Simple Summary
Predators drive prey trait diversification and promote ecological speciation. The impacts of predation are not only on the final state of antipredation traits, but also on the development of antipredation traits. Species of the dragonfly genus Leucorrhinia are distributed in both habitats dominated by predatory fish (fish lakes) and habitats dominated by predatory invertebrates (invertebrate lakes). In larval dragonflies, the spine is one of the most efficient traits deterring gape-limited fish predators. However, the spine is not useful in invertebrate lakes. In this study, we compared the developmental patterns of spines in both habitats. We constructed the scaling relationship between spine length and body size and compared the inflexion point on those curves in five species of Leucorrhinia dragonfly larvae. Here, we found that fish-lake Leucorrhinia species kept a higher spine growth rate than species from invertebrate lakes, and Leucorrhinia species from fish lakes displayed accelerated spine growth rate at larger body size compared to invertebrate-lake species. Our results highlight that development patterns, as well as the final states of antipredator traits, are essential to understanding predator–prey interactions.
Abstract
Predation is a major factor driving prey trait diversification and promoting ecological speciation. Consequently, antipredator traits are widely studied among prey species. However, comparative studies that examine how different predators shape the ontogenetic growth of antipredator traits are scarce. In larval dragonflies, abdominal spines are effective traits against predatory fish in fish lakes, which prefer larger prey. However, defensive spines increase mortality in habitats dominated by invertebrate predators (invertebrate lakes), which prefer smaller prey. Thus, species from fish lakes may accelerate spine growth at a later body size compared to species from invertebrate lakes when growing into the preferred prey size range of predatory fish. In this study, we constructed the allometric relationship between spine length and body size and compared the inflexion point of those growth curves in five species of Leucorrhinia dragonfly larvae. We found that fish-lake Leucorrhinia species accelerated spine growth at a larger body size than congenerics from invertebrate lakes. Further, rather than extending spine length constantly through development, fish-lake species rapidly accelerated spine growth at a larger body size. This is likely to be adaptive for avoiding invertebrate predation at an early life stage, which are also present in fish lakes, though in smaller numbers. Our results highlight that comparative studies of ontogenetic patterns in antipredator traits might be essential to develop an integrated understanding of predator–prey interactions.
1. Introduction
Predators do not just affect population and species abundances [1], but are well-known major selective drivers mediating species trait diversifications [2]. Thus, diversification of antipredator traits has been intensively studied in morphology, behavior, physiology, and life history [3,4,5,6,7]. However, less well-understood are ontogenetic growth trajectories of antipredator traits [8,9,10]. Understanding patterns of allometric growth in antipredator traits (i.e., allometry of defense) is especially important for understanding the diversity of vital morphological traits for survival [11,12], and thus permits a more specific understanding of alterations in the timing and rate of ontogenetic events of defensive traits [13,14].
Many aquatic animals separate along the predator-mediated habitat gradient [15] with permanent waters either dominated by predatory fish or invertebrate predators. Thereby, both predator types differ in a number of foraging and hunting attributes, leading to habitat-specific morphological, behavioral, physiological, and life history adaptations in a number of groups (e.g., amphibian larvae [16], mayflies [17], etc.). Evolutionary changes in morphological antipredator traits might occur either by modifying size or shifting proportions among different body parts during development [18]. For example, Leucorrhinia species that have adapted to predatory fish have developed wider abdomens than species that have adapted to invertebrate predators, given that swim escape speed to evade predatory fish is linked to increased muscle content [19]. Further, small turtles grow wider faster than they grow longer, which enables them to quickly achieve size refuge from gape-limited predators (e.g., dolphinfish) [20]. Diplodus fish species change growth trajectories of body shape (from rapid changes to slow changes) when they reach a size range at which a habitat transition (from pelagic to necto-benthic habitat) occurs [21]. Allometric growth of defensive organs will evidently alter the defensive ability and influence the defensive strategies in animals [22].
Defensive spines are prominent features across the animal kingdom [17,23,24,25,26]. Abdominal spines acting as defensive traits are particularly well-studied among dragonfly larvae [27,28,29,30,31]. However, species differ in the occurrence of abdominal spines. Ancestrally occurring in fish lakes, Leucorrhinia species reduced the length of their spines after inhabiting invertebrate lakes [32,33]. Among species of the dragonfly genus Leucorrhinia, species preferring habitats dominated by predatory fish (hereafter fish-lake species) possess long defensive abdominal spines. This is due to an increased survival with long spines [34]. However, spines in Leucorrhinia species preferring habitats dominated by invertebrate top-predators (hereafter invertebrate-lake species) became either lost or reduced, contrasting to the species from lakes with predatory fish [32]. This is due to an increased mortality of larvae with long spines in the presence of large invertebrate predators [35].
As spine expressions differ distinctly between fish-lake species and invertebrate-lake species, ontogenetic growth of spines might show different trajectories. Invertebrate predators prefer smaller prey than predatory fish [36]. Thus, prey in invertebrate-lake species could decelerate growth of spines at an early developmental size because of spine-mediated mortality (see above), saving production costs of spines [37]. In contrast, predatory fish prefer larger prey, with prey often growing into the preferred size range of predatory fish during later development stages [38]. Long spines enlarge the body volume of dragonfly larvae, resulting in decreased mortality [28,34,39]. However, even though fish lakes are dominated by predatory fish, they also have invertebrate predators, though at low density [40]. Thus, when dragonfly larvae are too tiny to be detected by predatory fish but face the pressure of invertebrate predators in fish lakes, they might keep a low growth rate of the spines at early age; in contrastive, when they grow larger and are easily detected by fish, they might grow defensive spines rapidly.
Although the importance of defensive spines as an effective antipredator trait is known, evolutionary change of ontogenetic patterns of spine growth have not been examined in detail. Here, we investigated the evolutionary divergence on ontogenetic growth trajectories of lateral spine length over body size in five larval European Leucorrhinia species. Specifically, we hypothesized that larvae of fish-lake Leucorrhinia species should express defensive spines in a later developmental stage and have a larger growth rate of defensive spines than invertebrate-lake species.
2. Materials and Methods
2.1. Collecting and Larvae Breeding
At least two egg clutches for each Leucorrhinia species were collected during June 2016 (details in Figure 1). The common predatory fish species for Leucorrhinia in the sampling area include perch, crucian carp, pike, common roach, bream, etc. The common large invertebrate predators include Anax, Aeshna larvae, etc. Egg clutches were kept separately in 500 mL containers filled with 400 mL dechlorinated tap water until they hatched (egg hatching takes around 2 weeks in all species). From the end of June until the middle of July 2016, hatched larvae from the different clutches of same species were mixed and put into 10 L big buckets filled with 9 L dechlorinated tap water. Larvae were fed with Daphnia sp. ad libitum every second day. All buckets were kept outside on the campus of the Freie Universität Berlin [52°31′ N and 13°24′ E].

Figure 1.
Phylogeny of five European Leucorrhinia species modified from Hovmöller and Johansson [32]. Fish and dragonfly larvae symbols indicate the preferred top predator (fish predator and large invertebrate predator, respectively) for each species. Sample locations and the number of egg clutches collected are given for each species.
2.2. Growth Inspection and Measurements
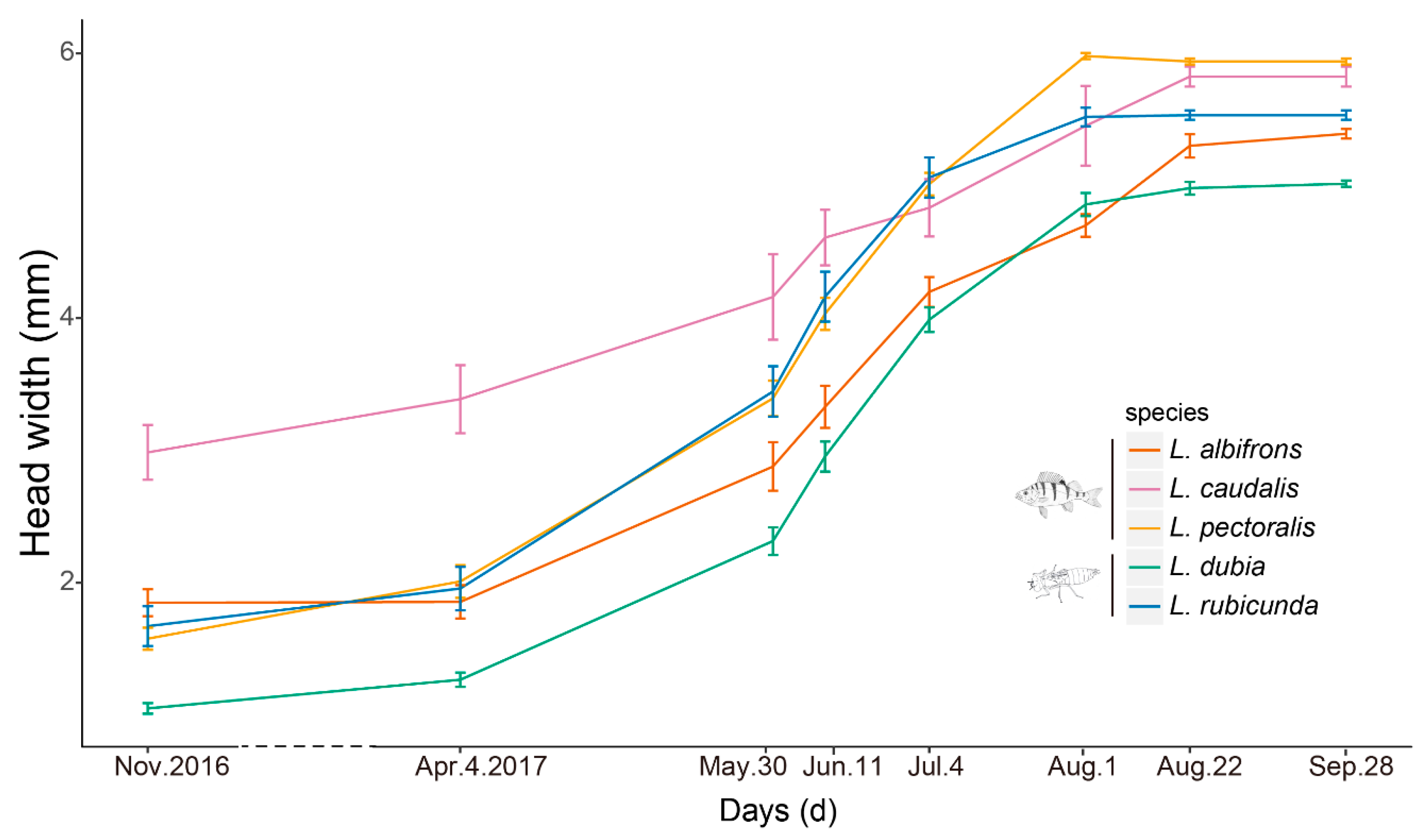
Larvae were measured from November 2016 to September 2017 (December 2016 to March 2017 were excluded because they barely grow during this cold period). For each time point, we counted the number of larvae. Head and lateral spines on segment 8 and 9 of the abdomen were photographed for each larvae using an Olympus digital microscope SZX16 (Hamburg, Germany). We could only measure lateral spines by photographing larvae from above, because early larvae do not survive when handled with forceps to put them into the correct position. We concentrated on lateral spines and excluded dorsal abdominal spines because larvae do not cope well with the necessary handling for photographing the dorsal site at an early stage. We used photos to measure head width and lateral spines in segments 8 and 9 with the free software ImageJ 1.50 g (National Institutes of Health, Bethesda, MD, USA 2016). Head width was used as a surrogate for body size [41].
2.3. Data Analysis
All the analyses were carried out under R 4.3.0 [42]. Head width differences as well as lateral spine lengths of abdominal segments 8 and 9 in last instar larvae were analyzed using separate phylogenetic generalized least squares (PGLS) in the “caper” package [43] with predator regime (fish lake vs. invertebrate lake) as an independent variable and the average of each measurement in each species as a dependent variable (the significance level was 0.05). We incorporated branch length from a pruned Leucorrhinia phylogeny (Hovmöller pers. communication, Figure 1) [32].
Allometric relationships of lateral spines (length of spines in segments 8 and 9 measured in each time point of each larva, separately) over head width were fitted with different growth models (linear models and non-linear models, model description in supplementary material Table S1 following Paine et al. [44]). New sets of nonlinear allometries, such as logistic or Gompertz growth models, can be used to analyze the changes of the growth curves during the evolution of the phenotype [18,44]. Inflexion point indicates the point with the highest growth rate, in which the second derivative becomes “zero”. Before the inflexion point, the trait has a fast growth rate; after the inflexion point, the growth rate decelerates [13].
The most suitable models were selected according to AIC values. The head width values on the inflexion points of the curves for each spine (hereafter inflexion point) and the maximum slope for the growth of spines (hereafter maximum slope) were extracted from the best fitting models.
We tested whether predator regime (fish lake vs. invertebrate lake) affected the inflexion point and maximum slope using PGLS with predator regime as an independent variable (see the above PGLS analyses).
3. Results
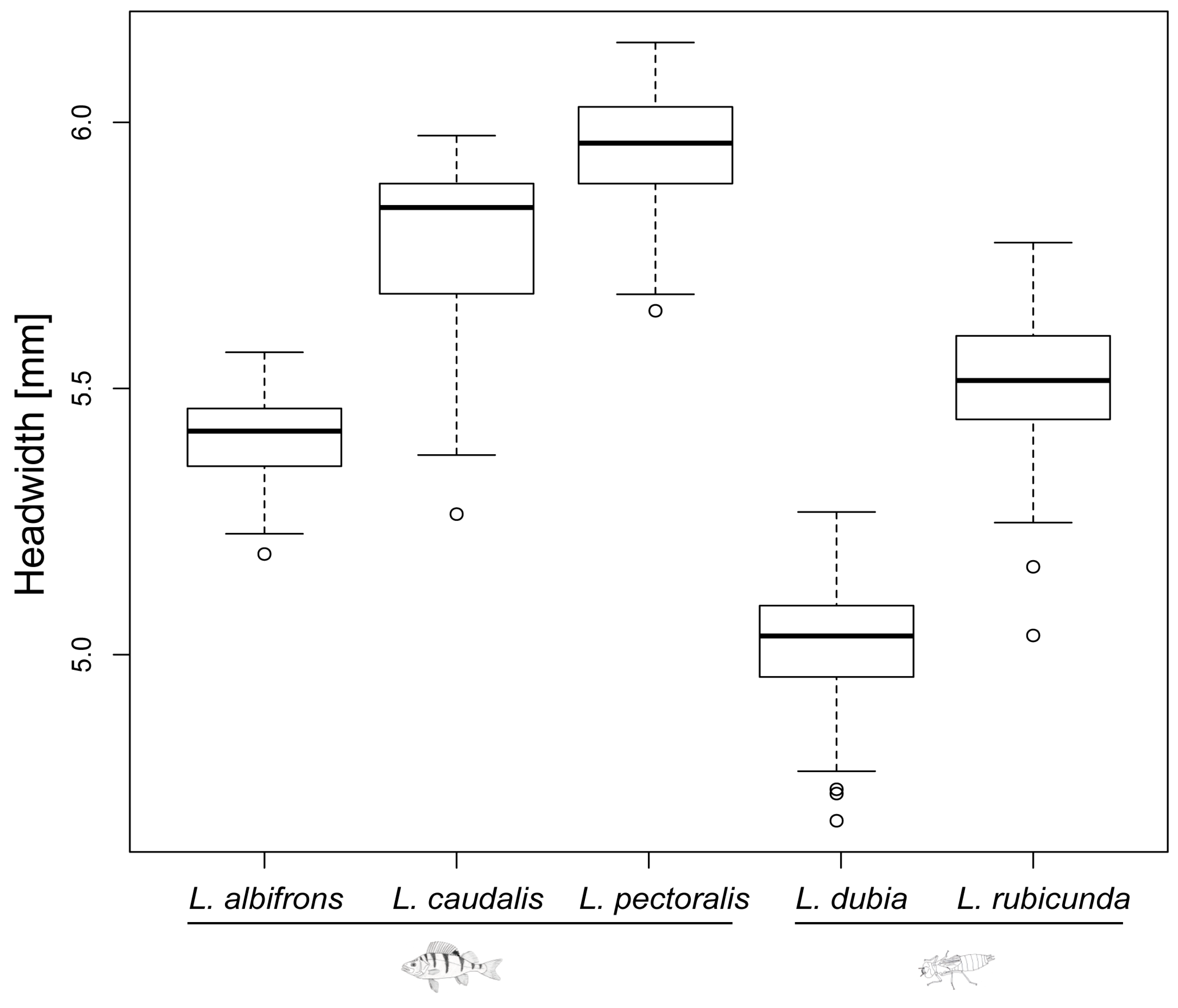
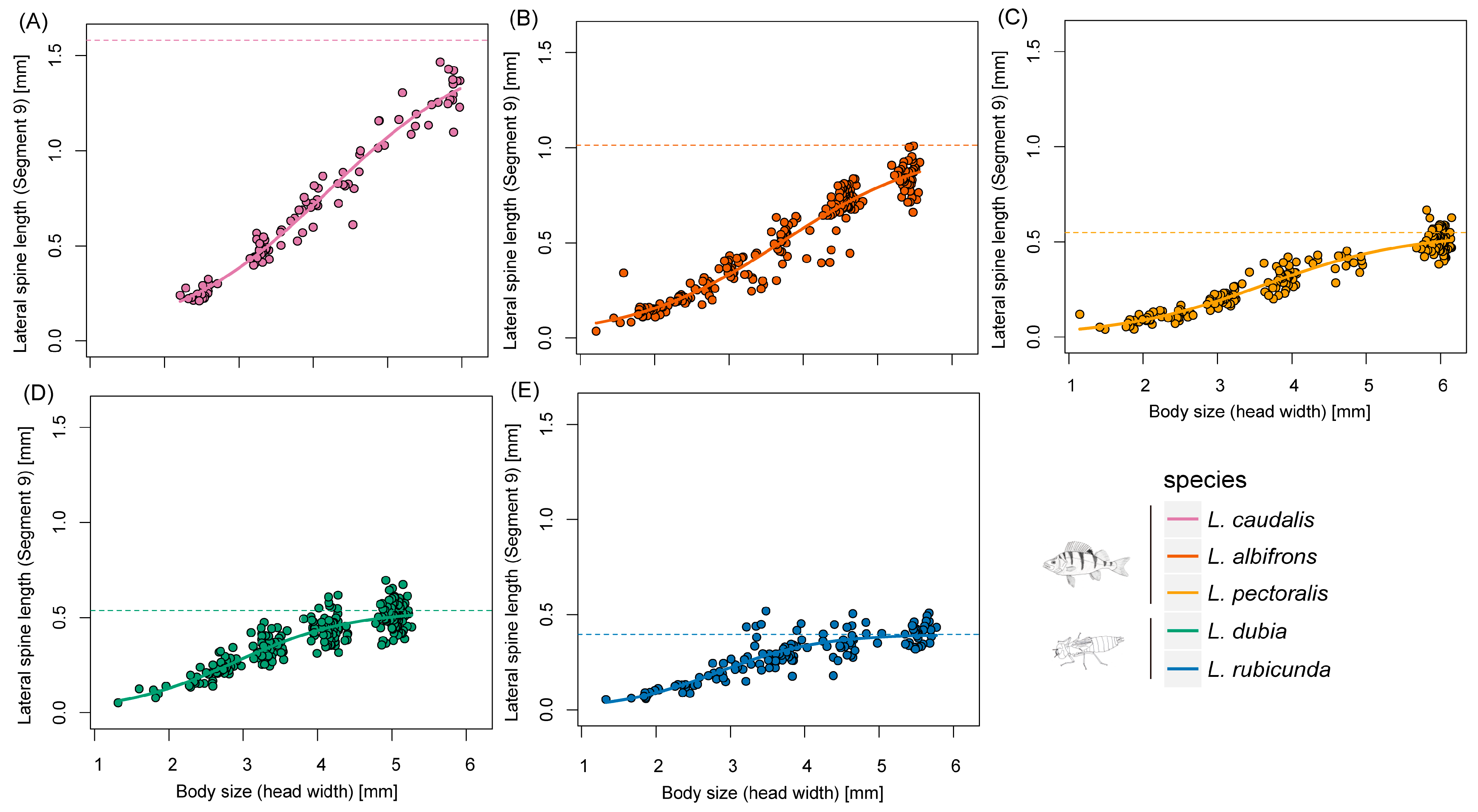
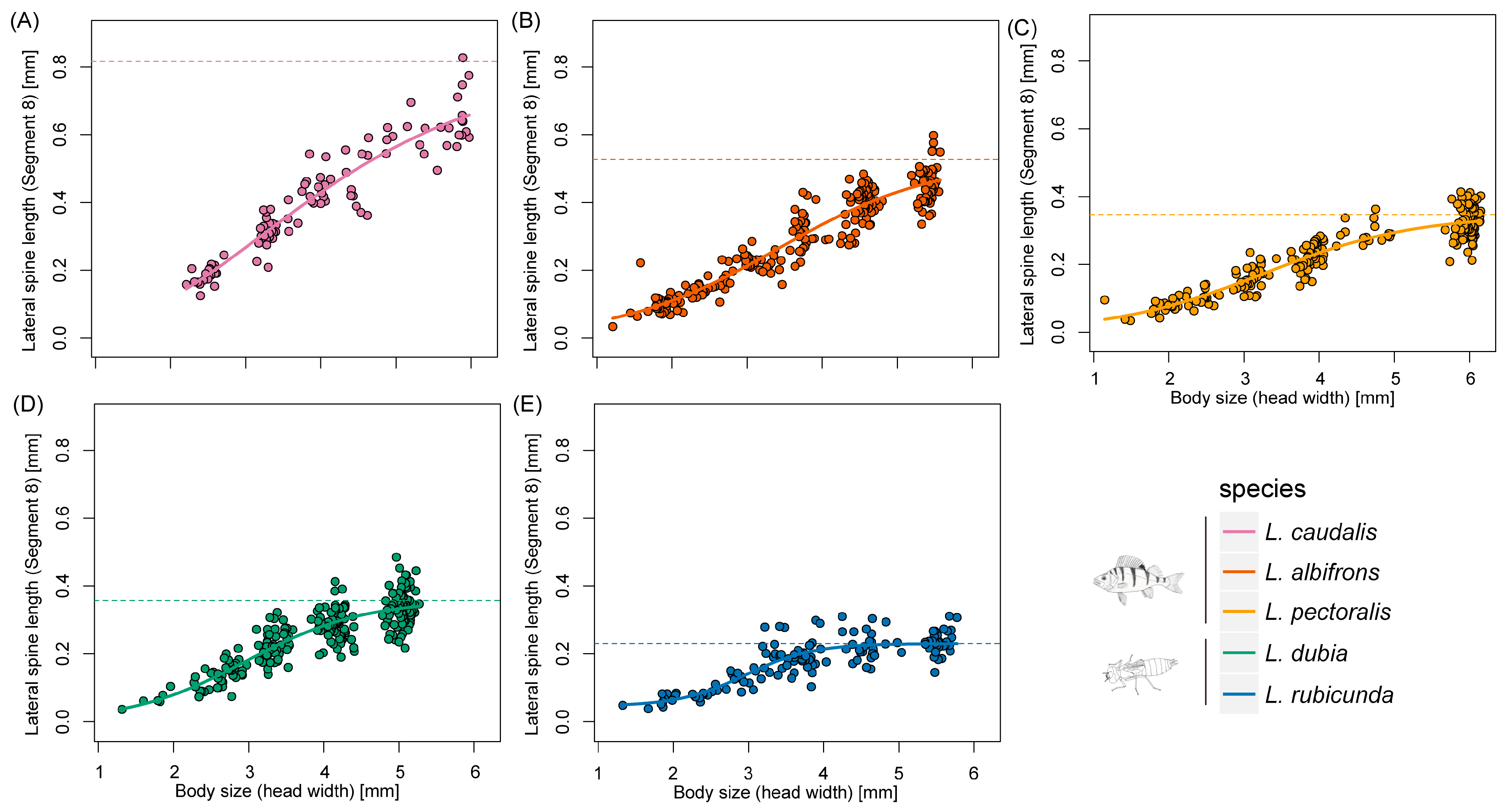
Last instar larvae of species from fish lakes were larger than from invertebrate lakes (F1,3 = 12.34, p = 0.039) (Figure 2 and Figure 3). Last instar lateral spines 9 (F1,3 = 6.77, p = 0.080) and spines 8 (F1,3 = 8.66, p = 0.060) only showed a tendency for being longer in fish lakes than invertebrate lakes (Figure 4 and Figure 5).
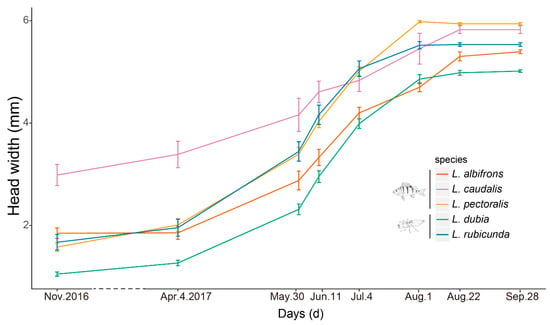
Figure 2.
Examined time points and growth lines of each species. Presented are mean ± 1.95 SE (95% confidence intervals). Species and the top predator in their preferred habitat (fish predator and large invertebrate predator, respectively) are also indicated with fish and dragonfly larvae symbols. The dash line indicates the long time intervals during winter.
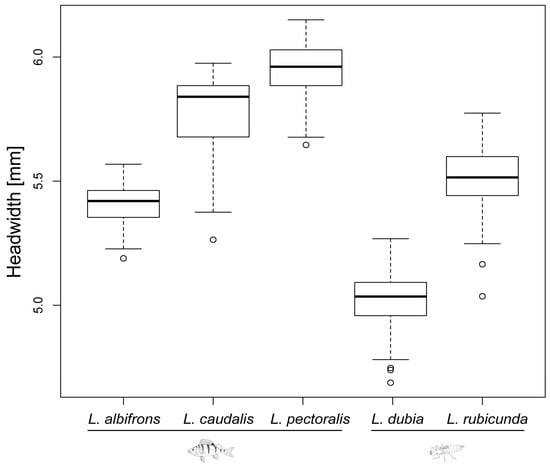
Figure 3.
Head width of last instar larvae for all 5 Leucorrhinia species. Fish and dragonfly larvae symbols indicate the preferred top predator (fish predator and large invertebrate predator, respectively) for each species.
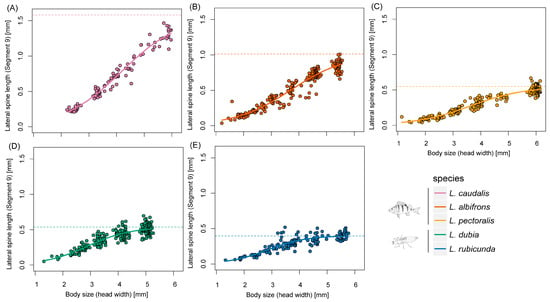
Figure 4.
Scaling relationships of lateral spine length in segment 9 and body size during larval developing time. (A–E) represent different Leucorrhinia species, which are shown with different colors.
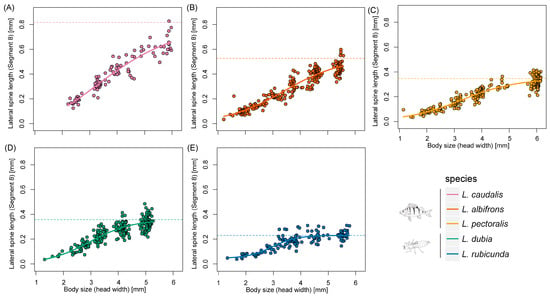
Figure 5.
Scaling relationships of lateral spine length in segment 8 and body size during larval developing time. (A–E) represent different Leucorrhinia species, which are shown with different colors.
According to AIC, the three-parameter logistic model was the most suitable model for the allometric relationship between spines at segment 9 and head width across all species (Table 1). For spines at segment 8, four-parameter logistic model was the most suitable model in L. caudalis and the Gompertz model was best in L. rubicunda, while the other species were all fit with the three-parameter logistic model (Table 1 and Table S2).

Table 1.
AIC values of fitting models for lateral spines in segment 8 and 9 across Leucorrhinia species.
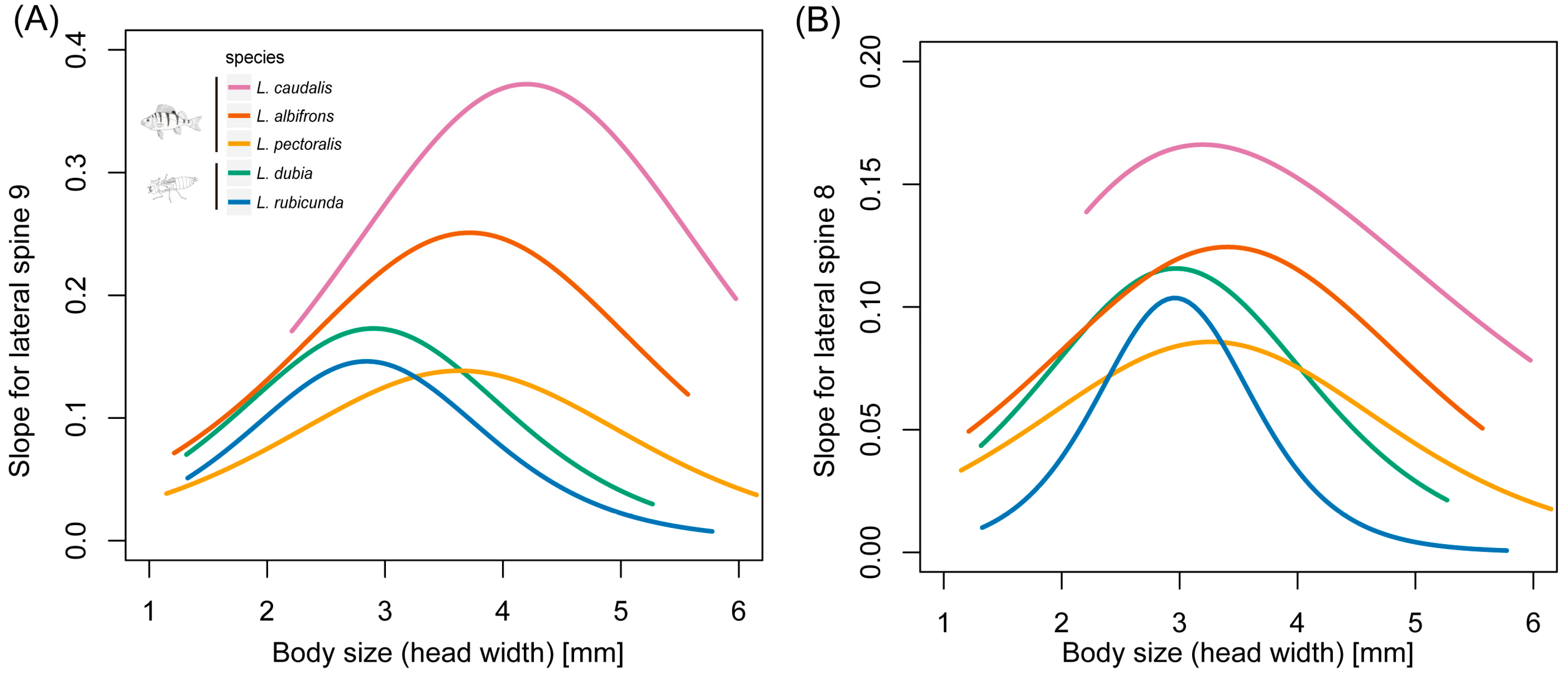
Comparative analyses with PGLS showed that the inflexion point was significantly larger in fish-lake species than invertebrate-lake species in lateral spines at segment 9 (F1,3 = 23.19, p = 0.017) and 8 (F1,3 = 278.63, p < 0.001) (Figure 6, Table S2). The maximum slope for spines at segment 9 (F1,3 = 10.47, p = 0.048) and 8 (F1,3 = 10.58, p = 0.047) was also significantly larger in fish-lake species than invertebrate-lake species.
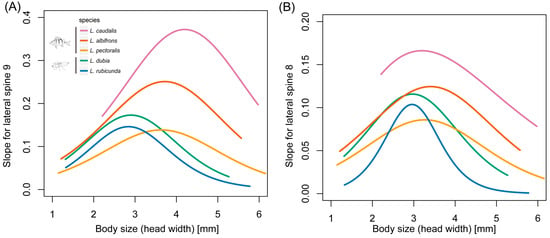
Figure 6.
Slopes of the scaling relationship curves of lateral spine length and body size during larval developing time in 5 Leucorrhinia species. (A) Lateral spines in segment 9 and (B) lateral spines in segment 8.
4. Discussion
Our study demonstrates that predator preferences caused evolutionarily shifts in ontogenetic growth trajectories of antipredator spines with growth of spines in fish-lake species accelerating at a larger body size and higher growth rate than in invertebrate-lake species. Thus, predators do not only select for more or less pronounced antipredator features, but also for a fine-scaled ontogenetic framework of antipredator traits supporting efficient defenses.
Predatory fish are gape-limited predators [45]. Prey that have evolved with large body dimensions can always survive better [46]. Preys enlarge their body dimensions in two ways: either enlarging their body size or growing longer spines. Our results show that fish-lake species evolve to have a larger body size than invertebrate-species, which indicate that large body size might be the important trait against gape-limited fish predators in Leucorrhinia species. The existence of spines could help to enlarge the body dimensions and increase predator handling time [39,47]. In this study, larvae of species from fish lakes exhibited only mildly longer lateral spines than larvae of species from invertebrate lakes. Longer spines in larval Leucorrhinia species from fish lakes have been shown several times [28,31,34]; these studies usually investigated the combined dorsal and lateral abdominal spines. However, in this study, we only measured lateral spines (see methods) and note that the change in dorsal and lateral spine length are positively correlated [33], and selection by predation on defensive spines is stronger in dorsal than in lateral spines [34], explaining why we only had a tendency for longer spines in fish-lake species.
Defensive traits are highly related and evolving as a whole [48]. Former experiments showed that in freshwater snails, behavioral and shape-based defenses were positively correlated [48]. Fish-lake species L. caudalis has the longest spine and a relatively large body size, which could be seen as trait cospecialization (Figure 3, Figure 4 and Figure 5). However, a compensatory relationship between spine length and body size was found in L. pectoralis. L. pectoralis possesses the shortest spine but the largest final body size among fish-lake species. Therefore, even under the same selection pressure from gape-limited fish predators, species can acquire different tactics to achieve large body dimensions.
Fish-lake species accelerated larval spine growth at a larger body size than invertebrate-lake species. This is likely to be evolutionary adaptation. Enlarging body dimension becomes an effective trait in defending against those gape-limited predators [39,49]. Thus, when fish-lake larvae grow into the size-relevant body size for predatory fish, elongated spines are an efficient way to avoid predation by increasing body volume [34]. Further, elongated larval spines in fish-lake species come with a larger final body size in fish-lake species (see results), which in combination increases body volume even further. However, spine length growth was not employed from an early developmental stage, but increased rather rapidly later in life. This might be due to the fact that predatory fish-dominated lakes also keep a low number of invertebrate predators [15,40]. Invertebrate predators prefer smaller prey size than predatory fish [36] and spines facilitate prey capture [35]. Consequently, larval Leucorrhinia in fish lakes should avoid development of long spines at an early stage, when they are not yet in focus by predatory fish, to prevent increased predation by invertebrate predators. Thus, growth differentiation of defensive spine seems strictly synchronized with potential costs and benefits of the defense at different life stages [10].
Spine trait divergence due to predators could stem either from a long evolutionary adaptation or simply from phenotypic plasticity. L. dubia [28] and L. pectoralis [31] could plastically develop long spines in the presence of fish predators. However, because of long-dispersing ability of adults [50], fish-lake species could also lay their eggs in invertebrate lakes, and vice versa. Therefore, local adaptation in response to the current predation habitat seems unlikely. Further, we raised all larvae without any predators’ cues; thus, plasticity within this generation could be excluded. The divergence of spine ontogenetic patterns most probably evolves as a long-term adaptation based on their preferred top predators. Moreover, a close inspection on the cross-generation coevolution of Leucorrhinia larvae and co-existing predators will be greatly helpful in understanding the evolution of ontogenetic patterns of defensive spines. However, although we could tell the presence of predatory fish by sampling in recent years, the detailed information on the historical changes of predator composition in the lakes could not be found. Therefore, much more effort needs to go into clearly unveiling the evolutionary mechanisms of ontogenetic pattern diversification in Leucorrhinia spines.
Overall, our results showed that different predators drive differentiation of ontogenetic growth patterns in an antipredator traits and growth of defensive spines is most pronounced during ontogeny when the potential benefits seem largest. The impacts from predators are most often obvious in the final morphological stage but studying ontogenetic patterns of antipredator traits clearly increases our understanding how selection shapes expression of antipredator traits.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects14080712/s1, Table S1: Models used for fitting scaling relationship between spines (S) and body size (H for head width); Table S2: The head width on the inflexion points (HIP) of the curves and its SE for each spine in Leucorrhinia species.
Author Contributions
Conceptualization, B.J. and D.J.M.; methodology, B.J. and Y.Y.; software, B.J. and Y.Y.; validation, B.J., Y.Y. and D.J.M.; formal analysis, B.J.; investigation, R.M. and D.J.M.; resources, R.M.; data curation, Y.Y.; writing—original draft preparation, B.J.; writing—review and editing, D.J.M.; visualization, Y.Y.; supervision, R.M. and D.J.M.; project administration, R.M.; funding acquisition, B.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Anhui Provincial Natural Science Foundation (Youth project, 2008085QC148), Special Funds for Supporting Innovation and Entrepreneurship for Returned Oversea-students in Anhui Province (2020LCX035), Anhui Provincial Key Laboratory of Molecular Enzymology and Mechanism of Major Diseases in Anhui Normal University (fzmx202007).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Acknowledgments
I would like to thank Jens Rolff and Guozhi Yu for providing valuable comments on the earlier manuscript. I would also like to thank editors and three anonymous reviewers for their valuable comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McPeek, M.A. Determination of Species Composition in the Enallagma damselfly assemblages of permanent lakes. Ecology 1990, 71, 83–98. [Google Scholar] [CrossRef]
- Sheriff, M.J.; Peacor, S.D.; Hawlena, D.; Thaker, M. Non-consumptive predator effects on prey population size: A dearth of evidence. J. Anim. Ecol. 2020, 89, 1302–1316. [Google Scholar] [CrossRef] [PubMed]
- Schluter, D. Evidence for ecological speciation and its alternative. Science 2009, 323, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Benard, M.F. Predator-induced phenotypic plasticity in organisms with complex life histories. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 651–673. [Google Scholar] [CrossRef]
- Mitchell, M.D.; Bairos-Novak, K.R.; Ferrari, M.C.O. Mechanisms underlying the control of responses to predator odours in aquatic prey. J. Exp. Biol. 2017, 220, 1937–1946. [Google Scholar] [CrossRef] [PubMed]
- Lass, S.; Spaak, P. Chemically induced anti-predator defences in plankton: A review. Hydrobiologia 2003, 491, 221–239. [Google Scholar] [CrossRef]
- Tollrian, R.; Harvell, C.D. The ecology and Evolution of Inducible Defenses; Princeton University Press: Princeton, NJ, USA, 1999. [Google Scholar]
- Jiang, B.; Mikolajewski, D.J. Shift in predation regime mediates diversification of foraging behaviour in a dragonfly genus. Ecol. Èntomol. 2018, 43, 525–533. [Google Scholar] [CrossRef]
- Hoverman, J.T.; Relyea, R.A. How flexible is phenotypic plasticity? developmental windows for trait induction and reversal. Ecology 2007, 88, 693–705. [Google Scholar] [CrossRef]
- West-Eberhard, M.J. Developmental Plasticity and Evolution; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Arnqvist, G.; Johansson, F. Ontogenetic reaction norms of predator-induced defensive morphology in dragonfly larvae. Ecology 1998, 79, 1847–1858. [Google Scholar] [CrossRef]
- Kishida, O.; Trussell, G.C.; Mougi, A.; Nishimura, K. Evolutionary ecology of inducible morphological plasticity in predator–prey interaction: Toward the practical links with population ecology. Popul. Ecol. 2009, 52, 37–46. [Google Scholar] [CrossRef]
- Boege, K.; Marquis, R.J. Facing herbivory as you grow up: The ontogeny of resistance in plants. Trends Ecol. Evol. 2005, 20, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Sun, Y.; Wei, J.; Gu, L.; Zhang, L.; Yang, Z.; Huang, Y. Toxic Microcystis aeruginosa alters the resource allocation in Daphnia mitsukuri responding to fish predation cues. Environ. Pollut. 2021, 278, 116918. [Google Scholar] [CrossRef]
- Alberch, P.; Gould, S.J.; Oster, G.F.; Wake, D.B. Size and shape in ontogeny and phylogeny. Paleobiology 1979, 5, 296–317. [Google Scholar] [CrossRef]
- Wellborn, G.A.; Skelly, D.K.; Werner, E.E. Mechanisms creating community structure across a freshwater habitat gradient. Annu. Rev. Ecol. Syst. 1996, 27, 337–363. [Google Scholar] [CrossRef]
- Relyea, R.A. The lasting effects of adaptive plasticity: Predator-induced tadpoles become long-legged frogs. Ecology 2001, 82, 1947–1955. [Google Scholar] [CrossRef]
- Dahl, J.; Peckarsky, B.L. Induced morphological defenses in the wild: Predator effects on a mayfly, Drunella Coloradensis. Ecology 2002, 83, 1620–1634. [Google Scholar] [CrossRef]
- Nijhout, H.F.; German, R.Z. Developmental causes of allometry: New models and implications for phenotypic plasticity and evolution. Integr. Comp. Biol. 2012, 52, 43–52. [Google Scholar] [CrossRef]
- Mikolajewski, D.J.; Scharnweber, K.; Jiang, B.; Leicht, S.; Mauersberger, R.; Johansson, F. Changing the habitat: The evolution of intercorrelated traits to escape from predators. J. Evol. Biol. 2016, 29, 1394–1405. [Google Scholar] [CrossRef]
- Salmon, M.; Scholl, J. Allometric growth in juvenile marine turtles: Possible role as an antipredator adaptation. Zoology 2014, 117, 131–138. [Google Scholar] [CrossRef]
- Loy, A.; Bertelletti, M.; Costa, C.; Ferlin, L.; Cataudella, S. Shape changes and growth trajectories in the early stages of three species of the genus Diplodus (Perciformes, Sparidae). J. Morphol. 2001, 250, 24–33. [Google Scholar] [CrossRef]
- Schroeder, L.; Huber, R. Fight strategies differ with size and allometric growth of claws in crayfish, Orconectes rusticus. Behaviour 2001, 138, 1437–1449. [Google Scholar] [CrossRef]
- Murphy, S.M.; Leahy, S.M.; Williams, L.S.; Lill, J.T. Stinging spines protect slug caterpillars (Limacodidae) from multiple generalist predators. Behav. Ecol. 2009, 21, 153–160. [Google Scholar] [CrossRef]
- Vamosi, S.M.; Schluter, D. Character shifts in the defensive armor of sympatric sticklebacks. Evolution 2004, 58, 376–385. [Google Scholar]
- Laske, S.M.; Rosenberger, A.E.; Kane, W.J.; Wipfli, M.S.; Zimmerman, C.E. Top-down control of invertebrates by Ninespine Stickleback in Arctic ponds. Freshw. Sci. 2017, 36, 124–137. [Google Scholar] [CrossRef]
- Zhang, H.; Hollander, J.; Hansson, L.-A. Bi-directional plasticity: Rotifer prey adjust spine length to different predator regimes. Sci. Rep. 2017, 7, 10254. [Google Scholar] [CrossRef]
- Johansson, F.; Mikolajewski, D.J. Evolution of Morphological Defences, in Dragonflies and Damselflies: Model Organisms for Ecological and Evolutionary Research; Cordoba-Aguilar, A., Ed.; Oxford University Press Inc.: New York, NY, USA, 2008; pp. 127–139. [Google Scholar]
- Mikolajewski, D.J.; Johansson, F. Morphological and behavioral defenses in dragonfly larvae: Trait compensation and cospecialization. Behav. Ecol. 2004, 15, 614–620. [Google Scholar] [CrossRef]
- Šigutová, H.; Šigut, M.; Dolný, A. Phenotypic plasticity in specialists: How long-spined larval Sympetrum depressiusculum (Odonata: Libellulidae) responds to combined predator cues. PLoS ONE 2018, 13, e0201406. [Google Scholar] [CrossRef]
- Fleck, G.; Brenk, M.; Misof, B. Larval and molecular characters help to solve phylogenetic puzzles in the highly diverse dragonfly family Libellulidae (Insecta: Odonata: Anisoptera): The Tetrathemistinae are a polyphyletic group. Org. Divers. Evol. 2008, 8, 1–16. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Y.; Mauersberger, R.; Schiel, F.; Mikolajewski, D.J. Predation promotes diversification in the mean and variance of antipredator traits. Ecol. Èntomol. 2022, 47, 679–688. [Google Scholar] [CrossRef]
- Hovmöller, R.; Johansson, F. A phylogenetic perspective on larval spine morphology in Leucorrhinia (Odonata: Libellulidae) based on ITS1, 5.8S, and ITS2 rDNA sequences. Mol. Phylogenetics Evol. 2004, 30, 653–662. [Google Scholar] [CrossRef]
- Petrin, Z.; Schilling, E.G.; Loftin, C.S.; Johansson, F. Predators shape distribution and promote diversification of morphological defenses in Leucorrhinia, Odonata. Evol. Ecol. 2010, 24, 1003–1016. [Google Scholar] [CrossRef]
- Mikolajewski, D.J.; Rolff, J. Benefits of morphological defence demonstrated by direct manipulation in larval dragonflies. Evol. Ecol. Res. 2004, 6, 619–626. [Google Scholar]
- Mikolajewski, D.J.; Johansson, F.; Wohlfahrt, B.; Stoks, R. Invertebrate predation selects for the loss of a morphological antipredator trait. Evolution 2006, 60, 1306–1310. [Google Scholar]
- Warren, P.H.; Lawton, J.H. Invertebrate predator-prey body size relationships: An explanation for upper triangular food webs and patterns in food web structure? Oecologia 1987, 74, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Walls, M.; Ketola, M. Effects of predator-induced spines on individual fitness in Daphnia pulex. Limnol. Oceanogr. 1989, 34, 390–396. [Google Scholar] [CrossRef]
- Gill, A.B. The dynamics of prey choice in fish: The importance of prey size and satiation. J. Fish Biol. 2003, 63, 105–116. [Google Scholar] [CrossRef]
- Price, S.A.; Friedman, S.T.; Wainwright, P.C. How predation shaped fish: The impact of fin spines on body form evolution across teleosts. Proc. R. Soc. B: Boil. Sci. 2015, 282, 20151428. [Google Scholar] [CrossRef]
- Benke, A.C. A Method for comparing individual growth Rates of Aquatic Insects with Special Reference to the Odonata. Ecology 1970, 51, 328–331. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: http://www.R-project.org (accessed on 21 April 2023).
- Orme, D. The Caper Package: Comparative Analysis of Phylogenetics and Evolution in R; R Development Core Team, 2013. [Google Scholar]
- Paine, C.E.T.; Marthews, T.R.; Vogt, D.R.; Purves, D.; Rees, M.; Hector, A.; Turnbull, L.A. How to fit nonlinear plant growth models and calculate growth rates: An update for ecologists. Methods Ecol. Evol. 2011, 3, 245–256. [Google Scholar] [CrossRef]
- Hambright, K.D. Experimental analysis of prey selection by largemouth bass: Role of predator mouth width and prey body depth. Trans. Am. Fish. Soc. 1991, 120, 500–508. [Google Scholar] [CrossRef]
- Moodie, G.E.E. Predation, natural selection and adaptation in an unusual threespine stickleback. Heredity 1972, 28, 155–167. [Google Scholar] [CrossRef]
- Nilsson, P.A.; Brönmark, C. Prey vulnerability to a gape-size limited predator: Behavioural and morphological impacts on northern pike piscivory. Oikos 2000, 88, 539–546. [Google Scholar] [CrossRef]
- Dewitt, T.J.; Sih, A.; Hucko, J.A. Trait compensation and cospecialization in a freshwater snail: Size, shape and antipredator behaviour. Anim. Behav. 1999, 58, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Relyea, R.A. Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology 2001, 82, 523–540. [Google Scholar] [CrossRef]
- McCauley, S.J. The effects of dispersal and recruitment limitation on community structure of odonates in artificial ponds. Ecography 2006, 29, 585–595. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).