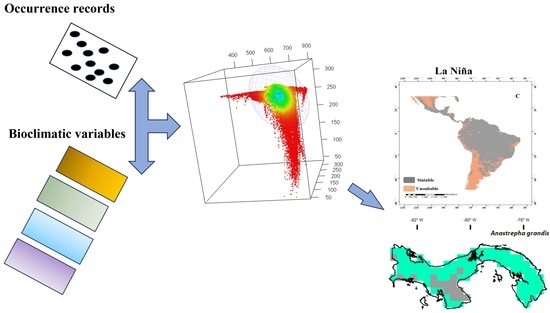
Evaluation of the Effect of the ENSO Cycle on the Distribution Potential of the Genus Anastrepha of Horticultural Importance in the Neotropics and Panama
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
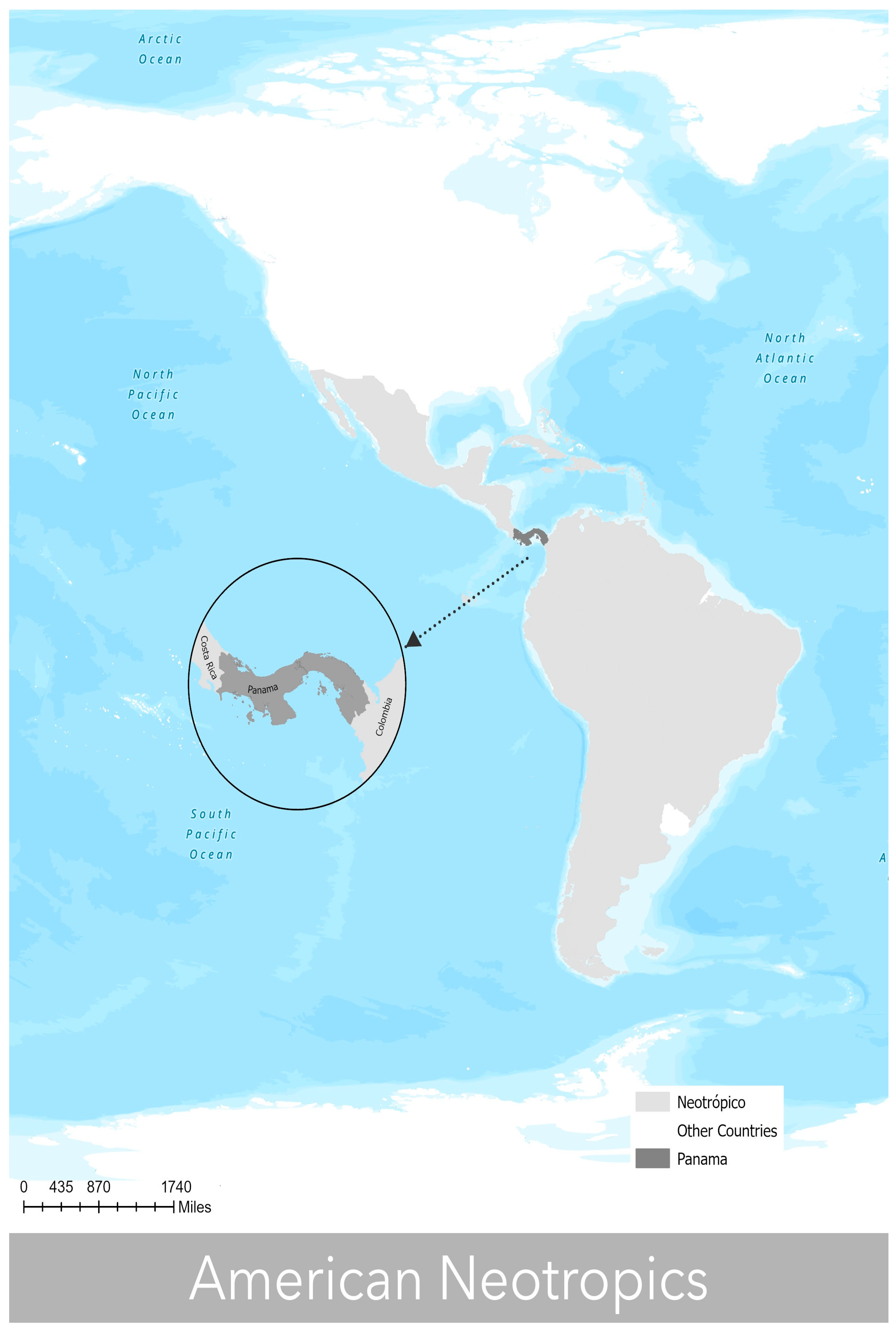
2.1. Study Area
2.2. Presence Records
2.3. Climate Data
2.4. Construction of the Models
2.5. Analysis of Geographic Space
3. Results
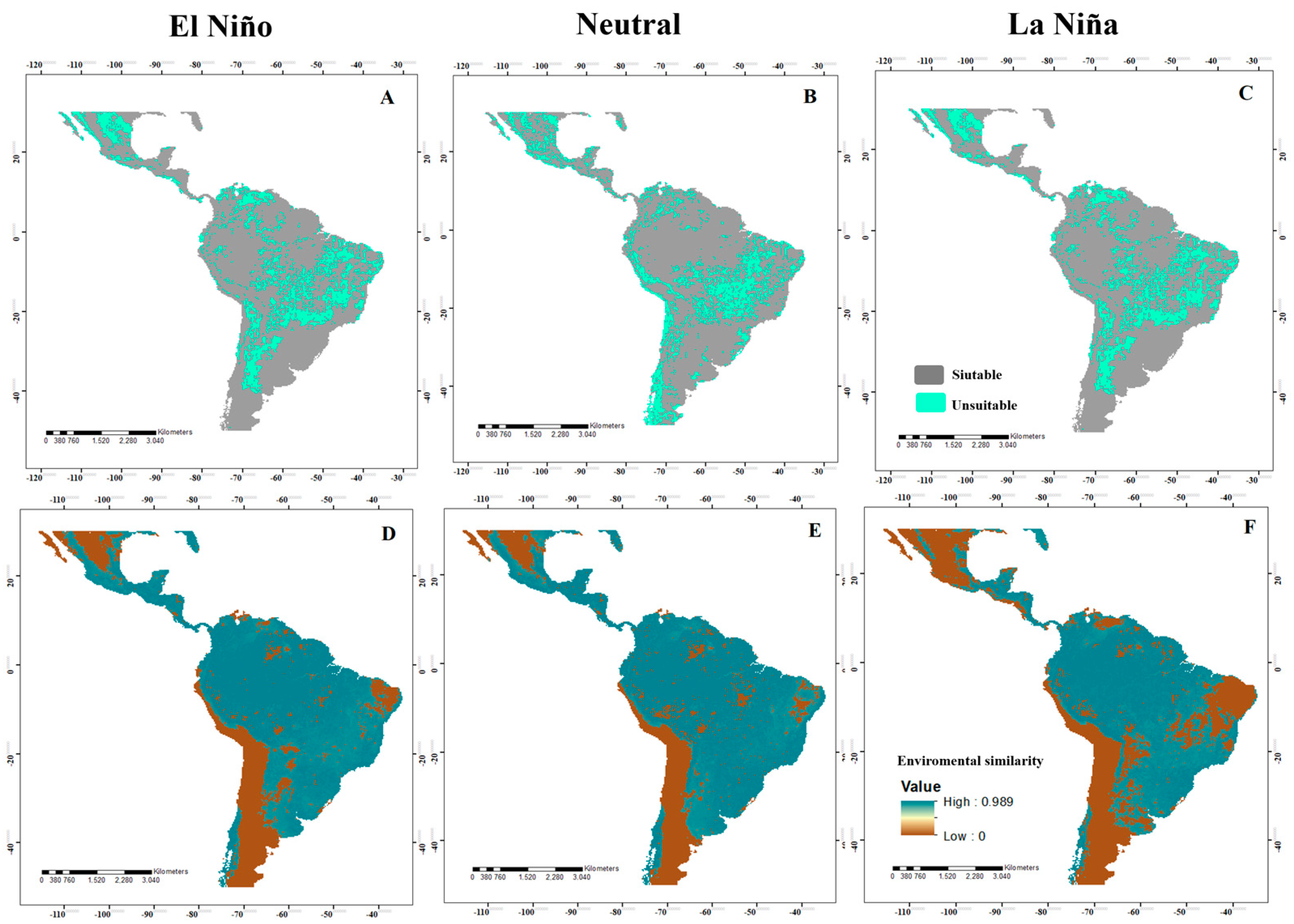
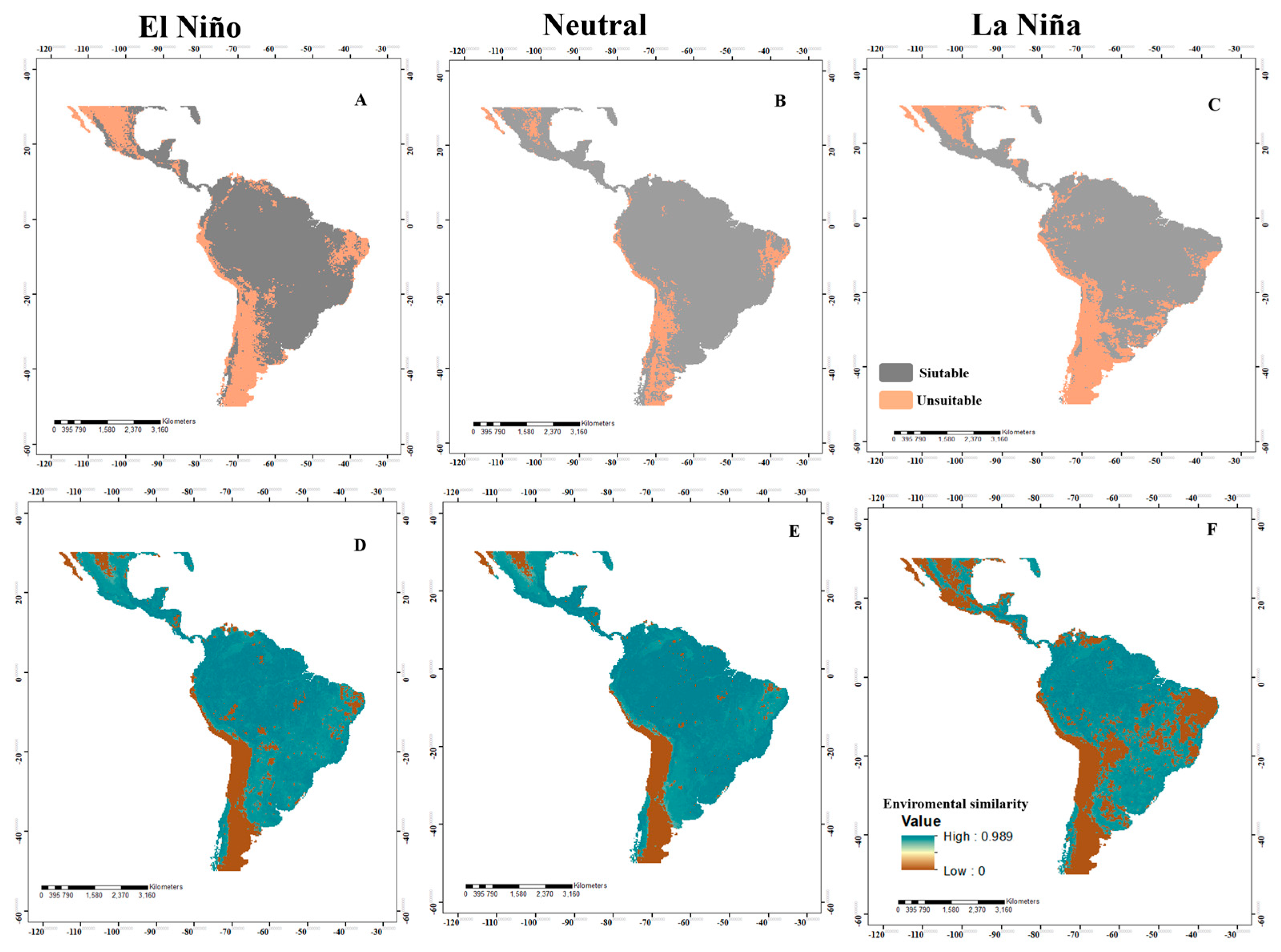
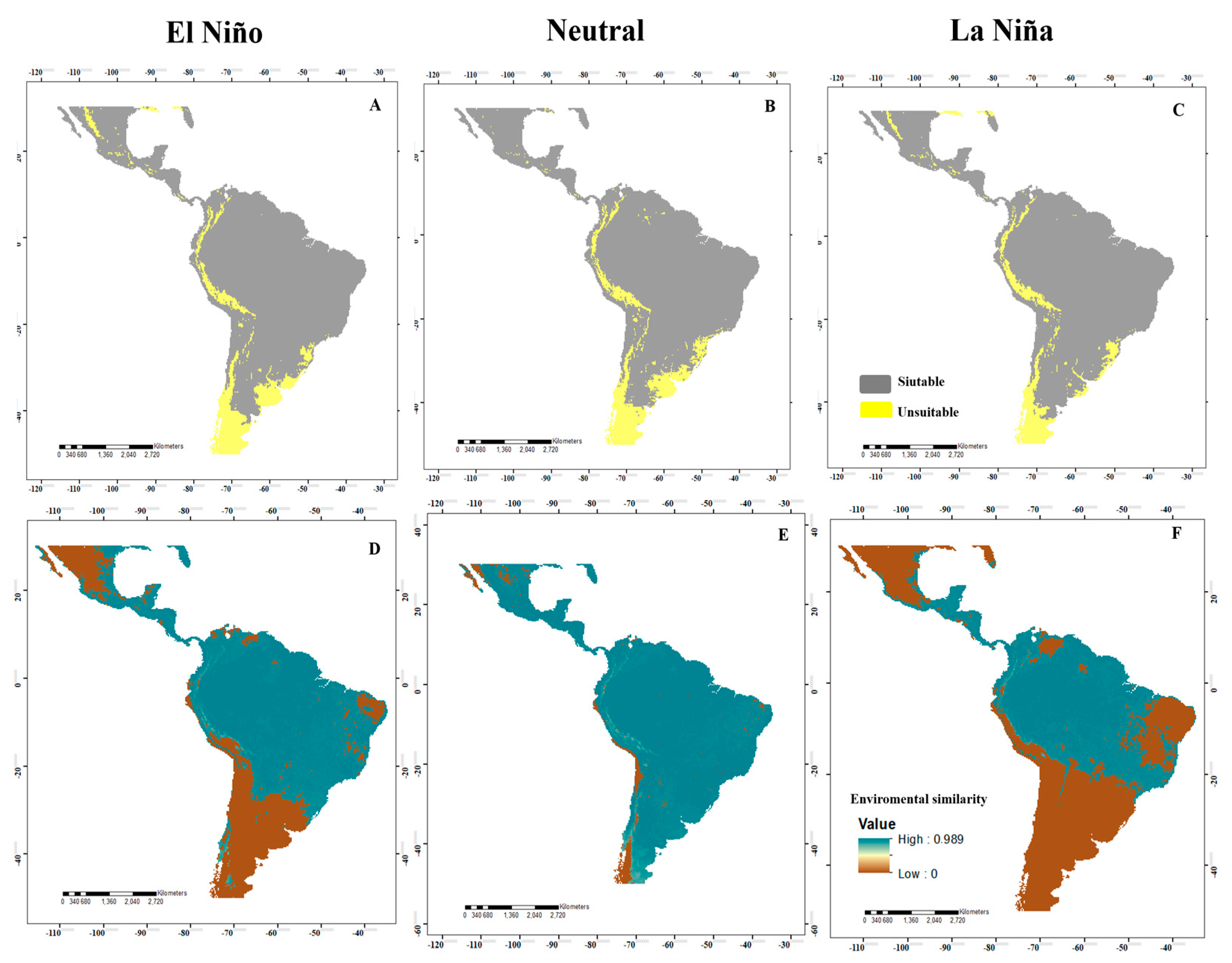
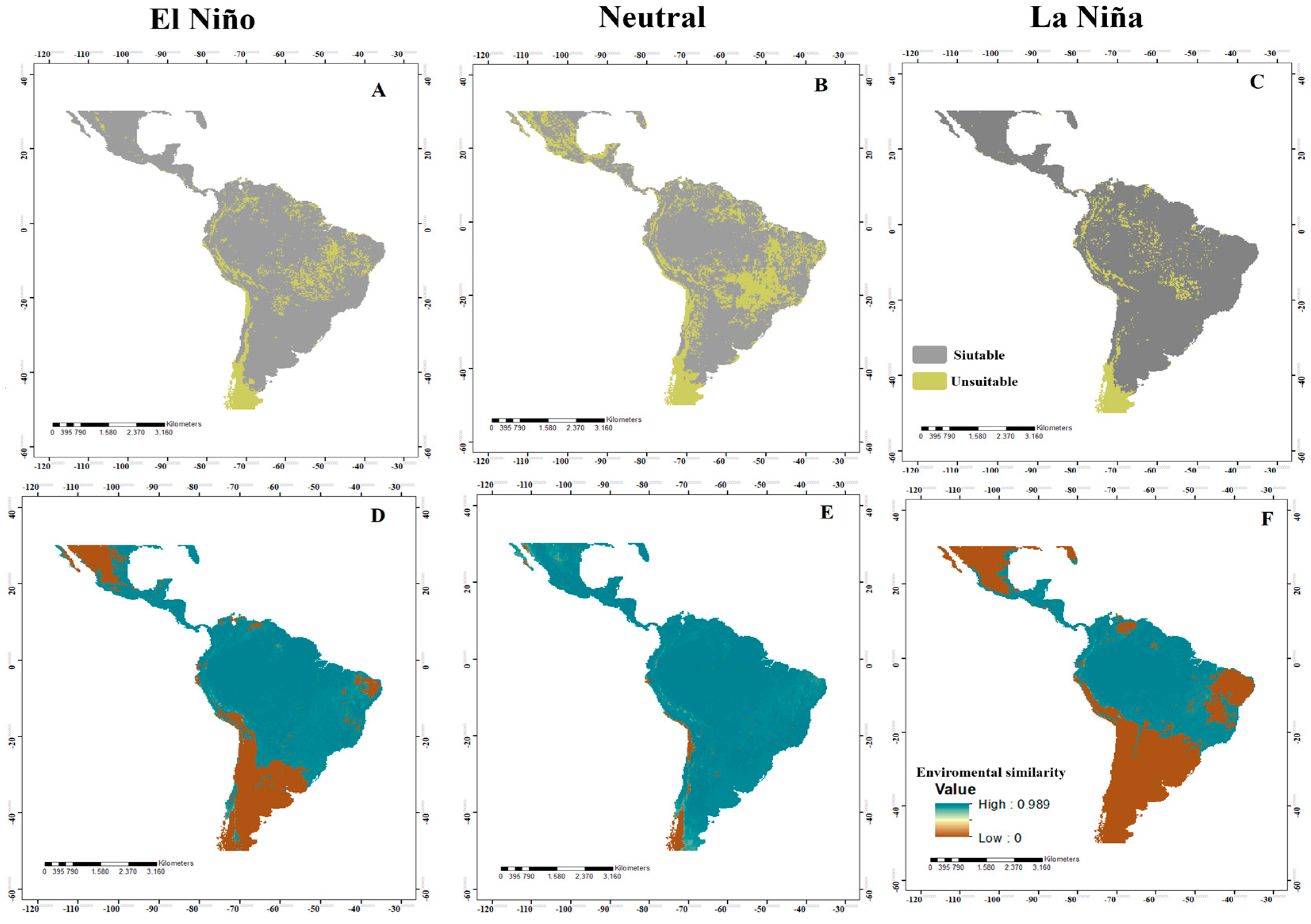
3.1. Potential Distribution in the Neotropics
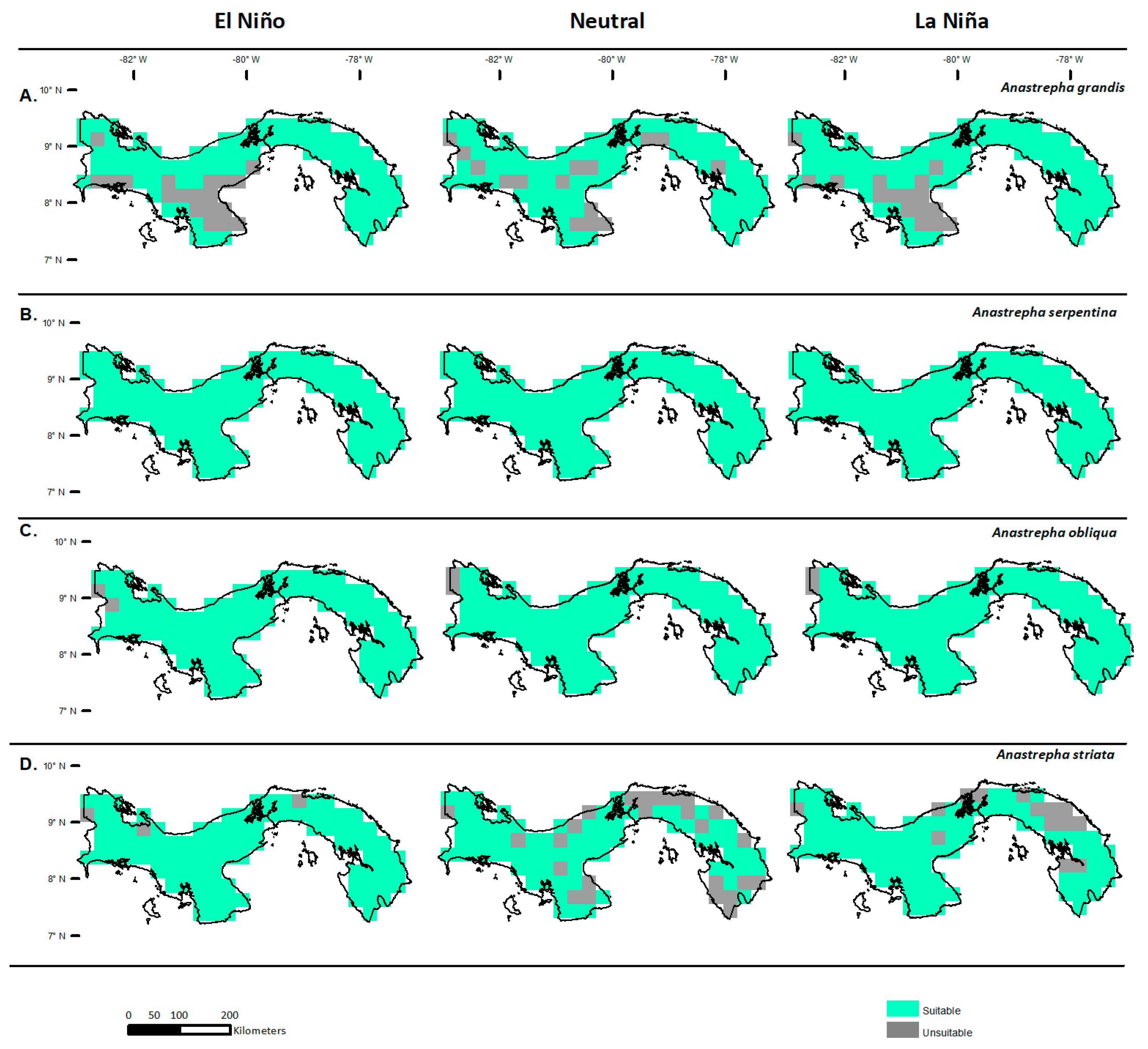
3.2. Potential Distribution in Panama
3.3. Movement-Oriented Parity Analysis (MOP)
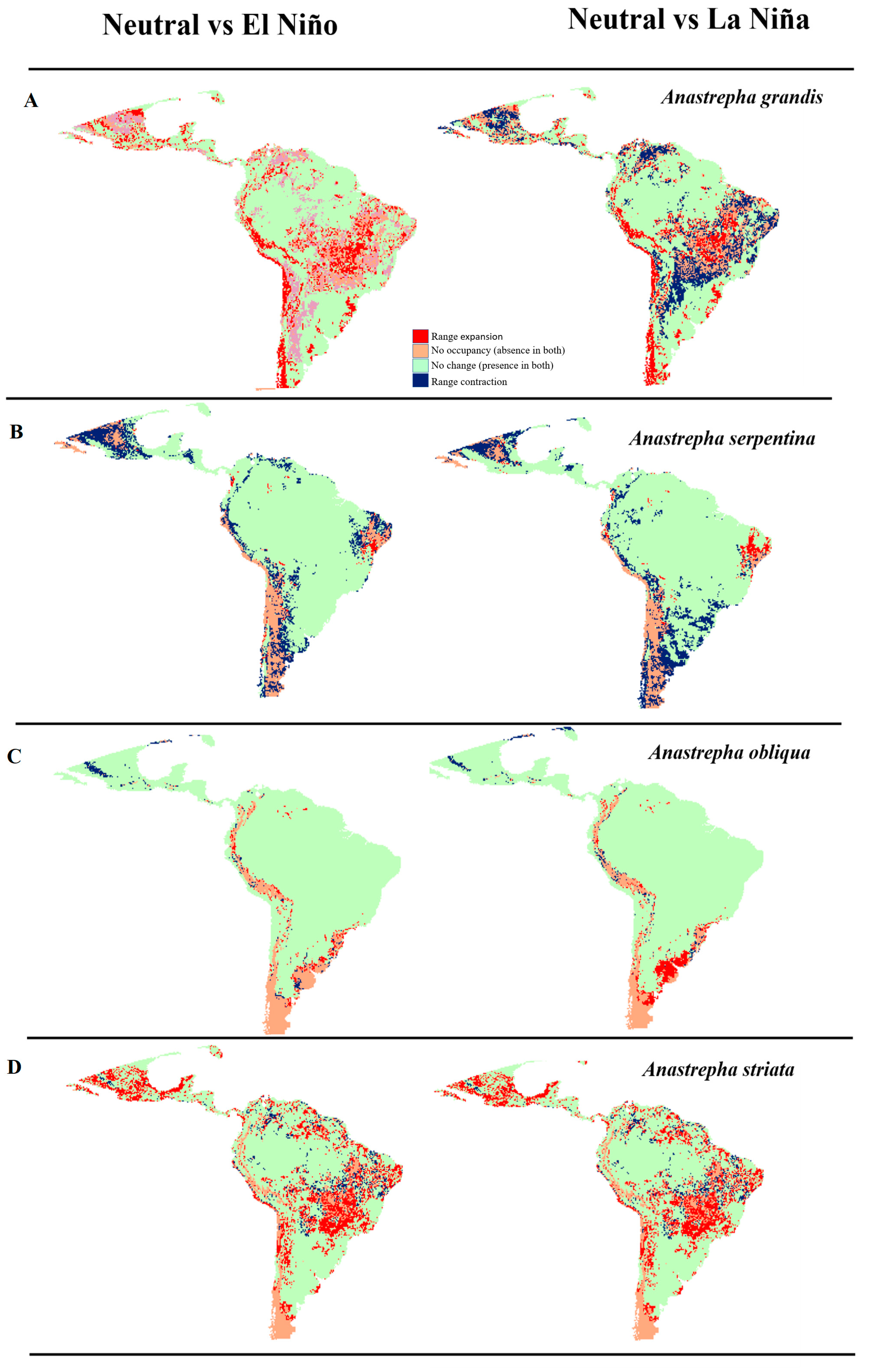
3.4. Analysis of Changes in Environmental Suitability in Different Scenarios
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montealegre, J.; Pabon, J. La Variabilidad Climática Interanual asociada al ciclo El Niño-La Niña–Oscilación del Sury su efecto en el patrón pluviométrico de Colombia. Meteorol. Colombina 2000, 2, 7–21. [Google Scholar]
- Trenberth, K.E.; Fasullo, J.T.; Kiehl, J. Earth’s global energy budget. Bull. Am. Meteorol. Soc. 2009, 90, 311–324. [Google Scholar] [CrossRef]
- Organización de las Naciones Unidas. Convención Marco De Las Naciones Unidas Sobre El Cambio Climático; Organización de las Naciones Unidas: NewYork, NY, USA, 2015; p. 40. Available online: https://unfccc.int/resource/docs/2015/cop21/spa/l09s.pdf (accessed on 1 March 2023).
- D’Antoni, H.L. Cambio global. Procesos naturales e intervención humana. Acta Bioquímica Clínica Latinoam. 2012, 46, 1–76. [Google Scholar]
- Moyano, E.; Paniagua, Á.; Lafuente, R. Políticas ambientales, cambio climático y opinión pública en escenarios regionales. El caso de Andalucía. Rev. Int. Sociol. 2009, 67, 681–699. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Climate Change, Impact, Adaptation and Vulnerability, Summary for Policymakers; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2014; p. 40. Available online: https://www.ipcc.ch/site/assets/uploads/2018/02/SYR_AR5_FINAL_full_es.pdf (accessed on 3 March 2023).
- Gonzales, G.F.; Zevallos, A.; Gonzales, C.C.; Nuñez, D.; Gastañaga, C.; Cabezas, C.; Steenland, K. Environmental pollution, climate variability and climate change: A review of health impacts on the peruvian population. Rev. Peru. Med. Exp. Salud Publica 2014, 31, 547–556. [Google Scholar]
- Zotelo, C. Variabilidad climática y ciclos naturales. In Jornada sobre “Evolución y Futuro del Desarrollo de Producciones Agrícola-Ganaderas en el SO Bonaerense”; Universidad Nacional de la Plata: Bahía Blanca, Argentina, 2011; pp. 374–381. Available online: http://sedici.unlp.edu.ar/handle/10915/27824 (accessed on 5 March 2023).
- Tobón, C.J. Evaluación de los Impactos Potenciales de la Variabilidad Climática y el Cambio Climático en Algunos Indicadores para Seguridad Alimentaria en Zonas Productoras de Mercados Campesinos; Trabajo de Grado-Mestria, Universidad de Colombia: Bogotá, Colombia, 2014. [Google Scholar]
- Krishnamurthy, L.; Vecchi, G.A.; Msadek, R.; Murakami, H.; Wittenberg, A.; Zeng, F. Impact of strong ENSO on regional tropical cyclone activity in a high-resolution climate model in the North Pacific and North Atlantic Oceans. J. Clim. 2016, 29, 2375–2394. [Google Scholar] [CrossRef]
- Collins, M.; CMIP Modeling Groups. El Niño- or La Niña-like climate change? Clim. Dyn. 2005, 24, 89–104. [Google Scholar] [CrossRef]
- Balvanera, P.; Astier, M.; Gurri, F.D.; Zermeño, H.I. Resiliencia, vulnerabilidad y sustentabilidad de sistemas socioecológicos en México. Rev. Mex. Biodivers. 2017, 88, 141–149. [Google Scholar] [CrossRef]
- Gouveia, S.F.; Hortal, J.; Tejedo, M.; Duarte, H.; Cassemiro, F.A.; Navas, C.A.; Diniz, F.J.A. Climatic niche at physiological and macroecological scales: The thermal tolerance-geographical range interface and niche dimensionality. Blackwell Publishing. Glob. Ecol. Biogeogr. 2014, 23, 446–456. [Google Scholar] [CrossRef]
- Lehmann, P.; Ammunét, T.; Barton, M.; Battisti, A.; Eigenbrode, S.D.; Jepsen, J.U.; Kalinkat, G.; Neuvonen, S.; Niemelä, P.; Terblanche, J.S.; et al. Complex responses of global insect pestss to climate warming. Front. Ecol. Environ. 2020, 18, 141–150. [Google Scholar] [CrossRef]
- Comisión Económica para América Latina. La economía del Cambio Climático en América Latina y el Caribe: Paradojas y Desafíos del Desarrollo Sostenible. Programa EUROCLIMA Cambio Climático, Componente Socioeconómico 2015. (CEC/10/001). Available online: https://hdl.handle.net/11362/37311 (accessed on 10 March 2023).
- Sanchez, R.R. Respuestas Urbanas al Cambio Climático en América Latina. Documentos de Proyectos e Investigación. CEPAL-Naciones Unidas. Instituto Interamericano para la Investigación del Cambio Global No. 563. 2013. Available online: https://hdl.handle.net/11362/36622 (accessed on 15 March 2023).
- Magrin, G. Adaptación al Cambio Climático en América Latina y el Caribe. Documentos de Proyectos e Investigación. CEPAL-Naciones Unidas. Instituto Interamericano para la Investigación del Cambio Global No. 692. 2015. Available online: https://hdl.handle.net/11362/39842 (accessed on 15 March 2023).
- Rascón, V.A.E.; Cervantes, R.E. Vulnerabilidad social y clima extremo en estudios de América Latina. 2000–2019. Tlalli. Rev. Investig. Geogr. 2022, 8, 6–32. [Google Scholar] [CrossRef]
- Sheldon, K.S. Climate change in the tropics: Ecological and evolutionary responses at low latitudes. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 303–333. [Google Scholar] [CrossRef]
- Régnière, J.; Powell, J.; Bentz, B.; Nealis, V. Effects of temperature on development, survival and reproduction of insects: Experimental design, data analysis and modeling. J. Insect Physiol. 2012, 58, 634–647. [Google Scholar] [CrossRef]
- Freeman, B.G.; Scholer, M.N.; Ruiz, V.; Fitzpatrick, J.W. Climate change causes upslope shifts and mountaintop extirpations in a tropical bird community. Proc. Natl. Acad. Sci. USA 2018, 115, 11982–11987. [Google Scholar] [CrossRef]
- Soberón, J. Grinnellian and Eltonian niches and geographic distributions of species. Ecol. Lett. 2007, 10, 1115–1123. [Google Scholar] [CrossRef]
- Powell, A.J.; Logan, A.J. Insect seasonality: Circle map analysis of temperature-driven life cycles. Theor. Popul. Biol. 2005, 3, 161–179. [Google Scholar] [CrossRef]
- Peterson, A.T.; Papeş, M.; Soberón, J. Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol. Modell. 2008, 213, 63–72. [Google Scholar] [CrossRef]
- Peterson, A.; Soberón, J.; Pearson, R.; Anderson, R.; Martínez, M.E.; Nakamura, M.; Araújo, M. Ecological Niches and Geographic Distributions (MPB-49); Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Beckler, A.A.; French, B.W.; Chandler, L.D. Using GIS in areawide pests management: A case study in South Dakota. Trans. GIS 2005, 9, 109–127. [Google Scholar] [CrossRef]
- Aluja, M.; Birke, A.; Ceymann, M.; Guillén, L.; Arrigoni, E.; Baumgartner, D.; Pascacio, C.; Villafán, J. Agroecosystem resilience to an invasive insect species that could expand its geographical range in response to global climate change. Agric. Ecosyst. Environ. 2014, 186, 54–63. [Google Scholar] [CrossRef]
- Peterson, A.T. Uses and requirements of ecological niche models and related distributional models. Biodivers. Inform. 2006, 3, 59–72. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Modell. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Aluja, M.; Mangan, R.L. Fruit fly Diptera: Tephritidae host status determination: Critical conceptual, methodological, and regulatory considerations. Annu. Rev. Entomol. 2008, 53, 473–502. [Google Scholar] [CrossRef]
- Norrbom, A.L. Host Plant Database for anastrepha and toxotrypana (Diptera: Tephritidae: Toxotrypanini). Diptera Data Dissemination Disk. CD—Not a Journal. 2004. Available online: https://www.ars.usda.gov/research/publications/publication/?seqNo115=108854. (accessed on 17 March 2023).
- Norrbom, A.L.; Neder, L.E. New neotropical species of Trupanea Diptera: Tephritidae with unusual wing patterns. Zootaxa 2014, 3821, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, O.V. El género Anastrepha Schiner en México Diptera: Tephritidae, taxonomía, distribución y sus plantas huéspedes. La Cienc. Y El Hombre 1992, 12, 190–191. [Google Scholar]
- Hernández, O.V.; Bartolucci, A.F.; Morales, V.P.; Frías, D.; Selivon, D. Cryptic species of the Anastrepha fraterculus complex: A multivariate approach for the recognition of South American morphotypes. Ann. Entomol. Soc. 2012, 105, 305–318. [Google Scholar] [CrossRef]
- Norrbom, A.L.; Korytkowski, C.A. New species of Anastrepha Diptera: Tephritidae, with a key for the species of the megacantha clade. Systematic Entomology Lab., USDA, ARS, c/o Smithsonian Institution. Zootaxa 2012, 34781, 11. [Google Scholar] [CrossRef]
- Cruz, B.; Bacca, M.L.; Nelson, A. Diversidad de las Moscas de las frutas Diptera: Tephritidae y sus parasitoides en siete minicipios del departamento de Narino. Boletín Científico. Cent. Museos. Mus. Hist. Nat. 2017, 21, 81–98. [Google Scholar] [CrossRef]
- Hernández, O.V.; Gómez, A.J.; Sánchez, A.; McPheron, B.; Aluja, M. Morphometric analysis of Mexican and South American populations of the Anastrepha fraterculus complex (Diptera: Tephritidae) and recognition of a distinct Mexican morphotype. Bull. Entomol. Res. 2004, 94, 487–499. [Google Scholar] [CrossRef]
- Selivon, D.; Perondini, A.L.; Morgante, J.S. A Genetic–Morphological Characterization of Two Cryptic Species of the Anastrepha fraterculus Complex (Diptera: Tephritidae). Ann. Entomol. Soc. 2005, 98, 367–381. [Google Scholar] [CrossRef]
- Silva, J.C.; Dutra, S.V.; Santos, M.S.; Silva, N.M.O.; Vidal, D.V.; Nink, R.V.; Guimarães, J.G.; Araujo, E.L. Diversity of Anastrepha spp. (Diptera: Tephritidae) and Associated Braconid Parasitoids from Native and Exotic Hosts in Southeastern Bahia, Brazil. Environ. Entomol. 2021, 39, 1457–1465. [Google Scholar] [CrossRef]
- Nolasco, N.; Iannacone, J. Fluctuación estacional de moscas de la fruta Anastrepha spp. y Ceratitis capitata (Wiedemann, 1824) (Diptera: Tephritidae) en trampas McPhail en Piura y en Ica, Perú. Acta Zool. Mex. 2008, 24, 33–44. [Google Scholar] [CrossRef]
- Aluja, M.; Rull, J.; Sivinski, J.; Allen, L.; Norrbom, A.; Wharton, R.M.; Díaz, F.; López, M. Fruit Flies of the Genus Anastrepha (Diptera: Tephritidae) and Associated Native Parasitoids (Hymenoptera) in the Tropical Rainforest Biosphere Reserve of Montes Azules, Chiapas, Mexico. Environ. Entomol. 2003, 32, 1377–1385. [Google Scholar] [CrossRef]
- Diznarda, S.B.; Flores, R.; Terrazas, G.G.; Leyva, R.E. Evaluación Económica de la Campaña Nacional Contra las Moscas de la Fruta en los Estados de Baja California, Guerrero Nuevo León, Sinaloa, Sonora y Tamaulipas; IICA-Ciudad de Mexico D.F: México city, Mexico, 2010; Available online: http://repiica.iica.int/docs/B2041e/B2041e.pdf (accessed on 17 March 2023).
- Valderrama, J.K.; Serrano, M.S.; Fischer, G. Mortalidad de larvas de Anastrepha fraterculus (Wiedemann) (Diptera: Tephritidae) en frutos de feijoa (Acca sellowiana [O. Berg] Burret) sometidos a un tratamiento cuarentenario de frio. Rev. Colomb. Entomol. 2005, 31, 171–176. [Google Scholar] [CrossRef]
- Saavedra, D.J.; Galeano, O.P.; Canal, N. Ecological relationships between host fruits, frugivorous flies and parasitoids in a fragment of tropical dry forest. Rev. Sci. Agric. 2017, 34, 32–49. [Google Scholar] [CrossRef]
- Sequeira, R.; Millar, L.; Bartels, D. Identification of Susceptible Areas for the Establishment of Anastrepha spp. Fruit Flies in the United States and Analysis of Selected Pathways; USDA-APHISPPQ Center for Plant Health Science and Technology: Raleigh, North Carolina, 2001; p. 47. Available online: https://www.aphis.usda.gov/plant_health/plant_pests_info/fruit_flies/downloads/isa.pdf (accessed on 18 March 2023).
- Godefroid, M.; Cruaud, A.; Rossi, J.P.; Rasplus, J.Y. Assessing the Risk of Invasion by Tephritid Fruit Flies: Intraspecific Divergence Matters. PLoS ONE 2015, 10, 8. [Google Scholar] [CrossRef]
- Fu, L.Z.H.; Huang, G.S.; Wu, X.X.; Ni, W.L.; Qü, W.W. The current and future potential geographic range of West Indian fruit fly, Anastrepha obliqua (Diptera: Tephritidae). Insect Sci. 2014, 21, 234–244. [Google Scholar] [CrossRef]
- Vázquez, P.; Escalona, A.H.; Segura, G.; Esparza, O.L.G. Modelación de la distribución geográfica potencial de dos especies de psitácidos neotropicales utilizando variables climáticas y topográficas. Acta Zool. Mex. 2014, 30, 471–490. [Google Scholar] [CrossRef]
- Jiménez, M.E.; Núñez, M.R.G.; Maradiaga, B.E.J. Distribución temporal de insectos asociados a maracuyá (Passiflora edulis Sims) en Matagalpa, Nicaragua. La Calera 2020, 20, 10–19. [Google Scholar] [CrossRef]
- Altamiranda, S.M.; Gutiérrez, D.J.; Araque, A.; Valencia, D.J.; Gutiérrez, R.; Martínez, R.A. Effect of El Niño Southern Oscillation cycle on the potential distribution of cutaneous leishmaniasis vector species in Colombia. PLoS Negl. Trop. Diseases 2020, 14, e0008324. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the Unites Nations, Fondo Internacional de Desarrollo Agricola, Organization Mundial de la Salud, Programa Mundial de Alimentos y Fondo de las Naciones Unidas por la Infancia. Versión Resumida de El Estado de la Seguridad Alimentaria y la Nutrición en el Mundo. Transformación de los Sistemas Alimentarios para que Promuevan Dietas Asequibles y Saludables. Roma. 2020. Available online: https://www.fao.org/documents/card/en/c/ca9699es (accessed on 18 March 2023).
- Food and Agriculture Organization of the Unites Nations; Centro de Cooperacion Internacional en Investigación Agrícola para el Desarrollo; Frutas y Hortalizas—Oportunidades y Desafíos para la Agricultura Sostenible a Pequeña Escala. Roma. 2021. Available online: https://www.fao.org/documents/card/en/c/cb4173es (accessed on 19 March 2023).
- Food and Agriculture Organization of the Unites Nations. Análisis del Mercado de las Principales Frutas Tropicales en Roma. 2022. Available online: https://www.fao.org/3/cb6897es/cb6897es.pdf (accessed on 19 March 2023).
- Instituto Interamericano de Cooperación para la Agricultura. La Fruticultura en Panamá: Su Potencial Socioeconómico e Iniciativas para su Desarrollo; Ministerio de Desarrollo Agropecuario, Instituto de Innovación Agropecuaria de Panamá, Ciudad de Panamá: Ancon, Panamá, 2008; Volume 167, p. 23. Available online: http://repiica.iica.int/docs/b0760e/b0760e.pdf (accessed on 19 March 2023).
- Ministerio de Desarrollo Agropecuario. Panamá. Cierre Agrícola Nacional. Dirección de Agricultura. Documento Panamá. 2020–21. Available online: https://mida.gob.pa/ (accessed on 20 March 2023).
- Olson, D.; Dinerstein, E.; Wikram, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial Ecoregions of the World: A New Map of Life on Earth. Biosci. J. 2001, 51, 933. [Google Scholar] [CrossRef]
- Dinerstein, E.; Olson, D.; Joshi, A.; Vynne, C.; Burgess, N.B.; Wikramanayake, E.; Hahn, N.; Palminteri, S.; Hansen, M.; Locke, M.; et al. An Ecoregion-Based Approach to Protecting Half the Terrestrial Realm. Biosci. J. 2017, 67, 534–545. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.; Mittermeier, C. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Metz, B.; Davidson, O.; Bosch, P.; Dave, R.; Meyer, L. Climate Change-Mitigation of Climate Change; OSTI Identifier: 21017235; Intergovernmental Panel on Climate Change, Geneva (Switzerland). Working Group III; U.S. Department of Energy Office of Scientific and Technical Information: Oak Ridge, TN, USA, 2007. Available online: https://www.osti.gov/biblio/21017235 (accessed on 3 June 2023).
- Jarvis, A.; Hijmans, R. The effect of climate change on crop wild relatives. Agric. Ecosyst. Environ. 2008, 126, 13–23. [Google Scholar] [CrossRef]
- Ramírez, O.; Ruiz, G.; Corral, J.A.; Pérez, M.C.; Villavicencio, G.R.; Mena, M.; Puga, N. Impactos del cambio climático en la distribución geográfica de Gossypium hirsutum L. en México. Rev. Mex. Cienc. 2014, 5, 1885–1895. [Google Scholar]
- Morrone, J.J. Biogeographical regionalisation of the Neotropical region. Zootaxa 2014, 3782, 1–110. [Google Scholar] [CrossRef] [PubMed]
- Jaén, S.O. Geografía de Panamá. Estudio Introductorio y Antología; Universidad de Panamá, Cuidad de Panama, Campus Central: Panama City, Panama, 1985; p. 472. Available online: https://books.google.com.pa/books?id=WlBrAAAAMAAJ&source=gbs_navlinks_s (accessed on 5 March 2023).
- Makay, A. Cien años de Geografía en Panamá. Universidad de Panamá. Articulo. 2003. Available online: http://bdigital.binal.ac.pa/bdp/artpma/cienanosdegeografia.pdf (accessed on 25 March 2023).
- Uchoa, A.M. Fruit Flies (Diptera: Tephritoidea): Biology, Host Plants, Natural Enemies, and the Implications to Their Natural Control; IntechOpen: London, UK, 2012; Volume 12, pp. 271–300. [Google Scholar] [CrossRef]
- Alvarado, G.L.; Medianero, E. Especies de parasitoides asociados a moscas de la fruta del género Anastrepha (Diptera: Tephritidae) en Panamá, República de Panamá. Scientia 2021, 25, 47–62. [Google Scholar]
- Uchôa, M.A.; Nicácio, J. New records of Neotropical fruit flies (Tephritidae), lance flies (Lonchaeidae) (Diptera: Tephritoidea), and their host plants in the South Pantanal and adjacent areas, Brazil. Ann. Entomol. Soc. 2010, 103, 723–733. [Google Scholar] [CrossRef]
- Cobos, M.E.; Jiménez, L.; Nuñez, P.C.; Romero, A.D.; Simoes, M. Sample data and training modules for cleaning biodiversity information. Biodiversity 2018, 13, 49–50. [Google Scholar] [CrossRef]
- Lammens, A.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Shaw, J.G.; Sanchez, M.; Spishakoff, L.M.; Trujillo, G.F.; Loppez, D. Dispersal and Migration of Tepa-Sterilized Mexican Fruit Flies. J. Econ. Entomol. 1967, 60, 992–994. [Google Scholar] [CrossRef]
- Chambers, D.L.; OConnell, T.B. A Flight Mill for Studies with the Mexican Fruit Fly. Ann. Entomol. Soc. 1969, 62, 917–920. [Google Scholar] [CrossRef]
- Mayara, R.; Dos Santos, M.D.; Martins, M.; Fornazier, J.M.; Uramoto, K.; Ferreira, F.; Zucchi, R.A.; Conde, W.A. Aggregation and spatio-temporal dynamics of fruit flies (Diptera, Tephritidae) in papaya orchards associated with different area delimitations in Brazil. Acta Sci. 2020, 44. [Google Scholar] [CrossRef]
- Japan Meteorological Agency. El Nino Monitoring and Outlook /TCC; Japan Meteorological Agency: Tokyo, Japan, 2019; Available online: https://www.data.jma.go.jp/multi/index.html?lang=es (accessed on 13 September 2019).
- Bureau of Meteorology. Climate Influences Timeline. Australian Government. 2018. Available online: http://www.bom.gov.au/ (accessed on 13 November 2018).
- National Weather Service. National Oceanic and Atmospheric Administration; NOAA’s Climate Prediction Center, National Weather Service: Silver Spring, MD, USA, 2018. Available online: https://www.weather.gov/ (accessed on 18 November 2018).
- Dupin, J.; Smith, S.D. Integrating historical biogeography and environmental niche evolution to understand the geographic distribution of Datureae. Am. J. Bot. 2019, 106, 667–678. [Google Scholar] [CrossRef]
- Wei, R.; Chan, K.W.; So, W.W.M. A systematic review of remote laboratory work in science education with the support of visualizing its structure through the Hist Cite and Cite Space software. Int. J. Sci. Math. Educ. 2017, 15, 1217–1236. [Google Scholar] [CrossRef]
- Moo-Llanes, D.A.; Arenas, C.Y.; Baak, B.C. Shifts ecological niche of Lutzomyia peruensis under climate change scenarios in Peru. Med. Vet. Entomol. 2017, 31, 123–131. [Google Scholar] [CrossRef]
- NASA, MO DIS Web 2018. Available online: https://modis.gsfc.nasa.gov/ (accessed on 19 December 2018).
- NOAA National Oceanic and Atmospheric Administration, What Are El Nino and La Nina? 2020. Available online: https://oceanservice.noaa.gov/facts/ninonina.html (accessed on 20 December 2020).
- Acker, J.; Leptoukh, G. Online analysis improves the use of NASA earth science data. Eos Trans. Am. Geophys. Union 2007, 88, 14–17. [Google Scholar] [CrossRef]
- Lobo, J.; Jiménez, V.A.; Real, R. AUC: Erratum: Predicting species distribution: Offering more than simple habitat models. Glob. Ecol. Biogeogr. 2008, 17, 145–151. [Google Scholar] [CrossRef]
- Carrington, A.M.; Manuel, D.G.; Fieguth, P.W.; Ramsay, T.; Osmani, V.; Wernly, B.; Bennett, C.; Hawken, S.; Magwood, O.; Sheikh, Y.; et al. Deep ROC Analysis and AUC as Balanced Average Accuracy, for Improved Classifier Selection, Audit and Explanation. IEEE Trans. Pattern Anal. Mach. Intell. 2023, 45, 329–341. [Google Scholar] [CrossRef]
- Barve, N.; Barve, V.; Jiménez, V.A.; Lira, N.A.; Maher, S.P.; Peterson, A.T.; Soberón, J.; Villalobos, F. The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecol. Modell. 2011, 222, 1810–1819. [Google Scholar] [CrossRef]
- Cruz, C.G.; Villaseñor, J.L.; López, M.L.; Martínez, M.E.; Ortiz, E. Selección de predictores ambientales para el modelado de la distribución de especies en Maxent. Rev. Chapingo. Ser. 2014, 20, 187–201. [Google Scholar]
- Díaz, A.C.J.; Romero, A.L.V.; Miranda, E.D.R. Neotropical páramos as biogeographic units. Biol. Trop. 2020, 68, 503–516. [Google Scholar] [CrossRef]
- Anderson, R.P.; Peterson, T. Evaluating predictive models of species’ distributions: Criteria for selecting optimal models. Ecol. Modell. 2003, 162, 211–232. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Mateo, R.; Felcisimo, Á.M.; Muñoz, J. Species distributions models: A synthetic revisión. Rev. Chil. Hist. Nat. 2011, 84, 217–240. [Google Scholar] [CrossRef]
- Moo, L.D.A.; López, O.T.; Torres, M.J.A.; Mosso, G.C.; Casas, M.M.; Samy, A.M. Assessing the Potential Distributions of the Invasive Mosquito Vector Aedes albopictus and Its Natural Wolbachia Infections in México. Insects 2021, 12, 143. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Cobos, M.E.; Peterson, A.T.; Osorio, O.L.; Jiménez, G.D. An exhaustive analysis of heuristic methods for variable selection in ecological niche modeling and species distribution modeling. Ecol. Inform. 2019, 53. [Google Scholar] [CrossRef]
- Pliscoff, P.; Fuentes, T. Modelación de la distribución de especies y ecosistemas en el tiempo y en el espacio: Una revisión de las nuevas herramientas y enfoques disponibles. Rev. Geogr. Norte Gd. 2011, 48, 61–79. [Google Scholar] [CrossRef]
- Owens, H.L.; Campbell, L.P.; Dornak, L.L.; Saupe, E.E.; Barve, N.; Soberón, J.; Ingenloff, K.; Lira, N.A.; Hensz, C.M.; Myers, C.E.; et al. Constraints on interpretation of ecological niche models by limited environmental ranges on calibration areas. Ecol. Modell. 2013, 263, 10–18. [Google Scholar] [CrossRef]
- Brown, J.L. SDM toolbox: A python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- Brown, J.L.; Bennett, J.R.; Connor, M. SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Peterson, A.T. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2006, 34, 102–117. [Google Scholar] [CrossRef]
- Pinto, J.N.; Bares, C.J. Predicting species distributions and community composition using satellite remote sensing predictors. Sci. Rep. 2021, 11, 16448. [Google Scholar] [CrossRef] [PubMed]
- Paini, D.R.; Sheppard, A.W.; Cook, D.C.; De Barro, P.J.; Worner, S.P.; Thomas, M.B. Global threat to agriculture from invasive species. Proc. Natl. Acad. Sci. USA 2016, 113, 7575–7579. [Google Scholar] [CrossRef]
- Lódola, A. Contratistas, Cambios Tecnológicos y Organizacionales en el Agro Argentino, Documentos de Proyectos No. 176. Available online: https://hdl.handle.net/11362/36772 (accessed on 25 April 2023).
- Atencio, V.R.; Collantes, G.R.; Caballero, E.M.; Hernández, A.P.; Vaña, H.M. Impacto de los Insectos en la Seguridad Alimentaria en Panamá. Sci. Agropecu 2021, 36, 139–165. [Google Scholar]
- Amat, E.; Altamiranda, S.M.; Canal, N.; Gómez, P.L. Changes in the potential distribution of the guava fruit fly Anastrepha striata (Diptera, Tephritidae) under current and possible future climate scenarios in Colombia. Bull. Entomol. Res. 2022, 112, 469–480. [Google Scholar] [CrossRef]
- Machado, T.C.; Krüger, P.; Edson, N.D.; Mello, G.F. Potential global distribution of the south American cucurbit fruit fly Anastrepha grandis (Diptera: Tephritidae). Crop Prot. 2021, 45, 0261–2194. [Google Scholar] [CrossRef]
- Silva, J.G. Biologia e Comportamento de Anastrepha Grandis (Macquart, 1846) (Diptera: Tephritidae); Dissertação Mestrado. Universidade de São Paulo: São Paulo, Brazil, 1991; Available online: https://repositorio.usp.br/item/000733447. (accessed on 1 March 2023).
- Topón, R.L.M. Ciclo Biológico de la Mosca de la Fruta del Género (anastrepha spp.) a dos temperaturas, Universidad Tecníca de Cotopaxi. Latacunga Tesis en Ingieneria Agronomica, Av. Simón Rodríguez, Latacunga, Ecuador. 2020, p. 93. Available online: http://repositorio.utc.edu.ec/handle/27000/7050. (accessed on 15 April 2023).
- Fúnez, X.I. Características Biológicas de Anastrepha Grandis (Macuart,1846) en Relación con su Hospedero Natural, Fevillea Cordifolia en Darién, Panamá. Maestría Thesis, Universidad de Panamá, Ciudad de Panamá, Panamá, 2014. Available online: http://up-rid.up.ac.pa/id/eprint/348. (accessed on 15 April 2023).
- Bonebrake, T.C.; Deutsch, C.A. Climate heterogeneity modulates impact of warming on tropical insects. Ecology 2012, 93, 449–455. [Google Scholar] [CrossRef]
- De La Vega, G.J.; Schilman, P.E. La importancia de la fisiología en la distribución geográfica de los insectos. Rev. Soc. Entomol. Arg. 2015, 74, 101–108. [Google Scholar]
- Addo, B.A.; Chown, S.L.; Gaston, K.J. Thermal tolerance, climatic variability and latitude. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2000, 267, 739–745. [Google Scholar] [CrossRef]
- Esparza, M. La sequía y la escasez de agua en México: Situación actual y perspectivas futuras. Secuencia 2014, 89, 193–219. [Google Scholar] [CrossRef]
- González, O.D.; Córdoba, A.A.; Dáttilo, W.; Noriega, L.A.; Sánchez, G.R.A.; Villalobos, F. Insect responses to heat: Physiological mechanisms, evolution and ecological implications in a warming world. Biol. Rev. 2020, 95, 802–821. [Google Scholar] [CrossRef] [PubMed]
- Bruniard, E.D. La diagonal árida Argentina: Un límite climático real. Rev. Geográfica 1982, 95, 5–20. [Google Scholar]
- Mason, S.J.; Goddard, L. Probabilistic Precipitation Anomalies Associated with EN SO. Research Paper. Bull. Am. Meteorol. Soc. 2001, 82, 619–638. [Google Scholar] [CrossRef]
- Karlin, M.S. Cambios temporales del clima en la subregión del Chaco Árido. Multequina 2012, 21, 3–16. [Google Scholar]
- Fava, G.A.; Acosta, J.C.; Blanco, G.M. The effects of seasonality and precipitation in the avifauna of the Argentine Southern Chaco Serrano. Rev. Biol. Trop. 2017, 65, 953–961. [Google Scholar] [CrossRef]
- Flores, A.R.; Kazuz, Y.E.M.; García, V.E.; Ayala, B.A.; Garrido, R.E.R.; Aceves, M.A.C.; Sánchez, O.M.A.; Tejacal, A.I. Control de Anastrepha serpentina (Wiedemann) y calidad de los frutos de zapote mamey Pouteria sapota (Jacq) Moore & Stearn tratados con aire caliente forzado. Rev. Chapingo. Ser. Cienc. 2009, 15, 9–15. [Google Scholar]
- Pinson, E.P.; Tejada, L.O.; Toledo, J.; Enkerlin, W.; Hurtado, C.H.; Valle, J.; Pérez, J.N.; Liedo, P. Caracterización de la adaptación de anastrepha serpentina (wied.) (diptera: Tephritidae) a condiciones de cría masiva. Fol. Entomol. Mex. 2006, 45, 97–112. [Google Scholar]
- Alexander, M.; Kulminski, F.M.; Irina, V.; Culminskaya, K.G.; Arbeev, S.V.; Ukraintseva, J.R.; Carey, A.I. Date of eclosion modulates longevity: Insights across dietary-restriction gradients and female reproduction in the mexfly Anastrepha ludens. Exp. Gerontol. 2009, 44, 718–726. [Google Scholar] [CrossRef]
- Alonso, E.H. Sincronía Biológica, Relación Interespecífica y Análisis de Calidad Hospedera de Pouteria Buenaventurensis (Sapotacea) con Anastrepha Serpentina y Anastrepha Intermedia, n.sp. en Altos de Pacora. Maestría Thesis, Universidad de Panamá, Ciudad de Panamá, Panamá, 2000. Available online: http://up-rid.up.ac.pa/id/eprint/3949 (accessed on 15 April 2023).
- Omkar, R.S.; Pandey, P. Effect of temperature on development and immature survival of Zygogramma bicolorata (Coleoptera: Chrysomelidae) under laboratory conditions. Int. J. Trop. Insect Sci. 2008, 28, 130–135. [Google Scholar] [CrossRef]
- Chaverri, L.G.; Soto, M.J.; Jirón, L.F. Biology and ecology of Anastrepha obliqua (diptera: Tephritidae), plague of Anacardiaceae plants in tropical America. II. Mature stages. Agron. Mesoam. 2006, 10, 99–102. [Google Scholar] [CrossRef]
- Soto, M.J.; Chaverri, L.G.; Jirón, L.F. Notes on the biology and ecology of Anastrepha obliqua (Diptera: Tephritidae), pests of plants in Tropical América. I. Imnature forms. Agron. Mesoam. 2016, 8, 116–120. [Google Scholar] [CrossRef]
- Hernández, E.; Ruiz, M.L.; Toledo, J.; Montoya, P.; Liedo, P.; Aceituno, M.M.; Perales, H. A comparison of sexual competitiveness and demographic traits of Anastrepha obliqua (Macquart) (Diptera: Tephritidae) among fruit-associated populations. Bull. Entomol. Res. 2019, 109, 333–341. [Google Scholar] [CrossRef]
- Candanedo, M.; Villarreal, D.; Bernal, S. Uso de registros de temperatura máxima promedio de las estaciones meteorológicas de ETESA, para la creación de mapas de temperatura mediante el uso de programa ArcGIS. Rev. De Iniciación Científica 2020, 6, 9–14. [Google Scholar] [CrossRef]
- Kemp, W.P.; Bosch, J. Effect of Temperature on Osmia lignaria (Hymenoptera: Megachilidae) Prepupa–Adult Development, Survival, and Emergence. J. Econ. Entomol. 2005, 98, 1917–1923. [Google Scholar] [CrossRef]
- Bateman, M.A. The Ecology of Fruit Flies. Annu. Rev. Entomo. 1972, 17, 493–518. [Google Scholar] [CrossRef]
- Cruz, L.L.; Malo, E.A.; Rojas, J.C. Sex Pheromone of Anastrepha striata. J. Chem. Ecol. 2015, 41, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Péres, D.; Aluja, M. Anastrepha striata (Diptera: Tephritidae) Females That Mate with Virgin Males Live Longer. Ann. Entomol. Soc. Am. 2004, 97, 1336–1341. [Google Scholar] [CrossRef]
- García, A.E.C.; Martínez, A.J.O.; Gómez, P.L.M. Distribución Geográfica Potencial de Anastrepha Striata (Schiner 1868)(Diptera: Tephritidae) en Colombia; Documento de conferencia, III Congreso Colombiano de Zoología: Medellín, Colombia, 2010; Available online: https://dspace.tdea.edu.co/handle/tdea/1462 (accessed on 12 May 2023).
- Ferrer, S.Y.; Jacho, S.A.A.; Wilmer, R.; Zambrano, U.; Absalo, P.J.A.; Vázquez, P.F.; Herminio, A.; Zambrano, M.G.J.; Castillo, M.M.J.; Rosado, C.A.; et al. Invasiones Biológicas en Agroecosistemas de Ecuador Continental: Nicho Ecológico de Especies Exóticas y Cultivos Agrícolas Bajo Riesgo. Acta Biol. Colomb. 2021, 26, 352–364. [Google Scholar] [CrossRef]
| Species | Records | Train | Selected Model | Partial ROC | Omission Rate (<5%) | AICc | ∆AICc |
|---|---|---|---|---|---|---|---|
| Anastrepha grandis | 39 | 32 | M_0.9_F_t_Set7 | 0 | 0 | 392.882 | 0.000 |
| Anastrepha serpentina | 88 | 70 | M_0.4_F_lq_Set8 | 0 | 0 | 1017.849 | 0.000 |
| Anastrepha obliqua | 93 | 74 | M_4_F_l_Set7 | 0 | 0 | 1080.475 | 0.000 |
| Anastrepha striata | 150 | 125 | M_3_F_lt_Set7 | 0 | 0 | 1854.480 | 0.000 |
| A. grandis Neutral vs. Niño | A. grandis Neutral vs. Niña | A. serpentina Neutral vs. Niño | A. serpentina Neutral vs. Niña | A. obliqua Neutral vs. Niño | A. obliqua Neutral vs. Niña | A. striata Neutral vs. Niño | A. striata Neutral vs. Niña | |
|---|---|---|---|---|---|---|---|---|
| Range expansion | 14.72 | 11.74 | 1.17 | 1.98 | 1.99 | 3.72 | 18.91 | 22.17 |
| No occupancy | 9.41 | 12.38 | 10.14 | 9.33 | 9.77 | 8.04 | 9.82 | 6.56 |
| No change | 61.53 | 59.38 | 74.57 | 74.29 | 86.4 | 86.59 | 66.84 | 68.44 |
| Range contraction | 14.33 | 16.47 | 14.11 | 14.38 | 1.77 | 1.63 | 4.41 | 2.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degracia, A.B.; Jiménez, J.Á.; Alvarado, A.B.; Valdespino, R.A.; Altamiranda-Saavedra, M. Evaluation of the Effect of the ENSO Cycle on the Distribution Potential of the Genus Anastrepha of Horticultural Importance in the Neotropics and Panama. Insects 2023, 14, 714. https://doi.org/10.3390/insects14080714
Degracia AB, Jiménez JÁ, Alvarado AB, Valdespino RA, Altamiranda-Saavedra M. Evaluation of the Effect of the ENSO Cycle on the Distribution Potential of the Genus Anastrepha of Horticultural Importance in the Neotropics and Panama. Insects. 2023; 14(8):714. https://doi.org/10.3390/insects14080714
Chicago/Turabian StyleDegracia, Arturo Batista, Julián Ávila Jiménez, Anovel Barba Alvarado, Randy Atencio Valdespino, and Mariano Altamiranda-Saavedra. 2023. "Evaluation of the Effect of the ENSO Cycle on the Distribution Potential of the Genus Anastrepha of Horticultural Importance in the Neotropics and Panama" Insects 14, no. 8: 714. https://doi.org/10.3390/insects14080714
APA StyleDegracia, A. B., Jiménez, J. Á., Alvarado, A. B., Valdespino, R. A., & Altamiranda-Saavedra, M. (2023). Evaluation of the Effect of the ENSO Cycle on the Distribution Potential of the Genus Anastrepha of Horticultural Importance in the Neotropics and Panama. Insects, 14(8), 714. https://doi.org/10.3390/insects14080714