The L1014F Knockdown Resistance Mutation Is Not a Strong Correlate of Phenotypic Resistance to Pyrethroids in Florida Populations of Culex quinquefasciatus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
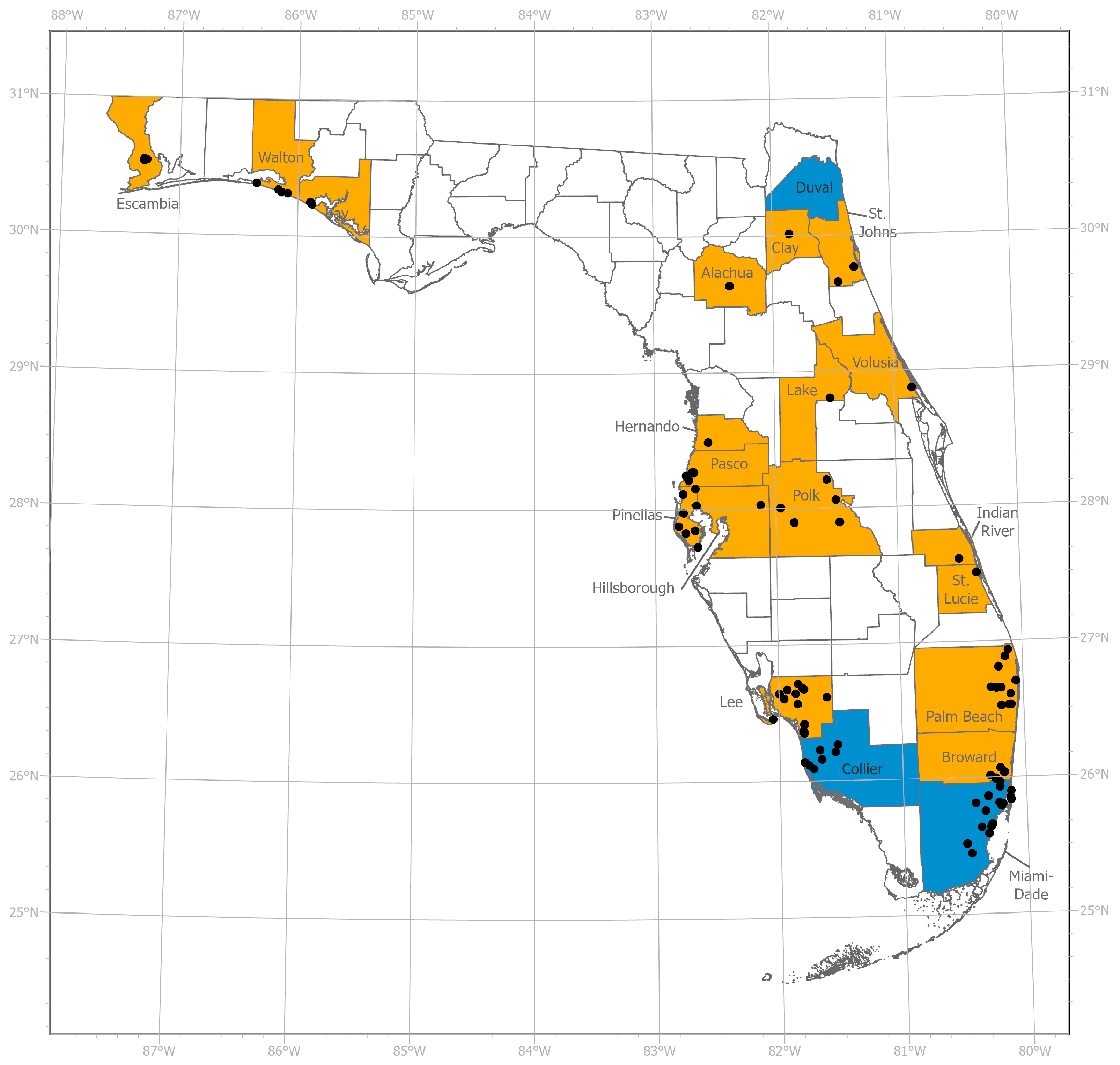
2.1. Mosquito Collections
2.2. Phenotypic Resistance Testing
2.3. Topical Application
2.4. Knockdown Resistance Genotyping
3. Results
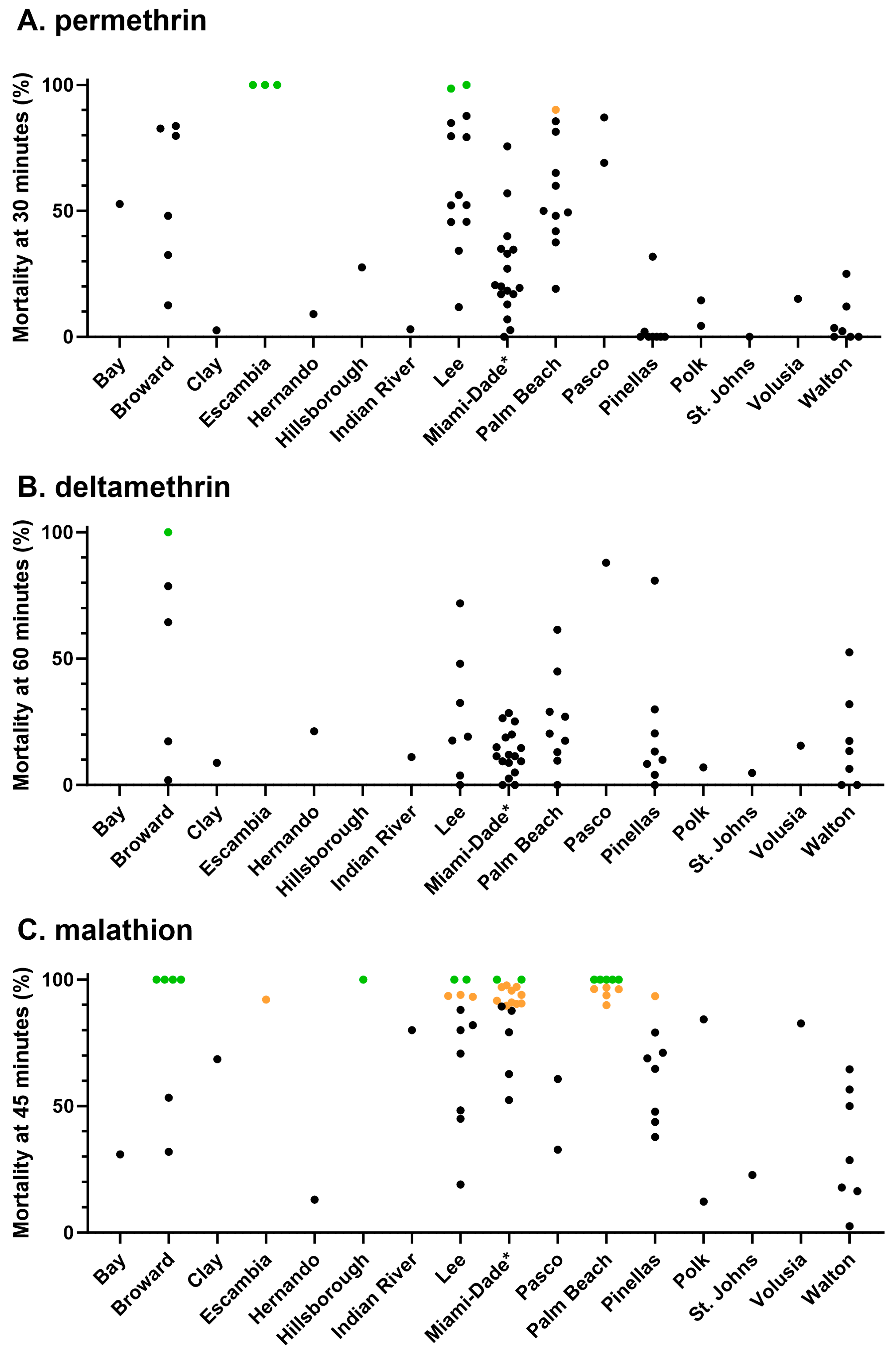
3.1. Phenotypic Resistance Testing
3.1.1. CDC Bottle Bioassay
3.1.2. Topical Application
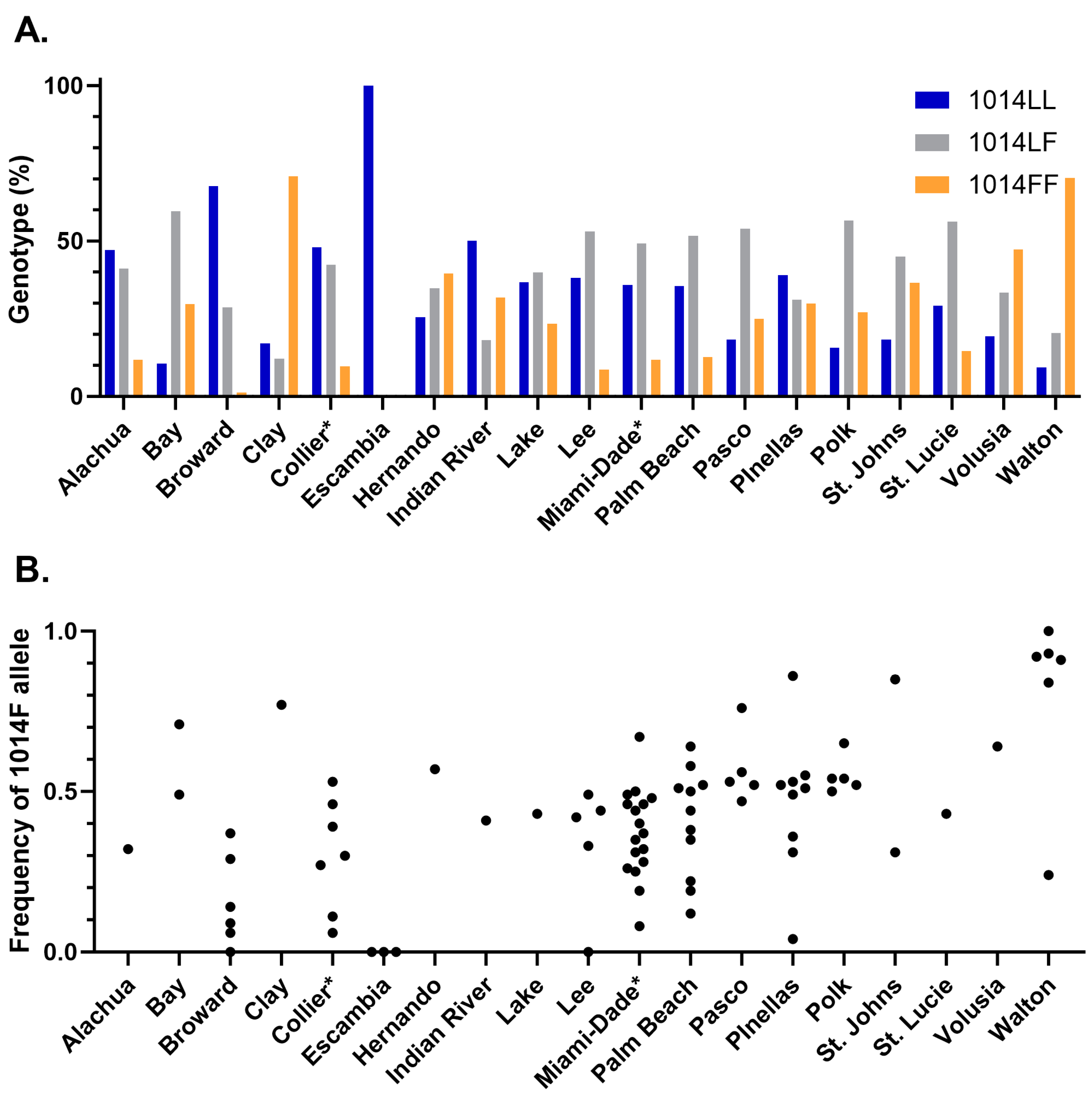
3.2. Knockdown Resistance Testing
3.3. Correlation between Bottle Bioassay and kdr Genotype or Allele Frequency
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farajollahi, A.; Fonseca, D.M.; Kramer, L.D.; Kilpatrick, A.M. “Bird biting” mosquitoes and human disease: A review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect. Genet. Evol. 2011, 11, 1577–1585. [Google Scholar] [CrossRef]
- Muturi, E.J.; Muriu, S.; Shililu, J.; Mwangangi, J.M.; Jacob, B.G.; Mbogo, C.; Githure, J.; Novak, R.J. Blood-feeding patterns of Culex quinquefasciatus and other culicines and implications for disease transmission in Mwea rice scheme, Kenya. Parasitol. Res. 2008, 102, 1329–1335. [Google Scholar] [CrossRef]
- Nitatpattana, N.; Apiwathnasorn, C.; Barbazan, P.; Leemingsawat, S.; Yoksan, S.; Gonzalez, J. First Isolation of Japanese Encephalitis from Culex quinquefasciatus in Thailand. S. Asian J. Trop. Med. Public Health 2005, 36, 875. [Google Scholar]
- Molaei, G.; Andreadis, T.G.; Armstrong, P.M.; Bueno, R.; Dennett, J.A.; Real, S.V.; Sargent, C.; Bala, A.; Randle, Y.; Guzman, H.; et al. Host feeding pattern of Culex quinquefasciatus (Diptera: Culicidae) and its role in transmission of West Nile virus in Harris County, Texas. Am. J. Trop. Med. Hyg. 2007, 77, 73–81. [Google Scholar] [CrossRef]
- American Mosquito Control Association. Available online: https://www.mosquito.org/assets/pdf/hr_november_2021_amca_bmp_ma/ (accessed on 4 February 2024).
- United States Environmental Protection Agency. Available online: https://www.epa.gov/mosquitocontrol/joint-statement-mosquito-control-united-states (accessed on 4 February 2024).
- World Health Organization. Available online: https://iris.who.int/bitstream/handle/10665/69745/WHO_HTM_NTD_VEM_2008.2_eng.pdf?sequence=1 (accessed on 4 February 2024).
- Gentry, J.W.; Hubert, A.A. Resistance of Culex quinquefasciatus to Chlorinated Hydrocarbons on Okinawa. Mosq. News 1957, 17, 92–93. [Google Scholar]
- Levi-Castillo, R. The Appearance of Resistance to Residual Insecticides in Culex pipiens quinquefasciatus [fatigans] in the City of Guayaquil. Rev. Ecuat. Entomol. Parasitol. 1953, 1, 7–16. [Google Scholar]
- Acosta, M. Physiological resistance to insecticides in Culex pipiens fatigans. Rev. Med. Peru. 1961, 30, 53–56. [Google Scholar]
- Smith, A. Dieldrin resistance in Culex pipiens fatigans in north eastern Tanganyika. Indian J. Malariol. 1959, 12, 341–343. [Google Scholar]
- Micks, D.W.; Cox, W.M.; McNeill, J.C. The Status of Insecticide Resistance in some Mosquito Species of Texas. Mosq. News 1961, 21, 229–232. [Google Scholar]
- Pennell, J.T.; Hoskins, W.M. The monofactorial inheritance of resistance to dieldrin in larval and adult Culex quinquefasciatus Say. Bull. World Health Organ. 1964, 31, 669–677. [Google Scholar] [PubMed]
- Brown, A.W.A. Meeting the resistance problem: Vector control prospects in the light of present knowledge of insecticide resistance. Bull. World Health Organ. 1963, 29, 41–50. [Google Scholar]
- Brown, A.W.A. The present status of control of Culex pipiens fatigans. Bull. World Health Organ. 1967, 37, 297–299. [Google Scholar]
- Georghiou, G.P.; Ariaratnam, V.; Pasternak, M.E.; Lin, C.S. Organophosphorus multiresistance in Culex pipiens quinquefasciatus in California. J. Econ. Entomol. 1975, 68, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Priester, T.M.; Georghiou, G.P. Induction of high resistance to permethrin in Culex pipiens quinquefasciatus. J. Econ. Entomol. 1978, 71, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Priester, T.M.; Georghiou, G.P. Cross-resistance spectrum in pyrethroid-resistant Culex quinquefasciatus. Pestic. Sci. 1980, 11, 617–624. [Google Scholar] [CrossRef]
- Priester, T.M.; Georghiou, G.P. Penetration of permethrin and knockdown in larvae of pyrethroid-resistant and-susceptible strains of the southern house mosquito. J. Econ. Entomol. 1980, 73, 165–167. [Google Scholar] [CrossRef]
- Magnin, M.; Marboutin, E.; Pasteur, N. Insecticide resistance in Culex quinquefasciatus (Diptera: Culicidae) in West Africa. J. Med. Entomol. 1988, 25, 99–104. [Google Scholar] [CrossRef]
- Amin, A.M.; Hemingway, J. Preliminary investigation of the mechanisms of DDT and pyrethroid resistance in Culex quinquefasciatus Say (Diptera: Culicidae) from Saudi Arabia. Bull. Entomol. Res. 1989, 79, 361–366. [Google Scholar] [CrossRef]
- Nayar, J.K.; Knight, J.W.; Munstermann, L.E. Temporal and geographic genetic variation in Culex pipiens quinquefasciatus (Diptera: Culicidae) from Florida. J. Med. Entomol. 2003, 40, 882–889. [Google Scholar] [CrossRef]
- Liu, H.; Cupp, E.W.; Micher, K.M.; Guo, A.; Liu, N. Insecticide resistance and cross-resistance in Alabama and Florida strains of Culex quinquefaciatus. J. Med. Entomol. 2004, 41, 408–413. [Google Scholar] [CrossRef]
- Zhou, L.; Lawrence, G.G.; Vineis, J.H.; McAllister, J.C.; Wirtz, R.A.; Brogdon, W.G.3. Detection of broadly distributed sodium channel alleles characteristic of insect pyrethroid resistance in West Nile virus vector Culex pipiens complex mosquitoes in the United States. J. Med. Entomol. 2009, 46, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Xu, Q.; Li, T.; He, L.; Zhang, L. Permethrin resistance and target site insensitivity in the mosquito Culex quinquefasciatus in Alabama. J. Med. Entomol. 2009, 46, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Lucas, K.J.; Bales, R.B.; McCoy, K.; Weldon, C. Oxidase, esterase, and KDR-associated pyrethroid resistance in Culex quinquefasciatus field collections of Collier County, Florida. J. Am. Mosq. Control Assoc. 2020, 36, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.G.; Yoshimizu, M.H.; Kasai, S. Pyrethroid resistance in Culex pipiens mosquitoes. Pest Biochem. Physiol. 2015, 120, 68–76. [Google Scholar] [CrossRef]
- Weill, M.; Lutfalla, G.; Mogensen, K.; Chandre, F.; Berthomieu, A.; Berticat, C.; Pasteur, N.; Philips, A.; Fort, P.; Raymond, M. Insecticide resistance in mosquito vectors. Nature 2003, 423, 136–137. [Google Scholar] [CrossRef] [PubMed]
- Weill, M.; Malcolm, C.; Chandre, F.; Mogensen, K.; Berthomieu, A.; Marquine, M.; Raymond, M. The unique mutation in ace-1 giving high insecticide resistance is easily detectable in mosquito vectors. Insect Mol. Biol. 2004, 13, 1–7. [Google Scholar] [CrossRef]
- Estep, A.S.; Sanscrainte, N.D.; Waits, C.M.; Bernard, S.J.; Lloyd, A.M.; Lucas, K.J.; Buckner, E.A.; Vaidyanathan, R.; Morreale, R.; Conti, L.A.; et al. Quantification of permethrin resistance and kdr alleles in Florida strains of Aedes aegypti (L.) and Aedes albopictus (Skuse). PLoS Negl. Trop. Dis. 2018, 12, e0006544. [Google Scholar] [CrossRef]
- Mack, L.K.; Kelly, E.T.; Lee, Y.; Brisco, K.K.; Shen, K.V.; Zahid, A.; van Schoor, T.; Cornel, A.J.; Attardo, G.M. Frequency of sodium channel genotypes and association with pyrethrum knockdown time in populations of Californian Aedes aegypti. Parasites Vectors 2021, 14, 141. [Google Scholar] [CrossRef]
- Estep, A., III; Kissoon, K.; Saldana, M.; Fredregill, C. Persistent variation in insecticide resistance intensity in container breeding Aedes (Diptera: Culicidae) co-collected in Houston, TX. J. Med. Entomol. 2023, 60, 631–636. [Google Scholar] [CrossRef]
- Unlu, I.; Buckner, E.A.; Medina, J.; Vasquez, C.; Cabrera, A.; Estep, A.S. Insecticide resistance of Miami-Dade Culex quinquefasciatus populations and initial field efficacy of a new resistance-breaking formulation. PLoS ONE 2024, 19, e0296046. [Google Scholar] [CrossRef]
- Watkins, A.S.; Babcock, E.; Lucas, K.J. Ornamental bromeliads of local botanical gardens serve as production sites for pyrethroid-resistant Culex quinquefasciatus (Say) in Collier County, Florida. J. FL Mosq. Control Assoc. 2020, 68, 14–23. [Google Scholar] [CrossRef]
- Richards, S.L.; Balanay, J.A.G.; Fields, M.; Vandock, K. Baseline insecticide susceptibility screening against six active ingredients for Culex and Aedes (Diptera: Culicidae) mosquitoes in the United States. J. Med. Entomol. 2017, 54, 682–695. [Google Scholar] [CrossRef]
- Pridgeon, J.W.; Pereira, R.M.; Becnel, J.J.; Allan, S.A.; Clark, G.G.; Linthicum, K.J. Susceptibility of Aedes aegypti, Culex quinquefasciatus Say, and Anopheles quadrimaculatus Say to 19 pesticides with different modes of action. J. Med. Entomol. 2008, 45, 82–87. [Google Scholar] [CrossRef] [PubMed]
- CONUS Manual for Evaluating Insecticide Resistance in Mosquitoes Using the CDC Bottle Bioassay Kit. Available online: https://www.cdc.gov/mosquitoes/pdfs/CONUS-508.pdf (accessed on 5 February 2024).
- Jensen, B.M.; Althoff, R.A.; Rydberg, S.E.; Royster, E.N.; Estep, A.; Huijben, S. Topical application bioassay to quantify insecticide toxicity for mosquitoes and fruit flies. J. Vis. Exp. 2022, 179, e63391. [Google Scholar] [CrossRef]
- Burgess, E.R., IV; Lopez, K.; Irwin, P.; Jaeger, C.P.; Estep, A.S. Assessing pyrethroid resistance status in the Culex pipiens complex (Diptera: Culicidae) from the northwest suburbs of Chicago, Illinois using Cox regression of bottle bioassays and other detection tools. PLoS ONE 2022, 17, e0268205. [Google Scholar] [CrossRef] [PubMed]
- Liu, N. Insecticide resistance in mosquitoes: Impact, mechanisms, and research directions. Ann. Rev. Entomol. 2015, 60, 537–559. [Google Scholar] [CrossRef] [PubMed]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.H. Biostatistics 104: Correlational analysis. Singap. Med. J. 2003, 44, 614–619. [Google Scholar]


| Strain 1 | Permethrin LD50 ± 95% CI (ng AI/mg Mosquito) | Resistance Ratio 2 | R2 |
|---|---|---|---|
| CMAVE | 1.39 (1.16–1.64) | 1.0 | 0.7165 |
| Pasco F4 | 56.47 (42.25–74.14) | 40.6 | 0.9169 |
| Cross Bayou F0 | 28.31 (24.06–33.75) | 20.3 | 0.9450 |
| North Highway F0 | 32.24 (21.17–50.23) | 23.2 | 0.7067 |
| Oldsmar Sewer F0 | 30.86 (22.98–42.68) | 22.2 | 0.8291 |
| Clearwater Nursery F0 | 32.98 (27.00–40.83) | 23.7 | 0.9186 |
| Keller F1 | 48.54 (39.62–58.86) | 34.9 | 0.9223 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estep, A.S.; Sanscrainte, N.D.; Stuck, J.; Unlu, I.; Prasauskas, A.; Mundis, S.J.; Cotter, N.; Romero-Weaver, A.L.; Fedirko, T.J.; Kendziorski, N.L.; et al. The L1014F Knockdown Resistance Mutation Is Not a Strong Correlate of Phenotypic Resistance to Pyrethroids in Florida Populations of Culex quinquefasciatus. Insects 2024, 15, 197. https://doi.org/10.3390/insects15030197
Estep AS, Sanscrainte ND, Stuck J, Unlu I, Prasauskas A, Mundis SJ, Cotter N, Romero-Weaver AL, Fedirko TJ, Kendziorski NL, et al. The L1014F Knockdown Resistance Mutation Is Not a Strong Correlate of Phenotypic Resistance to Pyrethroids in Florida Populations of Culex quinquefasciatus. Insects. 2024; 15(3):197. https://doi.org/10.3390/insects15030197
Chicago/Turabian StyleEstep, Alden S., Neil D. Sanscrainte, Jason Stuck, Isik Unlu, Agne Prasauskas, Stephanie J. Mundis, Nicholas Cotter, Ana L. Romero-Weaver, Troy J. Fedirko, Natalie L. Kendziorski, and et al. 2024. "The L1014F Knockdown Resistance Mutation Is Not a Strong Correlate of Phenotypic Resistance to Pyrethroids in Florida Populations of Culex quinquefasciatus" Insects 15, no. 3: 197. https://doi.org/10.3390/insects15030197
APA StyleEstep, A. S., Sanscrainte, N. D., Stuck, J., Unlu, I., Prasauskas, A., Mundis, S. J., Cotter, N., Romero-Weaver, A. L., Fedirko, T. J., Kendziorski, N. L., Kosinski, K. J., Ramirez, D., & Buckner, E. A. (2024). The L1014F Knockdown Resistance Mutation Is Not a Strong Correlate of Phenotypic Resistance to Pyrethroids in Florida Populations of Culex quinquefasciatus. Insects, 15(3), 197. https://doi.org/10.3390/insects15030197





