1. Introduction
The brown planthopper (BPH),
Nilaparvata lugens (Stål) (Hemiptera: Delphacidae), is a severe migratory rice pest in the Yangtze River Valley, in South and Southwest China [
1,
2]. There are three main ways in which the BPH damages rice plants. Firstly, the BPH ingests the rice sap through its mouthparts, which hinders the transport of nutrients and water in the rice plants. Secondly, females pierce the rice leaf sheaths with their spawning needles to dissipate plant water when spawning. Thirdly, the BPH transmits rice virus diseases such as rice grassy stunt virus and rice ragged shunt virus [
3,
4]. The BPH has a high innate capacity for proliferation, strong adaptability to the environment, and a long-distance migration ability, which makes it insidious, sudden, violent, and destructive. Therefore, the BPH presents a significant challenge for rice production in many Asian countries [
5,
6]. Chemical pesticides are still the main methods with which to control
N. lugens. However, as a result of the long-term, widespread, and unscientific use of pesticides,
N. lugens have developed high resistance levels to neonicotinoids, organophosphates, carbamates, and insect growth regulators [
7,
8,
9]. Among them, imidacloprid, buprofezin, and thiamethoxam have been suspended for the control of
N. lugens in China owing to their high resistance levels to them.
Pymetrozine is a pyridine azomethine insecticide with an excellent control effect against sucking pests [
10]. It has been gradually recommended as one of the main insecticides for controlling BPHs in China since 2008 [
9]. Pymetrozine can disrupt the normal function of the chordotonal mechanoreceptors, thereby affecting the insect’s sense of gravity, hearing, and coordination [
11,
12,
13]. This, in turn, affects the feeding and reproductive behavior of the target insects. Our previous study demonstrated that pymetrozine inhibited the reproductive behavior of
N. lugens by disrupting male initiative courtship, female abdominal vibration, and female oviposition. In addition, in
Drosophila, a significant reduction in the male courtship index and female receptivity was observed after pymetrozine treatment [
14]. Therefore, pymetrozine may significantly inhibit the reproductive behavior of
N. lugens by interfering with hearing, thus effectively inhibiting the number of next-generation insects. However, the effects of pymetrozine’s systemic and contact activities on the fecundity of
N. lugens have not been systematically studied.
In order to ensure the continuity of resistance monitoring and to take into account the actual operability of monitoring a large number of field populations, scientific research institutes in China have long been using the rice-stem-dipping method to monitor pymetrozine resistance in BPHs. This method evaluates the BPH’s toxicity to pymetrozine by introducing the third-instar nymphs onto rice stems treated with different pymetrozine concentrations and then assessing mortality after 7 days. The monitoring results showed that during 2012–2021,
N. lugens field populations in China reached moderate to high resistance levels to pymetrozine, except for a few populations in 2012, which had sensitive to low resistance levels [
9,
15,
16]. The LC
50 values determined by the rice-stem-dipping method could only reflect the sensitivity of contemporary
N. lugens to pymetrozine but could not reflect the pymetrozine inhibition in the next-generation
N. lugens population. Hence, pymetrozine toxicity and
N. lugens resistance could not be fully assessed.
Consequently, the Insecticide Resistance Action Committee (IRAC) and scientists from Japan have established bioassay methods to evaluate the activity of pymetrozine against
N. lugens based on treating contemporary
N. lugens with pymetrozine while investigating the number of next-generation nymphs [
17]. The IRAC no.005 method (IRAC no.005) introduces adult BPH females and males onto potted rice plants dipped in different concentrations of pymetrozine solution. Then, they are removed after 7 days of spawning, and the number of offspring is counted after 18 days. This method can investigate pymetrozine’s effect on contemporary and next-generation test insects under both systemic and contact modes of action. However, this method demands a large space and has a long test cycle due to the preparation of potted rice plants. Tsujimoto et al. only examined the contact effect of pymetrozine. Different doses of pymetrozine were topically applied to female brown planthoppers using a micro-applicator, and the males were not treated. Thereafter, they were transferred to rice seedlings in a test tube to spawn for 7–8 days and then removed. After 15–16 days, the number of offspring was counted. This method can only detect pymetrozine’s effect on female receptivity and oviposition behavior, but cannot reflect the pymetrozine’s effect on male courtship, male fertility, or the systemic activity of pymetrozine. In summary, the above two methods were used for test insects that had mated for many days and each replication introduced multiple females and males. However, there are certain limitations to the study. Firstly, the age and time of death of each female in each replication could not be determined, thus making it impossible to ensure that each female spawned for the same duration. Secondly, it was not possible to accurately determine whether each female mated or not, and thus, it was impossible to accurately assess the effects of pymetrozine on courtship, receptivity, and oviposition in brown planthoppers. Therefore, there is still a relative lack of effective resistance monitoring technology to assess pymetrozine resistance in
N. lugens. In addition, the real resistance level of field
N. lugens populations to pymetrozine still needs to be established, and these data, when available, will significantly affect resistance monitoring and the management of pymetrozine in relation to
N. lugens. Accordingly, in order to comprehensively evaluate the effect of pymetrozine on
N. lugens mating and female reproduction, the two fecundity assay methods established in our research used newly emerged unmated
N. lugens for single-pair pairing. In addition, only treatments with surviving females after 7 days were counted in order to exclude the effects of oviposition duration on the number of offspring and to improve the accuracy of the test results. The fecundity assay method can comprehensively reflect pymetrozine’s persistence, its characteristics in terms of inhibiting reproductive behavior, and more objectively reflect pymetrozine’s toxicity against BPHs.
In this study, we found that pymetrozine could significantly inhibit the reproductive behavior of N. lugens. Pymetrozine treatment can significantly inhibit the fecundity of N. lugens in nymphs and adults. On this basis, a more accurate bioassay method, i.e., the fecundity assay method, was established to reflect the pymetrozine resistance of N. lugens. In addition, this method was used to monitor pymetrozine resistance in N. lugens both in laboratory and field populations. These results are valuable for the monitoring and management of pymetrozine resistance.
2. Materials and Methods
2.1. Insects
The susceptible strain (Pym-S) of BPH was initially collected from Hangzhou, Zhejiang Province, in 1995 and has been maintained in the laboratory without exposure to any insecticides since. The pymetrozine-resistant strain (Pym-R) was collected from Jinhua, Zhejiang Province, in 2013 and was selected for resistance to pymetrozine for more than 90 generations. Two field populations (QS21 and YZ21) were collected from Qianshan (30°37′ N, 116°34′ E), Anhui Province, and Yizheng (32°16′ N, 119°11′ E), Jiangsu Province, in September 2021, respectively. All the populations were reared on indica rice seedlings (Taichung Native 1, TN1) under standard conditions of 27 ± 1 °C, 70–80% relative humidity, and a 16 h light/8 h dark photoperiod.
2.2. Insecticides
Technical-grade pymetrozine (95%) was supplied by Jiangsu Anpon Electrochemical Co., Ltd. (Huaian, China). The technical-grade pymetrozine was dissolved in acetone (for topical application) or N,N-dimethylformamide (for systemically application) as stock solution. Then, a serial dilution was prepared using acetone for the topical application bioassay and water containing 0.1% Triton X-100 for the rice-stem-dipping bioassay.
2.3. Bioassays
2.3.1. Rice-Stem-Dipping Bioassay Method
The rice-stem-dipping bioassay method was used to evaluate the LC
15, LC
50, and LC
85 estimations [
18]. Briefly, pymetrozine solution was diluted 4–5 concentrations in equal proportion and 3–4 replicates for each concentration. Three rice stems were grouped together and dipped in pymetrozine solutions for 30 s and then air-dried at room temperature. The roots of rice stems were wrapped with water-impregnated cotton and put into plastic cups. Fifteen insects (third-instar nymphs) were introduced onto rice stems for each replicate. Control rice stems were treated with 0.1% Triton X-100 water solution only. The plastic cups with treated insects were maintained under standard conditions of 27 ± 1 °C and 70–80% relative humidity, with a 16 h light/8 h dark photoperiod. Mortality was assessed after 168 h of exposure to pymetrozine.
2.3.2. Fecundity Assay Bioassay Method
The rice-seedling-dipping fecundity assay method was used to evaluate the EC50 estimations. The no.005 method was referred to and modified (IRAC no.005). Firstly, 30-day-old rice seedlings (approximate) were dipped in a series of concentrations of pymetrozine for 30 s each. Then, each newly emerged virgin female adult was paired with a single male onto the rice seedlings. After 7 days, the adults were removed, and their survival was counted. After 15 days, the number of nymphs (only counting the replicates of female adults surviving after 7 days) was counted, and the inhibition rate of offspring under the treatment of the corresponding pymetrozine concentration was calculated. There were 30 replicates for each concentration and 4–5 doses of pymetrozine. Control rice stems were treated with 0.1% Triton X-100 water solution only. All treatments were maintained under standard conditions of 27 ± 1 °C and 70–80% relative humidity, with a 16 h light/8 h dark photoperiod.
The topical application fecundity assay method was used to evaluate the ED
50 estimations. The method by Katsuhiko Tsujimoto that combines the topical application and the determination of offspring number was referred to and modified [
17]. Newly emerged virgin female adults were anesthetized with carbon dioxide for 10 s. A droplet (0.2 μL) of insecticide acetone solution was applied topically to the prothorax notum of each individual female with a hand-held micro-applicator (Hamilton Repeating Applicator, Burkard Manufacturing Co., Ltd., Rickmansworth, UK). Males were not treated, and control groups were treated with acetone. Then, each treated female adult was paired with a single male onto the 30-day-old rice seedlings (approximate). The remaining steps and experiment conditions were consistent with rice-seedling-dipping fecundity assay method.
The inhibition rate (%) was calculated using the following formula:
2.4. Effects of Pymetrozine Treatment at Third-Instar Nymph Stage on the Fecundity of N. lugens
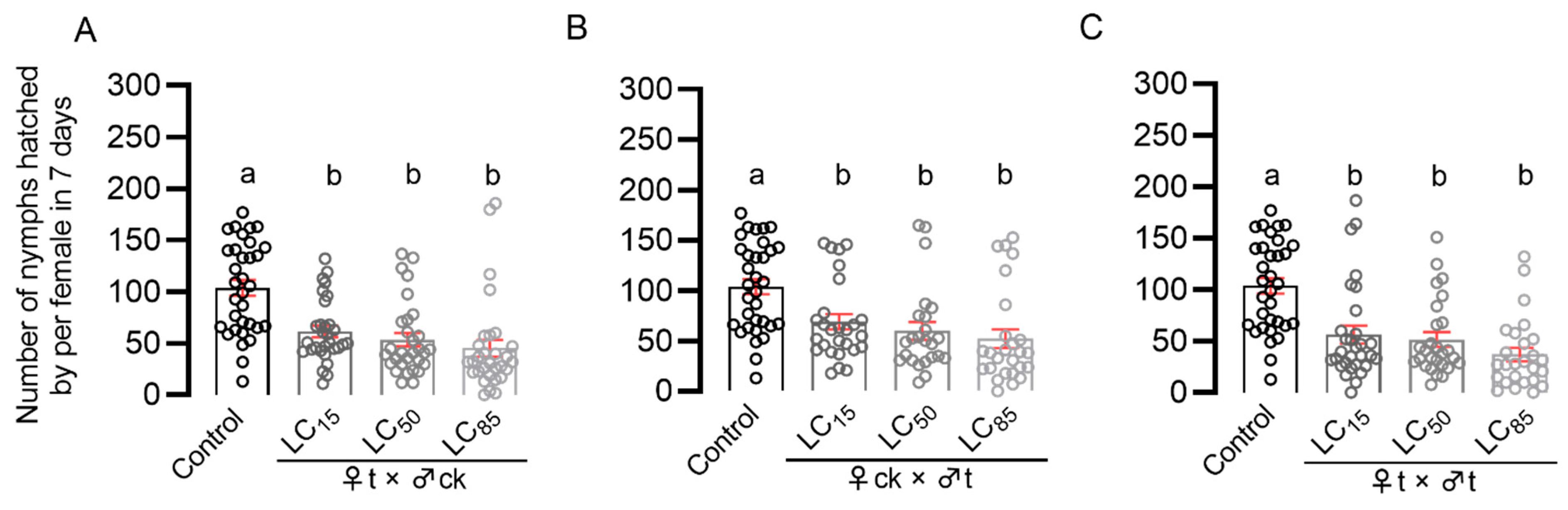
The LC15, LC50, and LC85 concentrations of pymetrozine in the susceptible strain (Pym-S) of BPH was determined by rice-stem-dipping bioassay method. Additionally, then the third-instar nymphs were introduced onto rice seedlings treated with pymetrozine solutions at LC15, LC50, and LC85 concentrations. After 7 days, the nymphs were transferred to new rice seedlings without pymetrozine treatment and reared until they emerged as adults. The newly emerged unmated N. lugens adults were paired according to the following combinations: ♀t (treated female) × ♂ck (untreated male); ♀ck (untreated female) × ♂t (treated male); and ♀t (treated female) × ♂t (treated male). Single pairs were introduced onto 30-day-old TN-1 rice seedlings to spawn for 7 days, and the number of offspring was counted after 15 days. ♀ck (untreated female) × ♂ck (untreated male) were regarded as the control group. There were no less than 30 replicates for each treatment.
2.5. Effects of Pymetrozine Treatment at Adult Stage on the Fecundity of N. lugens
The rice-seedling-dipping treatment and the susceptible strain (Pym-S) were used to evaluate the systemic toxicity of pymetrozine in BPHs. To this end, 30-day-old TN-1 rice seedlings (approximate) were dipped in 10 mg/L pymetrozine solution for 30 s and then air-dried at room temperature. Control rice seedlings were treated with 0.1% Triton X-100 water solution only. Pymetrozine’s effect on the fecundity of N. lugens was studied according to two treatments. The first treatment involved introducing single pairs of newly emerged unmated female and male adults in order to investigate the effects of pymetrozine on mating and reproduction in N. lugens. The second treatment involved introducing single N. lugens females that had emerged 2 to 3 days previous and were fully mated in order to investigate the effects of pymetrozine on oviposition in BPHs. In both of the above treatments, we removed the adults after 7 days, and the number of offspring was counted after 15 days. There were no less than 30 replicates for each treatment.
The topical application treatment and the susceptible strain (Pym-S) were used to evaluate the contact toxicity. N. lugens adults were anesthetized with carbon dioxide for 10 s. A droplet (0.2 μL) of 0.1 mg/L pymetrozine solution (0.02 ng/adult) was topically applied to the prothorax notum of the following adults with a hand-held micro-applicator (Hamilton Repeating Applicator, Burkard Manufacturing Co., Ltd., Rickmansworth, UK): (1) unmated females; (2) unmated males; (3) unmated females and males; and (4) fully mated pregnant female adults. Corresponding control groups were treated with acetone only. The topically applied N. lugens was introduced onto 30-day-old TN-1 rice seedlings as follows: unmated females paired with untreated males (♀t × ♂ck); unmated males paired with untreated females (♀ck × ♂t); unmated females paired with unmated males (♀t × ♂t); mated pregnant females (♀t). We also removed the adults after 7 days, and the number of offspring was counted after 15 days. There were no less than 30 replicates for each treatment.
2.6. Data Analysis
The sub-lethal (LC
15), median lethal (LC
50) and highly lethal (LC
85) concentrations and their 95% fiducial limits (FL) were calculated using the POLO-plus program (Version 2.0) (LeOra Software LLC, Berkeley, CA, USA) for BPHs. The median effective concentrations (EC
50) and the median effective dose (ED
50) were estimated using the GraphPad Prism 8 Software (GraphPad Software Inc., San Diego, CA, USA). Data statistical analyses were conducted using the GraphPad Prism 8.0 software (GraphPad Software Inc., San Diego, CA, USA). Data from multiple groups were assessed using one-way ANOVA with post hoc Tukey HSD, and the data between two groups fitted with normal distribution were assessed using Student’s
t-test, and the Mann–Whitney test was used to evaluate non-parametric. The resistance ratio (RR) was calculated by dividing the LC
50, EC
50, or ED
50 value of a resistant strain by that of the susceptible strain. Insecticide resistance of the field populations was classified as follows: RR < 5-fold as susceptible; RR = 5–10-fold as low resistance; RR = 10–100-fold as medium resistance; and RR > 100-fold as high resistance [
9].
4. Discussion
Pymetrozine has been recommended as an alternative insecticide to control BPH, which has developed resistance to imidacloprid, thiamethoxam, and buprofezin [
9,
19]. Using the rice-stem-dipping bioassay method, it was shown that BPHs have developed a strong resistance to pymetrozine [
9,
16]. However, pymetrozine still exhibits a good control effect against BPHs in paddy fields. Therefore, it is necessary to establish another bioassay method to evaluate the current resistance of BPHs to pymetrozine. On the basis of the impact of pymetrozine on BPHs reproductive behavior, we established a fecundity assay bioassay method and found that BPHs only developed low to moderate resistance to pymetrozine. We also found that pymetrozine can inhibit the fecundity of
N. lugens.Establishing a standard bioassay method can help us obtain the relationship between the insecticide dose and pest mortality, thus contributing to our understanding of the evolution of insecticide resistance and helping formulate strategies to delay the development of resistance [
20]. The mortality rate is not the only criterion in the insecticide bioassay. In this study, the fecundity assay method was used to calculate insecticide toxicity according to the offspring inhibition rate. For certain kinds of carbamates, benzoylureas, and pyrethroids, people calculate toxicity values according to egg-hatching inhibition rates to determine their ovicidal activity against pests such as
Plutella xylostella (L.) and
Spodoptera frugiperda [
21,
22]. However, an effective resistance monitoring method for pymetrozine needs to assess both its systemic and contact activities. The rice-stem-dipping bioassay method can reflect the two modes of action described above for pymetrozine, and the third-instar nymphs used in the test also meet the requirements concerning field prevention of lower-instar nymphs. The monitoring results of the rice-stem-dipping method showed that pymetrozine was less active against the contemporary
N. lugens and that
N. lugens had reached high levels of resistance to pymetrozine (194.6- to 212.8-fold). However, the results of this method did not fully reflect the field efficacy because of the long persistence of pymetrozine and the short duration of the treatment when rice-stem-dipping. The rice-seedling-dipping fecundity assay method examined both the systemic and contact effects of pymetrozine, taking into account its insecticidal mode and mechanism design. Using this method, we found that
N. lugens only developed moderate resistance to pymetrozine (11.2- to 12.4-fold). In this method, pymetrozine’s toxicity to
N. lugens was determined by calculating the inhibition rate after different pymetrozine doses from the number of
N. lugens offspring nymphs. Therefore, this method simultaneously evaluates pymetrozine toxicity in
N. lugens from the newly hatched nymphs to the third-instar nymphs. The topical application fecundity assay method can only examine the contact effects of pymetrozine. On the basis of this method, we found that
N. lugens developed low to moderate resistance to pymetrozine (5.4- to 10.8-fold). Both fecundity assay bioassay methods demonstrated that pymetrozine had a significant inhibitory effect on brown planthopper fecundity, and the resistance level monitored by the two methods was basically consistent at low to moderate. This is consistent with the fact that, although the short-term efficacy of pymetrozine against BPHs is decreasing in the current generation, it still has a good field control effect on the next generation due to its long persistence. The fecundity assay method was improved based on the no.005 method released by the IRAC and the topical application method reported by Tsujimoto et al. [
17]. This allowed for a more reasonable evaluation of pymetrozine toxicity in BPHs while also being subject to some constraints. As a result of the use of single-pair mating, the fecundity assay method had a large number of replications per concentration and required a large amount of work, which has certain limitations in terms of carrying large-scale resistance monitoring. Combined with the migration habits of
N. lugens, it is recommended to select three to five field populations of
N. lugens each year to monitor pymetrozine resistance using this method so as to accurately grasp the resistance dynamics of
N. lugens to pymetrozine.
In order to determine pymetrozine toxicity in BPHs using the fecundity assay method, this study comprehensively evaluated the effects of pymetrozine’s systemic and contact activity on fecundity in
N. lugens through rice-seedling-dipping treatment and topical application. As can be seen in our findings, the number of female individuals without offspring increased after pymetrozine treatment, and pymetrozine was shown to inhibit the mating of female and male brown planthoppers. Furthermore, pymetrozine can significantly inhibit the fecundity of
N. lugens. Wang et al. have confirmed that pymetrozine can activate the TRPV channels, i.e., the
nan and
iav complexes, of
N. lugens and inhibit the reproductive behavior of
N. lugens and
Drosophila melanogaster [
14,
23]. However, the specific effects of pymetrozine on the reproductive processes of female and male
N. lugens and the physiological, biochemical, and molecular mechanisms through which pymetrozine inhibits male courtship, female receptivity, and oviposition remain to be further studied.
In conclusion, pymetrozine can effectively inhibit the next-generation N. lugens population. Combined N. lugens control emphasizes the strategy of “suppressing the early population and controlling the late population”, therefore pymetrozine is still recommended for the control of migrating-generation N. lugens in order to control the offspring population. Nevertheless, the inhibitory effect of pymetrozine on the fecundity of N. lugens requires further attention. Once strong resistance is observed, the fecundity of N. lugens cannot be significantly inhibited in this manner. In these cases, the use of pymetrozine should be abandoned and other types of insecticides should be used.