Attraction of Sweet Potato Whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae), and Two Generalist Predators to Green Leaf Volatile Compounds
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Chemicals
2.3. Y-Tube Olfactometer
2.4. Statistical Analysis
3. Results
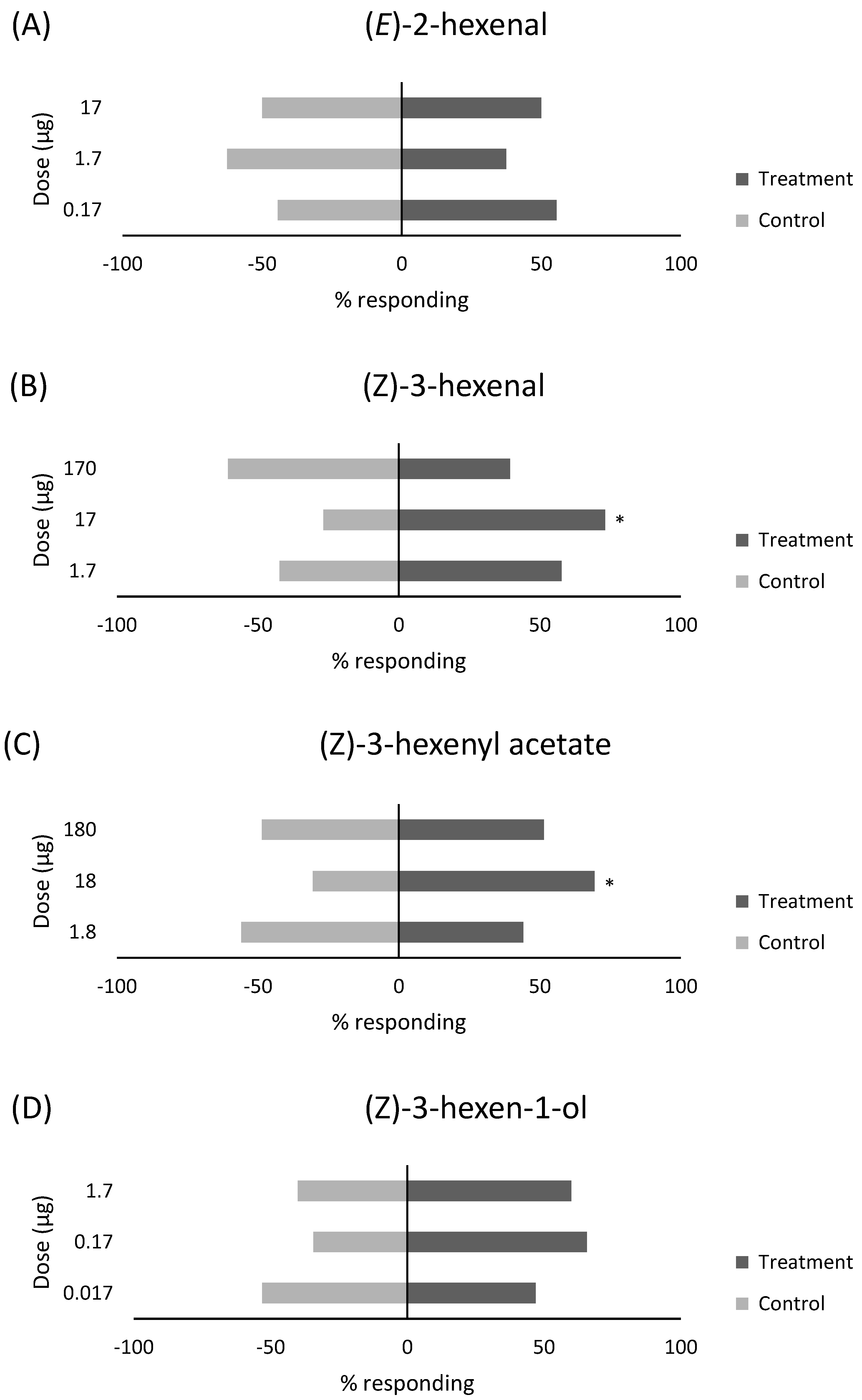
3.1. Response of Whiteflies to Single Compounds
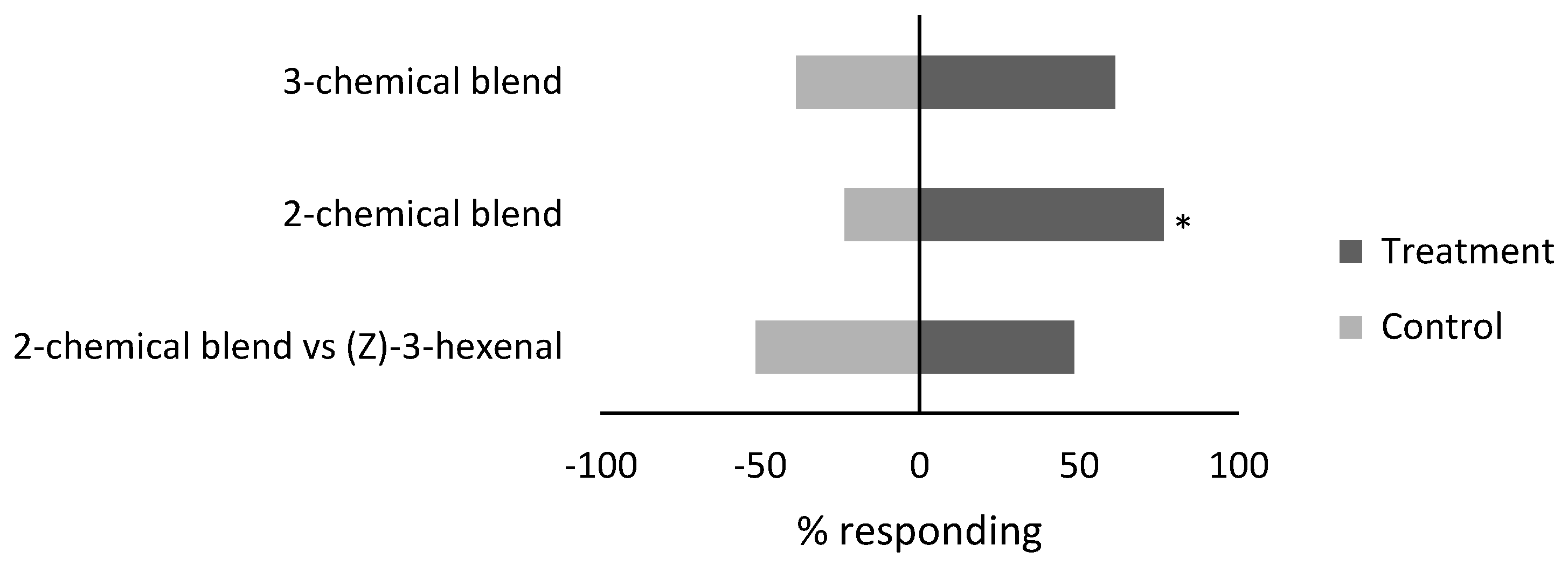
3.2. Response of Whiteflies to Compound Blends
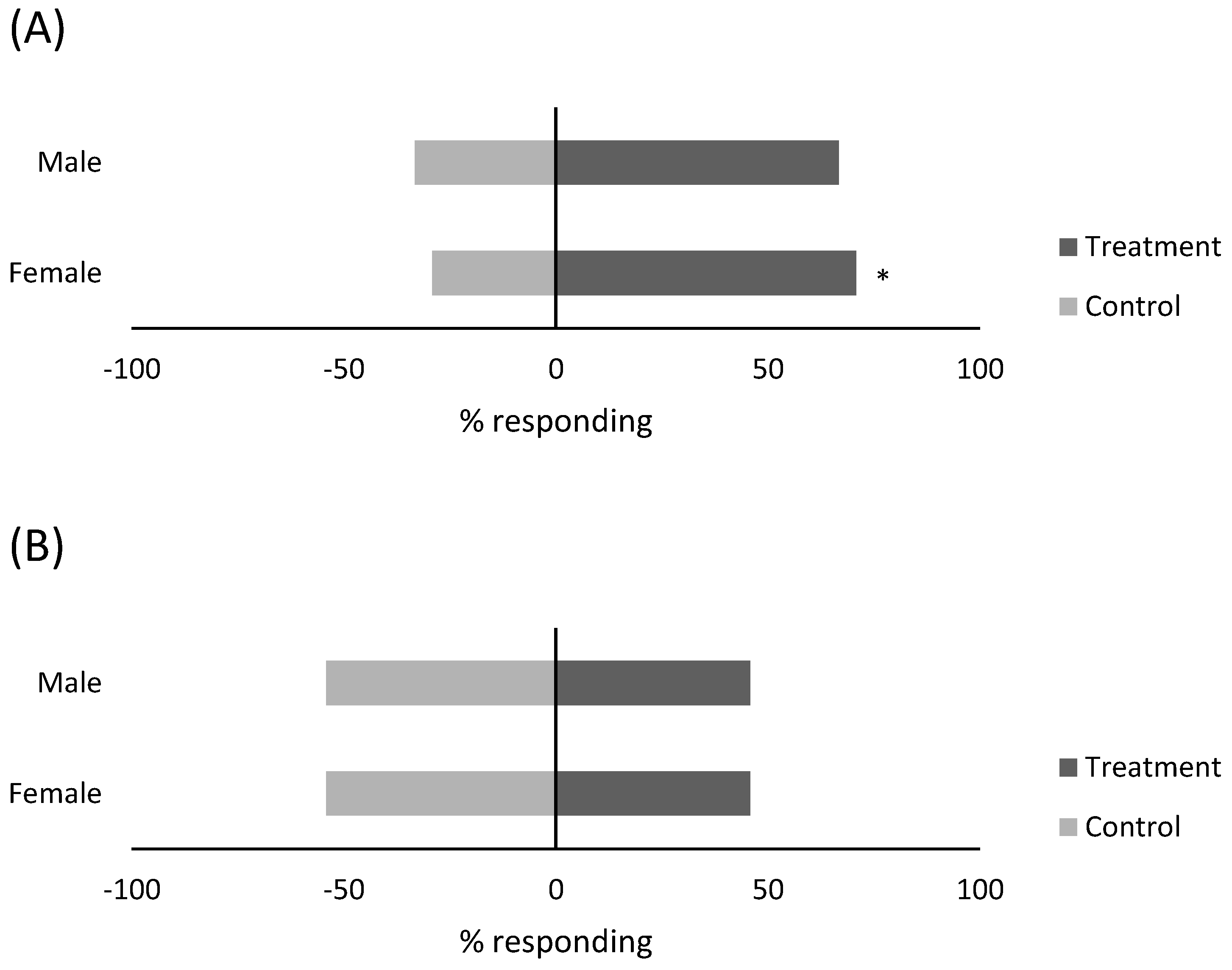
3.3. Response of Predators to Compound Blend
4. Discussions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oliveira, M.R.V.; Henneberry, T.E.; Anderson, P. History, current status, and collaborative research projects for Bemisia tabaci. Crop Prot. 2001, 20, 709–723. [Google Scholar] [CrossRef]
- Simmons, A.M.; Abd-Rabou, S. Population of the sweetpotato whitefly in response to different rates of three sulfur-containing fertilizers on ten vegetable crops. Int. J. Veg. Sci. 2008, 15, 57–70. [Google Scholar] [CrossRef]
- Barman, A.K.; Roberts, P.M.; Prostko, E.P.; Toews, M.D. Seasonal occurrence and reproductive suitability of weed hosts for sweetpotato whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae), in South Georgia. J. Entomol. Sci. 2022, 57, 1–11. [Google Scholar] [CrossRef]
- Jones, D.R. Plant viruses transmitted by whiteflies. Eur. J. Plant Pathol. 2003, 109, 195–219. [Google Scholar] [CrossRef]
- Birdsall, S.L.; Ritter, D.; Cason, P.L. Economic impact of the silverleaf whitefly in Imperial Valley. In Supplement to the 5-Year National Research and Action Plan; Henneberry, T.J., Toscano, N.C., Faust, R.M., Coppedge, J.R., Eds.; Third Annual Review, San Diego, CA, USA; National Technical Information Service: Springfield, VA, USA, 1995; p. 162. [Google Scholar]
- Ellsworth, P.C.; Tronstad, R.; Leser, J.; Goodell, P.B.; Godfrey, L.D.; Henneberry, T.J.; Hendrix, D.; Brushwood, D.; Naranjo, S.E.; Castle, S.; et al. Sticky Cotton Sources and Solutions; IPM Series No. 13; University of Arizona Cooperative Extension: Tucson, AZ, USA, 1999; Available online: https://cals.arizona.edu/crop/cotton/insects/wf/stickycss.pdf (accessed on 27 September 2024).
- Abubakar, M.; Koul, B.; Chandrashekar, K.; Raut, A.; Yadav, D. Whitefly (Bemisia tabaci) management (WFM) strategies for sustainable agriculture: A review. Agriculture 2022, 12, 1317. [Google Scholar] [CrossRef]
- Perier, J.D.; Cremonez, P.S.; Champagne, D.E.; Simmons, A.M.; Riley, D.G. Whiteflies at the intersection of polyphagy and insecticide resistance. Ann. Entomol. Soc. Amer. 2022, 115, 401–416. [Google Scholar] [CrossRef]
- Nyoike, T.W.; Liburd, O.E. Effect of living (buckwheat) and UV reflective mulches with and without imidacloprid on whiteflies, aphids and marketable yields of zucchini squash. Int. J. Pest. Manag. 2010, 56, 31–39. [Google Scholar] [CrossRef]
- Hilje, L.; Costa, H.S.; Stansly, P.A. Cultural practices for managing Bemisia tabaci and associated viral diseases. Crop Prot. 2001, 20, 801–812. [Google Scholar] [CrossRef]
- Eakteiman, G.; Moses-Koch, R.; Moshitzky, P.; Mestre-Rincon, N.; Vassão, D.G.; Luck, K.; Sertchook, R.; Malka, O.; Morin, S. Targeting detoxification genes by phloem-mediated RNAi: A new approach for controlling phloem-feeding insect pests. Insect Biochem. Mol. Biol. 2018, 100, 10–21. [Google Scholar] [CrossRef]
- Shukla, A.K.; Upadhyay, S.K.; Mishra, M.; Saurabh, S.; Singh, R.; Singh, H.; Thakur, N.; Rai, P.; Pandey, P.; Hans, A.L.; et al. Expression of an insecticidal fern protein in cotton protects against whitefly. Nat. Biotechnol. 2016, 34, 1046–1051. [Google Scholar] [CrossRef]
- Eslamizadeh, R.; Sajap, A.S.; Omar, D.; Binti Adam, N.A. Evaluation of different isolates of entomopathogenic fungus, Paecilomyces fumosoroseus (Deuteromycotina: Hyphomycetes) against Bemisia tabaci (Hemiptera: Aleyrodidae). Biocontrol Plant Prot. 2015, 2, 82–91. [Google Scholar]
- Mound, L.A. Studies on the olfaction and colour sensitivity of Bemisia tabaci (Genn.) (Homoptera, Aleyrodidae). Entomol. Exp. Appl. 1962, 5, 99–104. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, Y.; Zhang, H.; Li, D.; Zheng, Z.; Song, F. Overexpression of a rice defense-related F-box protein gene OsDRF1 in tobacco improves disease resistance through potentiation of defense gene expression. Physiol. plant. 2008, 134, 440–452. [Google Scholar] [CrossRef]
- Johnston, N.; Paris, T.; Paret, M.L.; Freeman, J.; Martini, X. Repelling whitefly (Bemisia tabaci) using limonene-scented kaolin: A novel pest management strategy. Crop Prot. 2022, 154, 105905. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, H.A.; Lee, H.J.; Choi, J.Y.; Lee, S.W.; Hong, S.S.; Jang, M.J. Push-pull strategy for control of sweet-potato whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae) in a tomato greenhouse. Korean. Soc. Appl. Entomol. 2020, 58, 209–218. [Google Scholar]
- Du, W.; Han, X.; Wang, Y.; Qin, Y. A primary screening and applying of plant volatiles as repellents to control whitefly Bemisia tabaci (Gennadius) on tomato. Sci. Rep. 2016, 6, 22140. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, W.; Zhang, S.; Chen, H.; Su, H.; Jing, T.; Yang, Y. Behavioral responses of Bemisia tabaci mediterranean cryptic species to three host plants and their volatiles. Insects 2022, 13, 703. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, S.; Qin, Y.; Zhang, S.; Gao, Z.; Dang, Z.; Pan, W. Identification of plant chemicals attracting and repelling whiteflies. Arthropod-Plant Inter. 2014, 8, 183–190. [Google Scholar] [CrossRef]
- Schlaeger, S.; Pickett, J.A.; Birkett, M.A. Prospects for management of whitefly using plant semiochemicals, compared with related pests. Pest. Manag. Sci. 2018, 74, 2405–2411. [Google Scholar] [CrossRef]
- Li, S.; Li, H.; Zhou, Q.; Zhang, F.; Desneux, N.; Wang, S.; Shi, L. Essential oils from two aromatic plants repel the tobacco whitefly Bemisia tabaci. J. Pest. Sci. 2022, 95, 971–982. [Google Scholar] [CrossRef]
- Deletre, E.; Matu, F.K.; Murungi, L.K.; Mohamed, S. Repellency potential of tomato herbivore-induced volatiles against the greenhouse whitefly (Trialeurodes vaporariorum) (Hemiptera: Aleyrodidae). J. Econ. Entomol. 2022, 115, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Wagan, T.A.; Cai, W.; Hua, H. Repellency, toxicity, and anti-oviposition of essential oil of Gardenia jasminoides and its four major chemical components against whiteflies and mites. Sci. Rep. 2018, 8, 9375. [Google Scholar] [CrossRef] [PubMed]
- Nomikou, M.; Meng, R.; Schraag, R.; Sabelis, M.W.; Janssen, A. How predatory mites find plants with whitefly prey. Exp. Appl. Acarol. 2005, 36, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; Zhao, C.; Wu, Z.Y.; Liu, G.F.; Yu, X.P.; Zhang, P.J. Whitefly-induced tomato volatiles mediate host habitat location of the parasitic wasp Encarsia formosa, and enhance its efficacy as a bio-control agent. Pest. Manage Sci. 2021, 77, 749–757. [Google Scholar] [CrossRef]
- Birkett, M.A.; Chamberlain, K.; Guerrieri, E.; Pickett, J.A.; Wadhams, L.J.; Yasuda, T. Volatiles from whitefly-infested plants elicit a host-locating response in the parasitoid, Encarsia formosa. J. Chem. Ecol. 2003, 29, 1589–1600. [Google Scholar] [CrossRef]
- Goolsby, J.A.; Hoelmer, K.A.; Gould, J.R. Biological Control of Silverleaf Whitefly in the United States. In Contributions of Classical Biological Control to the U.S. Food Security, Forestry, and Biodiversity; Van Driesche, R.G., Winston, R.L., Perring, T.M., Lopez, V.M., Eds.; FHAAST-2019-05; USDA Forest Service: Morgantown, WV, USA, 2022; pp. 59–72. Available online: https://bugwoodcloud.org/resource/files/23194.pdf (accessed on 1 January 2024).
- Gould, J.; Hoelmer, J.; Goolsby, J. Classical Biological Control of Bemisia tabaci in the United States: A Review of Interagency Research and Implementation; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Kheirodin, A.; Simmons, A.M.; Legaspi, J.C.; Grabarczyk, E.E.; Toews, M.D.; Roberts, P.M.; Chong, J.H.; Snyder, W.E.; Schmidt, J.M. Can generalist predators control Bemisia tabaci? Insects 2020, 11, 823. [Google Scholar] [CrossRef]
- Liu, T.X.; Stansly, P.A. Searching and feeding behavior of Nephaspis oculatus and Delphastus catalinae (Coleoptera: Coccinellidae), predators of Bemisia argentifolii (Homoptera: Aleyrodidae). Environ. Entomol. 1999, 28, 901–906. [Google Scholar] [CrossRef]
- Liu, T.X. Life history and life table analysis of the whitefly predator Delphastus catalinae (Coleoptera: Coccinellidae) on collards. Insect Sci. 2005, 12, 129–135. [Google Scholar] [CrossRef]
- Perring, T.M.; Stansly, P.A.; Liu, T.X.; Smith, H.A.; Andreason, S.A. Whiteflies: Biology, ecology, and management. In Sustainable Management of Arthropod Pests of Tomato; Wakil, W., Perring, T., Brust, G., Eds.; Academic Press: London, UK, 2018. [Google Scholar]
- Hoelmer, K.; Pickett, C. Geographic origin and taxonomic history of Delphastus spp.(Coleoptera: Coccinellidae) in commercial culture. Biocontrol Sci. Technol. 2003, 13, 529–535. [Google Scholar] [CrossRef]
- Pérez-Hedo, M.; Gallego, C.; Roda, A.; Kostyk, B.; Triana, M.; Alférez, F.; Stansly, P.A.; Qureshi, J.; Urbaneja, A. Biological traits of the predatory mirid Macrolophus praeclarus, a candidate biocontrol agent for the Neotropical region. Bull. Entomol. Res. 2021, 111, 429–437. [Google Scholar] [CrossRef]
- Roda, A.; Castillo, J.; Allen, C.; Urbaneja, A.; Pérez-Hedo, M.; Weihman, S.; Stansly, P.A. Biological control potential and drawbacks of three zoophytophagous mirid predators against Bemisia tabaci in the United States. Insects 2020, 11, 670. [Google Scholar] [CrossRef] [PubMed]
- Serra, C.; Lenteren, J.V. Biological control in the Dominican Republic. In Biological Control in Latin America and the Caribbean: Its rich History and Bright Future; CABI: Wallingford, UK, 2020; pp. 199–219. [Google Scholar]
- Roitberg, B.D. Why pest management needs behavioral ecology and vice versa. Entomol. Res. 2007, 37, 14–18. [Google Scholar] [CrossRef]
- Saad, K.A.; Mohamad, M.N.; Shukri, M.; Mirad, R.; Mansour, S.A.A.; Abuzid, I. Behavioral responses of whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae), in relation to sex and infestation status of their host plants. Acad. J. Entomol. 2013, 6, 95–99. [Google Scholar]
- Silva, D.B.; Urbaneja, A.; Pérez-Hedo, M. Response of mirid predators to synthetic herbivore-induced plant volatiles. Entomol. Exp. Et Appl. 2021, 169, 125–132. [Google Scholar] [CrossRef]
- Deletre, E.; Maelle, M.; Chantal, M.; Fabrice, C.; Thibaud, M. Behavioral Response of Bemisia tabaci (Hemiptera: Aleyrodidae) to 20 Plant Extracts. J. Econ. Entomol. 2015, 108, 1890–1901. [Google Scholar]
- Singh, G.; Aggarwal, N. Biology of whitefly, Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) on brinjal. J. Entomol. Res. 2023, 47, 563–568. [Google Scholar] [CrossRef]
- Veronesi, E.; Wratten, S.; van Koten, C.; Goldson, S. Potential of the mirid bug Engytatus nicotianae, and the parasitic wasp Tamarixia triozae for the biological control of the tomato-potato psyllid; a cage greenhouse assay. N. Z. J. Crop Hortic. Sci. 2022, 1–8. [Google Scholar] [CrossRef]
- Hadjistylli, M.; Roderick, G.; Brown, J. Global population structure of a worldwide pest and virus vector: Genetic diversity and population history of the Bemisia tabaci sibling species group. PLoS ONE 2016, 11, e0165105. [Google Scholar] [CrossRef]
- Jahan, S.; Lee, G.; Lee, S.; Lee, K. Acquisition of Tomato yellow leaf curl virus enhances attraction of Bemisia tabaci to green light emitting diodes. J. Asia-Pac. Entomol. 2014, 17, 79–82. [Google Scholar] [CrossRef]
- Anderson, P.; Anton, S. Experience-based modulation of behavioural responses to plant volatiles and other sensory cues in insect herbivores. Plant Cell Environ. 2014, 37, 1826–1835. [Google Scholar] [CrossRef]
- Cha, D.; Hesler, S.; Park, S.; Adams, T.; Zack, R.; Rogg, H.; Loeb, G.; Landolt, P. Simpler is better: Fewer non-target insects trapped with a four-component chemical lure vs. a chemically more complex food-type bait for D. rosophila suzukii. Entomol. Exp. Et Appl. 2015, 154, 251–260. [Google Scholar] [CrossRef]
- Xig, J.; Ono, M.; Kuroda, A.; Obi, K.; Sato, K.; Imamura, T. Kinetic study of the daytime atmospheric fate of (Z)-3-hexenal. J. Phys. Chem. 2012, 116, 8523–8529. [Google Scholar]
- Matsui, K.; Sugimoto, K.; Mano, J.I.; Ozawa, R.; Takabayashi, J. Differential metabolisms of green leaf volatiles in injured and intact parts of a wounded leaf meet distinct ecophysiological requirements. PLoS ONE 2012, 7, e36433. [Google Scholar] [CrossRef] [PubMed]
- Dumont, F.; Lucas, É.; Alomar, O. Oviposition behavior of the mirid Macrolophus pygmaeus under risk of intraguild predation and cannibalism. Insect Sci. 2021, 28, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Moayeri, H.; Ashouri, A.; Brødsgaard, H.; Enkegaard, A. Males of the predatory mirid bug Macrolophus caliginosus exploit plant volatiles induced by conspecifics as a sexual synomone. Entomol. Exp. Et Appl. 2007, 123, 49–55. [Google Scholar] [CrossRef]
- Legaspi, J.; Simmons, A.; Legaspi, B. Prey preference by Delphastus catalinae (Coleoptera: Coccinellidae) on Bemisia argentifolii (Homoptera: Aleyrodidae): Effects of plant species and prey stages. Fla. Entomol. 2006, 89, 218–222. [Google Scholar] [CrossRef]
| Compound | CAS Number | Dilution Factor | Dose (µg) | Responses | Attraction Index | ||
|---|---|---|---|---|---|---|---|
| Treatment | Control | No Response | |||||
| (E)-2-hexenal | 1335-39-3 | 0.01 | 17 | 15 | 15 | 12 | 0.00 |
| 0.001 | 1.7 | 12 | 20 | 8 | −0.25 | ||
| 0.0001 | 0.17 | 20 | 16 | 4 | 0.11 | ||
| (Z)-3-hexenal | 6789-80-6 | 0.1 | 170 | 13 | 20 | 7 | −0.21 |
| 0.01 | 17 | 41 | 15 | 4 | 0.46 | ||
| 0.001 | 1.7 | 30 | 22 | 8 | 0.15 | ||
| (Z)-3-hexenyl acetate | 1708-82-3 | 0.1 | 180 | 18 | 17 | 5 | 0.03 |
| 0.01 | 18 | 25 | 11 | 4 | 0.39 | ||
| 0.001 | 1.8 | 15 | 19 | 6 | −0.12 | ||
| (Z)-3-hexen-1-ol | 544-12-7 | 0.001 | 1.7 | 21 | 14 | 5 | 0.2 |
| 0.0001 | 0.17 | 23 | 12 | 5 | 0.31 | ||
| 0.00001 | 0.017 | 23 | 26 | 11 | −0.06 | ||
| Blend | |||||||
| 3-chemical blend vs. clean air | - | - | 19 | 12 | 9 | 0.23 | |
| 2-chemical blend vs. clean air | - | - | 26 | 8 | 6 | 0.53 | |
| 2-chemical blend vs. (Z)-3-hexenal | - | - | 17 | 18 | 5 | −0.03 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaffke, A.M.; Miller, N.W.; Sharma, A.; Allan, S.A. Attraction of Sweet Potato Whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae), and Two Generalist Predators to Green Leaf Volatile Compounds. Insects 2024, 15, 750. https://doi.org/10.3390/insects15100750
Gaffke AM, Miller NW, Sharma A, Allan SA. Attraction of Sweet Potato Whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae), and Two Generalist Predators to Green Leaf Volatile Compounds. Insects. 2024; 15(10):750. https://doi.org/10.3390/insects15100750
Chicago/Turabian StyleGaffke, Alexander M., Neil W. Miller, Anamika Sharma, and Sandra A. Allan. 2024. "Attraction of Sweet Potato Whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae), and Two Generalist Predators to Green Leaf Volatile Compounds" Insects 15, no. 10: 750. https://doi.org/10.3390/insects15100750









