Taxonomic and Functional Diversity of Flower-Visiting Insects in Coffee Crops
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Methodology
2.2.1. Taxonomic Diversity
2.2.2. Functional Diversity
2.2.3. Statistical Analyses
Taxonomic Diversity
Functional Diversity
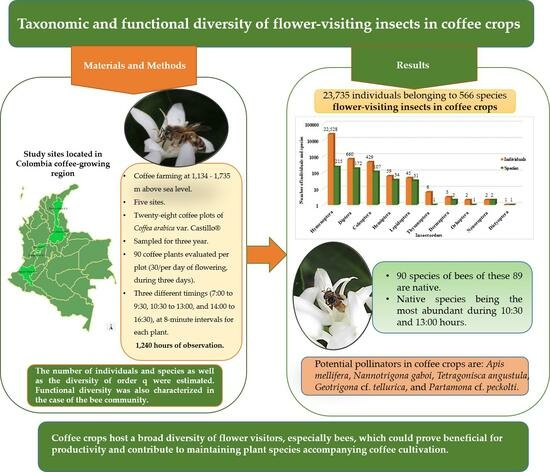
3. Results
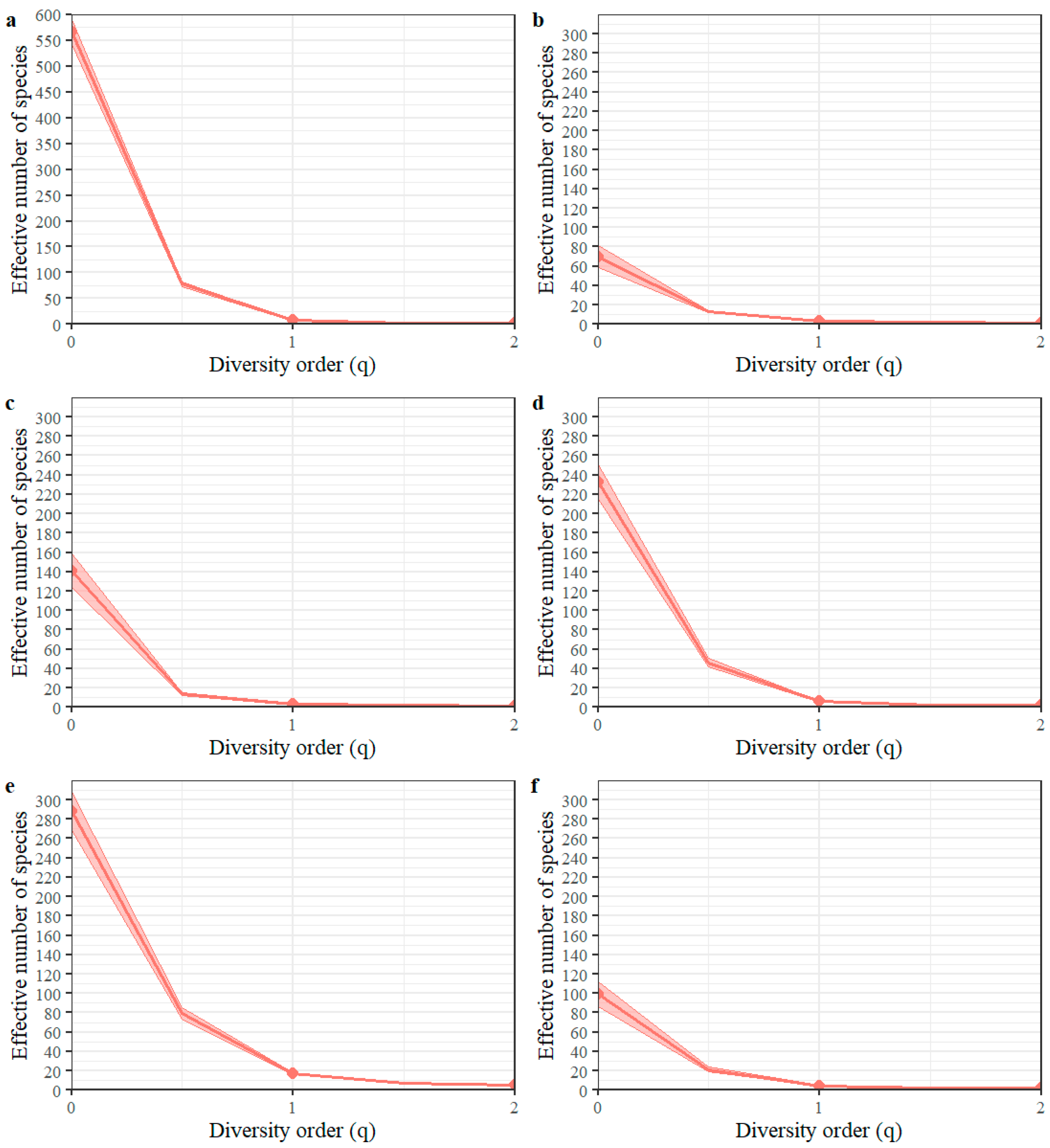
3.1. Taxonomic Diversity
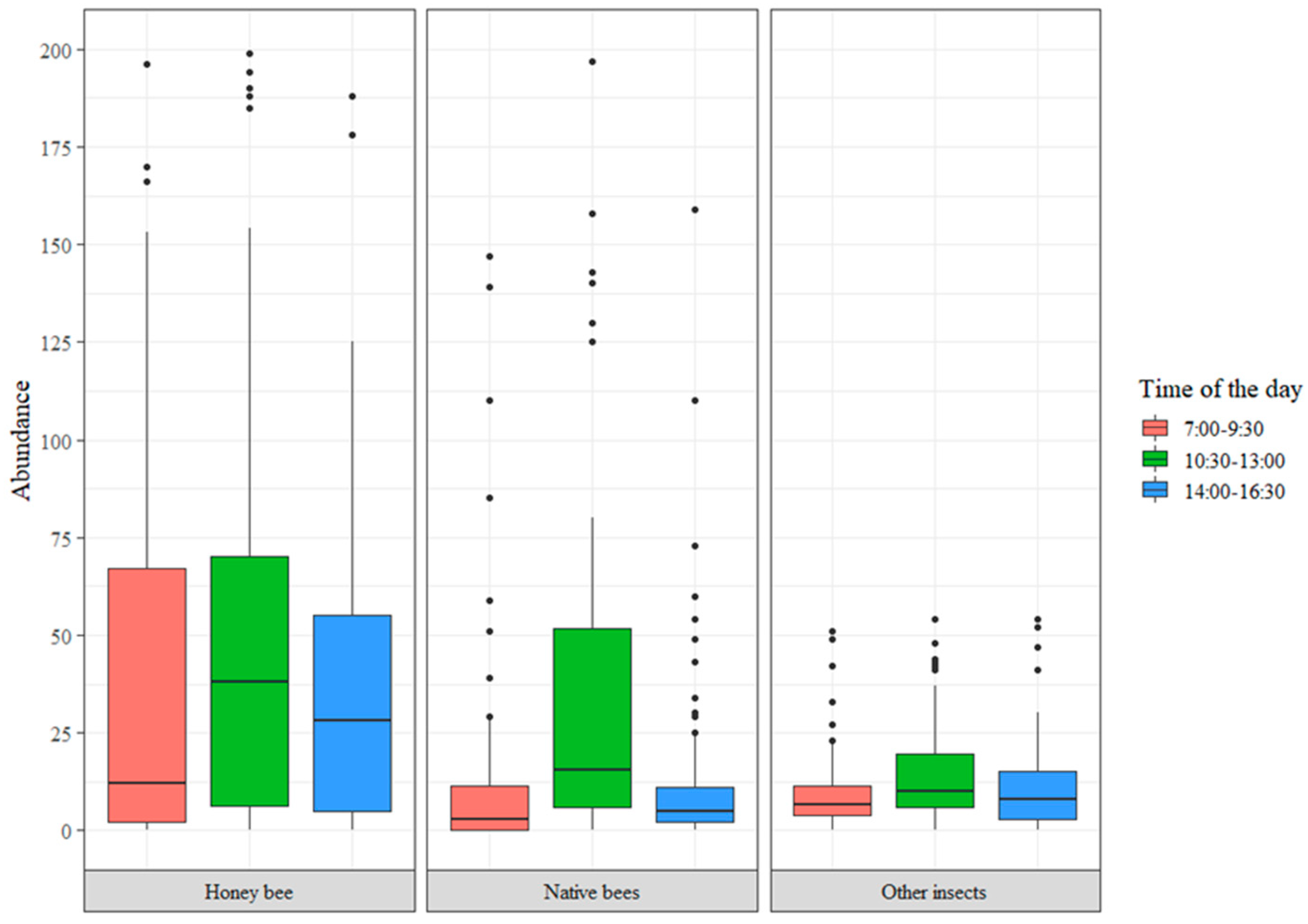
3.2. Functional Diversity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Order | Family | Specie | Study Sites |
|---|---|---|---|
| Blattodea | Blattodea sp. | C | |
| Coleoptera | Anthribidae | Anthribidae sp.1 | A |
| Anthribidae | Anthribidae sp.2 | A | |
| Anthribidae | Anthribidae sp.3 | D | |
| Cantharidae | Chauliognathus sp.1 | B, D | |
| Cantharidae | Chauliognathus sp.2 | E | |
| Cantharidae | Chauliognathus sp.3 | E | |
| Cantharidae | Chauliognathus sp.4 | E | |
| Cantharidae | Chauliognathus sp.5 | B | |
| Cantharidae | Chauliognathus sp.6 | E | |
| Cantharidae | Chauliognathus sp.7 | B | |
| Cantharidae | Chauliognathus sp.8 | B | |
| Cantharidae | Chauliognathus sp.9 | B | |
| Cantharidae | Chauliognathus sp.10 | B | |
| Cantharidae | Chauliognathus sp.11 | B | |
| Cantharidae | Chauliognathus sp.12 | D | |
| Cantharidae | Chauliognathus sp.13 | E | |
| Cantharidae | Discodon sp.1 | D | |
| Cantharidae | Discodon sp.2 | E | |
| Chrysomelidae | Chrysomelidae sp.1 | A, B, E | |
| Chrysomelidae | Chrysomelidae sp.2 | C, E | |
| Chrysomelidae | Chrysomelidae sp.3 | C | |
| Chrysomelidae | Chrysomelidae sp.4 | E | |
| Chrysomelidae | Chrysomelidae sp.5 | C, D | |
| Chrysomelidae | Chrysomelidae sp.6 | D | |
| Chrysomelidae | Chrysomelidae sp.7 | C, D | |
| Chrysomelidae | Chrysomelidae sp.8 | E | |
| Chrysomelidae | Chrysomelidae sp.9 | C, D | |
| Chrysomelidae | Chrysomelidae sp.10 | D | |
| Chrysomelidae | Chrysomelidae sp.11 | D | |
| Chrysomelidae | Chrysomelidae sp.12 | E | |
| Chrysomelidae | Chrysomelidae sp.13 | D | |
| Chrysomelidae | Chrysomelidae sp.14 | D | |
| Coleoptera | Chrysomelidae | Chrysomelidae sp.15 | E |
| Chrysomelidae | Chrysomelidae sp.16 | D | |
| Chrysomelidae | Chrysomelidae sp.17 | D | |
| Chrysomelidae | Chrysomelidae sp.18 | E | |
| Chrysomelidae | Chrysomelidae sp.19 | E | |
| Chrysomelidae | Chrysomelidae sp.20 | B | |
| Chrysomelidae | Chrysomelidae sp.21 | C | |
| Chrysomelidae | Chrysomelidae sp.22 | D | |
| Chrysomelidae | Chrysomelidae sp.23 | D | |
| Chrysomelidae | Chrysomelidae sp.24 | A | |
| Chrysomelidae | Chrysomelidae sp.25 | B | |
| Chrysomelidae | Chrysomelidae sp.26 | B | |
| Chrysomelidae | Chrysomelidae sp.27 | A | |
| Chrysomelidae | Chrysomelidae sp.28 | C, D | |
| Chrysomelidae | Chrysomelidae sp.29 | D, E | |
| Chrysomelidae | Chrysomelidae sp.30 | D, E | |
| Chrysomelidae | Chrysomelidae sp.31 | A, D, E | |
| Chrysomelidae | Chrysomelidae sp.32 | D | |
| Chrysomelidae | Chrysomelidae sp.33 | B | |
| Chrysomelidae | Chrysomelidae sp.34 | B | |
| Chrysomelidae | Chrysomelidae sp.35 | B | |
| Chrysomelidae | Chrysomelidae sp.36 | E | |
| Chrysomelidae | Chrysomelidae sp.37 | E | |
| Coccinellidae | Azya orbigera | C, D | |
| Coccinellidae | Coccinelldae sp.1 | B | |
| Coccinellidae | Coccinellidae sp.2 | A, B, D | |
| Coccinellidae | Coccinellidae sp.3 | D, E | |
| Coccinellidae | Coccinellidae sp.4 | D | |
| Coccinellidae | Coccinellidae sp.5 | B | |
| Coccinellidae | Coccinellidae sp.6 | B, D | |
| Coccinellidae | Coccinellidae sp.7 | D | |
| Coccinellidae | Coccinellidae sp.8 | D | |
| Coccinellidae | Coccinellidae sp.9 | D | |
| Coccinellidae | Coccinellidae sp.10 | C, D | |
| Coccinellidae | Coccinellidae sp.11 | B, D | |
| Curculionidae | Curculionidae sp.1 | C, D | |
| Curculionidae | Curculionidae sp.2 | D | |
| Curculionidae | Curculionidae sp.3 | C | |
| Curculionidae | Curculionidae sp.4 | B | |
| Curculionidae | Curculionidae sp.5 | D | |
| Curculionidae | Curculionidae sp.6 | D | |
| Curculionidae | Curculionidae sp.7 | C | |
| Curculionidae | Curculionidae sp.8 | B, C | |
| Coleoptera | Curculionidae | Curculionidae sp.9 | B |
| Curculionidae | Curculionidae sp.10 | E | |
| Curculionidae | Curculionidae sp.11 | C | |
| Curculionidae | Curculionmidae sp.12 | B | |
| Dascilidae | Dascilidae sp.1 | D | |
| Lagriidae | Lagriidae sp.1 | D | |
| Lampyridae | Lampyridae sp.1 | E | |
| Lycidae | Lycidae sp.1 | C, D | |
| Lycidae | Lycidae sp.2 | E | |
| Lycidae | Lycidae sp.3 | D | |
| Lycidae | Lycidae sp.4 | C | |
| Lycidae | Lycidae sp.5 | D | |
| Melyridae | Astylus lebasi | E | |
| Melyridae | Melyridae sp.1 | B | |
| Mordellidae | Mordellidae sp.1 | D | |
| Mordellidae | Mordellidae sp.2 | E | |
| Mordellidae | Mordellidae sp.3 | A | |
| Nitidulidae | Nitidulidae sp.1 | B | |
| Nitidulidae | Nitidulidae sp.2 | B | |
| Nitidulidae | Nitidulidae sp.3 | B | |
| Ptilodactylidae | Ptilodactylidae sp.1 | B | |
| Scarabaeidae | Macraspis sp.1 | C | |
| Scarabaeidae | Scarabaeidae sp.1 | C | |
| Scarabaeidae | Scarabaeidae sp.2 | C | |
| Scarabaeidae | Scarabaeidae sp.3 | E | |
| Scarabaeidae | Scarabaeidae sp.4 | E | |
| Staphylinidae | Staphylinidae sp.1 | C, D, E | |
| Staphylinidae | Staphylinidae sp.2 | C, D | |
| Staphylinidae | Staphylinidae sp.3 | D | |
| Staphylinidae | Staphylinidae sp.4 | C | |
| Staphylinidae | Staphylinidae sp.5 | A, B, C, D, E | |
| Staphylinidae | Staphylinidae sp.6 | C, D | |
| Dermaptera | Dermaptera spp. | A, B, D | |
| Diptera | Anthomyiidae | Anthomyiidae sp.1 | C |
| Anthomyiidae | Anthomyiidae sp.2 | C | |
| Anthomyiidae | Anthomyiidae sp.3 | C | |
| Bibionidae | Bibionidae sp.1 | C, D, E | |
| Bibionidae | Bibionidae sp.2 | B, C, D | |
| Bibionidae | Bibionidae sp.3 | C, D | |
| CalliPhoridae | CalliPhoridae sp.1 | D | |
| CalliPhoridae | CalliPhoridae sp.2 | C | |
| CalliPhoridae | CalliPhoridae sp.3 | B | |
| CalliPhoridae | CalliPhoridae sp.4 | B, D | |
| Diptera | CalliPhoridae | CalliPhoridae sp.5 | B, D |
| CalliPhoridae | CalliPhoridae sp.6 | D | |
| CalliPhoridae | Lucilia sp.1 | C | |
| CalliPhoridae | Lucilia sp.2 | C | |
| CalliPhoridae | Lucilia sp.3 | C | |
| CalliPhoridae | Lucilia sp.4 | D | |
| CalliPhoridae | Lucilia sp.5 | D | |
| CalliPhoridae | Lucilia sp.6 | C | |
| CalliPhoridae | Lucilia sp.7 | B | |
| CalliPhoridae | Lucilia sp.8 | D | |
| CalliPhoridae | Lucilia sp.9 | D | |
| Camillidae | Camillidae sp.1 | D | |
| Carnidae | Carnidae sp.2 | C | |
| Carnidae | Carnidae sp.3 | E | |
| Ceratopogonidae | Ceratopogonidae sp.1 | C, D | |
| Ceratopogonidae | Ceratopogonidae sp.2 | D | |
| Ceratopogonidae | Ceratopogonidae sp.3 | D | |
| Ceratopogonidae | Ceratopogonidae sp.4 | D | |
| Chamaemyiidae | Chamaemyiidae sp.1 | D | |
| Chamaemyiidae | Chamaemyiidae sp.2 | D | |
| Chloropidae | Chloropidae sp.1 | C, D, E | |
| Chloropidae | Chloropidae sp.2 | C | |
| Chloropidae | Chloropidae sp.3 | C | |
| Chloropidae | Chloropidae sp.4 | B | |
| Chloropidae | Chloropidae sp.5 | B, D | |
| Chloropidae | Chloropidae sp.6 | B | |
| Chloropidae | Chloropidae sp.7 | C, D | |
| Chloropidae | Chloropidae sp.8 | B | |
| Chloropidae | Chloropidae sp.9 | B | |
| Chloropidae | Chloropidae sp.10 | B | |
| Chloropidae | Chloropidae sp.11 | C, D, E | |
| Chloropidae | Chloropidae sp.12 | C, D | |
| Conopidae | Conopidae sp.1 | C | |
| Curtonotidae | Curtonotidae sp.1 | B | |
| Dolichopodidae | Dolichopodidae sp.1 | C | |
| Dolichopodidae | Dolichopodidae sp.2 | B, C | |
| Dolichopodidae | Dolichopodidae sp.3 | A | |
| Dolichopodidae | Dolichopodidae sp.4 | A | |
| Drosophilidae | Drosophilidae sp.1 | C | |
| Empididae | Empididae sp.1 | C | |
| Fanniidae | Fanniidae sp.1 | D | |
| Fanniidae | Fanniidae sp.2 | D | |
| Fanniidae | Fanniidae sp.3 | C, E | |
| Fanniidae | Fanniidae sp.4 | C | |
| Diptera | Heleomyzidae | Heleomyzidae sp.1 | D |
| Lauxaniidae | Lauxaniidae sp.1 | B, D | |
| Lauxaniidae | Lauxaniidae sp.2 | B | |
| Lauxaniidae | Lauxaniidae sp.3 | B | |
| Lauxaniidae | Lauxaniidae sp.4 | B, C, D | |
| Lauxaniidae | Lauxaniidae sp.5 | C, D | |
| Lauxaniidae | Lauxaniidae sp.6 | C | |
| Lauxaniidae | Lauxaniidae sp.7 | B | |
| Lauxaniidae | Lauxaniidae sp.8 | D | |
| Lauxaniidae | Lauxaniidae sp.9 | B | |
| Lauxaniidae | Lauxaniidae sp.10 | C, E | |
| Lauxaniidae | Lauxaniidae sp.11 | D | |
| Micropezidae | Micropezidae sp.1 | D | |
| Milichiidae | Milichiidae sp.1 | D | |
| Milichiidae | Milichiidae sp.2 | D, E | |
| Milichiidae | Milichiidae sp.3 | D, E | |
| Muscidae | Muscidae sp.1 | C, D | |
| Muscidae | Muscidae sp.2 | C | |
| Phoridae | Phoridae sp.1 | D, E | |
| Phoridae | Phoridae sp.2 | B | |
| Phoridae | Phoridae sp.3 | C | |
| Phoridae | Phoridae sp.4 | C | |
| Piophilidae | Piophilidae sp.1 | C | |
| Pipunculidae | Pipunculidae sp.1 | C | |
| Pipunculidae | Pipunculidae sp.2 | C | |
| Pipunculidae | Pipunculidae sp.3 | C | |
| Sarcophagidae | Sarcophagidae sp.1 | C, D, E | |
| Sarcophagidae | Sarcophagidae sp.2 | D | |
| Sarcophagidae | Sarcophagidae sp.3 | C | |
| Sarcophagidae | Sarcophagidae sp.4 | C, D, E | |
| Sarcophagidae | Sarcophagidae sp.5 | C, D | |
| Sarcophagidae | Sarcophagidae sp.6 | D | |
| Sarcophagidae | Sarcophagidae sp.7 | D | |
| Sarcophagidae | Sarcophagidae sp.8 | B, D | |
| Sarcophagidae | Sarcophagidae sp.9 | C, D | |
| Sarcophagidae | Sarcophagidae sp.10 | D | |
| Sciaridae | Sciaridae sp.1 | C, D | |
| Sciaridae | Sciaridae sp.2 | C, D | |
| Sciaridae | Sciaridae sp.3 | C | |
| Sciaridae | Sciaridae sp.4 | D | |
| Sciaridae | Sciaridae sp.5 | D | |
| Sciaridae | Sciaridae sp.6 | D | |
| Diptera | Sciaridae | Sciaridae sp.7 | D |
| Sciaridae | Sciaridae sp.8 | D | |
| Sciaridae | Sciaridae sp.9 | C, D | |
| Sciaridae | Sciaridae sp.10 | C, D | |
| Sciaridae | Sciaridae sp.11 | B, C, D | |
| Sciaridae | Sciaridae sp.12 | C, D | |
| Sciaridae | Sciaridae sp.13 | C, D | |
| Sciaridae | Sciaridae sp.14 | C | |
| Sciaridae | Sciaridae sp.15 | D | |
| Sciaridae | Sciaridae sp.16 | C | |
| Simulidae | Simulidae sp.1 | D | |
| Syrphidae | Copestylum sp.1 | C | |
| Syrphidae | Copestylum sp.2 | D | |
| Syrphidae | Copestylum sp.3 | C, D | |
| Syrphidae | Copestylum sp.4 | C | |
| Syrphidae | Copestylum sp.5 | D | |
| Syrphidae | Copestylum sp.6 | C, D | |
| Syrphidae | Eristalis sp.1 | A, D | |
| Syrphidae | Eristalis sp.2 | C | |
| Syrphidae | Ocyptamus (Hermesomyia) sp.1 | A | |
| Syrphidae | Ocyptamus sp.2 | C | |
| Syrphidae | Ocyptamus sp.3 | C | |
| Syrphidae | Ocyptamus sp.4 | B, C, D | |
| Syrphidae | Ocyptamus sp.5 | C, D | |
| Syrphidae | Ornidia major | E | |
| Syrphidae | Ornidia obesa | A, B, C, D, E | |
| Syrphidae | Palpada sp.1 | A, C, D | |
| Syrphidae | Palpada sp.2 | C, D | |
| Syrphidae | Palpada sp.3 | C, D | |
| Syrphidae | Palpada sp.4 | A, B, C, D, E | |
| Syrphidae | Palpada sp.5 | A, B, C, D | |
| Syrphidae | Palpada sp.6 | A, B, D | |
| Syrphidae | Palpada sp.7 | A, D | |
| Syrphidae | Palpada sp.8 | B | |
| Syrphidae | Palpada sp.9 | A | |
| Syrphidae | Pseudodorus clavatus | A | |
| Syrphidae | Pseudodorus aff. clavatus | B, C, D | |
| Syrphidae | Pseudodorus sp.3 | C | |
| Syrphidae | Toxomerus sp.1 | A, B, C, D, E | |
| Syrphidae | Toxomerus sp.2 | B, C, D | |
| Syrphidae | Toxomerus sp.3 | D | |
| Syrphidae | Toxomerus sp.4 | B, C, D | |
| Syrphidae | Toxomerus sp.5 | B, C, D | |
| Diptera | Syrphidae | Toxomerus sp.6 | B, C |
| Syrphidae | Toxomerus sp.7 | C | |
| Syrphidae | Toxomerus sp.8 | B, D | |
| Syrphidae | Xanthandrus sp.1 | E | |
| Syrphidae | Xanthandrus sp.2 | B | |
| Syrphidae | Xanthandrus sp.3 | B | |
| Tachinidae | Tachinidae sp.1 | C | |
| Tachinidae | Tachinidae sp.2 | D | |
| Tachinidae | Tachinidae sp.3 | D, E | |
| Tachinidae | Tachinidae sp.4 | A, D | |
| Tachinidae | Tachinidae sp.5 | B, C, D | |
| Tachinidae | Tachinidae sp.6 | D | |
| Tachinidae | Tachinidae sp.7 | D | |
| Tachinidae | Tachinidae sp.8 | C, D | |
| Tachinidae | Tachinidae sp.9 | D | |
| Tachinidae | Tachinidae sp.10 | C, D | |
| Tachinidae | Tachinidae sp.11 | C, D | |
| Tachinidae | Tachinidae sp.12 | B | |
| Tephritidae | Tephritidae sp.1 | C | |
| Tephritidae | Tephritidae sp.2 | C | |
| Tephritidae | Tephritidae sp.3 | B, C | |
| Tephritidae | Tephritidae sp.4 | C, D | |
| Tephritidae | Tephritidae sp.5 | D | |
| Tephritidae | Tephritidae sp.6 | C | |
| Tephritidae | Tephritidae sp.7 | A, B | |
| Tephritidae | Tephritidae sp.8 | C | |
| Tephritidae | Tephritidae sp.9 | B | |
| Tipulidae | Tipulidae sp.1 | C | |
| Tipulidae | Tipulidae sp.2 | D | |
| Tipulidae | Tipulidae sp.3 | C | |
| Tipulidae | Tipulidae sp.4 | D | |
| Ulidiidae | Ulidiidae sp.1 | E | |
| Ulidiidae | Ulidiidae sp.2 | C | |
| Ulidiidae | Ulidiidae sp.3 | B | |
| Hemiptera | Alydidae | Alydidae sp.1 | D |
| Coreidae | Coreidae sp.1 | A | |
| Coreidae | Coreidae sp.2 | B | |
| Coreidae | Sphictyrtus sp.3 | C, D | |
| Cydnidae | Cydnidae sp.1 | D | |
| Lygaeidae | Lygaeidae sp.1 | C | |
| Lygaeidae | Lygaeidae sp.2 | C | |
| Lygaeidae | Lygaeidae sp.3 | B | |
| Lygaeidae | Lygaeidae sp.4 | C | |
| Hemiptera | Lygaeidae | Lygaeidae sp.5 | C |
| Miridae | Miridae sp.1 | D | |
| Miridae | Miridae sp.2 | D | |
| Miridae | Miridae sp.3 | B | |
| Miridae | Miridae sp.4 | C | |
| Miridae | Miridae sp.5 | B, C, D, E | |
| Miridae | Miridae sp.6 | D | |
| Miridae | Miridae sp.7 | C, D | |
| Miridae | Miridae sp.8 | B | |
| Miridae | Miridae sp.9 | B, D | |
| Miridae | Miridae sp.10 | B, E | |
| Miridae | Miridae sp.11 | B | |
| Miridae | Miridae sp.12 | E | |
| Miridae | Miridae sp.13 | E | |
| Miridae | Miridae sp.14 | E | |
| Miridae | Miridae sp.15 | C | |
| Pentatomidae | Pentatomidae sp.1 | C, D | |
| Pentatomidae | Pentatomidae sp.2 | B, C | |
| Pentatomidae | Pentatomidae sp.3 | D | |
| Pyrrhocoridae | Pyrrhocoridae sp.1 | E | |
| Reduviidae | Reduviidae sp.1 | B, E | |
| Rhopalidae | Rhopalidae sp.1 | E | |
| Rhopalidae | Rhopalidae sp.2 | D | |
| Rhopalidae | Rhopalidae sp.3 | B | |
| Tingidae | Tingidae sp.1 | C | |
| Hymenoptera | Agaonidae | Agaonidae sp.1 | B |
| Apidae | Apis mellifera | A, B, C, D, E | |
| Apidae | Bombus melaleucus | B | |
| Apidae | Bombus pauloensis | B | |
| Apidae | Bombus pullatus | A | |
| Apidae | Ceratina (Calloceratina) sp.1 | A, C | |
| Apidae | Ceratina (Ceratinula) sp.2 | A | |
| Apidae | Ceratina (Crewella) sp.3 | A | |
| Apidae | Ceratina sp.4 | B | |
| Apidae | Ceratina sp.5 | A | |
| Apidae | Epicharis (Epicharana) sp.1 | C | |
| Apidae | Euglossa cf. dressleri | D | |
| Apidae | Eulaema cingulata | A, C, D | |
| Apidae | Eulaema polychroma | B, C | |
| Apidae | Exomalopsis aburraensis | A, C, D, E | |
| Apidae | Exomalopsis auropilosa | C, D, E | |
| Apidae | Exomalopsis digressa | C | |
| Apidae | Exomalopsis snowi | C | |
| Hymenoptera | Apidae | Exomalopsis sp.1 | C, D |
| Apidae | Exomalopsis sp.2 | A, B, C, D | |
| Apidae | Exomalopsis sp.3 | D | |
| Apidae | Geotrigona cf. tellurica | B | |
| Apidae | Geotrigona kaba | B | |
| Apidae | Nannotrigona gaboi | A, B | |
| Apidae | Nannotrigona pilosa | C, D | |
| Apidae | Nannotrigona tristella | C, D | |
| Apidae | Nasutopedia sp1 | D | |
| Apidae | Paratrigona eutaeniata | B, D | |
| Apidae | Paratrigona opaca | A, B | |
| Apidae | Partamona cf peckolti | B, C, D, E | |
| Apidae | Plebeia sp.1 | D | |
| Apidae | Plebeia sp.2 | B, D | |
| Apidae | Plebeia sp.3 | A | |
| Apidae | Scaptotrigona sp.1 | A | |
| Apidae | Tetragona perangulata | A, B, D | |
| Apidae | Tetragonisca angustula | A, B, D, E | |
| Apidae | Trigona fulviventris | A, B | |
| Apidae | Trigona nigerrima | A, B, E | |
| Apidae | Trigonisca cf. pediculana | C, D | |
| Apidae | Trigonisca cf. mepecheu | A | |
| Apidae | Xylocopa frontalis | C, D | |
| Apidae | Xylocopa sp.1 | C, D | |
| Bethylidae | Bethylidae sp.1 | D | |
| Braconidae | Braconidae sp.1 | E | |
| Braconidae | Braconidae sp.2 | C, D | |
| Braconidae | Braconidae sp.3 | C | |
| Braconidae | Braconidae sp.4 | D | |
| Braconidae | Braconidae sp.5 | C | |
| Braconidae | Braconidae sp.6 | D | |
| Braconidae | Braconidae sp.7 | B | |
| Braconidae | Braconidae sp.8 | B, C | |
| Braconidae | Braconidae sp.9 | D | |
| Braconidae | Braconidae sp.10 | C, D | |
| Braconidae | Braconidae sp.11 | B, D | |
| Chalcididae | Chalcididae sp.1 | C | |
| Chalcididae | Conura sp.1 | D | |
| Colletidae | Ptiloglossa sp.1 | C | |
| Crabronidae | Liris sp.1 | C | |
| Crabronidae | Tachytes sp.1 | D | |
| Encyrtidae | Encyrtidae sp.1 | D | |
| Eucharitidae | Eucharitidae sp.1 | C | |
| Hymenoptera | Eucharitidae | Eucharitidae sp.2 | B |
| Figitidae | Figitidae sp.1 | D | |
| Formicidae | Acropyga sp.1 | B | |
| Formicidae | Atta sp.1 | D | |
| Formicidae | Bothriomyrmex sp.1 | C, D | |
| Formicidae | Brachymyrmex brunneus | D | |
| Formicidae | Brachymyrmex cavernicola | B | |
| Formicidae | Brachymyrmex coactus | C, D | |
| Formicidae | Brachymyrmex heeri | D | |
| Formicidae | Brachymyrmex pilipes | A, C | |
| Formicidae | Brachymyrmex sculpturatus | D | |
| Formicidae | Brachymyrmex sossai | D | |
| Formicidae | Brachymyrmex sp.2 | B | |
| Formicidae | Brachymyrmex tristis | C, D | |
| Formicidae | Camponotus senex | C | |
| Formicidae | Camponotus sp.1 | A, D, E | |
| Formicidae | Camponotus sp.2 | A, D | |
| Formicidae | Camponotus sp.3 | A, B, C | |
| Formicidae | Camponotus sp.4 | B | |
| Formicidae | Camponotus sp.5 | B | |
| Formicidae | Carebara sp.1 | D | |
| Formicidae | Cephalotes sp.1 | C | |
| Formicidae | Crematogaster sp.1 | A, B | |
| Formicidae | Crematogaster sp.2 | D | |
| Formicidae | Dolichoderus sp.1 | D | |
| Formicidae | Dorymyrmex bicolor | C | |
| Formicidae | Dorymyrmex biconis | C, D, E | |
| Formicidae | Dorymyrmex brunneus | C, D | |
| Formicidae | Dorymyrmex insanus | C, D | |
| Formicidae | Ectatomma confine | B, D | |
| Formicidae | Ectatomma ruidum | C, D | |
| Formicidae | Ectatomma tuberculatum | D | |
| Formicidae | Linepithema angulatum | B, D | |
| Formicidae | Linepithema gallardoi | D | |
| Formicidae | Linepithema hirsutum | B | |
| Formicidae | Linepithema iniquum | C, E | |
| Formicidae | Linepithema neotropicum | B, C, E | |
| Formicidae | Linepithema sp.1 | B, D | |
| Formicidae | Megalomyrmex cuatiara | D | |
| Formicidae | Megalomyrmex incisus | D | |
| Formicidae | Megalomyrmex poatan | D, E | |
| Formicidae | Megalomyrmex sp.1 | D, E | |
| Formicidae | Megalomyrmex sp.2 | B, C, D | |
| Hymenoptera | Formicidae | Megalomyrmex sp.3 | B, D |
| Formicidae | Megalomyrmex sp.4 | E | |
| Formicidae | Megalomyrmex sp.5 | C, D | |
| Formicidae | Megalomyrmex sp.6 | D | |
| Formicidae | Myrcidris sp.1 | D | |
| Formicidae | Neivamyrmex sp.1 | C | |
| Formicidae | Nylanderia fulva | A | |
| Formicidae | Paratrechina fulva | B, D | |
| Formicidae | Paratrechina sp.1 | B, D | |
| Formicidae | Pseudomyrmex gracilis | D | |
| Formicidae | Pseudomyrmex oculatus | B | |
| Formicidae | Pseudomyrmex sp.1 | E | |
| Formicidae | Pseudomyrmex tenuis | A | |
| Formicidae | Solenopsis aff. geminata | C | |
| Formicidae | Solenopsis geminata | C, D | |
| Formicidae | Solenopsis sp.1 | C, D | |
| Formicidae | Solenopsis sp.2 | C, D, E | |
| Formicidae | Solenopsis sp.3 | C, D | |
| Formicidae | Solenopsis sp.4 | D | |
| Formicidae | Solenopsis sp.5 | C, E | |
| Formicidae | Solenopsis sp.6 | C, D | |
| Formicidae | Wasmannia auropunctata | D | |
| Formicidae | Wasmannia sp.2 | D | |
| Halictidae | Agapostemon sp.1 | C, D, E | |
| Halictidae | Augochlora sp.1 | D | |
| Halictidae | Augochlora sp.2 | D, E | |
| Halictidae | Augochlora sp.3 | E | |
| Halictidae | Augochlora sp.4 | D, E | |
| Halictidae | Augochlora sp.5 | D | |
| Halictidae | Augochlora sp.6 | D | |
| Halictidae | Augochlora sp.7 | C, D | |
| Halictidae | Augochlora sp.8 | A | |
| Halictidae | Augochlora sp.9 | A | |
| Halictidae | Augochlora sp.10 | A | |
| Halictidae | Augochlorella sp.1 | D | |
| Halictidae | Augochlorella sp.2 | C | |
| Halictidae | Augochlorella sp.3 | C | |
| Halictidae | Augochlorella sp.4 | D | |
| Halictidae | Augochlorella sp.5 | C | |
| Halictidae | Augochlorella sp.6 | C, E | |
| Halictidae | Augochlorella sp.7 | C | |
| Halictidae | Augochlorella sp.8 | C, D | |
| Halictidae | Augochloropsis sp.1 | B, D | |
| Hymenoptera | Halictidae | Augochloropsis sp.2 | C |
| Halictidae | Augochloropsis sp.3 | D | |
| Halictidae | Augochloropsis sp.4 | A | |
| Halictidae | Augochloropsis sp.5 | A | |
| Halictidae | Caenohalictus sp.1 | A, D | |
| Halictidae | Caenohalictus sp.2 | B, C, D | |
| Halictidae | Habralictus sp.1 | B, D, E | |
| Halictidae | Habralictus sp.2 | D, E | |
| Halictidae | Lasioglossum sp.1 | B, C, D, E | |
| Halictidae | Lasioglossum sp.2 | A, B, C, D, E | |
| Halictidae | Lasioglossum sp.3 | A, C, D, E | |
| Halictidae | Lasioglossum sp.4 | A, D, E | |
| Halictidae | Lasioglossum sp.5 | B, C | |
| Halictidae | Lasioglossum sp.6 | A | |
| Halictidae | Neocorynura sp.1 | E | |
| Halictidae | Neocorynura sp.2 | C | |
| Halictidae | Neocorynura sp.3 | D | |
| Halictidae | Neocorynura sp.4 | D | |
| Halictidae | Neocorynura sp.5 | D | |
| Halictidae | Neocorynura sp.6 | A | |
| Halictidae | Neocorynura sp.7 | D | |
| Halictidae | Pereirapis sp.1 | B, C, D | |
| Halictidae | Pereirapis sp.2 | D | |
| Halictidae | Pereirapis sp.3 | D | |
| Halictidae | Pereirapis sp.4 | D | |
| Halictidae | Pseudaugochlora sp.1 | D | |
| Halictidae | Pseudaugochlora sp.2 | D, E | |
| Ichneumonidae | Ichneumonidae sp.1 | E | |
| Ichneumonidae | Ichneumonidae sp.2 | D | |
| Ichneumonidae | Ichneumonidae sp.3 | E | |
| Ichneumonidae | Ichneumonidae sp.4 | C | |
| Ichneumonidae | Ichneumonidae sp.5 | D | |
| Megachilidae | Megachile sp.1 | D | |
| Megaspilidae | Megaspilidae sp.1 | E | |
| Platygastridae | Platygastridae sp.1 | C, D | |
| Platygastridae | Synopeas sp.1 | B, C | |
| Pompilidae | Aimatocare sp.1 | B | |
| Pompilidae | Entypus sp.1 | D | |
| Pompilidae | Entypus sp.2 | D | |
| Pompilidae | Priocnemis sp.1 | D, E | |
| Pompilidae | Priocnemis sp.2 | C, D, E | |
| Psenidae | Pseneo sp.1 | D | |
| Scoliidae | Campsomeris dorsata | C | |
| Hymenoptera | Scoliidae | Campsomeris peregrina | C, D |
| Scoliidae | Campsomeris servillei | A, D | |
| Scoliidae | Campsomeris sp.1 | E | |
| Tiphiidae | Tiphia sp.1 | C, D, E | |
| Tiphiidae | Tiphia sp.2 | D | |
| Tiphiidae | Tiphia sp.3 | C | |
| Vespidae | Agelaia areata | B, C | |
| Vespidae | Angiopolybia paraensis | A | |
| Vespidae | Brachygastra augusti | D | |
| Vespidae | Brachygastra baccalaurea | A | |
| Vespidae | Epipona guerini | C, D | |
| Vespidae | Pachodynerus nasidens | A | |
| Vespidae | Parachartergus colobopterus | A | |
| Vespidae | Parachartergus richardsi | C | |
| Vespidae | Parachartergus weyrauchi | A, B | |
| Vespidae | Polistes carnifex | C, D | |
| Vespidae | Polistes satan | B | |
| Vespidae | Polybia cf. simillima | C, D, E | |
| Vespidae | Polybia emaciata | B | |
| Vespidae | Polybia ignobilis | D | |
| Vespidae | Polybia occidentalis venezuelana | A, B | |
| Vespidae | Polybia rejecta | B, C | |
| Vespidae | Protopolybia acutiscutis | C, E | |
| Vespidae | Protopolybia fuscata | D | |
| Vespidae | Synoeca septentrionalis | A, B, C, D | |
| Lepidoptera | Erebidae | Erebidae sp.1. | C |
| Hesperiidae | Eutychide complana | D | |
| Hesperiidae | Hesperiidae sp.1 | C | |
| Hesperiidae | Hesperiidae sp.2 | C | |
| Hesperiidae | Hesperiidae sp.3 | C | |
| Hesperiidae | Hesperiidae sp.4 | C | |
| Hesperiidae | Hesperiidae sp.5 | C | |
| Hesperiidae | Hesperiidae sp.6 | C | |
| Hesperiidae | Hesperiidae sp.7 | C | |
| Hesperiidae | Hesperiidae sp.8 | C | |
| Hesperiidae | Hesperiidae sp.9 | C | |
| Hesperiidae | Hesperiidae sp.10 | C | |
| Hesperiidae | Hesperiidae sp.11 | C, E | |
| Hesperiidae | Hesperiidae sp.12 | D | |
| Hesperiidae | Hesperiidae sp.13 | C, E | |
| Hesperiidae | Hesperiidae sp.14 | D | |
| Hesperiidae | Thespieus sp.1 | C | |
| Hesperiidae | Urbanus procne | C | |
| Hesperiidae | Urbanus proteus | C | |
| Hesperiidae | Urbanus simplicius | D | |
| Hesperiidae | Urbanus teleus | C, D | |
| Lycaenidae | Rekoa palegon | C | |
| Lycaenidae | Strymon caldasensis | D | |
| Nymphalidae | Actinote anteas | C | |
| Nymphalidae | Anartia amathea | C | |
| Nymphalidae | Anartia jatrophae | C | |
| Nymphalidae | Dione juno | C | |
| Nymphalidae | Siproeta stelenes | C | |
| Papilionidae | Heraclides thoas nealces | D | |
| Pieridae | Ascia monuste | D | |
| Sphingidae | Sphingidae sp.1 | C | |
| Neuroptera | Chrysopidae | Chrysopidae sp.1 | B |
| Chrysopidae | Chrysopidae sp.2 | D | |
| Orthoptera | Orthoptera sp.1 | D, E | |
| Thysanoptera | Thysanoptera sp.1 | B, C, E |
Appendix B
| Species | Country | Number of Bee Species | Number of Species of Other Insects | Most Abundant Visitors (>5% of the Visits) | References |
|---|---|---|---|---|---|
| C. canephora | Papua New Guinea | 4 | NR | Trigona sp., Apis sp. Amegilla sapiens, Megachile frontalis, Syrphidae | [13] |
| C. canephora | Indonesia | 22 | NE | Lepidotrigona terminata, Heterotrigona sp., Apis cerana, Amegilla samarensis, Xylocopa dejeanii | [53] |
| C. canephora | Uganda | 25 | NE | Hypotrigona gribodoi, Axestotrigona ferrugínea, A. mellifera adansonii. | [54] |
| C. arabica | Jamaica | 4 | 14 | A. mellifera | [33] |
| C. arabica | Panama | 22 | ~29 | A. mellifera scutellata | [15] |
| C. arabica | Indonesia | ~15 | NE | Apis nigrocincta, Apis dorsata, A. cerana, L. terminata, Heterotrigona sp. 2, Halictidae, M. frontalis, Heriades sp. | [2] |
| C. arabica | Brazil | 5 | NR | A. mellifera scutellata | [55] |
| C. arabica | Brazil | 6 | NE | A. mellífera | [56] |
| C. arabica | Costa Rica | 14 | NE | A. mellifera, Plebeia jatiformis, Plebeia frontalis, Trigonisca sp. | [57] |
| C. arabica | Mexico | 5 | 22 | Trigona sp. 1, A. mellifera, Ceratina sp. 1, ceratina sp. 2, Brachymyrmex sp. 1, Brachymyrmex sp. 2, Crematogaster sp., Solenopsis sp. 1. | [34] |
| C. arabica | Ecuador | 8 | NE | A. mellifera scutellata, Cephalotrigona capitata, Melipona mimetica, Nannotrigona mellaria, Nannotrigona perilampoides, Partamona peckolti, T. angustula, Trigona amalthea | [58] |
| C. arabica | Mexico | 7 | 8 | A. mellifera, Scaptotrigona mexicana | [59] |
| C. arabica | Mexico | 7 | 8 | A. mellifera, Trigona corvina | [35] |
| C. arabica | Colombia | 13 | NE | A. mellifera, Paratrigona eutaeniata | [12] |
| C. arabica | Brazil | 22 | NE | A. mellifera, Trigona spinipes, Trigona hyalinata, Paratrigona subnuda | [60] |
| C. arabica | Brazil | 19 | 48 | A. mellifera, Trigona spinipes, Ceratitis capitata (Tephritidae) | [61] |
| C. arabica | Ethiopia | 27 | NE | A. mellifera, Lasioglossum aff. Atricrum, Zonalictus abessinicus | [62] |
References
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated by animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Klein, A.M.; Steffan-Dewenter, I.; Tscharntke, T. Fruit set of highland coffee increases with the diversity of pollinating bees. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Garibaldi, L.A.; Carvalheiro, L.G.; Vaissière, B.E.; Gemmill-Herren, B.; Hipólito, J.; Freitas, B.M.; Ngo, H.T.; Azzu, N.; Sáez, A.; Áström, J.; et al. Mutually beneficial pollinator diversity and crop yield outcomes in small and large farms. Science 2016, 351, 388–391. [Google Scholar] [CrossRef]
- Klein, A.M.; Müller, C.; Hoehn, P.; Kremen, C. Understanding the role of species richness for crop pollination services. In Biodiversity, Ecosystem Functioning, and Human Wellbeing; Naeem, S., Bunker, D.E., Hector, A., Loreau, M., Perrings, C., Eds.; Oxford University Press: New York, NY, USA, 2009; pp. 195–208. [Google Scholar]
- Hooper, D.U. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Federación Nacional de Cafeteros de Colombia (FNC). Informe del Gerente 90 Congreso Nacional de Cafeteros 2022. Available online: https://federaciondecafeteros.org/app/uploads/2022/12/Informe-del-Gerente-D.pdf (accessed on 2 November 2023).
- Flórez, C.P.; Ibarra, L.N.; Gómez, L.F.; Carmona, C.Y.; Castaño, Á.; Ortiz, A. Estructura y funcionamiento de la planta de café. In Federación Nacional de Cafeteros de Colombia, Manual del Cafetero Colombiano: Investigación y Tecnología para la Sostenibilidad de la Caficultura; LEGIS: Bogota, Colombia, 2013; Volume 1, pp. 123–168. [Google Scholar] [CrossRef]
- Ngo, H.T.; Mojica, A.C.; Packer, L. Coffee plant-pollinator interactions: A review. Can. J. Zool. 2011, 89, 647–660. [Google Scholar] [CrossRef]
- Moreaux, C.; Meireles, D.A.L.; Sonne, J.; Badano, E.I.; Classen, A.; González-Chaves, A.; Hipólito, J.; Klein, A.M.; Maruyama, P.K.; Metzger, J.P.; et al. The value of biotic pollination and dense forest for fruit set of Arabica coffee: A global assessment. Agric. Ecosyst. Environ. 2022, 323, 107680. [Google Scholar] [CrossRef]
- Jaramillo, A. Efecto de las Abejas Silvestres en la Polinización del Café (Coffea arabica: Rubiaceae) en tres Sistemas de Producción en el Departamento de Antioquia. Master’s Thesis, Universidad Nacional sede Medellín, Medellín, Colombia, 2012. Available online: https://repositorio.unal.edu.co/handle/unal/9860 (accessed on 28 May 2023).
- Cepeda-Valencia, J.; Gómez, P.D.; Nicholls, C. La estructura importa: Abejas visitantes del café y estructura agroecológica principal (EAP) en cafetales. Rev. Colomb. Entomol. 2014, 40, 241–250. [Google Scholar]
- Willmer, P.G.; Stone, G.N. Incidence of entomophilous pollination of lowland coffee (Coffea canephora): The role of leaf cutter bees in Papua New Guinea. Entomol. Exp. Appl. 1989, 50, 113–124. [Google Scholar] [CrossRef]
- Klein, A.-M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Landscape effects on crop pollination services: Are there general patterns? Ecol. Lett. 2008, 11, 499–515. [Google Scholar] [CrossRef]
- Roubik, D.W. Feral African bees augment neotropical coffee yield. In Pollinating Bees: The Conservation Link between Agriculture and Nature; Kevan, P.G., Imperatriz-Fonseca, V.L., Eds.; Ministry of Environment: Brasilia, Brazil, 2002; pp. 255–266. [Google Scholar]
- Garibaldi, L.A.; Bartomeus, I.; Bommarco, R.; Klein, A.M.; Cunningham, S.A.; Aizen, M.A.; Boreux, V.; Garratt, M.P.D.; Carvalheiro, L.G.; Kremen, C.; et al. Trait matching of flower visitors and crops predicts fruit set better than trait diversity. J. Appl. Ecol. 2015, 52, 1436–1444. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Garratt, M.P.D.; Powney, G.D.; Shaw, R.F.; Osborne, J.L.; Soroka, J.; Lindström, S.A.M.; Stanley, D.; Ouvrard, P.; Edwards, M.E.; et al. Meta-analysis reveals that pollinator functional diversity and abundance enhance crop pollination and yield. Nat. Commun. 2019, 10, 1481. [Google Scholar] [CrossRef]
- Arcila, P.J. Crecimiento y desarrollo de la planta de café. In Sistemas de Producción de Café en Colombia; Arcila, P.J., Farfán, V.F., Moreno, B.A.M., Salazar, G.L.F., Hincapié, G.E., Eds.; FNC-Cenicafé: Manizales, Colombia, 2007; pp. 21–61. [Google Scholar]
- Engel, M.S. Classification of the bee tribe Augochlorini (Hymenoptera: Halictidae). Bull. Am. Museum Nat. Hist. 2000, 2000, 1–89. [Google Scholar] [CrossRef]
- Triplehorn, C.A.; Johnson, N.F.; Borror, D.J. Borror and DeLong’s Introduction to the Study of Insects, 7th ed.; Thomson Brooks/Cole: Belmont, CA, USA, 2005; 864p. [Google Scholar]
- Fernandez, F.; Sharkey, M.J. Introdución a los Hymenoptera de la Región Neotropical; Sociedad Colombiana de Entomología y Universidad Nacional de Colombia: Bogotá, Colombia, 2006; 894p. [Google Scholar]
- Michener, C.D. The Bees of the World; The John Hopkins University Press: Baltimore, MD, USA, 2007; 953p. [Google Scholar]
- Normandin, É.; Vereecken, N.J.; Buddle, C.M.; Fournier, V. Taxonomic and functional trait diversity of wild bees in different urban settings. PeerJ 2017, 5, e3051. [Google Scholar] [CrossRef] [PubMed]
- Kratschmer, S.; Pachinger, B.; Schwantzer, M.; Paredes, D.; Guzmán, G.; Goméz, J.A.; Entrenas, J.A.; Guernion, M.; Burel, F.; Nicolai, A.; et al. Response of wild bee diversity, abundance, and functional traits to vineyard inter-row management intensity and landscape diversity across Europe. Ecol. Evol. 2019, 9, 4103–4115. [Google Scholar] [CrossRef] [PubMed]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of melissopalynology. Bee World 1970, 51, 125–138. [Google Scholar] [CrossRef]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- Colwell, R.K.; Elsensohn, J.A. EstimateS turns 20: Statistical estimation of species richness and shared species from samples, with non-parametric extrapolation. Ecography 2014, 37, 609–613. [Google Scholar] [CrossRef]
- Chao, A.; Ma, K.H.; Hsieh, T.C.; Chiu, C.H. SpadeR: Species-Richness Prediction and Diversity Estimation with R. Package ‘SpadeR’ Version 0.1.1. 2016. Available online: https://CRANR-project.org/package=SpadeR (accessed on 25 November 2023).
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Chao, A.; Shen, T.J. Nonparametric estimation of Shannon’s index of diversity when there are unseen species in sample. Environ. Ecol. Stat. 2003, 10, 429–443. [Google Scholar] [CrossRef]
- Chao, A.; Jost, L. Coverage based rarefaction and extrapolation: Standardizing samples by completeness rather than size. Ecology 2012, 93, 2533–2547. [Google Scholar] [CrossRef]
- Knop, E.; Gerpe, C.; Ryser, R.; Hofmann, F.; Menz, M.H.M.; Trösch, S.; Ursenbacher, S.; Zoller, L.; Fontaine, C. Rush hours in flower visitors over a day–night cycle. Insect Conserv. Divers. 2017, 11, 267–275. [Google Scholar] [CrossRef]
- Raw, A.; Free, J.B. The pollination of coffee (Coffea arabica) by honeybees. Trop. Agric. 1977, 54, 365–370. [Google Scholar]
- Philpott, S.M.; Uno, S.; Maldonado, J. The importance of ants and high-shade management to coffee pollination and fruit weight in Chiapas, Mexico. Biodivers. Conserv. 2006, 15, 487–501. [Google Scholar] [CrossRef]
- Vergara, C.H.; Badano, E.I. Pollinator diversity increases fruit production in Mexican coffee plantations: The importance of rusticmanagement systems. Agric. Ecosyst. Environ. 2009, 129, 117–123. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Sáez, A.; Aizen, M.A.; Fijen, T.; Bartomeus, I. Crop pollination management needs flower-visitor monitoring and target values. J. Appl. Ecol. 2020, 57, 664–670. [Google Scholar] [CrossRef]
- Hung, K.L.J.; Kingston, J.M.; Lee, A.; Holway, D.A.; Kohn, J.R. Non-native honey bees disproportionately dominate the most abundant floral resources in a biodiversity hotspot. Proc. R. Soc. B Biol. Sci. 2019, 286, 20182901. [Google Scholar] [CrossRef] [PubMed]
- Bänsch, S.; Tscharntke, T.; Gabriel, D.; Westphal, C. Crop pollination services: Complementary resource use by social vs solitary bees facing crops with contrasting flower supply. J. Appl. Ecol. 2021, 58, 476–485. [Google Scholar] [CrossRef]
- Hillebrand, H.; Bennett, D.M.; Cadotte, M.W. Consequences of dominance: A review of evenness effects on local and regional ecosystem processes. Ecology 2008, 89, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.-M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Moritz, R.F.A.; Härtel, S.; Neumann, P. Global invasions of the western honeybee (Apis mellifera) and the consequences for biodiversity. Ecoscience 2005, 12, 289–301. [Google Scholar] [CrossRef]
- Herrera, C.M. Daily patterns of pollinator activity, differential pollinating effectiveness, and floral resource availability, in a summer-flowering Mediterranean shrub. Oikos 1990, 58, 277. [Google Scholar] [CrossRef]
- McCall, C.; Primack, R.B. Influence of flower characteristics, weather, time of day, and season on insect visitation Rates in Three Plant Communities. Am. J. Bot. 1992, 79, 434–442. [Google Scholar] [CrossRef]
- Mason, N.W.H.; Mouillot, D.; Lee, W.G.; Wilson, J.B. Functional richness, functional evenness and functional divergence: The primary components of functional diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- Buchholz, S.; Egerer, M.H. Functional ecology of wild bees in cities: Towards a better understanding of trait-urbanization relationships. Biodivers. Conserv. 2020, 29, 2779–2801. [Google Scholar] [CrossRef]
- Mouchet, M.A.; Villéger, S.; Mason, N.W.H.; Mouillot, D. Functional diversity measures: An overview of their redundancy and their ability to discriminate community assembly rules. Funct. Ecol. 2010, 24, 867–876. [Google Scholar] [CrossRef]
- Arnan, X.; Cerdá, X.; Rodrigo, A.; Retana, J. Response of ant functional composition to fire. Ecography 2013, 36, 1182–1192. [Google Scholar] [CrossRef]
- Martins, K.T.; Gonzalez, A.; Lechowicz, M.J. Patterns of pollinator turnover and increasing diversity associated with urban habitats. Urban Ecosyst. 2017, 20, 1359–1371. [Google Scholar] [CrossRef]
- Thapa, K.B.; Davis, T.S.; Kondratieff, B. Livestock grazing is associated with seasonal reduction in pollinator biodiversity and functional dispersion but cheatgrass invasion is not: Variation in bee assemblages in a multiuse shortgrass prairie. PLoS ONE 2020, 15, e0237484. [Google Scholar] [CrossRef]
- Gómez, J.H.; Benavides, P.; Maldonado, J.D.; Jaramillo, J.; Acevedo, F.E.; Gil, Z.N. Flower-Visiting Insects Ensure Coffee Yield and Quality. Agriculture 2023, 13, 1392. [Google Scholar] [CrossRef]
- de Merxem, D.G.; Borremans, B.; de Jäger, M.L.; Johnson, T.; Jooste, M.; Ros, P.; Zenni, R.D.; Ellis, A.G.; Anderson, B. The importance of flower visitors not predicted by floral syndromes. S. Afr. J. Bot. 2009, 75, 660–667. [Google Scholar] [CrossRef]
- King, C.; Ballantyne, G.; Willmer, P.G. Why flower visitation is a poor proxy for pollination: Measuring single-visit pollen deposition, with implications for pollination networks and conservation. Methods Ecol. Evol. 2013, 4, 811–818. [Google Scholar] [CrossRef]
- Klein, A.M.; Dewenter, S.; Tscharntke, T. Pollination of Coffea canephora in relation to local and regional agroforestry management. J. Appl. Ecol. 2003, 40, 837–845. [Google Scholar] [CrossRef]
- Munyuli, T. Influence of functional traits on foraging behaviour and pollination efficiency of wild social and solitary bees visiting coffee (Coffea canephora) flowers in Uganda. Grana. 2014, 53, 69–89. [Google Scholar] [CrossRef]
- Malerbo-Souza, D.T.; Nogueira-Couto, R.H.; Couto, L.A.; de Souza, J.C. Atrativo para as abelhas Apis mellifera e polinização em café (Coffea arabica L.). Braz. J. Vet. Res. Anim. Sci. 2003, 40, 272–278. [Google Scholar] [CrossRef]
- De Marco Jr, P.; Monteiro, F. Services performed by the ecosystem: Forest remnants influence agricultural cultures’ pollination and production. Biodivers. Conserv. 2004, 13, 1245–1255. [Google Scholar] [CrossRef]
- Ricketts, T.H. Tropical forest fragments enhance pollinator activity in nearby coffee crops. Conserv. Biol. 2004, 18, 1262–1271. [Google Scholar] [CrossRef]
- Veddeler, D.; Klein, A.M.; Tscharntke, T. Contrasting responses of bee communities to coffee flowering at different spatial scales. Oikos 2006, 112, 594–601. [Google Scholar] [CrossRef]
- Vergara, C.; Contreras, J.; Ferrari, R.; Paredes, J. Polinización entomófila. In Agroecosistemas Cafetaleros de Veracruz: Biodiversidad, Manejo y Conservación; Manson, R.H., Hernández O, V., Gallina, S., Mehltreter, K., Eds.; Instituto Nacional de Ecología and Instituto de Ecología A.C.: Ciudad de México, Mexico, 2008; pp. 247–257. [Google Scholar]
- Saturni, F.T.; Jaffé, R.; Metzger, J.P. Landscape structure influences bee community and coffee pollination at different spatial scales. Agric. Ecosyst. Environ. 2016, 235, 1–12. [Google Scholar] [CrossRef]
- Hipólito, J.; Boscolo, D.; Viana, B.F. Landscape and crop management strategies to conserve pollination services and increase yields in tropical coffee farms. Agric. Ecosyst. Environ. 2018, 256, 218–225. [Google Scholar] [CrossRef]
- Geeraert, L.; Aerts, R.; Berecha, G.; Daba, G.; De Fruyt, N.; D´hollander, J.; Helsen, K.; Stynen, H.; Honnay, O. Effects of landscape composition on bee communities and coffee pollination in Coffea arabica production forests in southwestern Ethiopia. Agric. Ecosyst. Environ. 2020, 288, 106706–106717. [Google Scholar] [CrossRef]




| Study Sites | Location | Climate Conditions | Harvest Distribution | Area Cultivated in Coffee (Hectares) |
|---|---|---|---|---|
| (A) Pueblo Bello Experiment Station | Municipality of Pueblo Bello (department of Cesar), located in the Sierra Nevada de Santa Marta mountain range (10°25′ N; 73°34′ W); altitude: 1134 m above sea level; coffee ecotope: 402. | Average temperature of 27.9 °C; mean relative humidity of 78.3%; annual precipitation of 1727.7 mm; and 2302 h of sunshine/year. | Main harvest (90%): flowering between March and April; mid harvest (10%): flowering between August and September. | 25.6 |
| (B) San Antonio Experiment Station | Municipality of Floridablanca (department of Santander), Cordillera Oriental mountain range, western slope (07°06′ N; 73°04′ W); altitude: 1539 m above sea level; coffee ecotope: 302A. | Average temperature of 20.1 °C; average RH of 79.4%; annual precipitation of 1644 mm; 1155 h of sunshine/year. | Main harvest (90%): flowering between March and April; mid harvest (10%): flowering between August and September. | 3.22 |
| (C) Paraguaicito Experiment Station | Municipality of Buenavista (department of Quindío), Cordillera Central mountain range, western slope (04°4′ N; 75°44′ W); 1303 m above sea level; coffee ecotope: 211A. | Average temperature of 22.4 °C; average RH of 78.4%; annual precipitation of 1938 mm; 1541 h of sunshine/year. | Main harvest (55%): flowering between February and March; mid harvest (45%): flowering between August and September | 16.3 |
| (D) Naranjal Experiment Station | Municipality of Chinchiná (department of Caldas), Cordillera Central mountain range, western slope (4°58′ N; 75°39′ W); 1381 m above sea level; coffee ecotope: 206A. | Average temperature of 21.6 °C; average RH of 80.6%; annual precipitation of 2990 mm; 1537 h of sunshine/year. | Main harvest (75%): flowering between January and March; mid harvest (25%): flowering between August and September. | 48.7 |
| (E) Manuel Mejía Experiment Station | Municipality of El Tambo (department of Cauca), Cordillera Central, western slope (02°24′ N; 76°44′ W); altitude: 1735 m above sea level; coffee ecotope: 218A. | Average temperature of 19.8 °C; average RH of 81.1%; annual precipitation of 1826 mm; 1632 h sunshine/year. | Mid harvest (10%): flowering between February and March; main harvest (90%): flowering between August and September | 10.9 |
| Trait | Status of Trait | Type of Variable |
|---|---|---|
| Nesting habit | Pre-existing cavities in the ground, pre-existing cavities, new cavities in the ground, tree branches, decaying wood, dry logs and wood, and dry branches. | Nominal categorical |
| Degree of sociability | Eusocial, parasocial, solitary (subsocial and solitary), facultative parasocial, and facultative eusocial. | Nominal categorical |
| Size (mm) | Mean of the intertegular distance for collected individuals; maximum 20 individuals measured. | Continuous numeric |
| Stigma contact | Very frequent; always; occasional; never. | Nominal categorical (numeric for the FD package) |
| Location of pollen loading structure | Tibial, femorotibial, basitarsotibial, and gasteral. | Nominal categorical |
| Type of tongue | Short, long, and very long. | Ordinal categorical |
| Abundance from 7:00 to 9:30 | Number of individuals. | Whole numbers |
| Abundance from 10:30 to 13:00 | Number of individuals. | Whole numbers |
| Abundance from 14:00 to 16:30 | Number of individuals. | Whole numbers |
| Study Sites | Species Observed | Singletons | Sampling Coverage | Expected Species | Percentage Species in Sample | ||
|---|---|---|---|---|---|---|---|
| Chao1 | ACE | Chao1 | ACE | ||||
| Pueblo Bello E.S * | 70 | 36 | 0.991 | 133 | 155.8 | 52.6 | 44.9 |
| San Antonio E.S | 141 | 99 | 0.988 | 679.9 | 510.1 | 20.7 | 27.6 |
| Paraguaicito E.S | 233 | 125 | 0.973 | 405.2 | 439.2 | 57.5 | 53 |
| Naranjal E.S | 288 | 145 | 0.970 | 496.8 | 529.3 | 58.0 | 54.4 |
| Manuel Mejía E.S | 99 | 61 | 0.973 | 302.2 | 277.0 | 32.8 | 35.7 |
| General | 566 | 254 | 0.989 | 874.9 | 921.6 | 64.7 | 61.4 |
| Study Sites | Observed Species | Singletons | Sampling Coverage | Expected Species | Percentage Species in Sample | ||
|---|---|---|---|---|---|---|---|
| Chao1 | ACE | Chao1 | ACE | ||||
| Pueblo Bello E.S * | 30 | 11 | 0.997 | 48.3 | 55.5 | 62.1 | 54.1 |
| San Antonio E.S | 23 | 9 | 0.999 | 35.0 | 48.2 | 65.7 | 47.7 |
| Paraguaicito E.S | 33 | 10 | 0.997 | 37.1 | 41.1 | 88.9 | 80.3 |
| Naranjal E.S | 51 | 18 | 0.995 | 66.3 | 71.1 | 76.9 | 71.7 |
| Manuel Mejía E.S | 20 | 7 | 0.996 | 27.0 | 28.2 | 74.1 | 70.9 |
| General | 90 | 29 | 0.999 | 119 | 125.3 | 75.6 | 71.8 |
| Study Sites | Functional Species | FEve a | FDiv a | FDis a |
|---|---|---|---|---|
| Pueblo Bello E.S * | 30 | 0.37 | 0.98 | 0.18 |
| San Antonio E.S | 23 | 0.39 | 0.93 | 0.16 |
| Paraguaicito E.S | 33 | 0.43 | ~1.00 | 0.10 |
| Naranjal E.S | 51 | 0.39 | 0.99 | 0.27 |
| Manuel Mejía E.S | 20 | 0.45 | ~1.00 | 0.16 |
| General | 90 | 0.41 | 0.99 | 0.19 |
| Study Sites | Species | VR(A) a | PCC a | C a | PE a | PIV a |
|---|---|---|---|---|---|---|
| Pueblo Bello E.S | A. mellifera | 73.62 | 0.47 | 0.98 | 1.00 | 33.51 |
| N. gaboi | 4.12 | 0.53 | 0.94 | 0.92 | 1.91 | |
| T. angustula | 4.30 | 0.47 | 0.99 | 0.67 | 1.33 | |
| Scaptotrigona sp. 1 | 4.64 | 0.44 | 0.93 | 0.92 | 1.77 | |
| San Antonio E.S | A. mellifera | 73.29 | 0.47 | 0.95 | 1.00 | 32.81 |
| Geotrigona cf. tellurica | 17.24 | 0.53 | 0.97 | 0.92 | 8.10 | |
| Paraguaicito E.S | A. mellifera | 70.62 | 0.90 | 0.93 | 1.00 | 59.13 |
| Lasioglossum spp. | 2.64 | 0.10 | 0.64 | 0.65 | 0.11 | |
| Naranjal E.S | A. mellifera | 40.82 | 0.39 | 0.98 | 1.00 | 15.62 |
| N. tristella | 2.91 | 0.22 | 0.97 | 0.87 | 0.53 | |
| T. angustula | 15.27 | 0.39 | 0.99 | 0.67 | 3.93 | |
| Manuel Mejía E.S | A. mellifera | 69.27 | 0.33 | 0.94 | 1.00 | 21.75 |
| Partamona cf. peckolti | 7.12 | 0.35 | 0.86 | 0.93 | 2.00 | |
| T. angustula | 7.03 | 0.31 | 0.95 | 0.67 | 1.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maldonado-Cepeda, J.D.; Gómez, J.H.; Benavides, P.; Jaramillo, J.; Gil, Z.N. Taxonomic and Functional Diversity of Flower-Visiting Insects in Coffee Crops. Insects 2024, 15, 143. https://doi.org/10.3390/insects15030143
Maldonado-Cepeda JD, Gómez JH, Benavides P, Jaramillo J, Gil ZN. Taxonomic and Functional Diversity of Flower-Visiting Insects in Coffee Crops. Insects. 2024; 15(3):143. https://doi.org/10.3390/insects15030143
Chicago/Turabian StyleMaldonado-Cepeda, Juan Diego, Jesús Hernando Gómez, Pablo Benavides, Juliana Jaramillo, and Zulma Nancy Gil. 2024. "Taxonomic and Functional Diversity of Flower-Visiting Insects in Coffee Crops" Insects 15, no. 3: 143. https://doi.org/10.3390/insects15030143
APA StyleMaldonado-Cepeda, J. D., Gómez, J. H., Benavides, P., Jaramillo, J., & Gil, Z. N. (2024). Taxonomic and Functional Diversity of Flower-Visiting Insects in Coffee Crops. Insects, 15(3), 143. https://doi.org/10.3390/insects15030143