Efficacy and Residual Toxicity of Insecticides on Plutella xylostella and Their Selectivity to the Predator Solenopsis saevissima
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Crop Establishment
2.2. Insects
2.3. Insecticides
2.4. Bioassays
2.4.1. Efficacy of insecticides against P. xylostella
2.4.2. Physiological Selectivity to S. saevissima
2.4.3. Residual Toxicity against P. xylostella and S. saevissima
2.5. Statistical analyses
3. Results
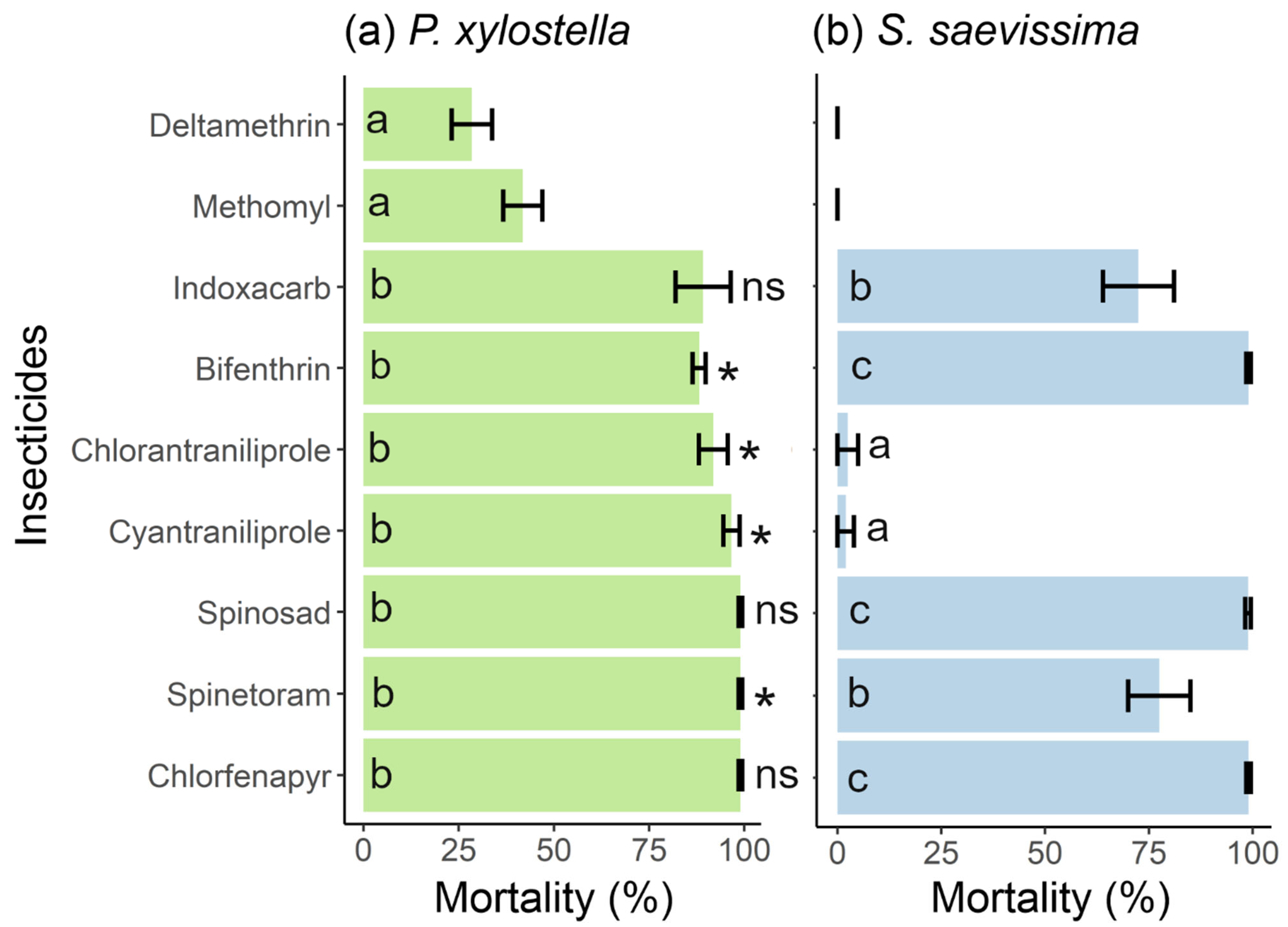
3.1. Efficacy of Insecticides for Controlling P. xylostella and Their Toxicity to S. saevissima
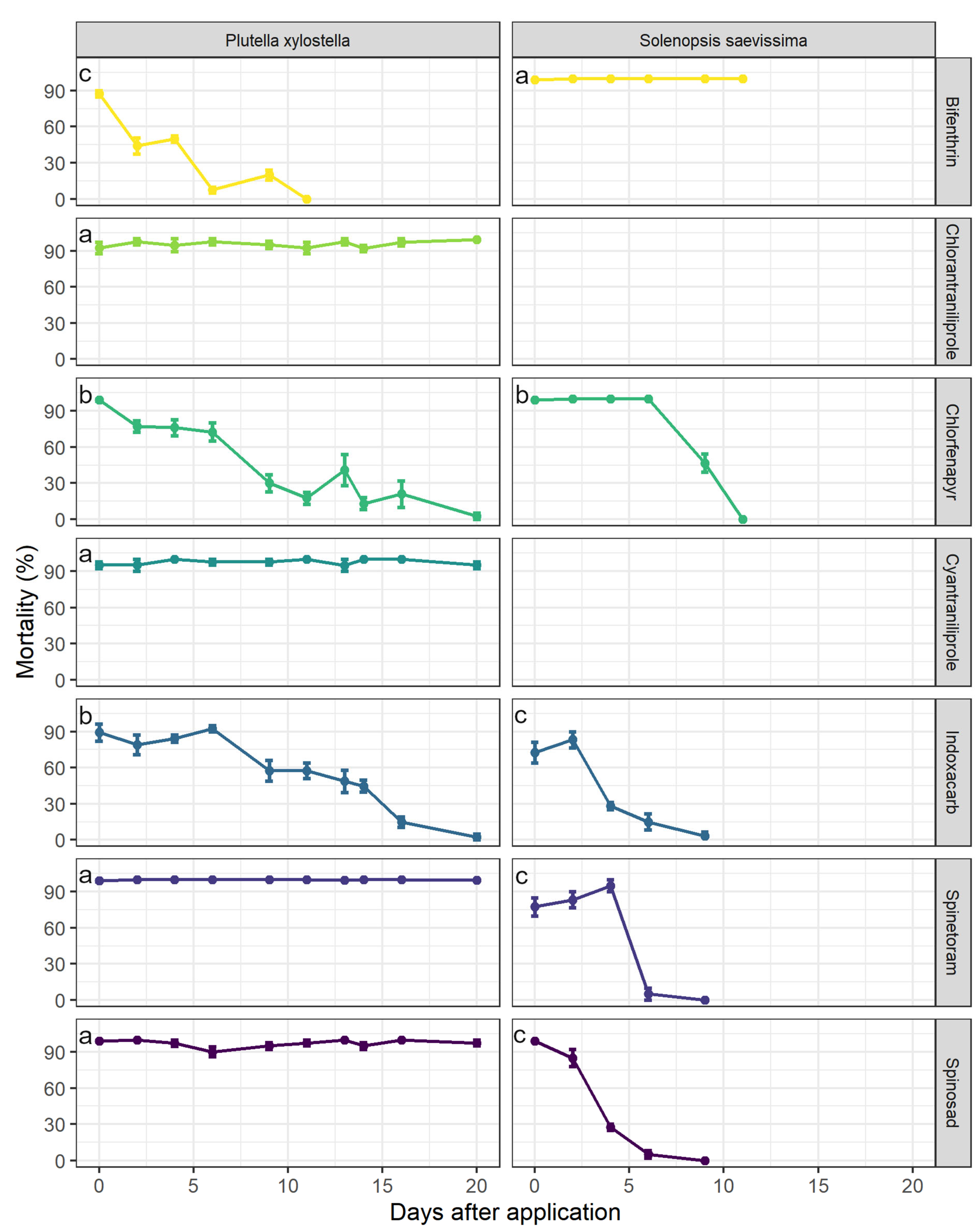
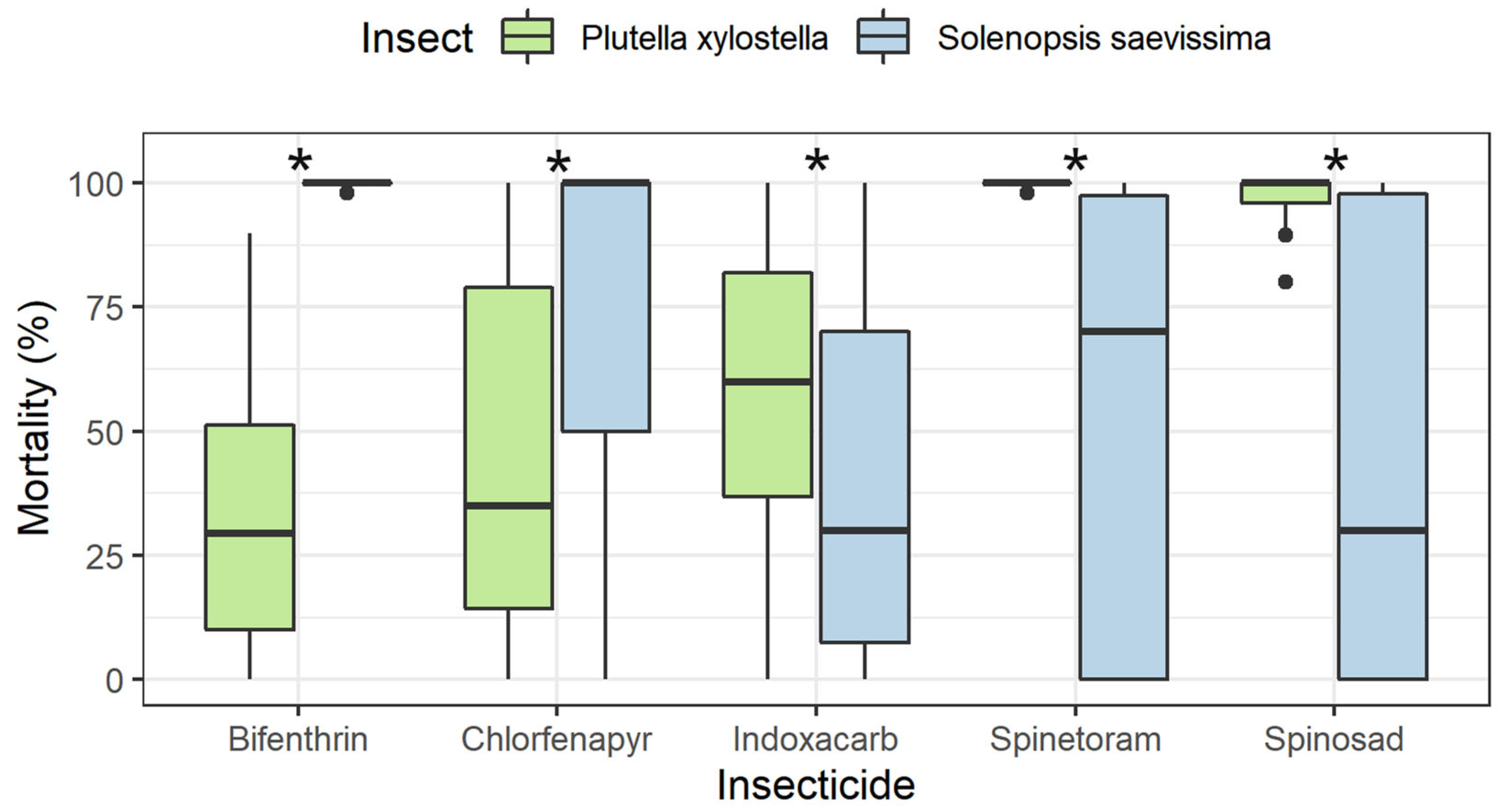
3.2. Residual Period of Control for P. xylostella and Selectivity to S. saevissima
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Matthews, G.A. Attitudes and Behaviours Regarding Use of Crop Protection Products—A Survey of More than 8500 Smallholders in 26 Countries. Crop Prot. 2008, 27, 834–846. [Google Scholar] [CrossRef]
- Tomenson, J.A.; Matthews, G.A. Causes and Types of Health Effects during the Use of Crop Protection Chemicals: Data from a Survey of over 6,300 Smallholder Applicators in 24 Different Countries. Int. Arch. Occup. Environ. Health 2009, 82, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Overton, K.; Hoffmann, A.A.; Reynolds, O.L.; Umina, P.A. Toxicity of Insecticides and Miticides to Natural Enemies in Australian Grains: A Review. Insects 2021, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.R.; Picanço, M.C.; Santana, P.A.; Moreira, S.S.; Guedes, R.N.C.; Corrêa, A.S. Insecticide Toxicity and Walking Response of Three Pirate Bug Predators of the Tomato Leaf Miner Tuta absoluta: Insecticide Impact on Predatory Bugs. Agr. Forest Entomol. 2014, 16, 293–301. [Google Scholar] [CrossRef]
- Ripper, W.E.; Greenslade, R.M.; Hartley, G.S. Selective Insecticides and Biological Control. BioControl 1951, 44, 448–459. [Google Scholar] [CrossRef]
- Goulart, R.M.; Volpe, H.X.; Vacari, A.M.; Thuler, R.T.; De Bortoli, S.A. Insecticide Selectivity to Two Species of Trichogramma in Three Different Hosts, as Determined by IOBC/WPRS Methodology. Pest. Manag. Sci. 2012, 68, 240–244. [Google Scholar] [CrossRef]
- Talekar, N.S.; Shelton, A.M. Biology, Ecology, and Management of the Diamondback Moth. Annu. Rev. Entomol. 1993, 38, 275–301. [Google Scholar] [CrossRef]
- Zalucki, M.P.; Shabbir, A.; Silva, R.; Adamson, D.; Shu-Sheng, L.; Furlong, M.J. Estimating the Economic Cost of One of the World’s Major Insect Pests, Plutella xylostella (Lepidoptera: Plutellidae): Just How Long Is a Piece of String? J. Econ. Entomol. 2012, 105, 1115–1129. [Google Scholar] [CrossRef]
- Liu, X.; Chen, M.; Collins, H.L.; Onstad, D.; Roush, R.; Zhang, Q.; Shelton, A.M. Effect of Insecticides and Plutella xylostella (Lepidoptera: Plutellidae) Genotype on a Predator and Parasitoid and Implications for the Evolution of Insecticide Resistance. J. Econ. Entomol. 2012, 105, 354–362. [Google Scholar] [CrossRef]
- Ramos, R.S.; Picanço, M.C.; da Silva Santana, P.A.E.M.; Bacci, L.; Gonring, A.H.R.; Silva, G.A. Natural Biological Control of Lepidopteran Pests by Ants. Sociobiology 2012, 59, 1389–1399. [Google Scholar]
- Farias, E.S.; Santos, R.C.; Carmo, D.G.; Soares, J.R.S.; Costa, T.L.; Santos, A.A.; Picanço, M.C. Life Tables for the Diamondback Moth (Plutella xylostella) in Southeast Brazil Indicate Ants and Spiders as Leading Mortality Factors. Ann. Appl. Biol. 2021, 178, 498–507. [Google Scholar]
- Santos, A.A.; Ribeiro, A.V.; Paes, J.S.; de Paula Silva, E.M.; Farias, E.S.; Picanço, M.C. Dry and Wet Seasons Do Not Affect the Mortality Caused by Ants to Larvae of Ascia monuste orseis (Lepidoptera: Pieridae). Biocontrol Sci. Technol. 2021, 31, 705–712. [Google Scholar]
- Tomm, G.; Wiethölter, S.; Dalmago, G.; Santos, H. Tecnologia Para Produção de Canola No Rio Grande Do Sul; Embrapa Trigo: Passo Fundo, Brazil, 2009. [Google Scholar]
- Ministério da Agricultura, Pecuária e Abastecimento. Manual de Procedimentos para Registro de Agrotóxicos; MAPA: Brasília, Brazil, 2012. [Google Scholar]
- Silva, G.A.; Picanço, M.C.; Bacci, L.; Crespo, A.L.B.; Rosado, J.F.; Guedes, R.N.C. Control Failure Likelihood and Spatial Dependence of Insecticide Resistance in the Tomato Pinworm, Tuta absoluta. Pest. Manag. Sci. 2011, 67, 913–920. [Google Scholar] [CrossRef]
- Galdino, T.V.; Picanço, M.C.; Morais, E.G.F.; Silva, N.R.; Silva, G.A.R.; Lopes, M.C. Bioassay Method for Toxicity Studies of Insecticide Formulations to Tuta absoluta (Meyrick, 1917). Cienc. Agrotec. 2011, 35, 869–877. [Google Scholar] [CrossRef]
- Sterk, G.; Hassan, S.A.; Baillod, M.; Bakker, F.; Bigler, F.; Bogenschütz, H.; Boller, E.; Bromand, B.; Brun, J.; Calis, J.N.M.; et al. Results of the Seventh Joint Pesticide Testing Progrmme Carried out by the IOBC/WPRS-Working Group ‘Pesticides and Beneficial Organisms’. BioControl 1999, 44, 99–117. [Google Scholar] [CrossRef]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Lenth, R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. GlmmTMB Balances Speed and Flexibility Among Packages for Zero-Inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Khaliq, A.; Attique, M.N.R.; Sayyed, A.H. Evidence for Resistance to Pyrethroids and Organophosphates in Plutella xylostella (Lepidoptera: Plutellidae) from Pakistan. Bull. Entomol. Res. 2007, 97, 191–200. [Google Scholar]
- De Oliveira, A.C.; de Siqueira, H.Á.A.; de Oliveira, J.V.; da Silva, J.E.; Michereff Filho, M. Resistance of Brazilian Diamondback Moth Populations to Insecticides. Sci. Agric. 2011, 68, 154–159. [Google Scholar] [CrossRef]
- Sayyed, A.H.; Attique, M.N.R.; Khaliq, A.; Wright, D.J. Inheritance of Resistance and Cross-Resistance to Deltamethrin in Plutella xylostella (Lepidoptera: Plutellidae) from Pakistan. Pest. Manag. Sci. 2005, 61, 636–642. [Google Scholar] [CrossRef]
- Santos, V.; De Siqueira, H.; Da Silva, J.; De Farias, M. Insecticide Resistance in Populations of the Diamondback Moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae), from the State of Pernambuco, Brazil. Neotrop. Entomol. 2011, 40, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Sial, A.A.; Brunner, J.F. Toxicity and Residual Efficacy of Chlorantraniliprole, Spinetoram, and Emamectin Benzoate to Obliquebanded Leafroller (Lepidoptera: Tortricidae). J. Econ. Entomol. 2010, 103, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Qiu, H.; Li, Q.; Tang, L.; Zeng, D.; Liu, K.; Gao, Y. Flower Injection of Imidacloprid and Spirotetramat: A Novel Tool for the Management of Banana Thrips Thrips hawaiiensis. J. Pest. Sci. 2020, 93, 1073–1084. [Google Scholar] [CrossRef]
- van Leeuwen, T.; Dermauw, W.; Van De Veire, M.; Tirry, L. Systemic Use of Spinosad to Control the Two-Spotted Spider Mite (Acari: Tetranychidae) on Tomatoes Grown in Rockwool. Exp. Appl. Acarol. 2005, 37, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Abouelghar, G.E.; Sakr, H.; Ammar, H.A.; Yousef, A.; Nassar, M. Sublethal Effects of Spinosad (Tracer®) on the Cotton Leafworm (Lepidoptera: Noctuidae). J. Plant Prot. Res. 2013, 53, 275–284. [Google Scholar] [CrossRef]
- Selby, T.P.; Lahm, G.P.; Stevenson, T.M.; Hughes, K.A.; Cordova, D.; Annan, I.B.; Barry, J.D.; Benner, E.A.; Currie, M.J.; Pahutski, T.F. Discovery of Cyantraniliprole, a Potent and Selective Anthranilic Diamide Ryanodine Receptor Activator with Cross-Spectrum Insecticidal Activity. Bioorg. Med. Chem. Lett. 2013, 23, 6341–6345. [Google Scholar] [CrossRef]
- Machado, A.V.A.; Potin, D.M.; Torres, J.B.; Silva Torres, C.S.A. Selective Insecticides Secure Natural Enemies Action in Cotton Pest Management. Ecotoxicol. Environ. Saf. 2019, 184, 109669. [Google Scholar] [CrossRef]
- Lofgren, C.S.; Banks, W.A.; Glancey, B.M. Biology and Control of Imported Fire Ants. Annu. Rev. Entomol. 1975, 20, 1–30. [Google Scholar] [CrossRef]
| Insecticide | Chemical Sub-Group | Manufacturer | Rate |
|---|---|---|---|
| Bifenthrin 100 CE | Pyrethroids | FMC Química do Brasil | 0.0500 |
| Cyantraniliprole 100 OD | Diamides | FMC Química do Brasil | 0.0125 |
| Chlorantraniliprole 100 SC | Diamides | FMC Química do Brasil | 0.0150 |
| Chlorfenapyr 240 SC | Pyrroles | BASF SA | 0.2400 |
| Deltamethrin 25 SC | Pyrethroids | Bayer SA | 0.0075 |
| Indoxacarb 300 WG | Oxadiazines | Du Pont do Brasil SA | 0.0300 |
| Methomyl 215 SL | Carbamates | Du Pont do Brasil SA | 0.2150 |
| Spinosad 480 SC | Spinosyns | Corteva | 0.0768 |
| Spinetoram 120 SC | Spinosyns | Corteva | 0.1200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
do Carmo, D.G.; Costa, T.L.; Santana Júnior, P.A.; Santana, W.C.; Marsaro Júnior, A.L.; Pereira, P.S.; Santos, A.A.; Picanço, M.C. Efficacy and Residual Toxicity of Insecticides on Plutella xylostella and Their Selectivity to the Predator Solenopsis saevissima. Insects 2023, 14, 98. https://doi.org/10.3390/insects14020098
do Carmo DG, Costa TL, Santana Júnior PA, Santana WC, Marsaro Júnior AL, Pereira PS, Santos AA, Picanço MC. Efficacy and Residual Toxicity of Insecticides on Plutella xylostella and Their Selectivity to the Predator Solenopsis saevissima. Insects. 2023; 14(2):98. https://doi.org/10.3390/insects14020098
Chicago/Turabian Styledo Carmo, Daiane G., Thiago L. Costa, Paulo A. Santana Júnior, Weyder C. Santana, Alberto L. Marsaro Júnior, Poliana S. Pereira, Abraão A. Santos, and Marcelo C. Picanço. 2023. "Efficacy and Residual Toxicity of Insecticides on Plutella xylostella and Their Selectivity to the Predator Solenopsis saevissima" Insects 14, no. 2: 98. https://doi.org/10.3390/insects14020098
APA Styledo Carmo, D. G., Costa, T. L., Santana Júnior, P. A., Santana, W. C., Marsaro Júnior, A. L., Pereira, P. S., Santos, A. A., & Picanço, M. C. (2023). Efficacy and Residual Toxicity of Insecticides on Plutella xylostella and Their Selectivity to the Predator Solenopsis saevissima. Insects, 14(2), 98. https://doi.org/10.3390/insects14020098






