A New Approach for Detecting Sublethal Effects of Neonicotinoids on Bumblebees Using Optical Sensor Technology
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Handling of Bumblebees
2.2. Pesticide Preparation
2.3. Flight Cages with Sensor Setup and Flight Recording
2.4. Acute Exposure Experiment
2.5. Chronic Exposure Experiment
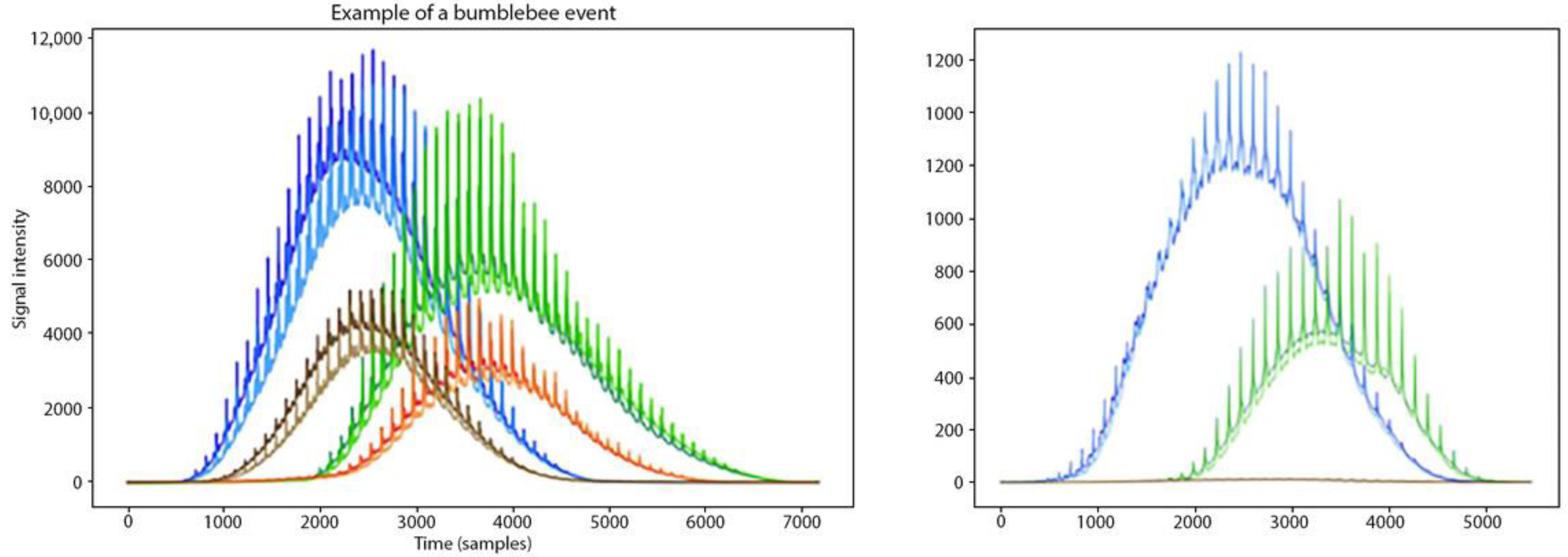
2.6. Data Analysis
3. Results
3.1. Acute Exposure Experiment
3.2. Chronic Exposure Experiment
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Williams, P.H. An annotated checklist of bumble bees with an analysis of patterns of description (Hymenoptera: Apidae, Bombini). Bull.-Nat. Hist. Mus. Entomol. Ser. 1998, 67, 79–152. [Google Scholar]
- Goulson, D. Bumblebees: Their Behaviour and Ecology; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Corbet, S.A.; Williams, I.H.; Osborne, J.L. Bees and the Pollination of Crops and Wild Flowers in the European Community. Bee World 2015, 72, 47–59. [Google Scholar] [CrossRef]
- Kleijn, D.; Winfree, R.; Bartomeus, I.; Carvalheiro, L.G.; Henry, M.; Isaacs, R.; Klein, A.-M.; Kremen, C.; M′Gonigle, L.K.; Rader, R.; et al. Delivery of crop pollination services is an insufficient argument for wild pollinator conservation. Nat. Commun. 2015, 6, 7414. [Google Scholar] [CrossRef] [PubMed]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Dicks, L.; Breeze, T.; Ngo, H.; Senapathi, D.; An, J.; Aizen, M.; Basu, P.; Buchori, D.; Galetto, L.; Garibaldi, L.; et al. A global assessment of drivers and risks associated with pollinator decline. Nat. Ecol. Evol. 2021, 5, 1453–1461. [Google Scholar] [CrossRef]
- Medrzycki, P.; Giffard, H.; Aupinel, P.; Belzunces, L.P.; Chauzat, M.P.; Claßen, C.; Colin, M.E.; Dupont, T.; Girolami, V.; Johnson, R.; et al. Standard methods for toxicology research in Apis mellifera. J. Apic. Res. 2013, 52, 1–60. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development (OECD). OECD Guidelines for the Testing of Chemicals Honeybees, Acute Contact Toxicity Test 214. OECD/OCDE 1998, 21, 1–7. [Google Scholar] [CrossRef]
- Sampson, B.; Gregorc, A.; Alburaki, M.; Werle, C.; Karim, S.; Adamczyk, J.; Knight, P. Sensitivity to imidacloprid insecticide varies among some social and solitary bee species of agricultural value. PLoS ONE 2023, 18, e0285167. [Google Scholar] [CrossRef]
- Cresswell, J.E.; Robert, F.X.L.; Florance, H.; Smirnoff, N. Clearance of ingested neonicotinoid pesticide (imidacloprid) in honey bees (Apis mellifera) and bumblebees (Bombus terrestris). Pest Manag. Sci. 2014, 70, 332–337. [Google Scholar] [CrossRef]
- Rundlöf, M.; Andersson, G.K.S.; Bommarco, R.; Fries, I.; Hederström, V.; Herbertsson, L.; Jonsson, O.; Klatt, B.K.; Pedersen, T.R.; Yourstone, J.; et al. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 2015, 521, 77–80. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development (OECD). Test No. 246: Bumblebees, Acute Contact Toxicity Test, OECD Guidelines for the Testing of Chemicals. OECD/OCDE 2017, 246, 1–11. [Google Scholar] [CrossRef]
- Decourtye, A.; Devillers, J. Ecotoxicity of Neonicotinoid Insecticides to Bees. Adv. Exp. Med. Biol. 2010, 683, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.J.; Baldock, K.C.R.; Brown, M.J.F.; Cresswell, J.E.; Dicks, L.V.; Fountain, M.T.; Garratt, M.P.; Gough, L.A.; Heard, M.S.; Holland, J.M.; et al. Protecting an Ecosystem Service: Approaches to Understanding and Mitigating Threats to Wild Insect Pollinators. Adv. Ecol. Res. 2016, 54, 135–206. [Google Scholar] [CrossRef]
- Goulson, D. Neonicotinoids and bees: What’s all the buzz? Significance 2013, 10, 6–11. [Google Scholar] [CrossRef]
- Tasei, J.N.; Lerin, J.; Ripault, G. Sub-lethal effects of imidacloprid on bumblebees, Bombus terrestris (Hymenoptera: Apidae), during a laboratory feeding test 2000. Pest Manag. Sci. 2000, 56, 784–788. [Google Scholar] [CrossRef]
- Tomizawa, M.; Casida, J.E. Selective Toxicity of Neonicotinoids Attributable to Specificity of Insect and Mammalian Nicotinic Receptors. Annu. Rev. Entomol. 2003, 48, 339–364. [Google Scholar] [CrossRef]
- Hopwood, J.; Vaughan, M.; Shepherd, M.; Biddinger, D.; Mader, E.; Black, S.H.; Mazzacano, C. Are neonicotinoids killing bees. In A Review of Research into the Effects of Neonicotinoid Insecticides on Bees, with Recommendations for Action; Xerces Society for Invertebrate Conservation: Portland, OR, USA, 2012. [Google Scholar]
- Botías, C.; David, A.; Horwood, J.; Abdul-Sada, A.; Nicholls, E.; Hill, E.; Goulson, D. Neonicotinoid Residues in Wildflowers, a Potential Route of Chronic Exposure for Bees. Environ. Sci. Technol. 2015, 49, 12731–12740. [Google Scholar] [CrossRef]
- Henry, M.; Béguin, M.; Requier, F.; Rollin, O.; Odoux, J.F.; Aupinel, P.; Aptel, J.; Tchamitchian, S.; Decourtye, A. A common pesticide decreases foraging success and survival in honey bees. Science 2012, 336, 348–350. [Google Scholar] [CrossRef]
- Schneider, C.W.; Tautz, J.; Grünewald, B.; Fuchs, S. RFID Tracking of Sublethal Effects of Two Neonicotinoid Insecticides on the Foraging Behavior of Apis mellifera. PLoS ONE 2012, 7, e30023. [Google Scholar] [CrossRef]
- Whitehorn, P.R.; O’Connor, S.; Wackers, F.L.; Goulson, D. Neonicotinoid Pesticide Reduces Bumble Bee Colony Growth and Queen Production. Science 2012, 336, 351–352. [Google Scholar] [CrossRef]
- APANET. Effects of Coated Maize Seed on Honeybees Report Based on Results Obtained From the Third Year Activity of the APENET Project; CRA-API: Bologna, Italy, 2011; Available online: http://www.reterurale.it/apenet (accessed on 1 May 2023).
- Carreck, N.L. A beekeeper’s perspective on the neonicotinoid ban. Pest Manag. Sci. 2017, 73, 1295–1298. [Google Scholar] [CrossRef]
- Schmuck, R. No causal relationship between Gaucho® seed dressing sunflowers and the French bee syndrome. Pflanzenschutz Nachrichten-Bayer-Engl. Ed. 1999, 52, 257–299. [Google Scholar]
- Regulation (EU) No 485/2013. Commission Implementing Regulation (EU) No 485/2013 of 24 May 2013 amending Implementing Regulation (EU) No 540/2011, as Regards the Conditions of Approval of the Active Substances Clothianidin, Thiamethoxam and Imidacloprid, and Prohibiting the Use and Sale of Seeds Treated with Plant Protection Products Containing Those Active Substances. 2013. Available online: http://data.europa.eu/eli/reg_impl/2013/485/oj (accessed on 1 May 2023).
- Regulation (EU) 2018/783. Commission Implementing Regulation (EU) 2018/783 of 29 May 2018 Amending Implementing Regulation (EU) No 540/2011 as Regards the Conditions of Approval of the Active Substance Imidacloprid. 2018. Available online: http://data.europa.eu/eli/reg_impl/2018/783/oj (accessed on 1 May 2023).
- Nicholls, E.; Botías, C.; Rotheray, E.L.; Whitehorn, P.; David, A.; Fowler, R.; David, T.; Feltham, H.; Swain, J.L.; Wells, P.; et al. Monitoring neonicotinoid exposure for bees in rural and peri-urban areas of the UK during the transition from pre-to post-moratorium. Environ. Sci. Technol. 2018, 52, 9391–9402. [Google Scholar] [CrossRef] [PubMed]
- Herbertsson, L.; Jonsson, O.; Kreuger, J.; Smith, H.G.; Rundlöf, M. Scientific note: Imidacloprid found in wild plants downstream permanent greenhouses in Sweden. Apidologie 2021, 52, 946–949. [Google Scholar] [CrossRef]
- Noleppa, S.; Cartsburg, M. Banning neonicotinoids in the European Union An ex-post assessment of economic and environmental costs. Banning neonicotinoids in the European Union: An Ex-Post Evaluation and Ex-Ante Assessment Considering the “Farm to Fork” and “Biodiversity” Strategies; HFFA Research GmbH: Berlin, Germany, 2021. [Google Scholar]
- LOI n° 2020-1578. LOI n° 2020-1578 du 14 Décembre 2020 Relative aux Conditions de Mise sur le Marché de Certains Produits Phytopharmaceutiques en Cas de Danger Sanitaire Pour les Betteraves Sucrières (1)-Légifrance n.d. Available online: https://www.legifrance.gouv.fr/jorf/id/JORFTEXT000042665456 (accessed on 11 October 2021).
- Camp, A.A.; Lehmann, D.M. Impacts of Neonicotinoids on the Bumble Bees Bombus terrestris and Bombus impatiens Examined through the Lens of an Adverse Outcome Pathway Framework. Environ. Toxicol. Chem. 2021, 40, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Bonmatin, J.M.; Moineau, I.; Charvet, R.; Fleche, C.; Colin, M.E.; Bengsch, E.R. LC/APCI-MS/MS Method for Analysis of Imidacloprid in Soils, in Plants, and in Pollens. Anal. Chem. 2003, 75, 2027–2033. [Google Scholar] [CrossRef]
- Stoner, K.A.; Eitzer, B.D. Movement of soil-applied imidacloprid and thiamethoxam into nectar and pollen of squash (Cucurbita pepo). PLoS ONE 2012, 7, e39114. [Google Scholar] [CrossRef]
- Switzer, C.M.; Combes, S.A. The neonicotinoid pesticide, imidacloprid, affects Bombus impatiens (bumblebee) sonication behavior when consumed at doses below the LD50. Ecotoxicology 2016, 25, 1150–1159. [Google Scholar] [CrossRef]
- Colgan, T.J.; Fletcher, I.K.; Arce, A.N.; Gill, R.J.; Rodrigues, A.R.; Stolle, E.; Chittka, L.; Wurm, Y. Caste- and pesticide-specific effects of neonicotinoid pesticide exposure on gene expression in bumblebees. Mol. Ecol. 2019, 28, 1964–1974. [Google Scholar] [CrossRef]
- Potts, R.; Clarke, R.M.; Oldfield, S.E.; Wood, L.K.; de Ibarra, N.H.; Cresswell, J.E. The effect of dietary neonicotinoid pesticides on non-flight thermogenesis in worker bumble bees (Bombus terrestris). J. Insect Physiol. 2018, 104, 33–39. [Google Scholar] [CrossRef]
- Martín-Blázquez, R.; Calhoun, A.C.; Sadd, B.M.; Cameron, S.A. Gene expression in bumble bee larvae differs qualitatively between high and low concentration imidacloprid exposure levels. Sci. Rep. 2023, 13, 9415. [Google Scholar] [CrossRef] [PubMed]
- Feltham, H.; Park, K.; Goulson, D. Field realistic doses of pesticide imidacloprid reduce bumblebee pollen foraging efficiency. Ecotoxicology 2014, 23, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.J.; Raine, N.E. Chronic impairment of bumblebee natural foraging behaviour induced by sublethal pesticide exposure. Funct. Ecol. 2014, 28, 1459–1471. [Google Scholar] [CrossRef]
- Kenna, D.; Cooley, H.; Pretelli, I.; Rodrigues, A.R.; Gill, S.D.; Gill, R.J. Pesticide exposure affects flight dynamics and reduces flight endurance in bumblebees. Ecol. Evol. 2019, 9, 5637. [Google Scholar] [CrossRef] [PubMed]
- Siviter, H.; Johnson, A.K.; Muth, F. Bumblebees Exposed to a Neonicotinoid Pesticide Make Suboptimal Foraging Decisions. Environ. Entomol. 2021, 50, 1299–1303. [Google Scholar] [CrossRef]
- Crall, J.D.; Switzer, C.M.; Oppenheimer, R.L.; Ford Versypt, A.N.; Dey, B.; Brown, A.; Eyster, M.; Guérin, C.; Pierce, N.E.; Combes, S.A.; et al. Neonicotinoid exposure disrupts bumblebee nest behavior, social networks, and thermoregulation. Science 2018, 362, 683–686. [Google Scholar] [CrossRef]
- Gill, R.J.; Ramos-Rodriguez, O.; Raine, N.E. Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature 2012, 491, 105–108. [Google Scholar] [CrossRef]
- Stanley, D.A.; Russell, A.L.; Morrison, S.J.; Rogers, C.; Raine, N.E. Investigating the impacts of field-realistic exposure to a neonicotinoid pesticide on bumblebee foraging, homing ability and colony growth. J. Appl. Ecol. 2016, 53, 1440–1449. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Blacquière, T.; Field, L.M.; Hails, R.S.; Petrokofsky, G.; Potts, S.G.; Raine, N.E.; Vanbergen, A.J.; McLean, A.R. A restatement of the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140558. [Google Scholar] [CrossRef]
- Fischer, D.; Lp, C.; Moriarty, T. Pesticide Risk Assessment for Pollinators: Summary of a SETAC Pellston Workshop Summary of the SETAC Pellston Workshop on Pesticide Risk Assessment for Pollinators; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Grootaert, P.; Pollet, M.; Dekoninck, W.; Van Achterberg, C. Chapter 15 Sampling Insects: General Techniques, Strategies and Remarks; ABC Taxa: Brussels, Belgium, 2010; pp. 377–399. [Google Scholar]
- Phung, Q.V.; Ahmad, I.; Habibi, D.; Hinckley, S. Automated Insect Detection Using Acoustic Features Based on Sound Generated from Insect Activities. Acoust. Aust. 2017, 45, 445–451. [Google Scholar] [CrossRef]
- Potamitis, I.; Rigakis, I.; Fysarakis, K. The Electronic McPhail Trap. Sensors 2014, 14, 22285–22299. [Google Scholar] [CrossRef] [PubMed]
- Preti, M.; Verheggen, F.; Angeli, S. Insect pest monitoring with camera-equipped traps: Strengths and limitations. J. Pest Sci. 2021, 94, 203–217. [Google Scholar] [CrossRef]
- Drake, V.A.; Hatty, S.; Symons, C.; Wang, H. Insect Monitoring Radar: Maximizing Performance and Utility. Remote Sens. 2020, 12, 596. [Google Scholar] [CrossRef]
- Osborne, J.L.; Clark, S.J.; Morris, R.J.; Williams, I.H.; Riley, J.R.; Smith, A.D.; Reynolds, D.; Edwards, A. A landscape-scale study of bumble bee foraging range and constancy, using harmonic radar. J. Appl. Ecol. 1999, 36, 519–533. [Google Scholar] [CrossRef]
- Jansson, S. Entomological Lidar Target Characterization and Field Applications. Ph.D. Thesis, Department of Physics, Lund University, Lund, Sweden, 2020. [Google Scholar]
- Rydhmer, K.; Bick, E.; Still, L.; Strand, A.; Luciano, R.; Helmreich, S.; Beck, B.D.; Grønne, C.; Malmros, L.; Poulsen, K.; et al. Automating insect monitoring using unsupervised near-infrared sensors. Sci. Rep. 2022, 12, 2603. [Google Scholar] [CrossRef]
- Gebru, A.; Jansson, S.; Ignell, R.; Kirkeby, C.; Prangsma, J.C.; Brydegaard, M. Multiband modulation spectroscopy for the determination of sex and species of mosquitoes in flight. J. Biophotonics 2018, 11, e201800014. [Google Scholar] [CrossRef]
- Blacquière, T.; Smagghe, G.; van Gestel, C.; Mommaerts, V. Neonicotinoids in bees: A review on concentrations, side-effects and risk assessment. Ecotoxicology 2012, 21, 973–992. [Google Scholar] [CrossRef]
- Hedenström, A.; Ellington, C.P.; Wolf, T.J. Wing wear, aerodynamics and flight energetics in bumblebees (Bombus terrestris): An experimental study. Funct. Ecol. 2001, 15, 417–422. [Google Scholar] [CrossRef]
- Soliman, M. Effects of uv-light, temperature and storage on the stability and biological effectiveness of some insecticides. J. Plant Prot. Res. 2012, 52, 275–280. [Google Scholar] [CrossRef]
- Farnworth, E.G. Effects of ambient temperature, humidity, and age on wing-beat frequency of Periplaneta species. J. Insect Physiol. 1972, 18, 827–839. [Google Scholar] [CrossRef]
- Domingos, P. A few useful things to know about machine learning. J. Mach. Learn. Res. 2012, 55, 78–87. [Google Scholar] [CrossRef]
- van Roy, J.; De Baerdemaeker, J.; Saeys, W.; De Ketelaere, B. Optical identification of bumblebee species: Effect of morphology on wingbeat frequency. Comput. Electron. Agric. 2014, 109, 94–100. [Google Scholar] [CrossRef]
- Cunningham, P.; Cord, M.; Delany, S.J. Supervised Learning. In Cognitive Technologies; Springer: Berlin/Heidelberg, Germany, 2008; pp. 21–49. [Google Scholar] [CrossRef]
- Sokolova, M.; Lapalme, G. A systematic analysis of performance measures for classification tasks. Inf. Process. Manag. 2009, 45, 427–437. [Google Scholar] [CrossRef]
- Amershi, S.; Chickering, M.; Drucker, S.M.; Lee, B.; Simard, P.; Suh, J. Modeltracker: Redesigning performance analysis tools for machine learning. In Proceedings of the 33rd Annual ACM Conference on Human Factors in Computing Systems, Seoul, Republic of Korea, 18–23 April 2015; pp. 337–346. [Google Scholar] [CrossRef]
- Van Der Maaten, L.; Hinton, G. Visualizing Data using t-SNE. J. Mach. Learn. Res. 2008, 9, 2579–2605. [Google Scholar]
- Stanley, D.A.; Smith, K.E.; Raine, N.E. Bumblebee learning and memory is impaired by chronic exposure to a neonicotinoid pesticide. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef]
- Bernardes, R.C.; Botina, L.L.; dos Araújo, R.S.; Guedes, R.N.C.; Martins, G.F.; Lima, M.A.P. Artificial Intelligence-Aided Meta-Analysis of Toxicological Assessment of Agrochemicals in Bees. Front. Ecol. Evol. 2022, 10, 425. [Google Scholar] [CrossRef]
- Tosi, S.; Burgio, G.; Nieh, J.C. A common neonicotinoid pesticide, thiamethoxam, impairs honey bee flight ability. Sci. Rep. 2017, 7, 1201. [Google Scholar] [CrossRef] [PubMed]
- Alkassab, A.T.; Kirchner, W.H. Sublethal exposure to neonicotinoids and related side effects on insect pollinators: Honeybees, bumblebees, and solitary bees. J. Plant Dis. Prot. 2017, 124, 1–30. [Google Scholar] [CrossRef]
- Abati, R.; Sampaio, A.R.; Maciel, R.M.A.; Colombo, F.C.; Libardoni, G.; Battisti, L.; Lozano, E.R.; Ghisi, N.D.C.; Costa-Maia, F.M.; Potrich, M. Bees and pesticides: The research impact and scientometrics relations. Environ. Sci. Pollut. Res. 2021, 28, 32282–32298. [Google Scholar] [CrossRef]
- More, S.J.; Auteri, D.; Rortais, A.; Pagani, S. EFSA is working to protect bees and shape the future of environmental risk assessment. EFSA J. 2021, 19, e190101. [Google Scholar] [CrossRef]
- Tosi, S.; Sfeir, C.; Carnesecchi, E.; vanEngelsdorp, D.; Chauzat, M.P. Lethal, sublethal, and combined effects of pesticides on bees: A meta-analysis and new risk assessment tools. Sci. Total Environ. 2022, 844, 156857. [Google Scholar] [CrossRef] [PubMed]
- Simon-Delso, N.; San Martin, G.; Bruneau, E.; Delcourt, C.; Hautier, L. The challenges of predicting pesticide exposure of honey bees at landscape level. Sci. Rep. 2017, 7, 3801. [Google Scholar] [CrossRef] [PubMed]
- Como, F.; Carnesecchi, E.; Volani, S.; Dorne, J.L.; Richardson, J.; Bassan, A.; Pavan, M.; Benfenati, E. Predicting acute contact toxicity of pesticides in honeybees (Apis mellifera) through a k-nearest neighbor model. Chemosphere 2017, 166, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Garber, K.; DeGrandi-Hoffman, G.; Curry, R.; Minucci, J.M.; Dawson, D.E.; Douglass, C.; Milone, J.P.; Purucker, S.T. Simulating the Effects of Pesticides on Honey Bee (Apis mellifera L.) Colonies with BeePop+. Ecologies 2022, 3, 275–291. [Google Scholar] [CrossRef]
- Barascou, L.; Godeau, U.; Pioz, M.; Martin, O.; Sené, D.; Crauser, D.; Le Conte, Y.; Alaux, C. Real-time monitoring of honeybee colony daily activity and bee loss rates can highlight the risk posed by a pesticide. Sci. Total Environ. 2023, 886, 163928. [Google Scholar] [CrossRef] [PubMed]
- Ingram, E.M.; Augustin, J.; Ellis, M.D.; Siegfried, B.D. Evaluating sub-lethal effects of orchard-applied pyrethroids using video-tracking software to quantify honey bee behaviors. Chemosphere 2015, 135, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Rigakis, I.; Potamitis, I.; Tatlas, N.A.; Psirofonia, G.; Tzagaraki, E.; Alissandrakis, E. A Low-Cost, Low-Power, Multisensory Device and Multivariable Time Series Prediction for Beehive Health Monitoring. Sensors 2023, 23, 1407. [Google Scholar] [CrossRef]
- Decourtye, A.; Devillers, J.; Aupinel, P.; Brun, F.; Bagnis, C.; Fourrier, J.; Gauthier, M. Honeybee tracking with microchips: A new methodology to measure the effects of pesticides. Ecotoxicology 2011, 20, 429–437. [Google Scholar] [CrossRef]
- Carreck, N.L.; Ratnieks, F.L. The dose makes the poison: Have “field realistic” rates of exposure of bees to neonicotinoid insecticides been overestimated in laboratory studies? J. Apic. Res. 2014, 53, 607–614. [Google Scholar] [CrossRef]
- Henry, M.; Cerrutti, N.; Aupinel, P.; Decourtye, A.; Gayrard, M.; Odoux, J.F.; Pissard, A.; Rüger, C.; Bretagnolle, V. Reconciling laboratory and field assessments of neonicotinoid toxicity to honeybees. Proc. R. Soc. B Biol. Sci. 2015, 282, 20152110. [Google Scholar] [CrossRef]
- Stanley, J.; Sah, K.; Jain, S.K.; Bhatt, J.C.; Sushil, S.N. Evaluation of pesticide toxicity at their field recommended doses to honeybees, Apis cerana and A. mellifera through laboratory, semi-field and field studies. Chemosphere 2015, 119, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Stoner, K.A. Current Pesticide Risk Assessment Protocols Do Not Adequately Address Differences between Honey Bees (Apis mellifera) and Bumble Bees (Bombus spp.). Front. Environ. Sci. 2016, 4, 79. [Google Scholar] [CrossRef]






| Repetition | Treatment | Number of Insect Events | Number of Data Points |
|---|---|---|---|
| 1 | imidacloprid-exposed | 6799 | 41,047 |
| control | 8449 | 50,855 | |
| 2 | imidacloprid-exposed | 15,399 | 84,587 |
| control | 8570 | 47,487 | |
| 3 | imidacloprid-exposed | 7319 | 59,350 |
| control | 8548 | 50,676 |
| Repetition | Treatment | Number of Insect Events | Number of Data Points |
|---|---|---|---|
| 1 | imidacloprid-exposed | 2057 | 11,789 |
| control | 3712 | 24,224 | |
| 2 | imidacloprid-exposed | 2523 | 16,373 |
| control | 3597 | 22,554 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzaki, V.; Montoro, M.; El-Rashid, R.; Jensen, A.B.; Lecocq, A. A New Approach for Detecting Sublethal Effects of Neonicotinoids on Bumblebees Using Optical Sensor Technology. Insects 2023, 14, 713. https://doi.org/10.3390/insects14080713
Chatzaki V, Montoro M, El-Rashid R, Jensen AB, Lecocq A. A New Approach for Detecting Sublethal Effects of Neonicotinoids on Bumblebees Using Optical Sensor Technology. Insects. 2023; 14(8):713. https://doi.org/10.3390/insects14080713
Chicago/Turabian StyleChatzaki, Vasileia, Marta Montoro, Rámi El-Rashid, Annette Bruun Jensen, and Antoine Lecocq. 2023. "A New Approach for Detecting Sublethal Effects of Neonicotinoids on Bumblebees Using Optical Sensor Technology" Insects 14, no. 8: 713. https://doi.org/10.3390/insects14080713
APA StyleChatzaki, V., Montoro, M., El-Rashid, R., Jensen, A. B., & Lecocq, A. (2023). A New Approach for Detecting Sublethal Effects of Neonicotinoids on Bumblebees Using Optical Sensor Technology. Insects, 14(8), 713. https://doi.org/10.3390/insects14080713







