Bogaert, F.; Meyer, S.; Boissinot, S.; Hugueney, P.; Ziegler-Graff, V.; et al. The aphid-transmitted turnip yellows virus differentially affects volatiles emission and subsequent vector behavior in two Brassicaceae plants. Int. J. Mol. Sci. 2018, 19, 2316. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Bello, V.H.; Ghanim, M. Transmission parameters of pepper whitefly-borne vein yellows virus (PeWBVYV) by Bemisia tabaci and identification of an insect protein with a putative role in polerovirus transmission. Virology 2021, 560, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Bello, V.H.; Ghosh, S.; Krause-Sakate, R.; Ghanim, M. Competitive interactions between whitefly and aphid transmitted poleroviruses within the plant host and the insect vectors. Phytopathology 2021, 111, 1042–1050. [Google Scholar] [CrossRef]
- Ghanim, M.; Morin, S.; Czosnek, H. Rate of Tomato yellow leaf curl virus translocation in the circulative transmission pathway of its vector, the whitefly Bemisia tabaci. Phytopathology 2001, 91, 188–196. [Google Scholar] [CrossRef]
- Gyan, N.M.; Yaakov, B.; Weinblum, N.; Singh, A.; Cna’ani, A.; Ben-Zeev, S.; Saranga, Y.; Tzin, V. Variation between three Eragrostis tef accessions in defense responses to Rhopalosiphum padi aphid infestation. Front. Plant Sci. 2020, 11, 1892. [Google Scholar] [CrossRef]
- Weinblum, N.; Cna’ani, A.; Yaakov, B.; Sadeh, A.; Avraham, L.; Opatovsky, I.; Tzin, V. Tomato cultivars resistant or susceptible to spider mites differ in their biosynthesis and metabolic profile of the monoterpenoid pathway. Front. Plant Sci. 2021, 12, 128. [Google Scholar] [CrossRef]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de BLima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, S.; Qin, Y.; Zhang, S.; Gao, Z.; Dang, Z.; Pan, W. Identification of plant chemicals attracting and repelling whiteflies. Arthropod. Plant. Interact. 2014, 8, 183–190. [Google Scholar] [CrossRef]
- Johnston, N.; Martini, X. The influence of visual and olfactory cues in host selection for Bemisia tabaci Biotype B in the Presence or Absence of Tomato Yellow Leaf Curl Virus. Insects 2020, 11, 115. [Google Scholar] [CrossRef]
- Fang, Y.; Jiao, X.; Xie, W.; Wang, S.; Wu, Q.; Shi, X.; Chen, G.; Su, Q.; Yang, X.; Pan, H. Tomato yellow leaf curl virus alters the host preferences of its vector Bemisia tabaci. Sci. Rep. 2013, 3, 2876. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Pan, H.; Xie, W.; Wang, S.; Wu, Q.; Fang, Y.; Shi, X.; Zhang, Y. Virus infection of a weed increases vector attraction to and vector fitness on the weed. Sci. Rep. 2013, 3, 2253. [Google Scholar] [CrossRef] [PubMed]
- Legarrea, S.; Barman, A.; Marchant, W.; Diffie, S.; Srinivasan, R. Temporal effects of a Begomovirus infection and host plant resistance on the preference and development of an insect vector, Bemisia tabaci, and implications for epidemics. PLoS ONE 2015, 10, e0142114. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Delafuente, A.; Garzo, E.; Moreno, A.; Fereres, A. A plant virus manipulates the behavior of its whitefly vector to enhance its transmission efficiency and spread. PLoS ONE 2013, 8, e61543. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.-Q.; Liu, W.-X.; Fan, Z.-N.; Wan, F.-H.; Cheng, L.-S. Behavioural responses of Bemisia tabaci B-biotype to three host plants and their volatiles. Acta Entomol. Sin. 2008, 51, 830–838. [Google Scholar]
- Togni, P.H.B.; Laumann, R.A.; Medeiros, M.A.; Sujii, E.R. Odour masking of tomato volatiles by coriander volatiles in host plant selection of Bemisia tabaci biotype B. Entomol. Exp. Appl. 2010, 136, 164–173. [Google Scholar] [CrossRef]
- Kong, H.; Zeng, Y.; Xie, W.; Wang, S.; Wu, Q.; Jiao, X.; Xu, B.; Zhang, Y. Differing behavioural responses of Bemisia tabaci MEAM1 and MED to cabbage damaged by conspecifics and heterospecifics. Sci. Rep. 2016, 6, 35095. [Google Scholar] [CrossRef]
- Shi, X.; Chen, G.; Pan, H.; Xie, W.; Wu, Q.; Wang, S.; Liu, Y.; Zhou, X.; Zhang, Y. Plants pre-infested with viruliferous MED/Q cryptic species promotes subsequent Bemisia tabaci infestation. Front. Microbiol. 2018, 9, 1404. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Maged, E.M. Tomato yellow leaf curl virus: Diagnosis and metabolites. Afr. J. Biotechnol. 2018, 17, 198–205. [Google Scholar] [CrossRef]
- Chang, X.; Wang, F.; Fang, Q.; Chen, F.; Yao, H.; Gatehouse, A.M.R.; Ye, G. Virus-induced plant volatiles mediate the olfactory behaviour of its insect vectors. Plant Cell Environ. 2021, 44, 2700–2715. [Google Scholar] [CrossRef]
- Birkett, M.A.; Chamberlaln, K.; Guerrieri, E.; Pickett, J.A.; Wadhams, L.J.; Yasuda, T. Volatiles from whitefly-infested plants elicit a host-locating response in the parasitoid, Encarsia formosa. J. Chem. Ecol. 2003, 29, 1589–1600. [Google Scholar] [CrossRef]
- Darshanee, H.L.C.; Ren, H.; Ahmed, N.; Zhang, Z.-F.; Liu, Y.-H.; Liu, T.-X. Volatile-mediated attraction of greenhouse whitefly Trialeurodes vaporariorum to tomato and eggplant. Front. Plant Sci. 2017, 8, 1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Q.; Yang, F.; Zhang, Q.; Tong, H.; Hu, Y.; Zhang, X.; Xie, W.; Wang, S.; Wu, Q.; Zhang, Y. Defence priming in tomato by the green leaf volatile (Z)-3-hexenol reduces whitefly transmission of a plant virus. Plant. Cell Environ. 2020, 43, 2797–2811. [Google Scholar] [CrossRef] [PubMed]
- Sadeh, D.; Nitzan, N.; Shachter, A.; Ghanim, M.; Dudai, N. Rosemary–whitefly interaction: A continuum of repellency and volatile combinations. J. Econ. Entomol. 2018, 112, 616–624. [Google Scholar] [CrossRef]
- Shi, X.; Chen, G.; Tian, L.; Peng, Z.; Xie, W.; Wu, Q.; Wang, S.; Zhou, X.; Zhang, Y. The salicylic acid-mediated release of plant volatiles affects the host choice of Bemisia tabaci. Int. J. Mol. Sci. 2016, 17, 1048. [Google Scholar] [CrossRef] [PubMed]
- Rajabaskar, D.; Wu, Y.; Bosque-Pérez, N.A.; Eigenbrode, S.D. Dynamics of Myzus persicae arrestment by volatiles from P otato leafroll virus-infected potato plants during disease progression. Entomol. Exp. Appl. 2013, 148, 172–181. [Google Scholar] [CrossRef]
- Dieng, H.; Satho, T.; Hassan, A.A.; Aziz, A.T.; Morales, R.E.; Hamid, S.A.; Miake, F.; Abubakar, S. Peroxidase Activity after Viral Infection and Whitefly Infestation in Juvenile and Mature Leaves of Solanum lycopersicum. J. Phytopathol. 2011, 159, 707–712. [Google Scholar] [CrossRef]
- Trevisan, M.T.S.; Scheffer, J.J.C.; Verpoorte, R. Peroxidase activity in hop plants after infestation by red spider mites. Crop Prot. 2003, 22, 423–424. [Google Scholar] [CrossRef]
- Flórez Menéndez, J.C.; Fernández Sánchez, M.L.; Fernández Martínez, E.; Sánchez Uría, J.E.; Sanz-Medel, A. Static headspace versus head space solid-phase microextraction (HS-SPME) for the determination of volatile organochlorine compounds in landfill leachates by gas chromatography. Talanta 2004, 63, 809–814. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, S.; Didi-Cohen, S.; Cna’ani, A.; Kontsedalov, S.; Lebedev, G.; Tzin, V.; Ghanim, M. Comparative Analysis of Volatiles Emitted from Tomato and Pepper Plants in Response to Infection by Two Whitefly-Transmitted Persistent Viruses. Insects 2022, 13, 840. https://doi.org/10.3390/insects13090840
Ghosh S, Didi-Cohen S, Cna’ani A, Kontsedalov S, Lebedev G, Tzin V, Ghanim M. Comparative Analysis of Volatiles Emitted from Tomato and Pepper Plants in Response to Infection by Two Whitefly-Transmitted Persistent Viruses. Insects. 2022; 13(9):840. https://doi.org/10.3390/insects13090840
Chicago/Turabian StyleGhosh, Saptarshi, Shoshana Didi-Cohen, Alon Cna’ani, Svetlana Kontsedalov, Galina Lebedev, Vered Tzin, and Murad Ghanim. 2022. "Comparative Analysis of Volatiles Emitted from Tomato and Pepper Plants in Response to Infection by Two Whitefly-Transmitted Persistent Viruses" Insects 13, no. 9: 840. https://doi.org/10.3390/insects13090840
APA StyleGhosh, S., Didi-Cohen, S., Cna’ani, A., Kontsedalov, S., Lebedev, G., Tzin, V., & Ghanim, M. (2022). Comparative Analysis of Volatiles Emitted from Tomato and Pepper Plants in Response to Infection by Two Whitefly-Transmitted Persistent Viruses. Insects, 13(9), 840. https://doi.org/10.3390/insects13090840








