Insecticidal and Repellent Activity of Essential Oils from Seven Different Plant Species against Tribolium castaneum (Coleoptera: Tenebrionidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Collection of Plant Materials and Extraction of Essential Oils
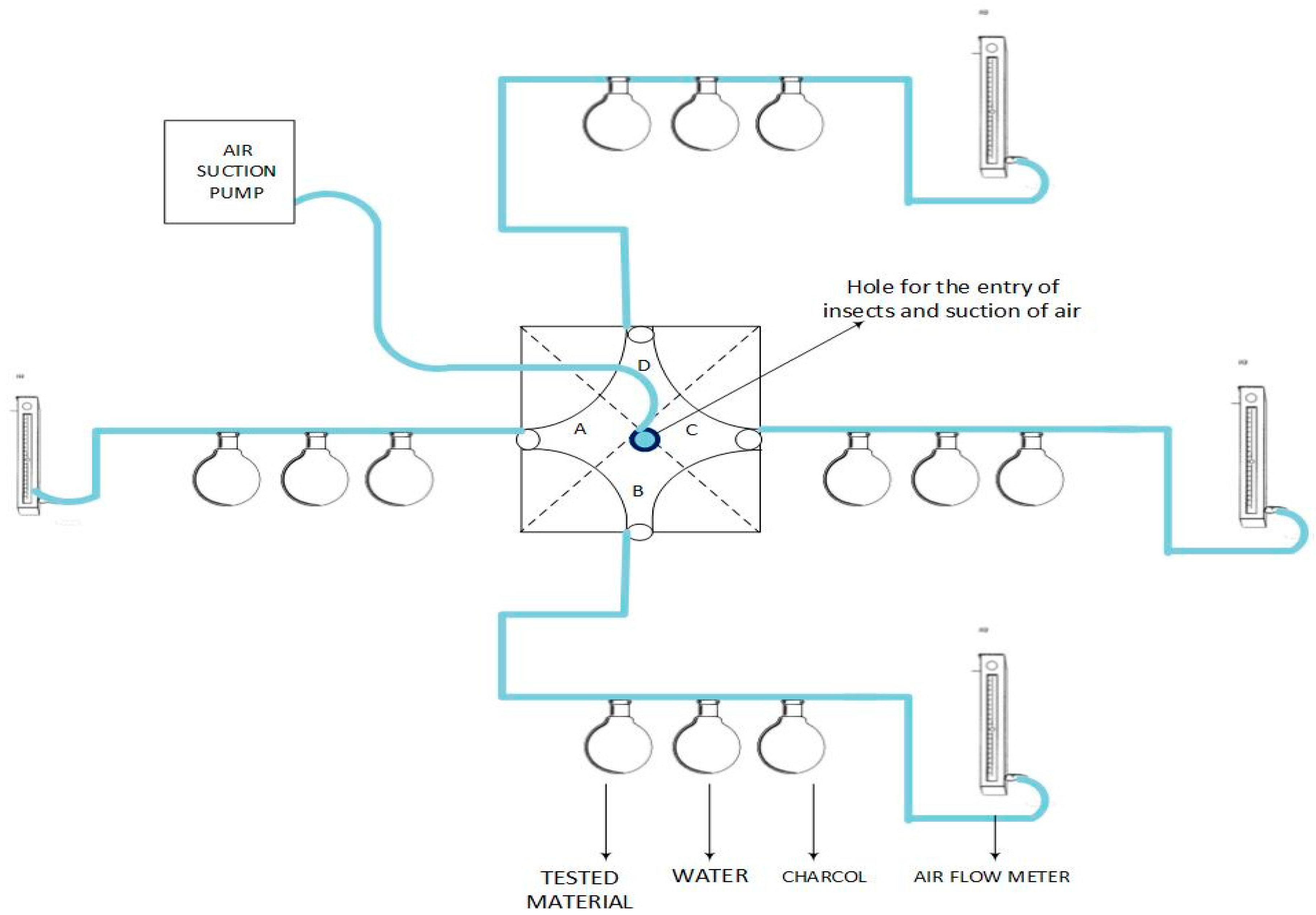
2.3. Fumigation Bioassay
2.4. Repellency Bioassays
2.5. Statistical Analysis
3. Results
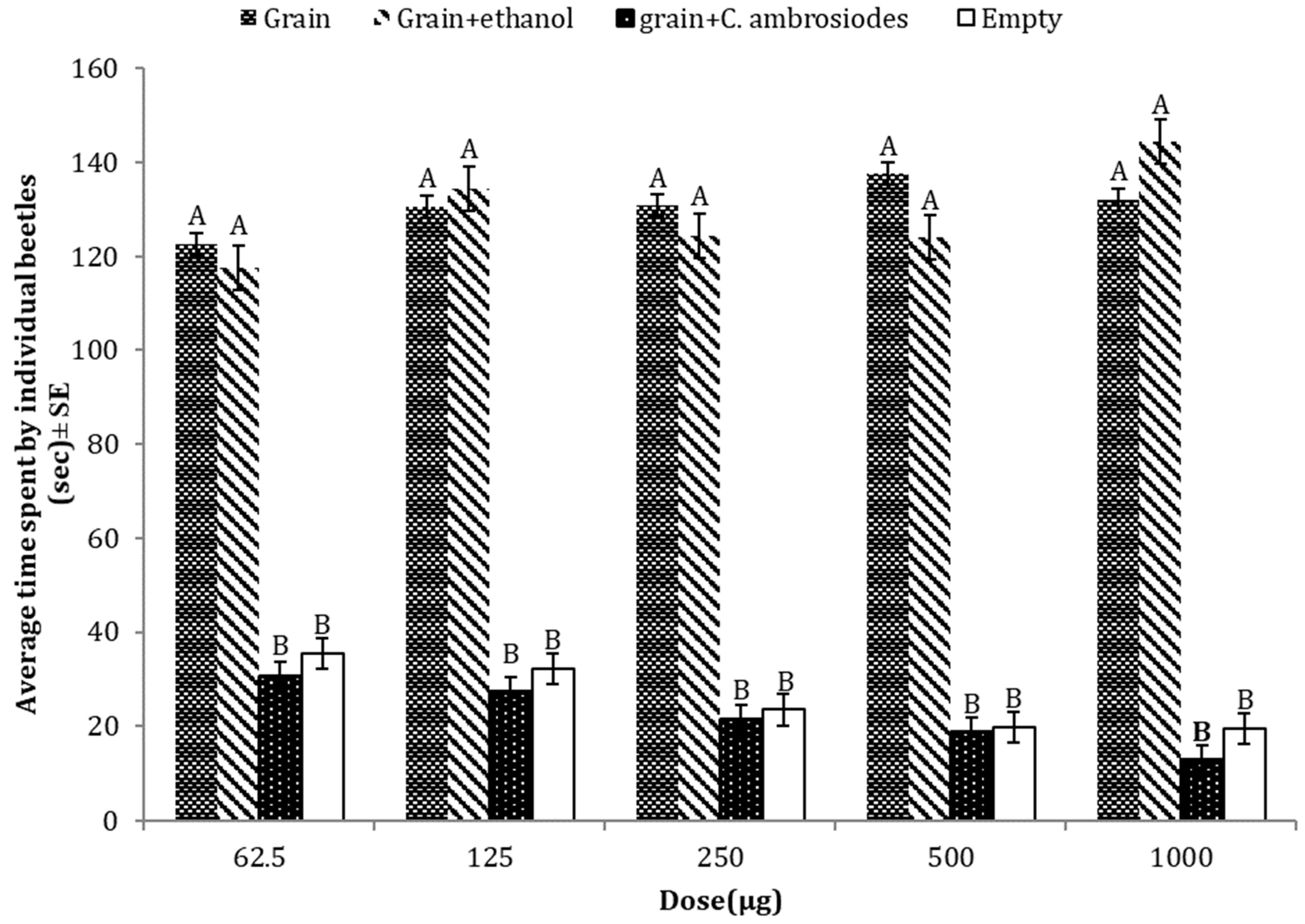
3.1. Repellent Effects of C. ambrosiodes against T. castaneum
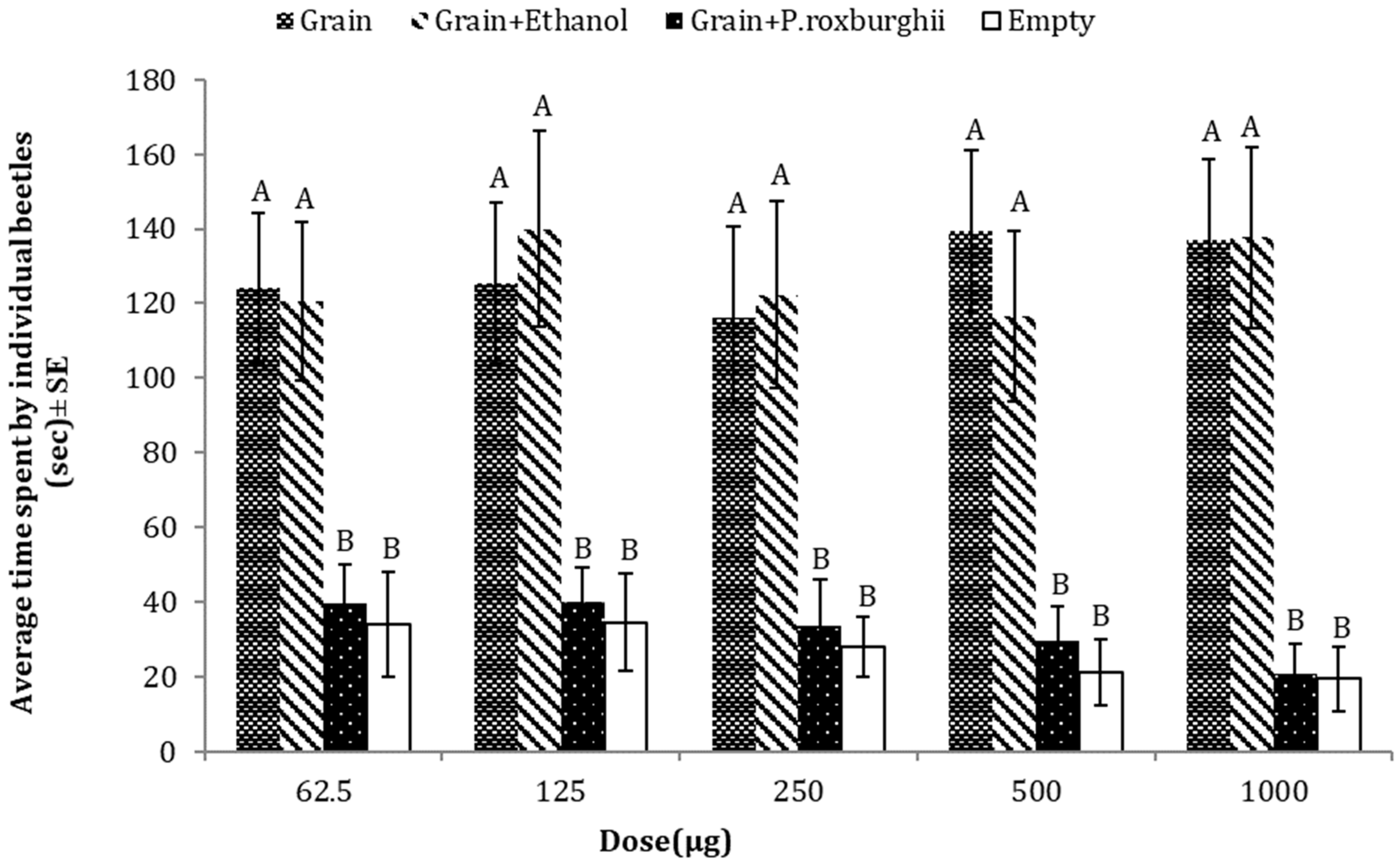
3.2. Repellent Effects of P. roxburghii against T. castaneum
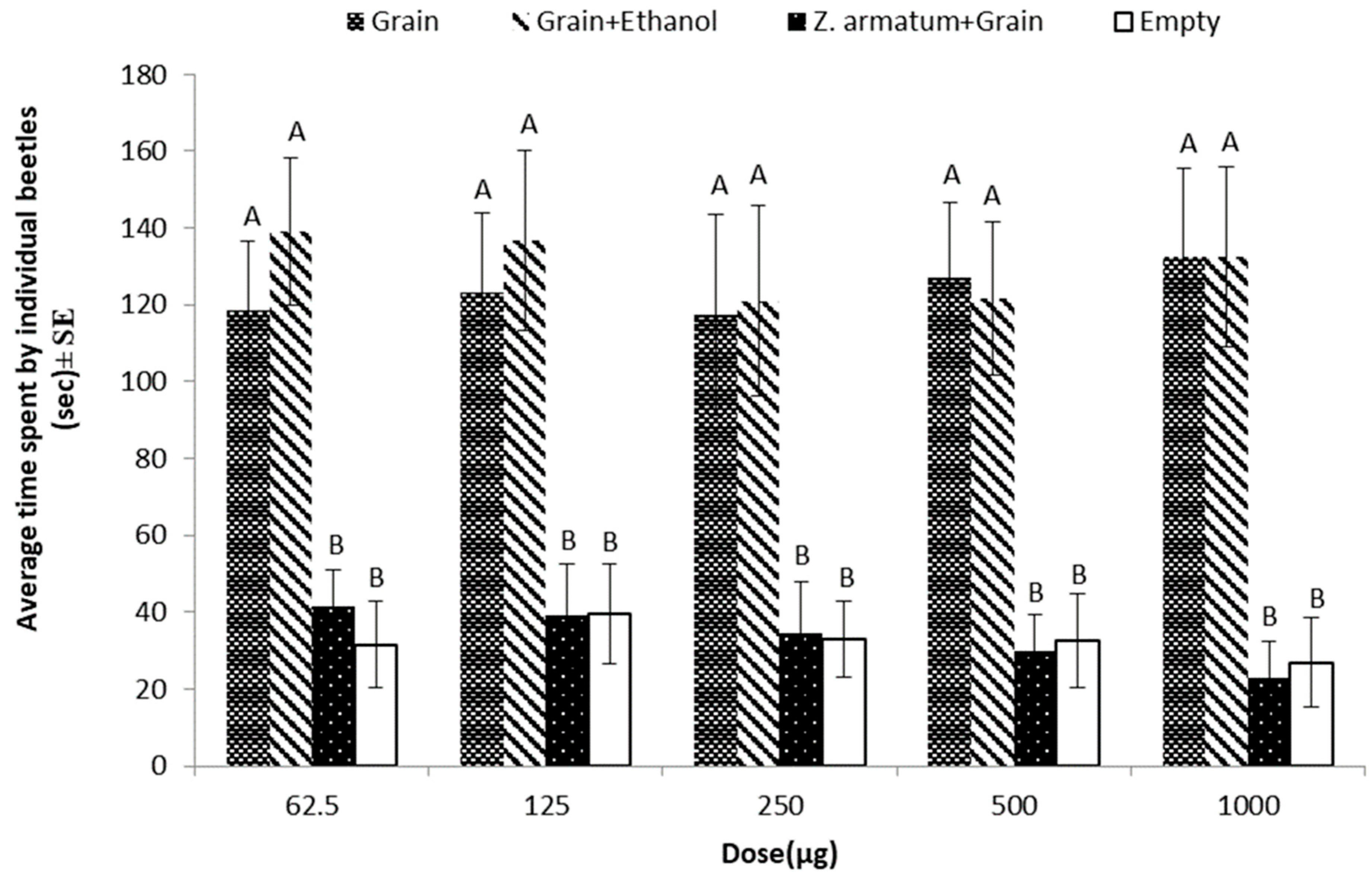
3.3. Repellent Effects of Z. armatum against T. castaneum
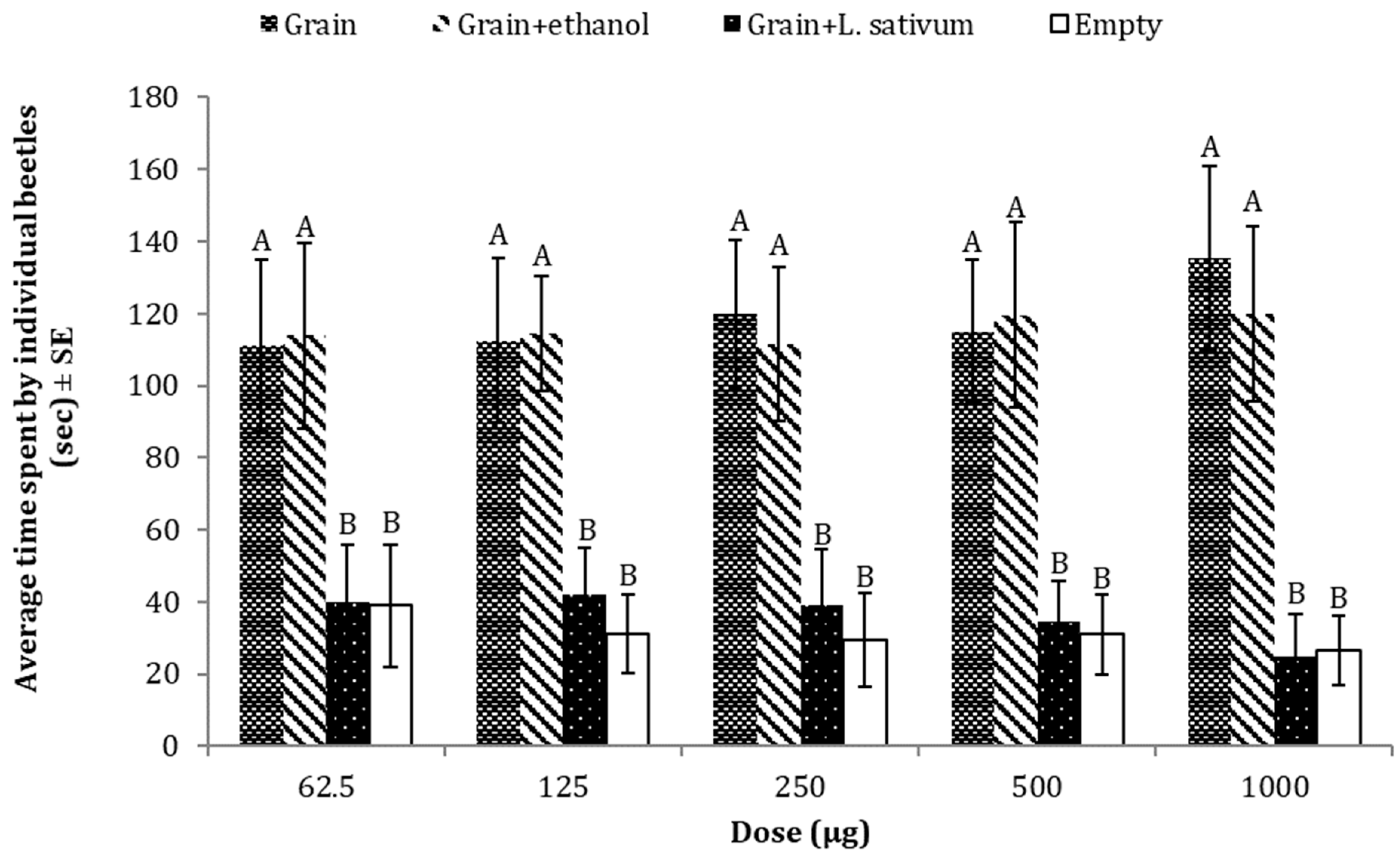
3.4. Repellent Effects of L. sativum against T. castaneum
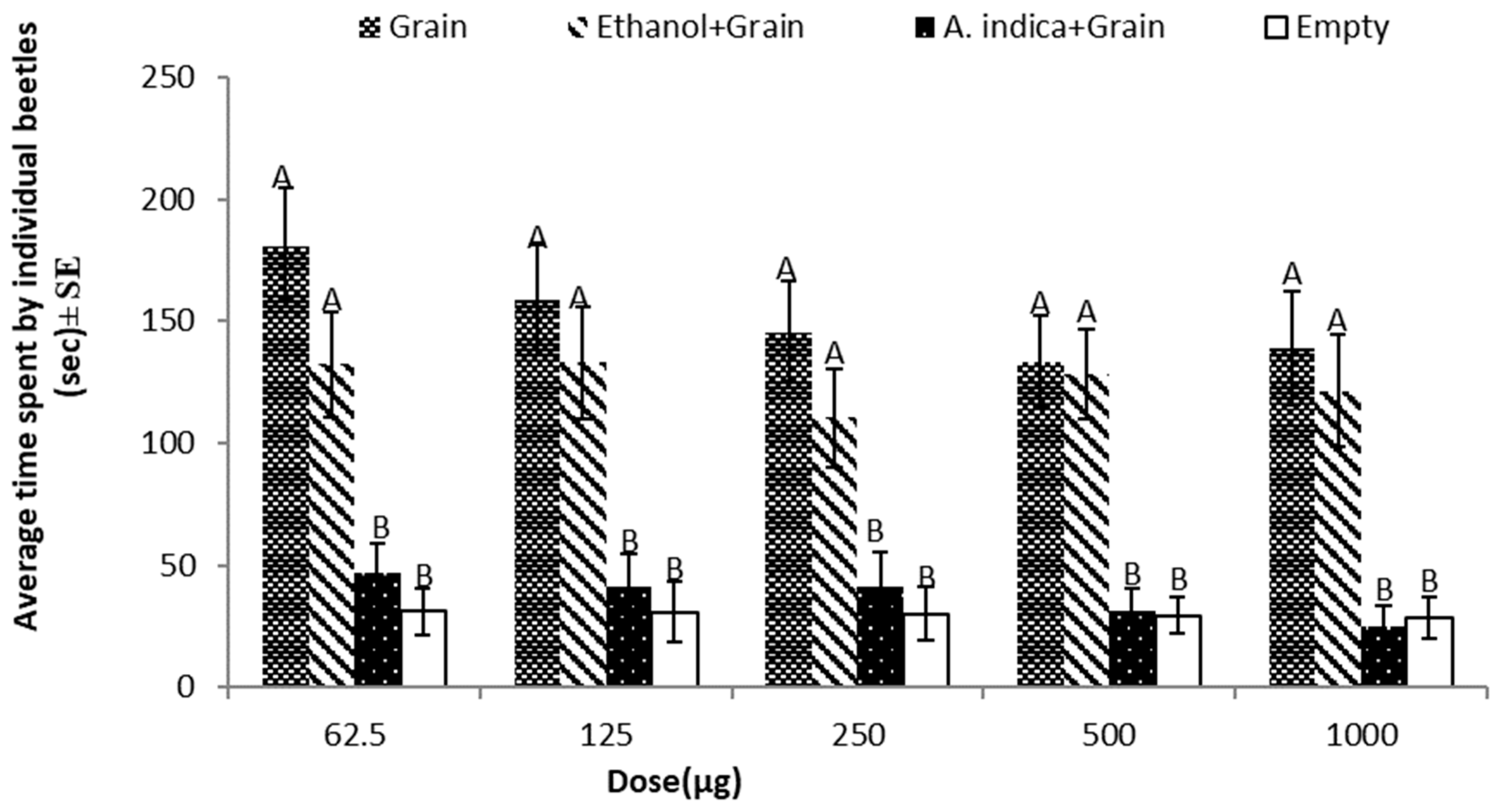
3.5. Repellent Effects of A. indica against T. castaneum
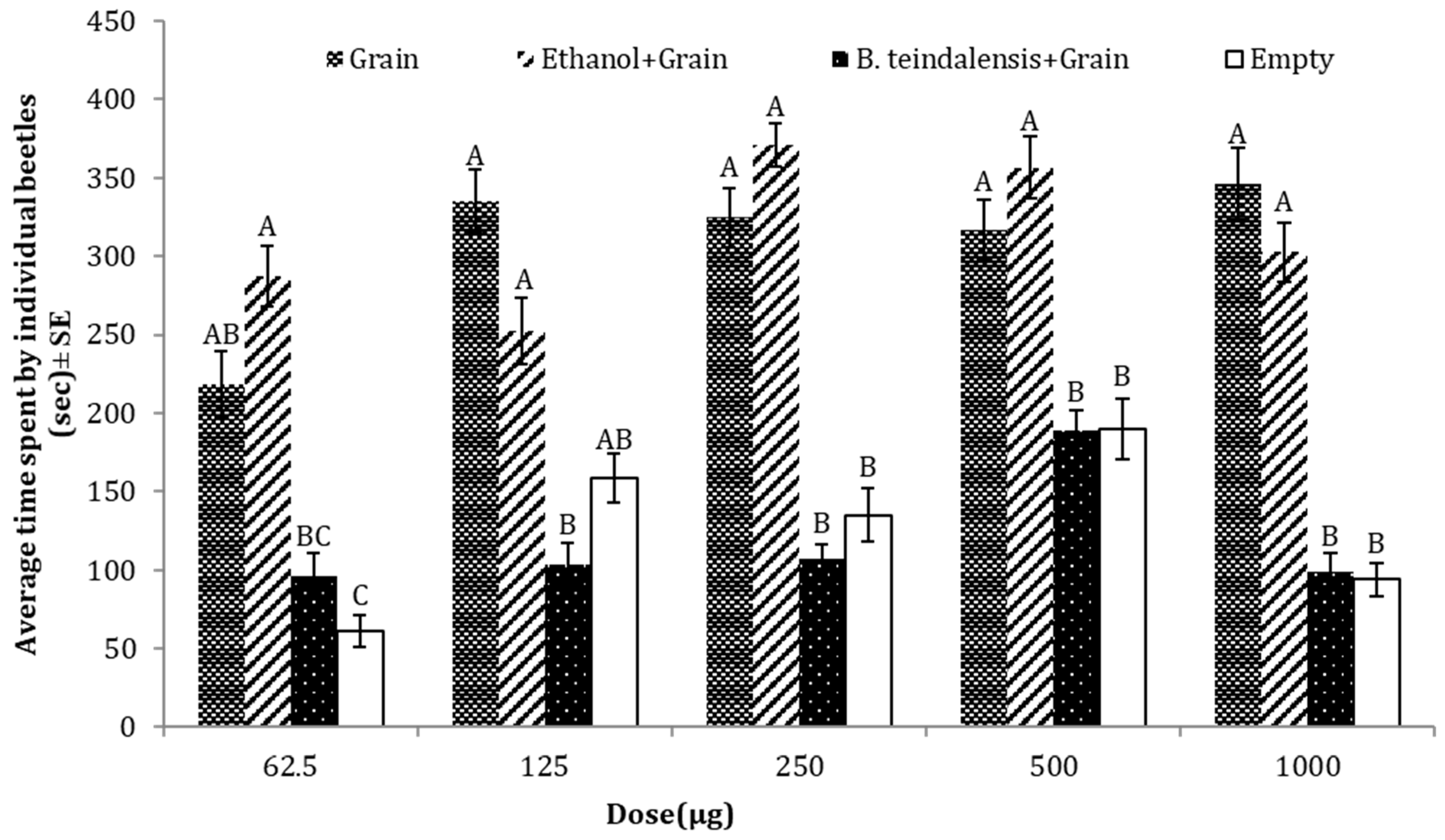
3.6. Repellent Effects of B. teindalensis against T. castaneum
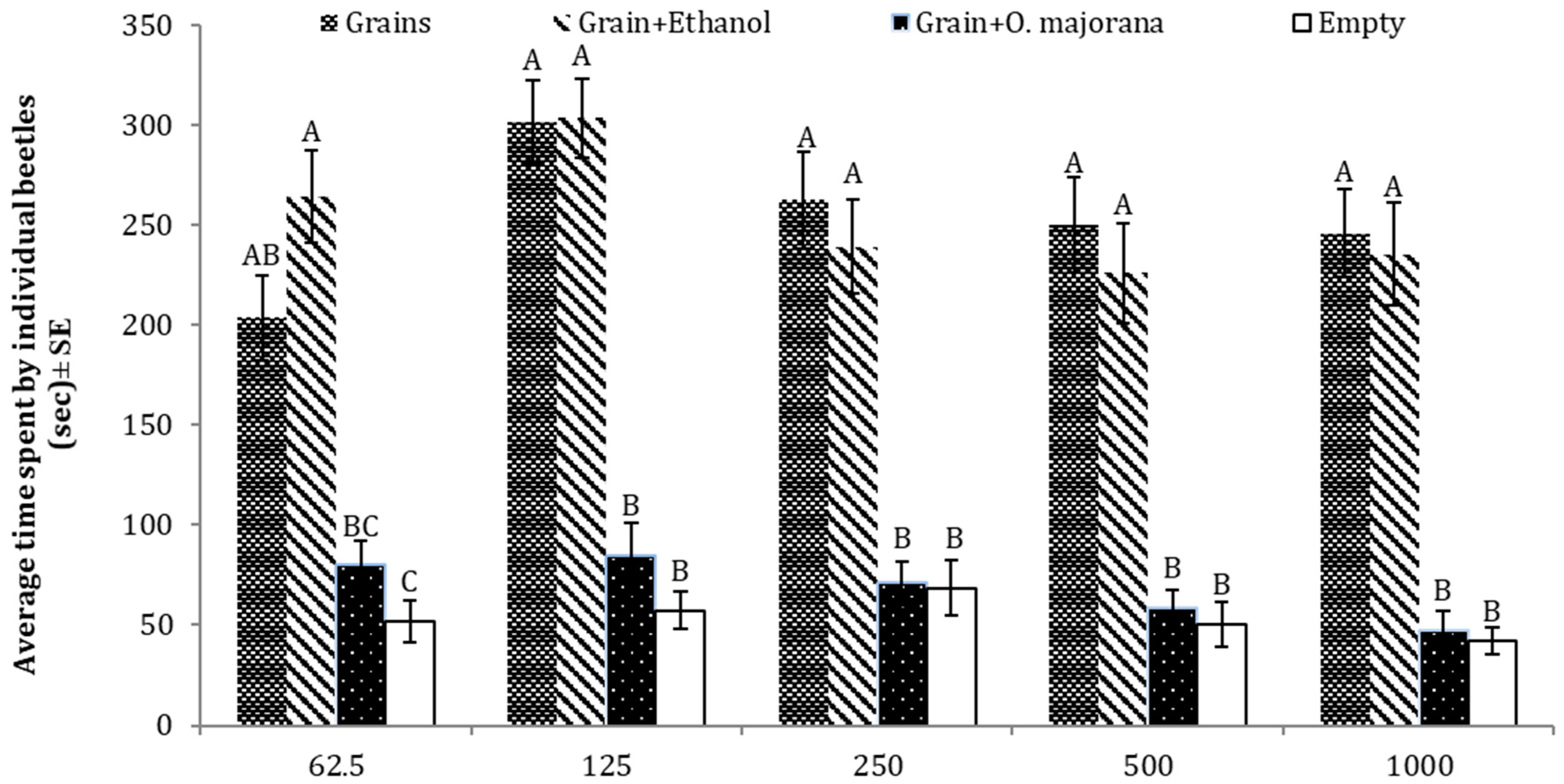
3.7. Repellent Effects of O. majorana against T. castaneum
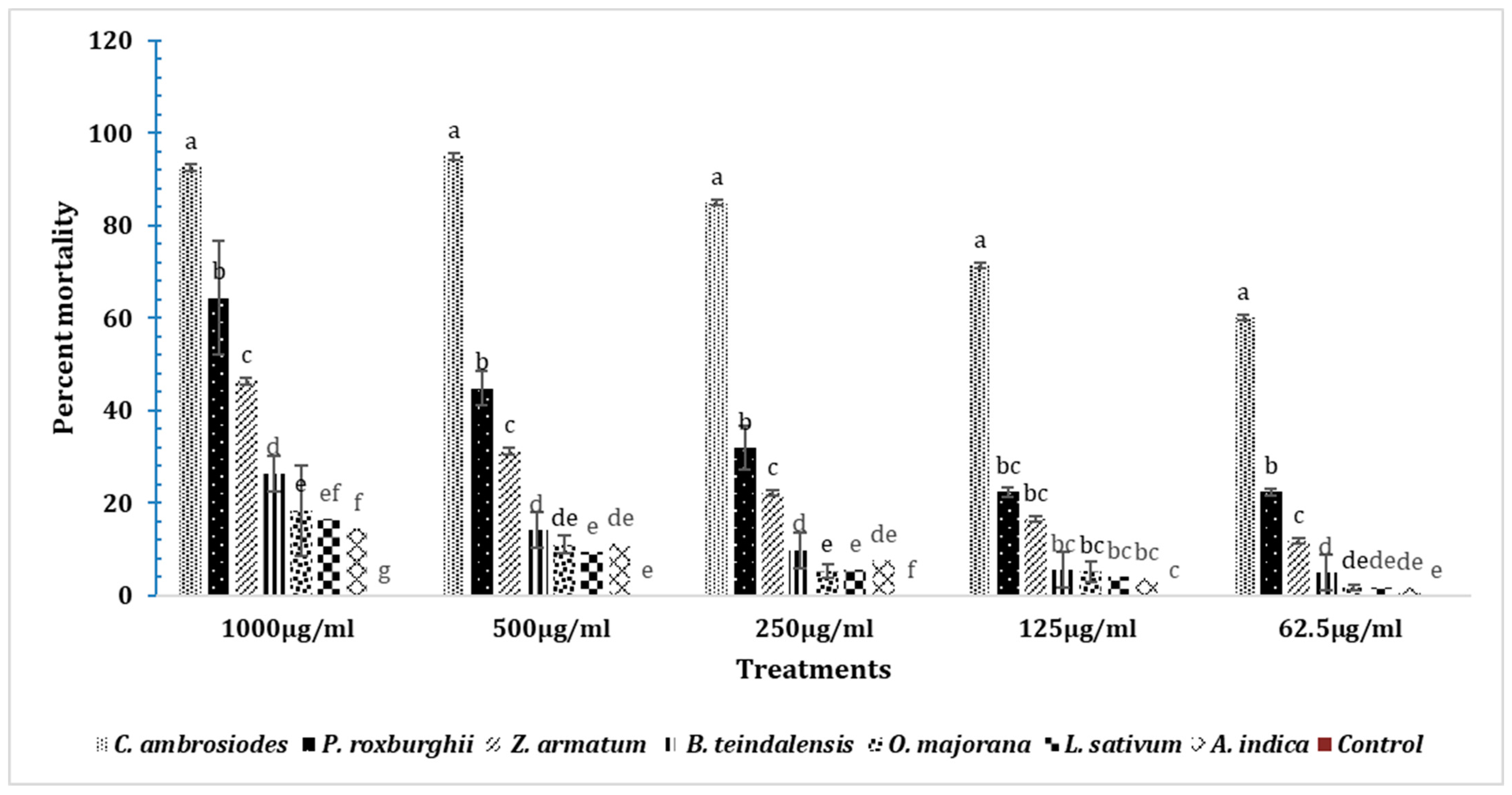
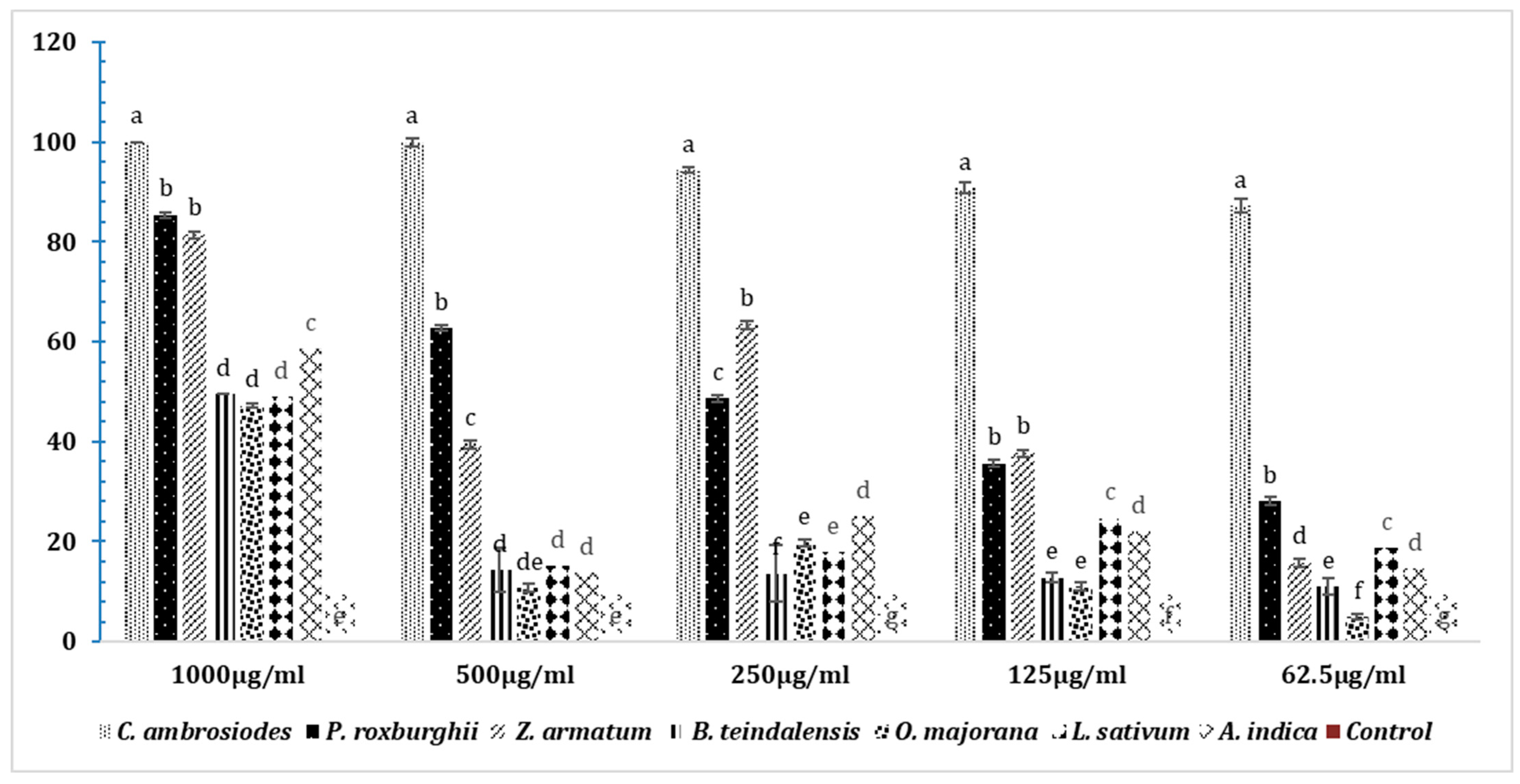
3.8. Insecticidal Effects of Essential Oils in T. castaneum after 24 h
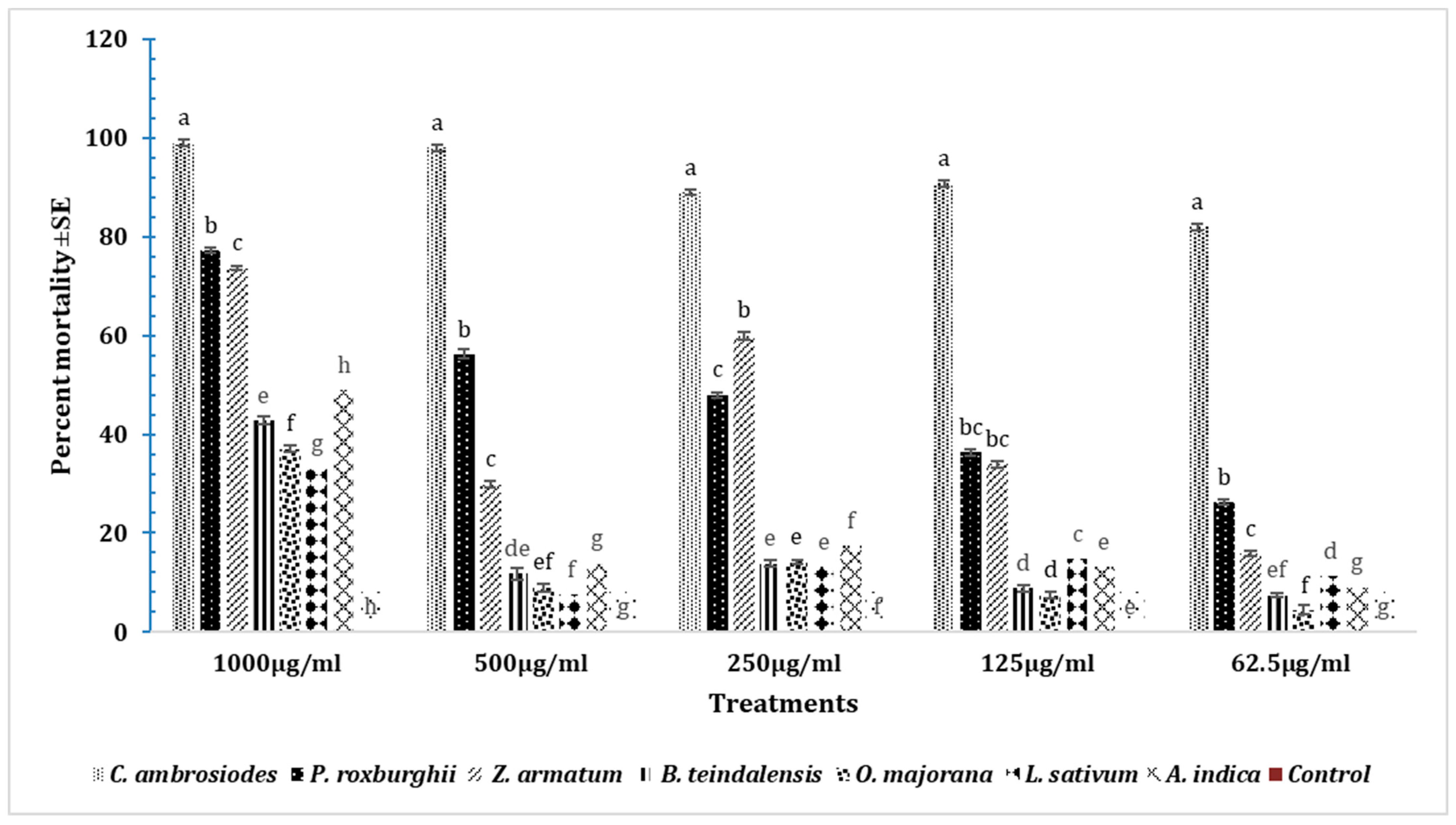
3.9. Insecticidal Effects of Essential Oils on T. castaneum after 48 h
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Semeao, A.A.; Campbell, J.F.; Whitworth, R.J.; Sloderbeck, P.E. Influence of environmental and physical factors on capture of Tribolium castaneum (Coleoptera: Tenebrionidae) in a flour mill. J. Econ. Entomol. 2012, 105, 686–702. [Google Scholar] [CrossRef] [PubMed]
- Mariadoss, A.; Umamaheswari, S. Feeding preference and development of red flour beetle, Tribolium castaneum (Herbst.) in different rice varieties stored for public distribution in India. J. Exp. Zool. India 2020, 23, 265–268. [Google Scholar]
- Rees, D. Insects of Stored Grain: A Pocket Reference; CSIRO Publishing: Melbourne, Australia, 2007. [Google Scholar]
- Trematerra, P.; Sciarreta, A.; Tamasi, E. Behavioural responses of Oryzaephilus surinamensis, Tribolium castaneum and Tribolium confusum to naturally and artificially damaged durum wheat kernels. Entomol. Exp. Appl. 2000, 94, 195–200. [Google Scholar] [CrossRef]
- Abdullahi, G.; Muhamad, R.; Sule, H. Biology, host range and management of red flour beetle Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae): A review. Taraba J. Agric. Res. 2019, 7, 48–64. [Google Scholar]
- Kumar, R. Insect Pests of Stored Grain: Biology, Behavior, and Management Strategies; Apple Academic Press: Palm Bay, FL, USA, 2017. [Google Scholar]
- Padın, S.; Dal Bello, G.; Fabrizio, M. Grain loss caused by Tribolium castaneum, Sitophilus oryzae and Acanthoscelides obtectus in stored durum wheat and beans treated with Beauveria bassiana. J. Stored Prod. Res. 2002, 38, 69–74. [Google Scholar] [CrossRef]
- Shafique, M.; Ahmad, M.; Chaudry, M.A. Feeding preference and development of Tribolium castaneum (Herbst.) in wheat products. Pak. J. Zool. 2006, 38, 27. [Google Scholar]
- Gao, F.; Qi, Y.; Hamadou, A.H.; Zhang, J.; Manzoor, M.F.; Guo, Q.; Xu, B. Enhancing wheat-flour safety by detecting and controlling red flour beetle Tribolium castaneum Herbst (Coleoptera: Tenebrionidae). J. Consum. Prot. Food Saf. 2022, 17, 113–126. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Ahmad, S. Management strategies for control of stored grain insect pests in farmer stores and public ware houses. World J. Agric. Sci. 2011, 7, 527–549. [Google Scholar]
- Bernardes, M.F.F.; Pazin, M.; Pereira, L.C.; Dorta, D.J. Impact of pesticides on environmental and human health. In Toxicology Studies—Cells, Drugs and Environment; BoD—Books on Demand: Norderstedt, Germany, 2015; pp. 195–233. [Google Scholar]
- Attia, M.A.; Wahba, T.F.; Shaarawy, N.; Moustafa, F.I.; Guedes, R.N.C.; Dewer, Y. Stored grain pest prevalence and insecticide resistance in Egyptian populations of the red flour beetle Tribolium castaneum (Herbst) and the rice weevil Sitophilus oryzae (L.). J. Stored Prod. Res. 2020, 87, 101611. [Google Scholar] [CrossRef]
- Budnik, L.T.; Kloth, S.; Velasco-Garrido, M.; Baur, X. Prostate cancer and toxicity from critical use exemptions of methyl bromide: Environmental protection helps protect against human health risks. Environ. Health 2012, 11, 5. [Google Scholar] [CrossRef]
- Kaushik, R. Methyl bromide: Risk assessment, environmental, and health hazard. In Hazardous Gases; Elsevier: Amsterdam, The Netherlands, 2021; pp. 239–250. [Google Scholar]
- Sciuto, A.M.; Wong, B.J.; Martens, M.E.; Hoard-Fruchey, H.; Perkins, M.W. Phosphine toxicity: A story of disrupted mitochondrial metabolism. Ann. N. Y. Acad. Sci. 2016, 1374, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.; Faroni, L.R.D.; Pimentel, M.; Guedes, R. Developmental and population growth rates of phosphine-resistant and-susceptible populations of stored-product insect pests. J. Stored Prod. Res. 2009, 45, 241–246. [Google Scholar] [CrossRef]
- Rozman, V.; Kalinović, I.; Korunić, Z. Toxicity of naturally occurring compounds of Lamiaceae and Lauraceae to three stored-product insects. J. Stored Prod. Res. 2007, 43, 349–355. [Google Scholar] [CrossRef]
- Isman, M. Pesticides based on plant essential oils. J. Pestic. Outlook 1999, 10, 68–72. [Google Scholar]
- Raja, N.; Albert, S.; Ignacimuthu, S.; Dorn, S. Effect of plant volatile oils in protecting stored cowpea Vigna unguiculata (L.) Walpers against Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) infestation. J. Stored Prod. Res. 2001, 37, 127–132. [Google Scholar] [CrossRef]
- Rao, V.P.; Pandey, D. Extraction of Essential Oil and Its Applications. Ph.D. Dissertation, Biju Patnaik Central Library, National Institute of Technology, Rourkela, Odisha, India, 2007. [Google Scholar]
- Adam, F.; Bertoncini, F.; Thiébaut, D.; Esnault, S.; Espinat, D.; Hennion, M. Towards comprehensive hydrocarbons analysis of middle distillates by LC-GC× GC. J. Chromatogr. Sci. 2007, 45, 643–649. [Google Scholar] [CrossRef][Green Version]
- Sarkar, M.; Akulwad, A.; Kshirsagar, R. A novel non-contact bioassay method for quantitative evaluation of vapour phase toxicity of insecticides against mosquitoes. J. Asia-Pac. Entomol. 2018, 21, 1315–1320. [Google Scholar] [CrossRef]
- Isman, M.B. Plant essential oils for pest and disease management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Singh, G.; Upadhyay, R. Essential oils-a potent source of natural pesticides. J. Sci. Ind. Res. 1993, 52, 676–683. [Google Scholar]
- Negahban, M.; Moharramipour, S.; Sefidkon, F. Fumigant toxicity of essential oil from Artemisia sieberi Besser against three stored-product insects. J. Stored Prod. Res. 2007, 43, 123–128. [Google Scholar] [CrossRef]
- Kim, S.-I.; Yoon, J.-S.; Jung, J.W.; Hong, K.-B.; Ahn, Y.-J.; Kwon, H.W. Toxicity and repellency of origanum essential oil and its components against Tribolium castaneum (Coleoptera: Tenebrionidae) adults. J. Asia-Pac. Entomol. 2010, 13, 369–373. [Google Scholar] [CrossRef]
- Lee, B.-H.; Annis, P.C.; Choi, W.-S. Fumigant toxicity of essential oils from the Myrtaceae family and 1, 8-cineole against 3 major stored-grain insects. J. Stored Prod. Res. 2004, 40, 553–564. [Google Scholar] [CrossRef]
- Kasali, A.A.; Ekundayo, O.; Paul, C.; König, W.A.; Eshilokun, A.O.; Ige, B. 1,2:3,4-diepoxy-p-menthane and 1,4-epoxy-p-menth-2-ene: Rare monoterpenoids from the essential oil of Chenopodium ambrosioides L. var ambrosioides leaves. J. Essent. Oil Res. 2006, 18, 13–15. [Google Scholar] [CrossRef]
- Chu, S.S.; Feng Hu, J.; Liu, Z.L. Composition of essential oil of Chinese Chenopodium ambrosioides and insecticidal activity against maize weevil, Sitophilus zeamais. Pest Manag. Sci. 2011, 67, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Tapondjou, L.; Adler, C.; Bouda, H.; Fontem, D. Efficacy of powder and essential oil from Chenopodium ambrosioides leaves as post-harvest grain protectants against six-stored product beetles. J. Stored Prod. Res. 2002, 38, 395–402. [Google Scholar] [CrossRef]
- Mackled, M.I.; EL-Hefny, M.; Bin-Jumah, M.; Wahba, T.F.; Allam, A.A. Assessment of the toxicity of natural oils from Mentha piperita, Pinus roxburghii, and Rosa spp. against three stored product insects. Processes 2019, 7, 861. [Google Scholar] [CrossRef]
- Kumar, R.; Tiwari, S.N. Fumigant toxicity of essential oils against four major storage insect pests. Indian J. Entomol. 2017, 79, 156–159. [Google Scholar] [CrossRef]
- Tiwary, M.; Naik, S.; Tewary, D.K.; Mittal, P.; Yadav, S. Chemical composition and larvicidal activities of the essential oil of Zanthoxylum armatum DC (Rutaceae) against three mosquito vectors. J. Vector Borne Dis. 2007, 44, 198. [Google Scholar]
- Wang, C.-F.; Zhang, W.-J.; You, C.-X.; Guo, S.-S.; Geng, Z.-F.; Fan, L.; Du, S.-S.; Deng, Z.-W.; Wang, Y.-Y. Insecticidal constituents of essential oil derived from Zanthoxylum armatum against two stored-product insects. J. Oleo Sci. 2015, 64, 861–868. [Google Scholar] [CrossRef]
- Hanif, C.M.S.; Ul-Hasan, M.; Shagger, M.; Saleem, S.; Akthar, S.; Ijaz, M. Insecticidal and repellent activities of essential oils of three medicinal plants towards insect pests of stored wheat. Bulg. J. Agric. Sci. 2016, 22, 470–476. [Google Scholar]
- Al-Fuhaid, N. Can a Garden Cress (Lepidium sativum: Cruciferae) Seeds be a Poisonous Bait for the Larvae of Trogoderma granarium Everts? World J. Agric. Res. 2018, 6, 31–36. [Google Scholar] [CrossRef]
- Salaheddine, S.; Zohra, B.; Cheikh, I.; Asma, L. Study of the toxicity of essential oils of Origanum majorana on Tribolium castaneum and Plodia interpunctella (stored product insects). Tunis. J. Med. Plants Nat. Prod. 2013, 9, 29–34. [Google Scholar]
- Padin, S.B.; Fuse, C.B.; Urrutia, M.I.; Dal Bello, G. Toxicity and Repellency of Nine Medicinal Plants against Tribolium castaneum in Stored Wheat. Bull. Insectol. 2013, 66, 45–49. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, M.; Khizar, M.; Alshaya, D.S.; Hameed, A.; Muhammad, N.; Binyameen, M.; Azeem, M.; Hussain, M.; Abbas, Q.; Attia, K.A.; et al. Insecticidal and Repellent Activity of Essential Oils from Seven Different Plant Species against Tribolium castaneum (Coleoptera: Tenebrionidae). Insects 2024, 15, 755. https://doi.org/10.3390/insects15100755
Khalil M, Khizar M, Alshaya DS, Hameed A, Muhammad N, Binyameen M, Azeem M, Hussain M, Abbas Q, Attia KA, et al. Insecticidal and Repellent Activity of Essential Oils from Seven Different Plant Species against Tribolium castaneum (Coleoptera: Tenebrionidae). Insects. 2024; 15(10):755. https://doi.org/10.3390/insects15100755
Chicago/Turabian StyleKhalil, Misha, Mishal Khizar, Dalal Suleiman Alshaya, Asifa Hameed, Noor Muhammad, Muhammad Binyameen, Muhammad Azeem, Mussurat Hussain, Qaisar Abbas, Kotb A. Attia, and et al. 2024. "Insecticidal and Repellent Activity of Essential Oils from Seven Different Plant Species against Tribolium castaneum (Coleoptera: Tenebrionidae)" Insects 15, no. 10: 755. https://doi.org/10.3390/insects15100755
APA StyleKhalil, M., Khizar, M., Alshaya, D. S., Hameed, A., Muhammad, N., Binyameen, M., Azeem, M., Hussain, M., Abbas, Q., Attia, K. A., & Shah, T. A. (2024). Insecticidal and Repellent Activity of Essential Oils from Seven Different Plant Species against Tribolium castaneum (Coleoptera: Tenebrionidae). Insects, 15(10), 755. https://doi.org/10.3390/insects15100755







