Simple Summary
The engrailed (en) and invected (inv) paralogs play a fundamental role in arthropod segmentation. Previous research suggests that knockdown of either en or inv in sequentially segmenting insects leads to an unexpected and variable loss of segments but does not mimic the segment polarity defects seen in Drosophila en mutants; the consequences for segmentation when both paralogs are lost have not been reported outside of Drosophila. We analyzed the phenotypes of single and double knockdowns in the flour beetle Tribolium castaneum. Unlike Drosophila, inv knockdowns are inviable, consistent with a functional divergence of the paralogs between Tribolium and Drosophila. We find the Tribolium paralogs are redundant and act synergistically to pattern trunk appendages and segments. The most common Tribolium double knockdown results in small, limbless larvae that suffer a loss of a portion of each trunk segment that shares characteristics with segment polarity mutants in Drosophila. Some of the double knockdown embryos arrest development before germband retraction, consistent with an underexplored early function for en and inv in the regulation of cell proliferation or death in sequentially segmenting insects.
Abstract
Engrailed (en) and invected (inv) encode paralogous transcription factors found as a closely linked tandem duplication within holometabolous insects. Drosophila en mutants segment normally, then fail to maintain their segments. Loss of Drosophila inv is viable, while loss of both genes results in asegmental larvae. Surprisingly, the knockdown of Oncopeltus inv can result in the loss or fusion of the entire abdomen and en knockdowns in Tribolium show variable degrees of segmental loss. The consequence of losing or knocking down both paralogs on embryogenesis has not been studied beyond Drosophila. To further investigate the relative functions of each paralog and the mechanism behind the segmental loss, Tribolium double and single knockdowns of en and inv were analyzed. The most common cuticular phenotype of the double knockdowns was small, limbless, and open dorsally, with all but a single, segmentally iterated row of bristles. Less severe knockdowns had fused segments and reduced appendages. The Tribolium paralogs appear to act synergistically: the knockdown of either Tribolium gene alone was typically less severe, with all limbs present, whereas the most extreme single knockdowns mimic the most severe double knockdown phenotype. Morphological abnormalities unique to either single gene knockdown were not found. inv expression was not affected in the Tribolium en knockdowns, but hh expression was unexpectedly increased midway through development. Thus, while the segmental expression of en/inv is broadly conserved within insects, the functions of en and inv are evolving independently in different lineages.
1. Introduction
It has long been known that most insect species add their segments sequentially, in contrast to the near-simultaneous process in dipteran insects such as Drosophila. The discovery of the developmental genetic network that regulates Drosophila melanogaster segmentation has opened the door to the comparative exploration of the molecular basis of segmentation in insect species lacking the same genetic tools. The Drosophila segmentation genes have become key tools for understanding the evolution of differences in the mechanisms of segmentation. Most, but not all, of the Drosophila segmentation genes, are readily identified across species, yet many of their expression patterns, functions, and regulatory interactions vary throughout arthropods (reviewed in [1,2]). One exception to this pattern is the class of genes referred to as segment polarity genes. Particularly noteworthy is the remarkable conservation of the expression patterns of the segment polarity genes engrailed (en) and/or invected (inv) that encode paralogous homeodomain-containing transcription factors.
In Drosophila, en and inv function to establish and maintain the posterior compartment in segments and appendages throughout development [3,4,5]. en and inv are also known to function in neurogenesis [6,7], axon targeting [8] wing venation, and butterfly wing coloration patterning [9,10,11,12]. Their expression has been widely examined, most frequently using the 4D9 antibody that typically recognizes the homeodomain of both proteins [13]. In all species examined, 4D9 expression is detected in a narrow stripe in the posterior compartment of each segment [14,15,16,17,18,19,20,21,22]. In those species for which both en and inv expression has been examined separately, their expression co-localizes during segmentation, with only minor variance in the onset of expression in antennal and mandibular segments in one species [15,16,23,24]. The timing of their embryonic expression is thought to coincide with the ‘phylotypic stage’ of insects—the segmented germband [1].
Further support for the conservation of their function came from comparative analyses of an early acting feedback loop between wingless (wg), en, and hedgehog (hh) known to maintain the architecture of the Drosophila segment. wg expressing cells are restricted to the anterior segmental compartment and secrete wg protein toward the posterior which maintains en in the cells within the posterior compartment. In turn, en regulates hh to maintain wg in the anterior compartment. Failure to maintain this feedback loop results in a failure to maintain the segment boundaries. Later, wg and en function independently [5,25,26,27,28,29]. This feedback loop is expected to be conserved in other species, and its conservation is supported by the fact that transplantation of en-expressing cells creates new boundaries when juxtaposed to cells in the anterior of Oncopeltus segments [30] and more directly by ectopic expression of wg via a baculovirus vector in Tribolium embryos that leads to induction of en expression in adjacent cells [31]. In addition, embryos and larvae with the most severe reduction in the function of Tribolium hh and wg share similarities with one another [31].
Given the widespread conservation of patterns and timing of expression during a conserved embryonic stage, as well as support for conserved regulatory interactions, it was generally assumed that the function of en/inv genes would also be conserved. Consequently, their function in early segmentation, either in single or double loss of function or knockdown experiments, has not been widely explored. Beyond Drosophila, there is a single report of Tribolium en knockdowns [32] and a single report of Oncopeltus fasciatus inv knockdowns [14], (see Supplemental Data Note). In both cases, the results differ from that expected from Drosophila. Drosophila en mutants are embryonic lethal and have an unusual phenotype displaying features that affect the cuticular pattern in both a pair-rule and segmental manner [4,33]. By contrast, Tribolium castaneum en knockdowns result in cuticles with incomplete or irregular segment boundaries, missing anywhere between 1–11 trunk segments [32]. Null mutations in the other Drosophila en family paralog inv are viable [34,35]. However, when inv is knocked- down in Oncopeltus fasciatus, the phenotype varies from segment fusions and poorly demarcated boundaries to larvae in which the entire abdomen is lost or fused, suggesting segment development from the growth zone might have been affected [14]. The Oncopletus inv and Tribolium en knockdown data suggest that en and inv function in segmental patterning may vary from their described roles in Drosophila.
The en gene has long served as a model for the fate of gene duplications [36]. Duplications within metazoan lineages have resulted in gene families of one to four copies, in which the paralogs have different degrees of divergence in expression and function [12,23,37,38]. For example, in zebrafish, subfunctionalization of en duplicates led to differential expression in pectoral appendages and neurons [36]. In contrast to this widespread divergence of function between en family paralogs, the degree to which the hexapod en/inv gene duplication serves redundant functions in segmentation is unknown. When both en and inv functions are lost in Drosophila, segmentation defects are more severe than those that result from the loss of either gene alone. The double mutant results in truncated embryos and a ‘lawn of denticles’, with no apparent segmentation, indicating redundancy of en and inv in maintaining the structure of the segment in the fruit fly [34]. The effect of the loss or reduction of function of both genes on segmentation has not been tested in any insect other than Drosophila.
In Tribolium, the consequences of a reduction in inv function have yet to be reported, and the consequences for embryonic development due to the knockdown of both en and inv have not been explored. Through single and double knockdowns of en and inv with embryonic RNAi (eRNAi), we find support for the previously reported en knockdown phenotypes and propose a novel interpretation for the loss of segment phenotype. Our double knockdowns have disruptions of both anterior-posterior and dorsal-ventral boundaries, segment fusions, as well as a complete loss of distal leg elements. Severely affected embryos that develop cuticles are dramatically reduced in size; these highly contracted embryos retain their full segment number within the CNS, as confirmed with antibody staining. Our results suggest that en and inv serve redundant functions in gnathic and leg development, and the late maintenance of segment integrity. Interestingly, a significant portion of the dsRNAi-treated embryos fail to form cuticles at all, with phenotypes consistent with a failure to grow. The Tribolium inv or en single RNAi knockdowns result in phenotypically similar larval defects that are, for the most part, significantly less severe than the RNAi knockdown of both en and inv. We did not find evidence for functions unique to either paralog. Our results are consistent with a model in which the en/inv paralogs act synergistically to redundantly pattern the embryo.
2. Materials and Methods
2.1. Husbandry, Egg Collections, and Injection Preparations
Tribolium castaneum beetles (Strain GA-1) were maintained on whole-wheat flour supplemented with yeast (5% whole volume) at 30 °C, 40–60% humidity. To collect embryos for injection, adult beetles were incubated at 30 °C on white flour for one hour. Eggs were collected using stainless-steel mesh sieves (710 μm and 300 μm). Embryos were prepared for injections after incubating for 4 h at 30 °C. They were transferred to a handmade dechorionation apparatus constructed from a 50 mL plastic centrifuge tube with a large hole cut in its lid and a piece of mesh tightly secured to allow the eggs to be agitated in a 5% bleach solution for 2 min and then rinsed in distilled water 3 to 5 times. Eggs were transferred to a microscope slide using a 5/0 paintbrush and oriented to enable the injection needle to enter the lateral flank of the egg. The dechorionated eggs adhere sufficiently to the slide to allow resistance to the injection needle without further manipulation.
2.2. Cloning and dsRNA Synthesis
PCR primers amplified a 477 bp region of en Tc-008952 and a 627 bp of inv Tc-009896 (see Supplemental Note on identification of en/inv orthologs from genome databases). Amplified fragments were designed to exonic regions, avoiding the homeobox (Figure 1). To avoid off-target sequences for inv cloning, we performed a BlastN search on NCBI of the cDNA for our genes, choosing the algorithm “somewhat similar sequences”. We examined the hits for stretches of base pairs longer than about 15 bp and chose our clones to exempt potential overlap with known genes, e.g., in the highly similar homeodomain area. Our potential Tc-inv RNA fragments were also run through the Deqor software, and no off-target hits were identified [39]. PCR primers were designed with an optimal size of roughly 500 bp (as defined by [40]). For the Tc-en RNA fragment, we chose to use a nearly identical fragment used by [32].
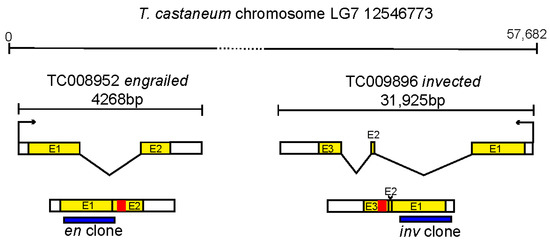
Figure 1.
Description of clones used in this study. Location of dsRNA fragments within the en and inv genes. The Tribolium en and inv gene are present in a closely linked tandem duplication shown at the top (as previously described in [24]). Exons (E1,2,3) are colored yellow, the homeobox encoding region is colored red, location of the regions cloned and used for dsRNA experiments is shown in blue.
The desired gene fragments were amplified via PCR from genomic DNA. Amplified fragments were cloned into the pSC-A-amp/kan PCR cloning vector (Agilent Stratagene TA Cloning Kit, Santa Clara, CA, USA) and verified by sequencing (UA Genomics Core, Tucson, AZ, USA). Standard PCR reactions were conducted using OneTaq reagents from New England Biolabs and primers designed to create a T7 primer sequence on either end of the Tc-en and Tc-in clones (for downstream dsRNA synthesis). dsRNA was synthesized and purified with the T7 MEGAscript kit (Ambion, Carlsbad, CA, USA). dsRNA solutions used for injection included 0.5, 1.0, 1.5 and 2.0 µg/µL each of Tc-en and Tc-in.
2.3. Microinjections and Needle Preparation
A Narishige Micromanipulator was used in conjunction with a 1.0 mm × 100 mm thin wall glass capillary needle (TW100F-4; WPI, Inc., Sarasota, FL, USA) shaped using a Model P-97 Sutter Instruments Co., (Novato, CA, USA) needle puller. Needles were broken under a dissecting microscope with fine-tip forceps. The injections were carried out using an aspirator tube assembly (Sigma, St. Louis, MI, USA) fitted with a solution-filled 3.5′ glass capillary tube (Drummond, Birmingham, AL, USA). The nitrogen gas pressure balance and pressure are first set to pBal = 0.5 PSI and pOut = 28 PSI and adjusted as needed. Microinjection was performed under a Leica dissecting scope. For embryonic injections, embryos were collected for one hour and then aged at 30 °C for 3 h prior to processing for injection. The embryos were injected without any covering. All solutions were injected into the lateral flank of each egg. Once injections were complete, embryos were kept on the injection slide, stored on petri dishes filled with 1% agarose inside a closed large Tupperware container, and raised to the desired age at 30 °C. To raise the embryos to hatchlings the Tupperware containers were opened after the 3rd day of incubation to decrease the humidity in the container.
2.4. qPCR Analysis
For Tc-en qPCR experiments, embryos were injected with either 1 or 2 ug/uL Tc-en at 4 h AEL and collected at later developmental stages, roughly (24, 31, 48, 50 h AEL). Total RNA was isolated from 50 or more buffer-injected controls and dsRNA-injected eggs using Trizol (Invitrogen), according to the manufacturer’s instructions. The aqueous phase was purified using the NEB Monarch RNA mini-prep kit (NEB #T2010). 10 ng of RNA was used in each reaction of the NEB Luna Universal One-Step RT-qPCR kit (cat # E3005). RT-qPCR reactions were carried out in triplicate, and melting curves were examined to ensure single products. Results were quantified using the CFX Maestro software from Biorad using the ‘‘delta-delta Ct” method and normalized to Histone 3 (H3) transcript levels (Supplemental Figure S2). Primer sequences used are: H3 F: 5′-CTGCCCTTCCAGAGATTGGT-3′; H3 R: 5′-GAACAGACCCACGAGGTACG-3′; en F: 5′-CGCAGGGACTCTACAACCAC-3′; en R: 5′-CGAGATTTGCCTTCGCTCTC-3′; inv F: 5′-GCAAGCCGAAGAAGGTTGTG-3′; inv R: 5′-TTCTTGACTCGCCTGGTTCG-3′; hh F: 5′-CACTGAAGGACGCATCGGAA-3′; hh R: 5′-GGTTCATCACCGAAATCGCC-3′.
2.5. Cuticle Preparation
Samples were mounted in 1:1 of Sigma Aldrich Lactic Acid ACS reagent ≥85% and Hoyer’s mounting solution and placed on a heat block set to 60 °C for 12–24 h. Samples were imaged using a Zeiss Axioplan2 and captured with AxioVision 4.8 software at either 10 or 20×. DIC/Nomarski imaging was performed using high-contrast settings.
2.6. Statistical Analysis
The statistical significance of the mean cuticle length was tested by one-way ANOVA, and statistical significance was defined as p < 0.05. The beetle categories were based on knockdown type and concentration of knockdown. Two groups were compared at a time to determine if there was a difference in mean length.
2.7. Embryo Fixation and Immunohistochemistry
Embryos were dechorionated in 5% bleach for 2 min, washed with distilled water to fully dilute the remaining bleach, and fixed in a 1:1 (v/v) ratio of n-heptane and 4% formaldehyde, for ~45 min. The fix solution was removed, replaced with ice-cold 100% MeOH, and shaken vigorously by hand for 2 min. to encourage devitellinization. Embryos that fell to the bottom were separated as devitellinized; embryos remaining at the interphase were either sonicated, dissected, or left as whole embryos and stored in 100% methanol. Immunohistochemistry followed the protocol as described in [13]. Antibodies used were: 4D9—Engrailed/Invected (DSHB) [13]; FP6.87-Ubx-abdA DSHB) [41]. We assume 4D9 detects both En and Inv proteins and FP6.87 detects both Ubx-abdA, although that has not been directly tested in Tribolium.
3. Results
3.1. Knockdown of Both en and inv Produces a Range of Morphological Defects
The larval cuticles resulting from Tc-en/inv dsRNA-injected embryos showed substantial phenotypic variability. To represent that variability, we grouped larvae into categories reflecting the severity of segment and appendage loss, bristle pattern, segment fusion, and dorsal closure defects (Figure 2, Figure 3 and Figure 4).
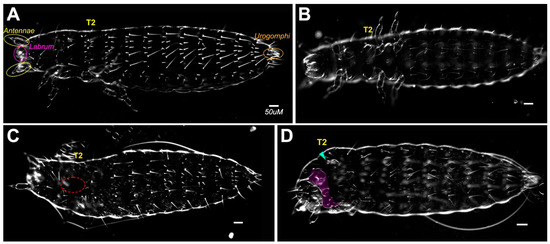
Figure 2.
Mild phenotypes resulting from double dsRNA knockdown of en and inv. (A) Dorsal side of buffer injected control; (B) ventral side of buffer injected control; (C) Category B larva, primarily normal, with incomplete dorsal closure on first and second thoracic segments (outlined in red); (D) Category B larva, primarily normal with missing (arrowhead) or abnormal appendages (highlighted in purple). All scale bars = 50 µm.
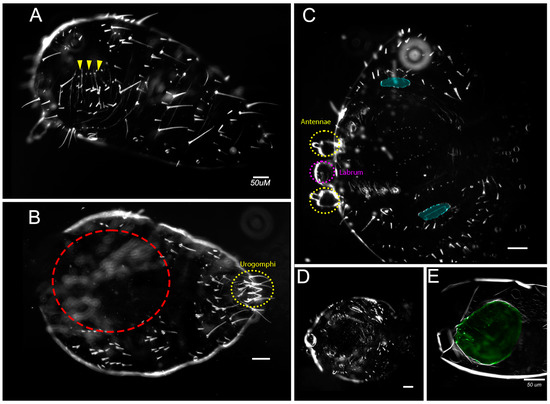
Figure 3.
Variety of severe phenotypes resulting from knockdown of both en and inv. (A) Category C larva with fusing and minimized gnathic and thoracic segments (arrowheads); (B) Category C larva with a reduced number of segments and incomplete dorsal closure (red circled area) but complete urogomphi (circled in red). (C) Category D asegmental larva with fused spiracles (blue). Moreover, note the absence of long bristles, mouthparts, and legs; antennae (circled in yellow) and labrum (circled in purple) were present. (D) Category D asegmental larva with similar cuticular features to C but more complete dorsal closure; (E) Category E larvae with secreted cuticle and minimal cuticular features. All larvae are oriented anterior to the left. Larvae had been injected with 0.5 µg/µL of both Tc-en and Tc-inv, except for C, which was injected with (1 µg/uL each of Tc-en and Tc-inv). Scale bar = 50 µm.

Figure 4.
Phenotypic categories resulting from increasing concentration of Tc-en/inv dsRNA injected. Category A: phenotypically normal; Category B: fully segmented, primarily normal with incomplete dorsal closure on the anterior thorax, reduced limbs, and minor disruptions of bristle pattern; Category C: larvae with fused and/or missing segments, a greater degree of incomplete dorsal closure, and missing appendages; Category D: asegmental larvae with fused tracheal pits, reduced bristle pattern, and failed dorsal closure; Category E: asegmental, unhatched larvae with minimal or no cuticular features. Sample sizes are indicated at the top of each bar; concentrations in µg/µL of dsRNA injected of either en/inv, en, or inv dsRNA are indicated below the bars.
3.1.1. Segment Number
Larvae varied from asegmental to fully segmented, although greater than 75% of the double knockdowns were asegmental (Figure 4). Category A larvae were phenotypically normal (Figure 2A,B). Category B larvae were fully segmented (Figure 2C,D). Category C larvae (Figure 3A,B) were missing between 1–9 trunk segments, with posterior segments less affected. Categories D and E (Figure 3C–E) were asegmental and included unhatched larvae.
To examine segmentation and to what degree en/inv were knocked down in the extremely reduced phenotypes, we fixed embryos at stages prior to the secretion of cuticle and examined the expression of En/Inv proteins using the 4D9 antibody ([13]; Figure 5A,B) and expression of Ultrabithorax/abdominal-A using the FP6.87 antibody [41]; Figure 5C–D’). Wildtype embryos (48–50 h AEL) show standard En/Inv stripes in the posterior of each segment; expression can be seen in the (3) gnathal, (3) thoracic, and (10) abdominal segments, as well as in all appendages. The double knockdown is much smaller and has no detectable En/Inv stripes. The CNS tissue occupies a more extensive amount of the embryo width, and the tissue has not begun to close dorsally (not shown in image). In the Ubx/abd-A specimens, a ridge of cells strongly expressing the Ubx/abd-A antigen (arrowhead in B) runs down the lateral flank of both sides of the embryo, likely correlating to the spiracles (bright expression surrounding each tracheal pit in the control). A similar ridge appears in every embryo analyzed and seems to delineate the ventral midline tissues (which have visible segmental structures) from the lateral flanks (with smoothened appearance), suggesting that segmental expression is still detectable in the CNS but segments in the lateral flank have fused/lost segmental boundaries. We compared individual z-slices to the z-max projection, confirming that at least 8 abdominal segments are present in these animals; the non-expressing tissue posterior to the final segment was identified as the protruding hindgut, slightly curled ventrally.
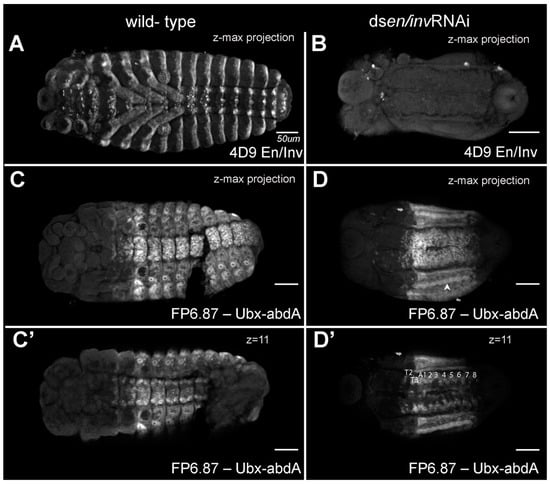
Figure 5.
En/Inv and Ubx-adbA protein expression in wild type and en/inv RNAi knockdowns. (A) En/Inv expression in wild-type and (B) en/inv RNAi knockdowns. (C,C’) Ubx-abdA expression in wild-type embryos. (D,D’) Ubx-abdA expression in en/inv RNAi knockdowns. Ubx-adbA protein expression extends from the posterior of the third thoracic segment through 8 abdominal segments in both wild-type and knockdown embryos. (C’,D’) The expression is shown in a single focal plane. The arrowhead in (D) points to fused spiracles. Both WT and knockdown embryos were reared to 48–50 h AEL. Scale bars = 50µm.
3.1.2. Larval Length
Larvae resulting from the Tc-en/inv dsRNA injections were significantly shorter (using p < 0.05) than buffer-injected controls along the AP axis (Figure 6). The reduction in size along the AP axis can be attributed to several independent features of the phenotype: (1) Intrasegmental tissue loss: the loss of bristle and naked cuticle pattern elements suggests a loss of a significant portion of many, if not most, of the segments (Figure 7). (2) Segment fusions: fusions were detected by the presence of fused tracheal pits on the ventral and lateral surfaces (Figure 3C,D, Figure 5B,D and Figure 7B). Further evidence of segment fusion was seen dorsally, where rows of bristles from two to four segments were observed to converge along the midline (not shown). Posterior segments fused less frequently: in category C, the segment fusions left the two most posterior abdominal segments unaffected or much less affected than the anterior abdominal and thoracic segments (Figure 3A,B). (3) Reduction in the size of the head: The head was severely reduced in size in all but Category B embryos.
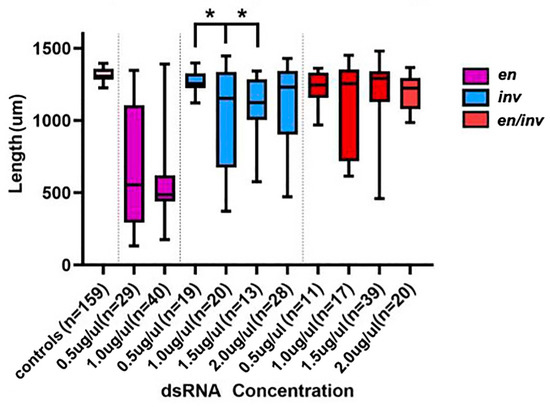
Figure 6.
Reduction in length as a function of dsRNA injected. All concentrations of dsRNA used in single or double knockdowns led to a significant reduction in length (p < 0.05) relative to controls. Within each group, only en 0.5 µg/µL relative to either en 1.0 µg/µL or en 1.5 µg/µL led to significant differences in length, indicated with an asterisk. No inv or en/inv dsRNA concentrations led to larvae significantly different in length from one another. dsRNA concentrations are shown in µg/µL.
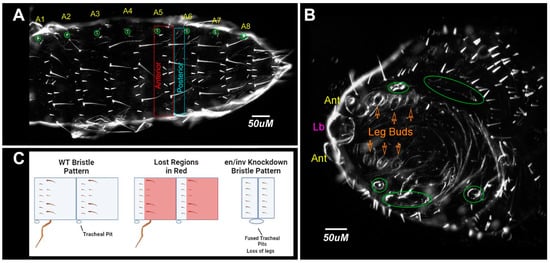
Figure 7.
Model for loss of repeated segmental elements and segment fusions. (A) Dorsal view, buffer-injected control. Spiracles are circled in green; (B) en/inv dsRNA injected (1 µg/µL) category D larvae with repeating rows of short bristles missing intervening regions of the naked cuticle. Fused spiracles are circled in green. Ant, antennae, Lb, labrum. (C) Diagram of loss of cuticular phenotypes in the severely affected double knockdowns.
3.1.3. Appendage Formation
Gnathal and thoracic appendages were either lost or significantly reduced in size in all categories (Figure 3, Figure 5 and Figure 7). The labrum and antennae were present but reduced in size (Figure 3, Figure 5 and Figure 7). Urogomphi were unaffected in all but the most severely affected larvae (e.g., Figure 3C). Appendage loss was independent of segment loss-appendages were lost, or present only as small buds, even in embryos with normal segmentation.
3.1.4. Bristle Patterns
Bristle patterns highlighted abnormalities in segment formation. A hallmark feature of Category D phenotypes was the repeated basiconic sensilla (as described in [41]) covering the lateral and dorsal cuticle, atypically uninterrupted by the naked cuticle (Figure 3C and Figure 7). In Category C larvae, rows of bristles were atypically discontinuous along the dorsal midline (Figure 3B). Category E (Figure 3E) larvae were covered in primarily naked cuticles and only a few bristles of uncertain segmental origin could be identified.
3.1.5. Dorsal Closure
The majority of larvae resulting from the Tc-en/inv eRNAi also failed to complete dorsal closure. The least affected larvae failed to close along the dorsal midline of the first thoracic segment (Figure 2C). The most severely affected larvae failed to close at any position along the AP axis. This could also be seen in the examination of late-stage embryos.
3.1.6. Failure to Form Cuticle
The most extremely affected embryos (Figure 8; on average 22.5% of double knockdowns; 10.25% of single knockdowns) lacked cuticles altogether. Embryonic tissue could be identified in these embryos but was frequently curled and spiraled within the vitelline membrane. Some embryos had an obvious anteroposterior polarity but had no distinguishable axial characteristics. Some appeared to have formed extensions orthogonal to the A/P axis (Figure 8D,F).
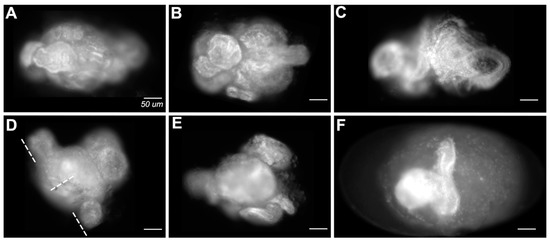
Figure 8.
Representative images of embryos that failed to secrete cuticle. (A–F) Embryos stained with DAPI. Embryo (F) is shown within the vitelline membrane, the remaining panels are cropped to isolate the embryo. Dotted lines in (D) indicate unusual orthogonal axes, also shown visibly in (F). Scale bars = 50 µm.
3.2. Single Knockdowns
Single knockdowns of either Tc-en or Tc-inv resulted in similar phenotypes, although the inv dsRNA-injected individuals were more frequently normal under all concentrations of dsRNA injected (Figure 4). Even at the highest concentrations of injected dsRNA for either gene, the larvae were primarily normal morphologically, with minor patterns of bristle fusions (Category A,B; Figure 9A,B). More severe phenotypes included primarily irregularly shaped appendages, with a bloated appearance (Figure 9C,D). The more severe phenotypes were overall smaller in size, with greater deformation of the appendages, and missing or fused segments (Figure 9E,F,H). A single inv dsRNA injected larva developed nearly normal gnathic and thoracic appendages but lacked an abdomen Figure 9H). Unlike the double knockdowns, except in the most extreme phenotypes (Figure 9G) appendages were present. Category D phenotypes (Figure 3C,D and Figure 7B) were not observed in the single knockdowns.
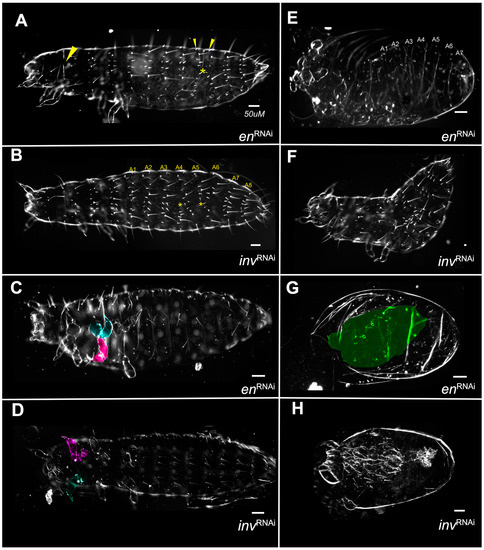
Figure 9.
No qualitative differences between Tc-en and Tc-inv single knockdowns. (A) 2.0 µg/µL Tc-en dsRNA injected larva with abdominal bristle fusions (yellow* and small arrowheads) and minor failure to complete dorsal closure (large arrowhead); (B) 1.5 µg/µL Tc-inv dsRNA injected larva with fusing segments A4-A5 and A5-A6 (yellow*); (C) 2.0 ug/uL Tc-en dsRNA injected larva with misshapen limbs (T2 legs colored); (D) 0.5 µg/µL inv dsRNA injected larva with misshapen limbs (T1 legs colored); (E) 2.0 µg/µL Tc-en larva with misshapen limbs, failed dorsal closure and missing abdominal segment; (F) 1.5 µg/µL Tc-inv dsRNA injected larva with segmental fusion, missing abdominal segment; (G) 2.0 µg/µL Tc-en dsRNA injected amorphous cuticle highlighted in green; (H) 1.5 µg/µL Tc-inv dsRNA injected larva very reduced in size, with the vestigial abdomen.
3.2.1. Asegmental embryos at High dsRNA Tc-en or Tc-inv Concentrations
In double knockdowns, the loss of all segmentation, bristle pattern, and morphology was observed in approximately 28% of samples injected with 1 µg/µL Tc-en/inv dsRNA (Category E). This phenotype was not observed in single gene knockdown larvae resulting from 0.5 or 1 µg/µL Tc-en or Tc-inv dsRNA injections, but became more prevalent with increasing concentrations of dsRNA injected: at 1.5 µg/µL Tc-inv dsRNA injected 3% of the sample were category E, at 2.0 µg/µL Tc-en dsRNA injected 11% of the sample were Category E. These larvae were inviable and lacked visible morphological structures. However, the cuticle preparations of these samples revealed that the cuticle was present, frequently with an axial polarity and a few random bristles (Figure 9G).
3.2.2. En Single Knockdowns Do Not Significantly Affect inv mRNA Levels
As noted above, Drosophila en mutations lead to the loss of inv function and some Drosophila en enhancer reporters fail to express in inv mutants [34,35]. Thus, a potential explanation for the asegmental phenotypes observed at high concentrations of dsRNA injected in the single knockdowns (Figure 9H) could be due to a regulatory interaction between en and inv in Tribolium. To test this idea, we performed qPCR on en eRNAi embryos at a series of stages (24, 31, and 48 h AEL) during germband retraction. All stages showed significant knockdown of en. We found no evidence of a significant modification of inv expression in the en knockdowns (Figure 10). We also measured changes in hh expression in the en knockdowns: hh levels would be maintained in the absence of en if Inv protein redundantly regulates hh in the absence of En protein. hh mRNA levels were unchanged at 24 or 48 h AEL but unexpectedly rose slightly at the 31 h AEL timepoint (Figure 10).
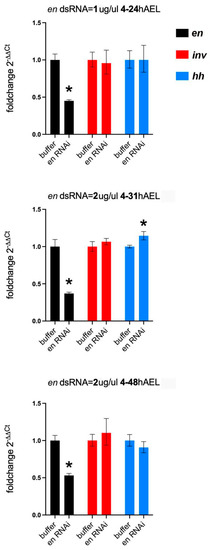
Figure 10.
Effects on expression of inv and hh in en knockdowns. en knockdown is initiated at 4 h AEL and examined at progressively later stages during which the phenotype is more pronounced. At each time point, en expression is reduced (black columns) and inv (red) is not affected, but hh (blue) levels are statistically increased in the 31 h sample (error bars are SD and significance (*) is p < 0.05).
4. Discussion
4.1. Paralog Redundancy—A Model for the Tribolium en/inv Paralogs Acting Synergistically
We find that the Tribolium en/inv paralogs have redundant functions in the embryo. Knockdown of either Tribolium en or inv function results in nearly identical phenotypes. Larvae from both single knockdowns are viable at lower concentrations of dsRNAi-injected but inviable at higher concentrations. Their functional redundancy is further supported by the qPCR analysis of en knockdowns. Based on the En-Hh/Wg feedback loop in Drosophila, the En protein would be expected to activate hh gene expression [42]. Transcription of hh did not show a decrease in the en knockdowns, consistent with the idea that inv can compensate for the loss of en function.
We do not find that en/inv are fully redundant. A small percentage of the knockdown of either gene alone induces an extreme phenotype with very little discernible pattern in the cuticle (red in Figure 4). A similar phenotype is also seen in the double knockdown, albeit at much higher penetrance than in the single knockdowns. This extreme phenotype does not seem to result from direct or indirect regulation between the paralogs, as is known to occur in Drosophila [29,34,35]. Our qPCR analysis of en knockdowns did not show a consequent decrease in the transcription of inv from 24 to 48 h AEL, as would have been expected from a requirement for either paralog in the continued transcription of the other. An alternate explanation to shared regulation is that the en/inv paralogs act synergistically. The double knockdown phenotype is observed when either gene or the combination of both genes, is reduced below a threshold. Similar synergistic effects between paralogs have been reported in Arabidopsis thaliana and Caenorhabditis elegans [43,44]. In our results, this postulated threshold is reached at lower concentrations of en dsRNA injected than inv dsRNA. We speculate that the different responses in the en and inv single knockdowns are primarily due to quantitative differences in expression. Because inv mRNA is expressed at much higher levels in the typically developing embryo (Supplemental Figure S3), it is likely more difficult to reach that threshold with inv-only dsRNA injections.
One caveat to our model is the absence of the most common double knockdown phenotype (Category D; Figure 3C,D and Figure 7B) from the single knockdowns. This remains unexplained. We note that [32] reports en knockdown larvae from pupal RNA that appear to have a similar phenotype, although the bristle pattern was not described.
Redundancy between the en/inv paralogs is also seen in Drosophila. The en/inv redundancy may contribute to both developmental robustness as has been suggested for other gene paralogs [45] as well as the observed evolutionary stability of the segment polarity pathway. While both species have maintained redundant functions of the en/inv paralogs, comparing the relative function of each paralog between the two species suggests the degree of genetic redundancy between the two paralogs varies between Tribolium and Drosophila.
4.2. En/Inv Are Redundant for Gnathal and Thoracic Appendage Formation
A requirement for an interaction between en and wg in the establishment of the proximal-distal axis of the leg has been well documented in flies [46,47,48]. Here we demonstrate that this interaction can be fulfilled redundantly by either en or inv in Tribolium. Gnathal and thoracic appendage formation was absent or severely reduced in the Tc-en/inv RNAi larvae but present, albeit at times misshapen, in the single knockdowns (except for the most extremely affected phenotypes). Similarly, appendages were present when inv function was knocked down in Oncopeltus [14], and in a separate study of en knockdowns in Tribolium [32]. All these results are consistent with Tc-en/inv having redundant roles in larval appendage development, a phenotype that would not be observed in legless Drosophila larvae.
The labrum and antennae were present, although sometimes with an irregular shape, in not only single knockdowns but also the double en/inv knockdowns that lacked legs. Similar differential effects on labrum and antennae vs. other appendages were reported for Tc-wg knockdowns [49] (note that [50] shows a weaker effect) and the Dll expression in the Tribolium labrum has been shown to be independent of either Tc-wg and Tc-hh signaling [50]. Interestingly, antennae are lost in Tc-hh pRNAi in both Tribolium [51] and Oncopeltus [52]. This suggests that Hh signaling is maintained in the antennae by transcriptional activity other than en/inv. Antennae have long been considered serially homologous to limbs [53,54,55,56,57] and it has been suggested that the labrum is also appendicular in origin (e.g., [58,59,60]). However, accumulating developmental evidence has been used to argue against considering the head and thoracic appendages as serial homologs [52]. Our evidence provides further developmental genetic support for the proposed lack of serial homology between head and thoracic appendages [52].
4.3. En/Hh-Wg—Regulatory Loop
In Drosophila, en and inv expression in the posterior of each segment expression is initiated by the transient activity of the upstream pair-rule genes, then regulated by a positive feedback loop with adjacent wingless (wg) expressing cells, mediated by the Hh signal, as described above. While our data do not directly address the feedback loop, careful analysis of the timing of misregulation in en/inv, hh, or wg knockdowns suggests additional players or regulatory interactions are involved in the maintenance of segment boundaries in Tribolium. While wg expression is lost early in Tribolium en knockdowns [32]. En/Inv expression (measured by 4D9 antibody) is maintained throughout gastrulation and germband extension in Tc-hh knockdowns [51]. This extended maintenance of the 4D9 stripes in the hh knockdowns suggests the presence of additional cues that function to maintain En prior to germband retraction. It is unlikely this is prolonged maintenance from most of the pair-rule genes as their expression has abated by this time. A second ortholog of the pair-rule gene, sloppy-paired2, has been shown to have an expression that resembles that of a segment polarity gene in the red flour beetle [61]. It is possible that slp-2 also functions in the maintenance of segment boundaries during germband extension. We also unexpectedly observed an increase in hh expression at 31 h AEL in en knockdowns. While this increase in hh remains unexplained, it implies a role for En in repressing hh expression at the completion of the germband extension. Further experiments are needed to resolve the regulatory interactions between En and hh over time and the complete gene regulatory network that maintains segment boundaries in Tribolium embryos.
4.4. Loss of Intersegmental Cellular Identity vs. Segment Polarity
We have found it curious that while the evidence suggests conservation of at least parts of the Wg-Hh feedback loop that maintains segment polarity, comparative analyses of the knockdown of segment polarity genes hh and wg in Tribolium have emphasized that these knockdowns do not manifest a change in the polarity of the Tribolium segmental pattern. Drosophila en mutations, while classified as segment polarity mutants, are an exception to the generality of intrasegmental polarity reversals within mutations of the segment polarity genes. The embryonic phenotype of some Drosophila en mutants have a significant deletion of the posterior region of even-numbered segments, which led to its initial characterization as a pair-rule gene [24]. However, Drosophila en mutants also affects the anterior margin of every segment, thus en was unique among the pair-rule genes by having both segmental and pair-rule phenotypes [33]. Stronger en mutant alleles result in embryos with apparent segment fusions accompanied by an increase in cell death in portions of the segment [4,62]. While we have not documented cell death in the phenotype, our analysis of the cuticular bristle pattern in the Tribolium en/inv double knockdowns demonstrates both segment fusions (Figure 4, Category C/D) and a deletion of a portion of the segment (Figure 4, Category D), consistent with a broadly defined segment polarity phenotype.
4.5. Is the Segment Addition Process Disrupted?
One of our original questions based on previously published knockdowns of Tribolium en and Oncopletus inv was what role, if any, en/inv play in the segment addition process in sequentially segmenting insects. Both previous reports document a loss of one-to-many segments in the RNAi knockdowns, which we confirm in our own single and double en/inv knockdowns. Our Ubx-abdA expression pattern verifies that, in at least one class of the knockdowns, all trunk segments were made but likely become reduced in size during germband retraction, as is the case in Drosophila en mutants [4]. Further analyses of embryo growth over time, with concomitant analyses of cell behaviors and segmentation gene expression, will resolve whether the other classes of embryos preferentially lose segments late in development or fail to add them initially.
We also found that a significant number of double knockdowns fail to form larval cuticle (Figure 8). These embryos have elongated but consist of significantly fewer cells than wild-type or less affected embryos. This failure to grow could result from a primary role for en/inv in cell division or a secondary consequence of increased cell death. That the tissue was frequently found coiled in a spiral is consistent with a disruption of cell adhesion, another proposed function of Drosophila en [15], and an early disruption of adhesion may have impacted subsequent growth. We speculate that the empty cuticle phenotype observed in both single and double en/inv knockdowns (Category E) may also be related to a failure to grow; embryos may have grown sufficiently enough to secrete cuticle but have insufficient growth to complete normal intrasegmental patterning that governs the bristle pattern.
A function for zygotic en in organizing the pre-cellular blastoderm, earlier than its function in patterning the posterior compartment, is known in Drosophila [62]. Drosophila en has also been shown to play a role in the control of growth in the wing disc in a Hh independent mechanism, although in that circumstance its loss of function results in an increase in cell proliferation [63]. Most curious in our phenotypes was the appearance of extensions orthogonal to the primary axis in these embryos. It is possible that the disrupted expression of en/inv is causing bifurcations from the primary axis such as that observed as a consequence of the disruption of en or wg in Drosophila imaginal discs [64,65,66]. Thus, as in Drosophila, en likely has more complex roles than maintaining identity in the cells in the posterior of the segment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects14080691/s1 Figure S1: Phylogenetic Tree of en/inv Ensembl hits. PhylML tree with bootstrap values made with ClustalW based on sequence alignment of en or inv-like hits from Ensembl aligned with putative orthologs from NCBI Blast hits; Figure S2: Cycle number of the H3 reference gene at different embryonic stages in Tribolium. Each timepoint represents the unmodified H3 cycle number from the qPCR averaged across both control and experimental trials. We found in this experiment (and many additional qPCR trials) that the H3 gene gave very consistent cycle numbers across stages and across trials; Figure S3: Relative expression levels of en and inv mRNA during the first 24 h of development. inv mRNA (red) has a slightly earlier onset of expression, a greater rate of increase, and ultimately reaches roughly 4× the level of expression than en mRNA (blue). At 30 °C at 10 h AEL the embryo is in the later blastoderm, when no stripes of En/Inv protein are detectable, by 13 h AEL the germband has formed and is fully extended, with a full complement of 10 abdominal segments by 24 h. These data are from unpublished work describing the wild-type Tribolium transcriptome at the onset of gastrulation through the end of segmentation. Source: [67,68,69].
Author Contributions
Conceptualization: L.M.N., S.B., S.O., T.A.W., H.G.-V. and T.C.; Supervision: S.O., H.G.-V., L.M.N. and T.A.W.; Project Administration: L.M.N. and T.A.W.; Investigation: S.O., H.G.-V., J.C., L.M.N., S.B., M.H. and T.C.; Formal Analysis: L.M.N., S.O., S.B., H.G.-V. and T.A.W.; Methodology: S.O., L.M.N., S.B., H.G.-V. and T.C.; Validation: L.M.N., S.O., S.B., H.G.-V. and T.A.W.; Data Curation: S.O., L.M.N., S.B., H.G.-V. and T.A.W.; Resources: L.M.N. and T.A.W.; Funding Acquisition: L.M.N. and T.A.W.; Writing—Original Draft Preparation: L.M.N., M.H., S.B., S.O. and T.C.; Writing—Review & Editing: J.C., H.G.-V., L.M.N., S.B., S.O. and T.A.W.; Visualization: S.O., H.G.-V., S.B., L.M.N. and T.A.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by NSF MCB grants 1817173 and 1817485 to L. M. Nagy and NSF MCB 1817873 to T. A. Williams.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We thank Rebekah Mitchell for editorial assistance, Katie Russell and Jahmiyes Wright for discussions about the data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Peel, A.D.; Chipman, A.D.; Akam, M. Arthropod segmentation: Beyond the Drosophila paradigm. Nat. Rev. Genet. 2005, 6, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Auman, T.; Chipman, A.D. The Evolution of Gene Regulatory Networks that Define Arthropod Body Plans. Integr. Comp. Biol. 2017, 57, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Morata, G.; Lawrence, P.A. Control of compartment development by the engrailed gene in Drosophila. Nature 1975, 255, 614–617. [Google Scholar] [CrossRef]
- Kornberg, T. Engrailed: A gene controlling compartment and segment formation in Drosophila. Proc. Natl. Acad. Sci. USA 1981, 78, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, P.A.; Struhl, G. Further studies of the engrailed phenotype in Drosophila. EMBO J. 1982, 1, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.H.; Martin-Blanco, E.; Coleman, K.G.; Poole, S.J.; Ellis, M.C.; Kornberg, T.B.; Goodman, C.S. Expression of engrailed proteins in arthropods, annelids, and chordates. Cell 1989, 58, 955–968. [Google Scholar] [CrossRef]
- Condron, B.G.; Patel, N.H.; Zinn, K. engrailed controls glial/neuronal cell fate decisions at the midline of the central nervous system. Neuron 1994, 13, 541–554. [Google Scholar] [CrossRef]
- Whitington, P.M.; Meier, T.; King, P. Segmentation, neurogenesis and formation of early axonal pathways in the centipede, Ethmostigmus rubripes (Brandt). Roux’s Arch. Dev. Biol. 1991, 199, 349–363. [Google Scholar] [CrossRef]
- Keys, D.N.; Lewis, D.L.; Selegue, J.E.; Pearson, B.J.; Goodrich, L.V.; Johnson, R.L.; Gates, J.; Scott, M.P.; Carroll, S.B. Recruitment of a hedgehog Regulatory Circuit in Butterfly Eyespot Evolution. Science 1999, 283, 532–534. [Google Scholar] [CrossRef]
- Brunetti, C.R.; E Selegue, J.; Monteiro, A.; French, V.; Brakefield, P.M.; Carroll, S.B. The generation and diversification of butterfly eyespot color patterns. Curr. Biol. 2001, 11, 1578–1585. [Google Scholar] [CrossRef]
- Monteiro, A.; Podlaha, O. Wings, horns, and butterfly eyespots: How do complex traits evolve? PLoS Biol. 2009, 7, e37. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, T.D.; Ramos, D.; Monteiro, A. Expression of Multiple engrailed Family Genes in Eyespots of Bicyclus anynana Butterflies Does Not Implicate the Duplication Events in the Evolution of This Morphological Novelty. Front. Ecol. Evol. 2020, 8, 227. [Google Scholar] [CrossRef]
- Patel, N.H.; Kornberg, T.B.; Goodman, C.S. Expression of engrailed during segmentation in grasshopper and crayfish. Development 1989, 107, 201–212. [Google Scholar] [CrossRef]
- Angelini, D.R.; Kaufman, T.C. Functional analyses in the milkweed bug Oncopeltus fasciatus (Hemiptera) support a role for Wnt signaling in body segmentation but not appendage development. Dev. Biol. 2005, 283, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Siegler, M.V.; Jia, X.X. engrailed negatively regulates the expression of cell adhesion molecules connectin and neuroglian in embryonic Drosophila nervous system. Neuron 1999, 22, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Marie, B.; Bacon, J.P. Two engrailed -related genes in the cockroach: Cloning, phylogenetic analysis, expression and isolation of splice variants. Dev. Genes Evol. 2000, 210, 436–448. [Google Scholar] [CrossRef]
- Williams, T.; Blachuta, B.; Hegna, T.A.; Nagy, L.M. Decoupling elongation and segmentation: Notch involvement in anostracan crustacean segmentation. Evol. Dev. 2012, 14, 372–382. [Google Scholar] [CrossRef]
- Janssen, R. Segment polarity gene expression in a myriapod reveals conserved and diverged aspects of early head patterning in arthropods. Dev. Genes Evol. 2012, 222, 299–309. [Google Scholar] [CrossRef]
- Green, J.; Akam, M. Evolution of the pair rule gene network: Insights from a centipede. Dev. Biol. 2013, 382, 235–245. [Google Scholar] [CrossRef]
- Gibert, J.M.; Mouchel-Vielh, E.; Quéinnec, E.; Deutsch, J.S. Barnacle duplicate engrailed genes: Divergent expression patterns and evidence for a vestigial abdomen. Evol. Dev. 2000, 2, 194–202. [Google Scholar] [CrossRef]
- Nakagaki, Y.; Sakuma, M.; Machida, R. Expression of engrailed -family genes in the jumping bristletail and discussion on the primitive pattern of insect segmentation. Dev. Genes Evol. 2015, 225, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, T.; Sidén, I.; O’Farrell, P.; Simon, M. The engrailed locus of Drosophila: In situ localization of transcripts reveals compartment-specific expression. Cell 1985, 40, 45–53. [Google Scholar] [CrossRef]
- Peterson, M.D.; Popadic, A.; Kaufman, T.C. The expression of two engrailed-related genes in an apterygote insect and a phylogenetic analysis of insect engrailed -related genes. Dev. Genes Evol. 1998, 208, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Peel, A.D.; Telford, M.J.; Akam, M. The evolution of hexapod engrailed-family genes: Evidence for conservation and concerted evolution. Proc. Biol. Sci. 2006, 273, 1733–1742. [Google Scholar] [CrossRef]
- Perrimon, N.; Mahowald, A.P. Multiple functions of segment polarity genes in Drosophila. Dev. Biol. 1987, 119, 587–600. [Google Scholar] [CrossRef]
- Baker, N.E. Embryonic and imaginal requirements for wingless, a segment-polarity gene in Drosophila. Dev. Biol. 1988, 125, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Bejsovec, A.; Martinez Arias, A. Roles of wingless in patterning the larval epidermis of Drosophila. Development 1991, 113, 471–485. [Google Scholar] [CrossRef]
- Bejsovec, A.; Wieschaus, E. Segment polarity gene interactions modulate epidermal patterning in Drosophila embryos. Development 1993, 119, 501–517. [Google Scholar] [CrossRef]
- Heemskerk, J.; DiNardo, S.; Kostriken, R.; O’Farrell, P.H. Multiple modes of engrailed regulation in the progression towards cell fate determination. Nature 1991, 352, 404–410. [Google Scholar] [CrossRef]
- Campbell, G.L.; Caveney, S. engrailed gene expression in the abdominal segment of Oncopeltus: Gradients and cell states in the insect segment. Development 1989, 106, 727–737. [Google Scholar] [CrossRef]
- Oppenheimer, D.I.; MacNicol, A.M.; Patel, N.H. Functional conservation of the wingless-engrailed interaction as shown by a widely applicable baculovirus misexpression system. Curr. Biol. 1999, 9, 1288–1296. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lim, J.; Choe, C.P. Functional analysis of engrailed in Tribolium segmentation. Mech. Dev. 2020, 161, 103594. [Google Scholar] [CrossRef]
- Nusslein-Volhard, C.; Wieschaus, E. Mutations affecting segment number and polarity in Drosophila. Nature 1980, 287, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Gustavson, E.; Goldsborough, A.S.; Ali, Z.; Kornberg, T.B. The Drosophila engrailed and invected genes: Partners in regulation, expression and function. Genetics 1996, 142, 893–906. [Google Scholar] [CrossRef]
- Cheng, Y.; Brunner, A.L.; Kremer, S.; DeVido, S.K.; Stefaniuk, C.M.; Kassis, J.A. Co-regulation of invected and engrailed by a complex array of regulatory sequences in Drosophila. Dev. Biol. 2014, 395, 131–143. [Google Scholar] [CrossRef]
- Force, A.; Lynch, M.; Pickett, F.B.; Amores, A.; Yan, Y.-L.; Postlethwait, J. Preservation of duplicate genes by complementary, degenerative mutations. Genetics 1999, 151, 1531–1545. [Google Scholar] [CrossRef]
- Ahzhanov, A.; Kaufman, T.C. Evolution of distinct expression patterns for engrailed paralogues in higher crustaceans (Malacostraca). Dev. Genes Evol. 2000, 210, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Marie, B.; Blagburn, J.M. Differential roles of engrailed paralogs in determining sensory axon guidance and synaptic target recognition. J. Neurosci. 2003, 23, 7854–7862. [Google Scholar] [CrossRef][Green Version]
- Henschel, A.; Buchholz, F.; Habermann, B. DEQOR: A web-based tool for the design and quality control of siRNAs. Nucleic Acids Res. 2004, 32 (Suppl. 2), W113–W120. [Google Scholar] [CrossRef]
- Miller, S.C.; Miyata, K.; Brown, S.J.; Tomoyasu, Y. Dissecting systemic RNA interference in the red flour beetle Tribolium castaneum: Parameters affecting the efficiency of RNAi. PLoS ONE 2012, 7, e47431. [Google Scholar] [CrossRef]
- Kelsh, R.; Weinzierl, R.O.J.; White, R.A.H.; Akam, M. Homeotic gene expression in the locust Schistocerca: An antibody that detects conserved epitopes in ultrabithorax and abdominal-A proteins. Dev. Genet. 1994, 15, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Tabata, T.; Eaton, S.; Kornberg, T.B. The Drosophila hedgehog gene is expressed specifically in posterior compartment cells and is a target of engrailed regulation. Genes Dev. 1992, 6, 2635–2645. [Google Scholar] [CrossRef] [PubMed]
- Nodine, M.D.; Yadegari, R.; Tax, F.E. RPK1 and TOAD2 are two receptor-like kinases redundantly required for arabidopsis embryonic pattern formation. Dev. Cell 2007, 12, 943–956. [Google Scholar] [CrossRef]
- Lambie, E.J.; Kimble, J. Two homologous regulatory genes, lin-12 and glp-1, have overlapping functions. Development 1991, 112, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Vanden Broek, K.; Han, X.; Hansen, D. Redundant mechanisms regulating the proliferation vs. differentiation balance in the C. elegans germline. Front. Cell Dev. Biol. 2022, 10, 960999. [Google Scholar] [CrossRef]
- Simcox, A.A.; Roberts, I.J.; Hersperger, E.; Gribbin, M.C.; Shearn, A.; Whittle, J.R. Imaginal discs can be recovered from cultured embryos mutant for the segment-polarity genes engrailed, naked and patched but not from wingless. Development 1989, 107, 715–722. [Google Scholar] [CrossRef]
- Cohen, B.; Simcox, A.A.; Cohen, S.M. Allocation of the thoracic imaginal primordia in the Drosophila embryo. Development 1993, 117, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M. Specification of limb development in the Drosophila embryo by positional cues from segmentation genes. Nature 1990, 343, 173–177. [Google Scholar] [CrossRef]
- Ober, K.A.; Jockusch, E.L. The roles of wingless and decapentaplegic in axis and appendage development in the red flour beetle, Tribolium castaneum. Dev. Biol. 2006, 294, 391–405. [Google Scholar] [CrossRef]
- Posnien, N.; Bashasab, F.; Bucher, G. The insect upper lip (labrum) is a nonsegmental appendage-like structure. Evol. Dev. 2009, 11, 480–488. [Google Scholar] [CrossRef]
- Farzana, L.; Brown, S.J. Hedgehog signaling pathway function conserved in Tribolium segmentation. Dev. Genes Evol. 2008, 218, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Lev, O.; Edgecombe, G.D.; Chipman, A.D. Serial Homology and Segment Identity in the Arthropod Head. Integr. Org. Biol. 2022, 4, obac015. [Google Scholar] [CrossRef] [PubMed]
- Snodgrass, R.E. Principles of Insect Morphology, 1st ed.; McGraw-Hill Publications in the Zoological Sciences: New York, NY, USA, 1935; Volume IX, 667p. [Google Scholar]
- Struhl, G. A homoeotic mutation transforming leg to antenna in Drosophila. Nature 1981, 292, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Rogers, B.T.; Kaufman, T.C. Structure of the insect head in ontogeny and phylogeny: A view from Drosophila. Int. Rev. Cytol. 1997, 174, 1–84. [Google Scholar]
- Scholtz, G.; Edgecombe, G.D. The evolution of arthropod heads: Reconciling morphological, developmental and palaeontological evidence. Dev. Genes Evol. 2006, 216, 395–415. [Google Scholar] [CrossRef]
- Ortega-Hernández, J.; Janssen, R.; Budd, G.E. Origin and evolution of the panarthropod head—A palaeobiological and developmental perspective. Arthropod Struct. Dev. 2017, 46, 354–379. [Google Scholar] [CrossRef]
- Haas, M.S.; Brown, S.J.; Beeman, R.W. Pondering the procephalon: The segmental origin of the labrum. Dev. Genes Evol. 2001, 211, 89–95. [Google Scholar] [CrossRef]
- Popadić, A.; Panganiban, G.; Rusch, D.; Shear, W.A.; Kaufman, T.C. Molecular evidence for the gnathobasic derivation of arthropod mandibles and for the appendicular origin of the labrum and other structures. Dev. Genes Evol. 1998, 208, 142–150. [Google Scholar] [CrossRef]
- Prpic, N.-M.; Wigand, B.; Damen, W.G.; Klingler, M. Expression of dachshund in wild-type and Distal-less mutant Tribolium corroborates serial homologies in insect appendages. Dev. Genes Evol. 2001, 211, 467–477. [Google Scholar] [CrossRef]
- Janssen, R. The embryonic expression pattern of a second, hitherto unrecognized, paralog of the pair-rule gene sloppy-paired in the beetle Tribolium castaneum. Dev. Genes Evol. 2020, 230, 247–256. [Google Scholar] [CrossRef]
- Karr, T.L.; Ali, Z.; Drees, B.; Kornberg, T. The engrailed locus of D. melanogaster provides an essential zygotic function in precellular embryos. Cell 1985, 43 Pt 2, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, A. Three distinct roles for the engrailed gene in Drosophila wing development. Curr. Biol. 1994, 4, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Tabata, T.; Schwartz, C.; Gustavson, E.; Ali, Z.; Kornberg, T.B. Creating a Drosophila wing de novo, the role of engrailed, and the compartment border hypothesis. Development 1995, 121, 3359–3369. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Struhl, G. Complementary and mutually exclusive activities of decapentaplegic and wingless organize axial patterning during Drosophila leg development. Cell 1996, 86, 401–409. [Google Scholar] [CrossRef]
- Johnston, L.A.; Schubiger, G. Ectopic expression of wingless in imaginal discs interferes with decapentaplegic expression and alters cell determination. Development 1996, 122, 3519–3529. [Google Scholar] [CrossRef] [PubMed]
- Panfilio, K.A.; Jentzsch, I.M.V.; Benoit, J.B.; Erezyilmaz, D.; Suzuki, Y.; Colella, S.; Robertson, H.M.; Poelchau, M.F.; Waterhouse, R.M.; Ioannidis, P.; et al. Molecular evolutionary trends and feeding ecology diversification in the Hemiptera, anchored by the milkweed bug genome. Genome Biol. 2019, 20, 64. [Google Scholar] [CrossRef]
- Brown, S.J.; Patel, N.H.; Denell, R.E. Embryonic expression of the single Tribolium engrailed homolog. Dev. Genet. 1994, 15, 7–18. [Google Scholar] [CrossRef]
- Goldman-Huertas, B.; Sagun, J.; Nagy, L.M.; Williams, T.A. Transcriptome of the segmenting embryo of Tribolium castaneum reveals contribution of alternative pathways to developmental regulation, manuscript in preparation. 2023. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).