The Intricate Role of Ecdysis Triggering Hormone Signaling in Insect Development and Reproductive Regulation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Structure, Function, and Regulation of ETH and ETHRs
3. Role of ETH in the Regulation of Larval Ecdysis in Holo- and Hemimetabolous Insects
4. Coordinated Endocrine Network of ETH Promotes JH Production and Reproductive Success in Adult Insects
5. Conclusions and Future Direction
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wadsworth, T.; Carriman, A.; Gutierrez, A.A.; Moffatt, C.; Fuse, M. Ecdysis behaviors and circadian rhythm of ecdysis in the stick insect, Carausius morosus. J. Insect Physiol. 2014, 71, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Žitňan, D.; Daubnerová, I. Chapter 85—Corazonin. In Handbook of Hormones; Takei, Y., Ando, H., Tsutsui, K., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 477–478. ISBN 978-0-12-801028-0. [Google Scholar]
- Zitnan, D.; Adams, M.E. Neuroendocrine regulation of insect ecdysis. In Comprehensive Insect Biochemistry, Physiology, Pharmacology, and Molecular Biology; Gilbert, L.I., Iatrou, K., Gill, S.S., Eds.; Elsevier Press: London, UK, 2005. [Google Scholar]
- Žitňan, D.; Kim, Y.-J.; Žitňanová, I.; Roller, L.; Adams, M.E. Complex steroid-peptide-receptor cascade controls insect ecdysis. Gen. Comp. Endocrinol. 2007, 153, 88–96. [Google Scholar] [CrossRef] [PubMed]
- White, B.H.; Ewer, J. Neural and hormonal control of postecdysial behaviors in insects. Annu. Rev. Entomol. 2014, 59, 363–381. [Google Scholar] [CrossRef] [PubMed]
- Loveall, B.J.; Deitcher, D.L. The essential role of bursicon during Drosophila development. BMC Dev. Biol. 2010, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.S.; Katayama, H.; Dircksen, H. New functions of arthropod bursicon: Inducing deposition and thickening of new cuticle and hemocyte granulation in the blue crab, Callinectes sapidus. PLoS ONE 2012, 7, e46299. [Google Scholar] [CrossRef] [PubMed]
- Zitnan, D.; Ross, L.S.; Zitnanova, I.; Hermesman, J.L.; Gill, S.S.; Adams, M.E. Steroid induction of a peptide hormone gene leads to orchestration of a defined behavioral sequence. Neuron 1999, 23, 523–535. [Google Scholar] [CrossRef]
- Žitňan, D.; Hollar, L.; Spalovská, I.; Takáč, P.; Žitňanová, I.; Gill, S.S.; Adams, M.E. Molecular cloning and function of ecdysis-triggering hormones in the silkworm Bombyx mori. J. Exp. Biol. 2002, 205, 3459–3473. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Zitnan, D.; Galizia, C.G.; Cho, K.-H.; Adams, M.E. A command chemical triggers an innate behavior by sequential activation of multiple peptidergic ensembles. Curr. Biol. 2006, 16, 1395–1407. [Google Scholar] [CrossRef]
- Yamanaka, N.; Yamamoto, S.; Žitňan, D.; Watanabe, K.; Kawada, T.; Satake, H.; Kaneko, Y.; Hiruma, K.; Tanaka, Y.; Shinoda, T.; et al. Neuropeptide Receptor Transcriptome reveals unidentified neuroendocrine pathways. PLoS ONE 2008, 3, e3048. [Google Scholar] [CrossRef]
- Park, Y.; Filippov, V.; Gill, S.S.; Adams, M.E. Deletion of the ecdysis-triggering hormone gene leads to lethal ecdysis deficiency. Development 2002, 129, 493–503. [Google Scholar] [CrossRef]
- Park, Y.; Kim, Y.-J.; Dupriez, V.; Adams, M.E. Two subtypes of ecdysis-triggering hormone receptor in Drosophila melanogaster. J. Biol. Chem. 2003, 278, 17710–17715. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Žitňan, D.; Cho, K.-H.; Schooley, D.A.; Mizoguchi, A.; Adams, M.E. Central peptidergic ensembles associated with organization of an innate behavior. Proc. Natl. Acad. Sci. USA 2006, 103, 14211–14216. [Google Scholar] [CrossRef]
- Arakane, Y.; Li, B.; Muthukrishnan, S.; Beeman, R.W.; Kramer, K.J.; Park, Y. Functional analysis of four neuropeptides, EH, ETH, CCAP and Bursicon, and their receptors in adult ecdysis behavior of the red flour beetle, Tribolium castaneum. Mech. Dev. 2008, 125, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Asuncion-Uchi, M.; Shawa, H.E.; Martin, T.; Fuse, M. Different actions of ecdysis-triggering hormone on the brain and ventral nerve cord of the hornworm, Manduca sexta. Gen. Comp. Endocrinol. 2010, 166, 54–65. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, Y.-J.; Adams, M.E. Endocrine regulation of airway clearance in drosophila. Proc. Natl. Acad. Sci. USA 2018, 115, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Predel, R.; Neupert, S.; Russell, W.K.; Scheibner, O.; Nachman, R.J. Corazonin in Insects. Peptides 2007, 28, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.-L.; Jiang, H.-B.; Gui, S.-H.; Chen, E.-H.; Wei, D.-D.; Li, H.-M.; Wang, J.-J.; Smagghe, G. A Role of Corazonin Receptor in Larval-Pupal Transition and Pupariation in the Oriental Fruit Fly Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Front. Physiol. 2017, 8, 77. [Google Scholar] [CrossRef]
- Žitňan, D.; Žitňanová, I.; Spalovská, I.; Takáč, P.; Park, Y.; Adams, M.E. Conservation of Ecdysis-Triggering Hormone Signalling in Insects. J. Exp. Biol. 2003, 206, 1275–1289. [Google Scholar] [CrossRef]
- Zitnan, D.; Kingan, T.G.; Hermesman, J.L.; Adams, M.E. Identification of ecdysis-triggering hormone from an epitracheal endocrine system. Science 1996, 271, 88–91. [Google Scholar] [CrossRef]
- Ikeda, E. Kimon Rimensen. In Experimental Anatomy and Physiology of Bombyx mori; EIkeda, Ed.; Meibundo: Tokyo, Japan, 1913; pp. 242–243. [Google Scholar]
- Zitnan, D.; Adams, M.E. 7—Neuroendocrine regulation of ecdysis. In Insect Endocrinology; Gilbert, L.I., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 253–309. ISBN 978-0-12-384749-2. [Google Scholar]
- Roller, L.; Žitňanová, I.; Dai, L.; Šimo, L.; Park, Y.; Satake, H.; Tanaka, Y.; Adams, M.E.; Žitňan, D. The ecdysis triggering hormone signaling in arthropods. Peptides 2010, 31, 429–441. [Google Scholar] [CrossRef]
- Lenaerts, C.; Cools, D.; Verdonck, R.; Verbakel, L.; Vanden Broeck, J.; Marchal, E. The ecdysis triggering hormone system is essential for successful moulting of a major hemimetabolous pest insect, Schistocerca gregaria. Sci. Rep. 2017, 7, 46502. [Google Scholar] [CrossRef] [PubMed]
- Meiselman, M.; Lee, S.S.; Tran, R.-T.; Dai, H.; Ding, Y.; Rivera-Perez, C.; Wijesekera, T.P.; Dauwalder, B.; Noriega, F.G.; Adams, M.E. Endocrine network essential for reproductive success in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2017, 114, E3849–E3858. [Google Scholar] [CrossRef] [PubMed]
- Daubnerová, I.; Roller, L.; Satake, H.; Zhang, C.; Kim, Y.J.; Žitňan, D. Identification and function of ETH receptor networks in the silkworm Bombyx mori. Sci. Rep. 2021, 11, 11693. [Google Scholar] [CrossRef]
- Adams, M.E.; Zitnan, D. Identification of ecdysis-triggering hormone in the silkworm Bombyx bori. Biochem. Biophys. Res. Commun. 1997, 230, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Iversen, A.; Cazzamali, G.; Williamson, M.; Hauser, F.; Grimmelikhuijzen, C.J.P. Molecular identification of the first insect ecdysis triggering hormone receptors. Biochem. Biophys. Res. Commun. 2002, 299, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wei, Z.; Nachman, R.J.; Kaczmarek, K.; Zabrocki, J.; Park, Y. Functional characterization of five different PRXamide receptors of the red flour beetle Tribolium castaneum with peptidomimetics and identification of agonists and antagonists. Peptides 2015, 68, 246–252. [Google Scholar] [CrossRef]
- Shi, Y.; Jiang, H.-B.; Gui, S.-H.; Liu, X.-Q.; Pei, Y.-X.; Xu, L.; Smagghe, G.; Wang, J.-J. Ecdysis triggering hormone signaling (ETH/ETHR-A) is required for the larva-larva ecdysis in Bactrocera dorsalis (Diptera: Tephritidae). Front. Physiol. 2017, 8, 587. [Google Scholar] [CrossRef]
- Oliphant, A.; Alexander, J.L.; Swain, M.T.; Webster, S.G.; Wilcockson, D.C. Transcriptomic analysis of crustacean neuropeptide signaling during the moult cycle in the green shore crab, Carcinus maenas. BMC Genom. 2018, 19, 711. [Google Scholar] [CrossRef]
- Fastner, S.; Predel, R.; Kahnt, J.; Schachtner, J.; Wegener, C. A Simple purification protocol for the detection of peptide hormones in the hemolymph of individual insects by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 23–28. [Google Scholar] [CrossRef]
- Cho, K.-H.; Daubnerová, I.; Park, Y.; Zitnan, D.; Adams, M.E. Secretory competence in a gateway endocrine cell conferred by the nuclear receptor ΒFTZ-F1 enables stage-specific ecdysone responses throughout development in drosophila. Dev. Biol. 2014, 385, 253–262. [Google Scholar] [CrossRef]
- Kingan, T.G.; Cardullo, R.A.; Adams, M.E. Signal transduction in eclosion hormone-induced secretion of ecdysis-triggering ormone. J. Biol. Chem. 2001, 276, 25136–25142. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Schroeder, A.J.; Helfrich-Förster, C.; Jackson, F.R.; Ewer, J. Targeted Ablation of CCAP Neuropeptide-containing neurons of drosophila causes specific defects in execution and circadian timing of ecdysis behavior. Development 2003, 130, 2645–2656. [Google Scholar] [CrossRef]
- Dai, L.; Adams, M.E. Ecdysis triggering hormone signaling in the yellow fever mosquito Aedes aegypti. Gen. Comp. Endocrinol. 2009, 162, 43–51. [Google Scholar] [CrossRef]
- Grunert, L.W.; Clarke, J.W.; Ahuja, C.; Eswaran, H.; Nijhout, H.F. A Quantitative analysis of growth and size regulation in Manduca sexta: The physiological basis of variation in size and age at metamorphosis. PLoS ONE 2015, 10, e0127988. [Google Scholar] [CrossRef]
- Krüger, E.; Mena, W.; Lahr, E.C.; Johnson, E.C.; Ewer, J. Genetic analysis of eclosion hormone action during drosophila larval ecdysis. Development 2015, 142, 4279–4287. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, N.; Rewitz, K.F.; O’Connor, M.B. Ecdysone control of developmental transitions: Lessons from drosophila research. Annu. Rev. Entomol. 2013, 58, 497–516. [Google Scholar] [CrossRef]
- Gammie, S.C.; Truman, J.W. Eclosion hormone provides a link between ecdysis-triggering hormone and crustacean cardioactive peptide in the neuroendocrine cascade that controls ecdysis behavior. J. Exp. Biol. 1999, 202, 343–352. [Google Scholar] [CrossRef]
- Ewer, J.; Gammie, S.C.; Truman, J.W. Control of insect ecdysis by a positive-feedback endocrine system: Roles of eclosion hormone and ecdysis triggering hormone. J. Exp. Biol. 1997, 200, 869–881. [Google Scholar] [CrossRef]
- Nässel, D.R. Substrates for neuronal cotransmission with neuropeptides and small molecule neurotransmitters in Drosophila. Front. Cell. Neurosci. 2018, 12, 83. [Google Scholar] [CrossRef]
- Clark, A.C.; del Campo, M.L.; Ewer, J. Neuroendocrine control of larval ecdysis behavior in drosophila: Complex regulation by partially redundant neuropeptides. J. Neurosci. 2004, 24, 4283–4292. [Google Scholar] [CrossRef]
- Chang, J.C.; Yang, R.B.; Adams, M.E.; Lu, K.H. Receptor guanylyl cyclases in Inka cells targeted by eclosion hormone. Proc. Natl. Acad. Sci. USA 2009, 106, 13371–13376. [Google Scholar] [CrossRef] [PubMed]
- Klowden, M. Physiological Systems in Insects, 2nd ed.; Academic Press: Burlington, MA, USA, 2008. [Google Scholar]
- Areiza, M.; Nouzova, M.; Rivera-Perez, C.; Noriega, F.G. Ecdysis triggering hormone ensures proper timing of juvenile hormone biosynthesis in pharate adult mosquitoes. Insect Biochem. Mol. Biol. 2014, 54, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Goodman, W.G.; Cusson, M. The juvenile hormones. In Insect Endocrinology; Gilbert, L.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 310–365. [Google Scholar]
- Riddiford, L.M. How does juvenile hormone control insect metamorphosis and reproduction? Gen. Comp. Endocrinol. 2012, 179, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Noriega, F.G. Juvenile hormone biosynthesis in insects: What is new, what do we know, and what questions remain? Int. Sch. Res. Notices 2014, 2014, 967361. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sheng, Z.; Liu, H.; Wen, D.; He, Q.; Wang, S.; Shao, W.; Jiang, R.-J.; An, S.; Sun, Y.; et al. Juvenile hormone counteracts the BHLH-PAS transcription factors MET and GCE to prevent caspase-dependent programmed cell death in Drosophila. Development 2009, 136, 2015–2025. [Google Scholar] [CrossRef]
- Gruntenko, N.E.; Wen, D.; Karpova, E.K.; Adonyeva, N.V.; Liu, Y.; He, Q.; Faddeeva, N.V.; Fomin, A.S.; Li, S.; Rauschenbach, I.Y. Altered juvenile hormone metabolism, reproduction and stress response in drosophila adults with genetic ablation of the corpus allatum cells. Insect Biochem. Mol. Biol. 2010, 40, 891–897. [Google Scholar] [CrossRef]
- Gujar, H.; Palli, S.R. Krüppel Homolog 1 and E93 mediate juvenile hormone regulation of metamorphosis in the common bed bug, Cimex lectularius. Sci. Rep. 2016, 6, 26092. [Google Scholar] [CrossRef]
- Wyatt, G.R. Juvenile Hormone in Insect Reproduction—A Paradox? EJE 2013, 94, 323–333. [Google Scholar]
- Wijesekera, T.P.; Saurabh, S.; Dauwalder, B. Juvenile hormone is required in adult males for drosophila courtship. PLoS ONE 2016, 11, e0151912. [Google Scholar] [CrossRef]
- Uryu, O.; Ameku, T.; Niwa, R. Recent progress in understanding the role of ecdysteroids in adult insects: Germline development and circadian clock in the fruit fly Drosophila melanogaster. Zool. Lett. 2015, 1, 32. [Google Scholar] [CrossRef]
- Jindra, M.; Uhlirova, M.; Charles, J.P.; Smykal, V.; Hill, R.J. Genetic evidence for function of the bHLH-PAS protein Gce/Met as a juvenile hormone receptor. PLoS Genet. 2015, 11, e1005394. [Google Scholar] [CrossRef]
- Abdou, M.A.; He, Q.; Wen, D.; Zyaan, O.; Wang, J.; Xu, J.; Baumann, A.A.; Joseph, J.; Wilson, T.G.; Li, S.; et al. Drosophila met and gce are partially redundant in transducing juvenile hormone action. Insect Biochem. Mol. Biol. 2011, 41, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Bai, H.; Dolezal, A.G.; Amdam, G.; Tatar, M. Juvenile hormone regulation of drosophila aging. BMC Biol. 2013, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Adams, M.E. Regulation of drosophila long-term courtship memory by ecdysis triggering hormone. Front. Neurosci. 2021, 15, 670322. [Google Scholar] [CrossRef]
- Meiselman, M.R.; Kingan, T.; Adams, M.E. Stress-induced reproductive arrest in Drosophila occurs through ETH deficiency-mediated suppression of oogenesis and ovulation. BMC Biol. 2018, 16, 18. [Google Scholar] [CrossRef]
- Soller, M.; Bownes, M.; Kubli, E. Control of oocyte maturation in sexually mature drosophila females. Dev. Biol. 1999, 208, 337–351. [Google Scholar] [CrossRef]
- Schwedes, C.C.; Carney, G.E. Ecdysone signaling in adult Drosophila melanogaster. J. Insect Physiol. 2012, 58, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Shlyueva, D.; Stampfel, G.; Stark, A. Transcriptional enhancers: From properties to genome-wide predictions. Nat. Rev. Genet. 2014, 15, 272–286. [Google Scholar] [CrossRef]
- Wayne, M.L.; Soundararajan, U.; Harshman, L.G. Environmental stress and reproduction in Drosophila melanogaster: Starvation resistance, ovariole numbers and early age egg production. BMC Evol. Biol. 2006, 6, 57. [Google Scholar] [CrossRef]
- Jindra, M.; Palli, S.R.; Riddiford, L.M. The juvenile hormone signaling pathway in insect development. Annu. Rev. Entomol. 2013, 58, 181–204. [Google Scholar] [CrossRef]
- Gauthier, S.A.; VanHaaften, E.; Cherbas, L.; Cherbas, P.; Hewes, R.S. Cryptocephal, the Drosophila melanogaster ATF4, is a specific coactivator for ecdysone receptor isoform B2. PLOS Genet. 2012, 8, e1002883. [Google Scholar] [CrossRef] [PubMed]
- Lenaerts, C.; Marchal, E.; Peeters, P.; Vanden Broeck, J. The ecdysone receptor complex is essential for the reproductive success in the female desert locust, Schistocerca gregaria. Sci. Rep. 2019, 9, 15. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Sonobe, H. Subchapter 98A-20-Hydroxyecdysone. In Handbook of Hormones; Takei, Y., Ando, H., Tsutsui, K., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 560-e598A–562. [Google Scholar]
- Schwartz, M.B.; Kelly, T.J.; Imberski, R.B.; Rubenstein, E.C. The effects of nutrition and methoprene treatment on ovarian ecdysteroid synthesis in Drosophila melanogaster. J. Insect Physiol. 1985, 31, 947–957. [Google Scholar] [CrossRef]
- Terashima, J.; Takaki, K.; Sakurai, S.; Bownes, M. Nutritional status affects 20-hydroxyecdysone concentration and progression of oogenesis in Drosophila melanogaster. J. Endocrinol. 2005, 187, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Terashima, J.; Bownes, M. E75A and E75B have opposite effects on the apoptosis/development choice of the Drosophila egg chamber. Cell Death Differ. 2006, 13, 454–464. [Google Scholar] [CrossRef]
- Lee, S.S.; Ding, Y.; Karapetians, N.; Rivera-Perez, C.; Noriega, F.G.; Adams, M.E. Hormonal signaling cascade during an early adult critical period required for courtship memory retention in drosophila. Curr. Biol. 2017, 27, 2798–2809.e3. [Google Scholar] [CrossRef] [PubMed]
- Hiruma, K.; Kaneko, Y. Hormonal regulation of insect metamorphosis with special reference to juvenile hormone biosynthesis. Curr. Top. Dev. Biol. 2013, 103, 73–100. [Google Scholar] [CrossRef]
- Areiza, M.; Nouzova, M.; Rivera-Perez, C.; Noriega, F.G. 20-Hydroxyecdysone stimulation of juvenile hormone biosynthesis by the mosquito corpora allata. Insect Biochem. Mol. Biol. 2015, 64, 100–105. [Google Scholar] [CrossRef]
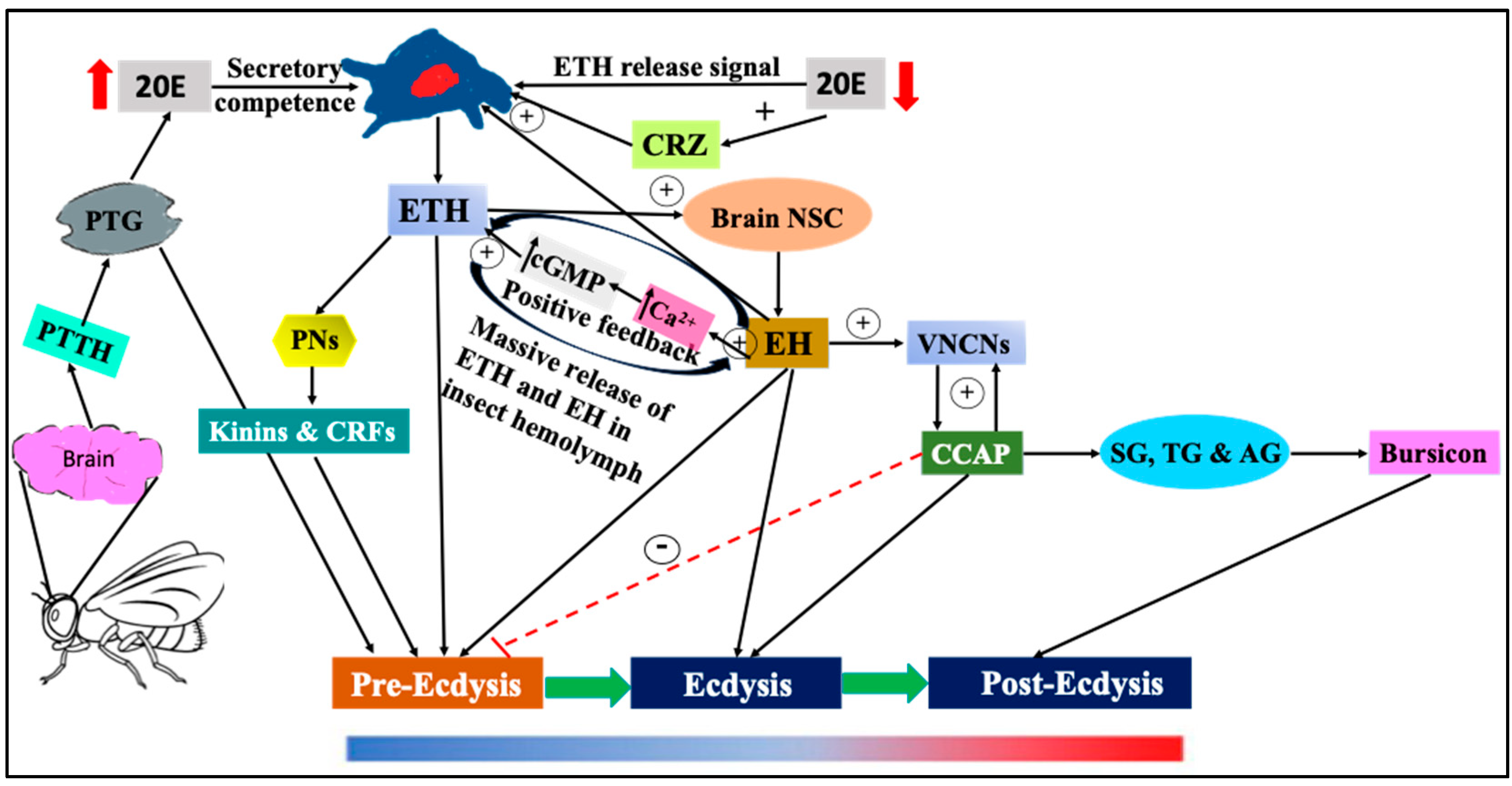


| Phases of Ecdysis | Hormones | Gland and Nerves/Cells Involved |
|---|---|---|
| Pre-ecdysis (I and II) | PTTH, ETH (II), EH, 20E, PETH(I), Kinins and CRFs, myoinhibitory peptides FMRFamide | PTG, epitracheal Inka cells (Trachea), neuro secretary cells of brain, and peptidergic neurons in the CNS. |
| Ecdysis | ETH, EH and CCAP, CRZ | PTG, epitracheal Inka cells (Trachea), neuro secretary cells of brain, ventral nerve cord neurons, lateral brain neurosecretory cells projecting to the corpora cardiaca-corpora allata (CC-CA) complex, and in neurons of the ventral nerve cord. |
| Post-ecdysis | Bursicon | Neurons of sub-esophageal, thoracic, and first segment of abdominal ganglion. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malhotra, P.; Basu, S. The Intricate Role of Ecdysis Triggering Hormone Signaling in Insect Development and Reproductive Regulation. Insects 2023, 14, 711. https://doi.org/10.3390/insects14080711
Malhotra P, Basu S. The Intricate Role of Ecdysis Triggering Hormone Signaling in Insect Development and Reproductive Regulation. Insects. 2023; 14(8):711. https://doi.org/10.3390/insects14080711
Chicago/Turabian StyleMalhotra, Pooja, and Saumik Basu. 2023. "The Intricate Role of Ecdysis Triggering Hormone Signaling in Insect Development and Reproductive Regulation" Insects 14, no. 8: 711. https://doi.org/10.3390/insects14080711





