A Long Photoperiod Promoted the Development, Reproduction, and Predation of Harmonia axyridis Pallas (Coleoptera: Coccinellidae) at an Average Greenhouse Temperature during the Winter
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Assays of Effects of Different Photoperiods on Development and Reproduction
2.3. Assays of Effect of Different Photoperiods on Predation by H. axyridis
2.3.1. Assay of Effects of Different Photoperiods on the Daily Average Amount of Predation
2.3.2. Assay of Effects of Different Photoperiods on Predation by H. axyridis
2.3.3. Assays of Effects of Different Photoperiods on Predation Parameters for H. axyridis in Greenhouse
2.4. Statistical Analysis
3. Results
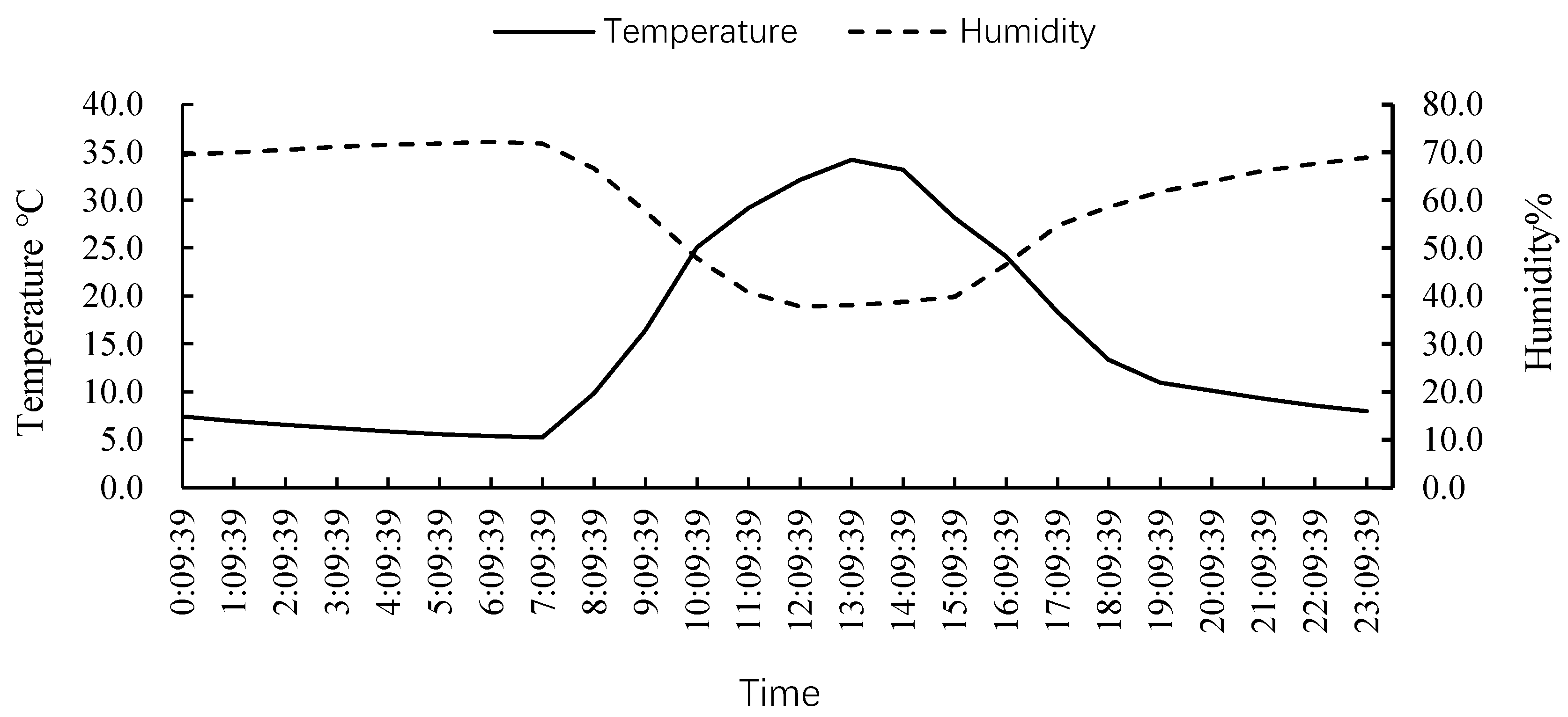
3.1. Daily Average Temperature in the Greenhouse during December 2021
3.2. Development and Reproduction Parameters for H. axyridis under Different Photoperiods
3.3. H. axyridis Population Parameters under Different Photoperiods
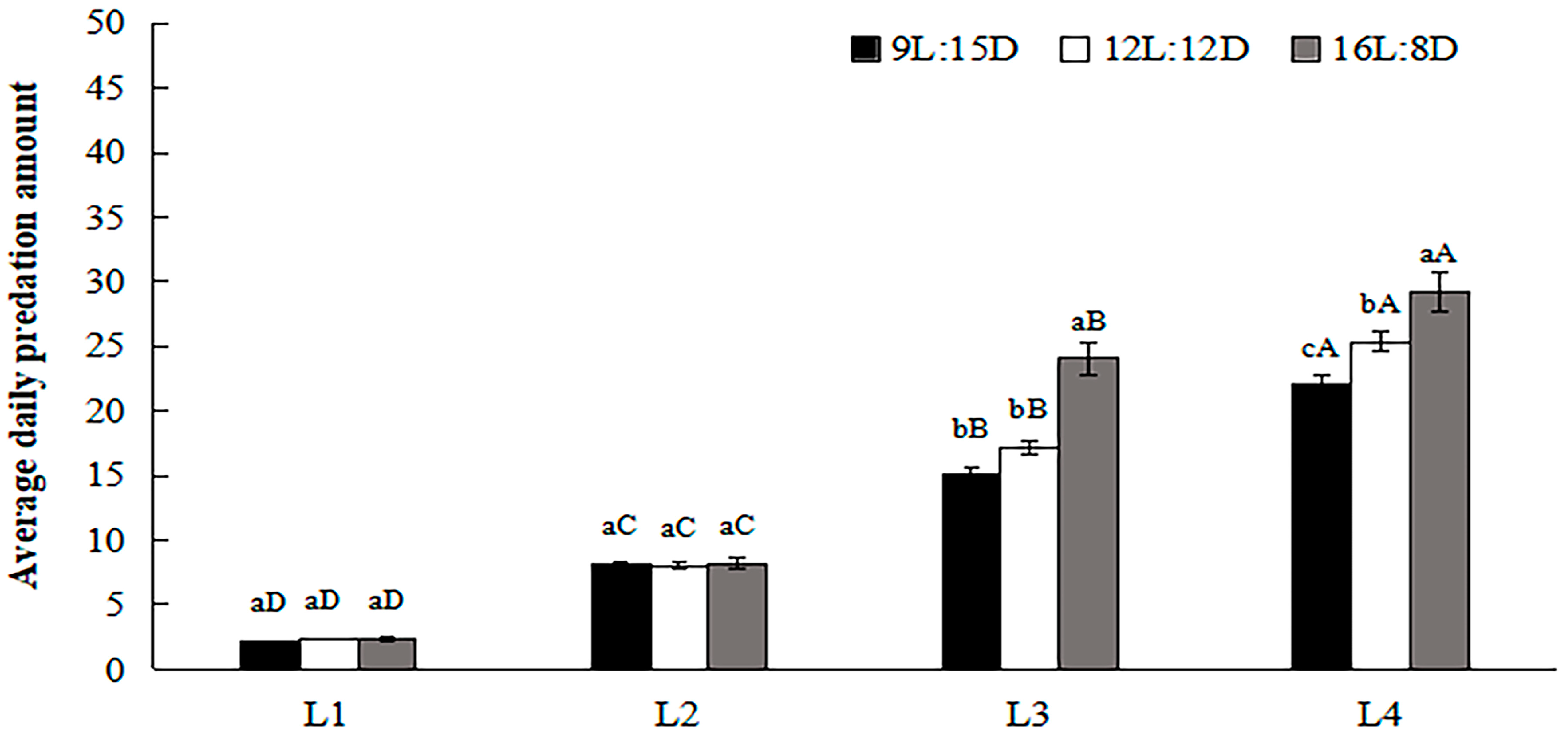
3.4. Daily Predation Amounts by H. axyridis under Different Photoperiods
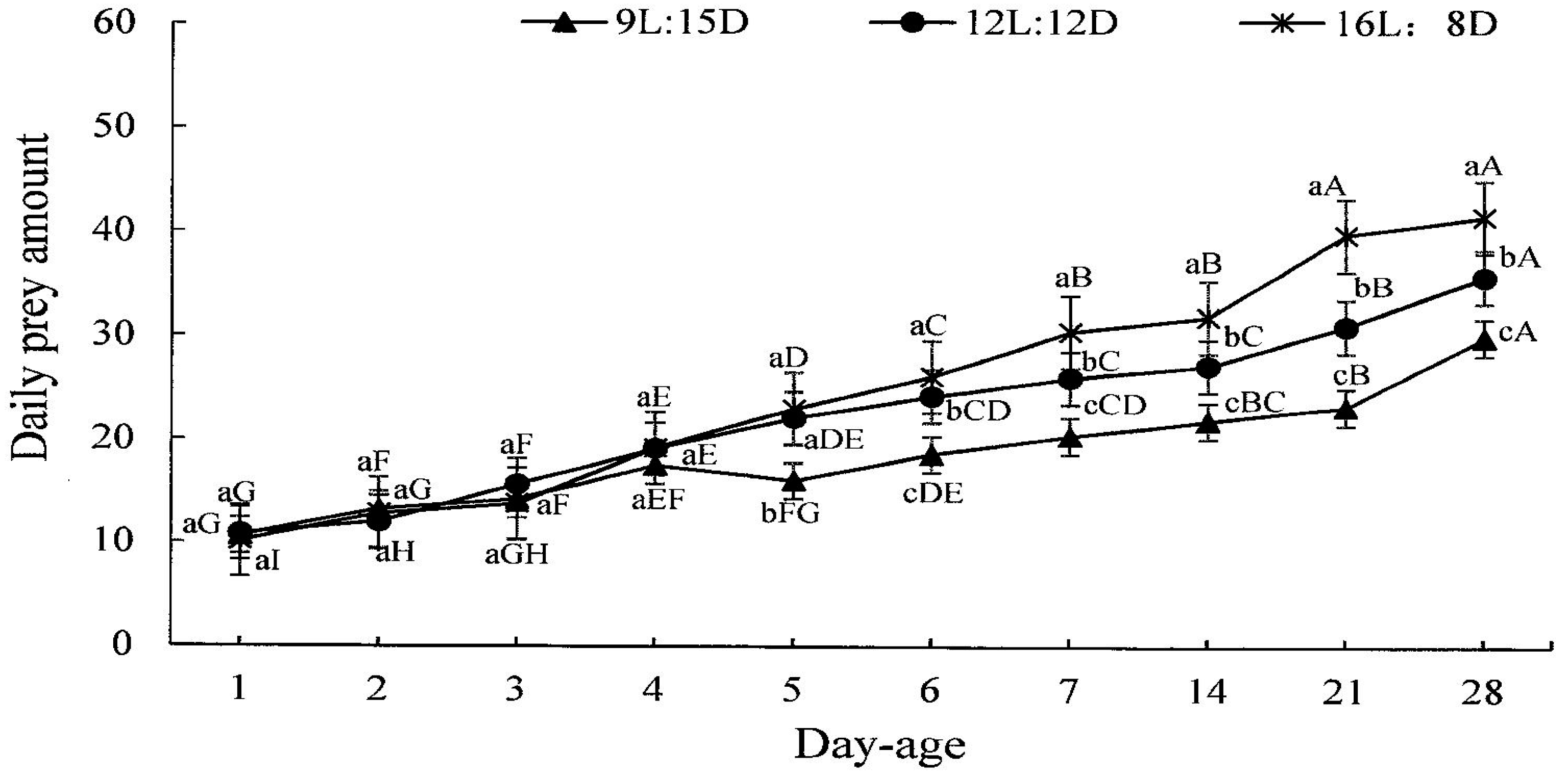
3.5. Regulatory Effect of Photoperiod on Predation by H. axyridis
3.6. Effects of Photoperiod on Predation Parameters of H. axyridis
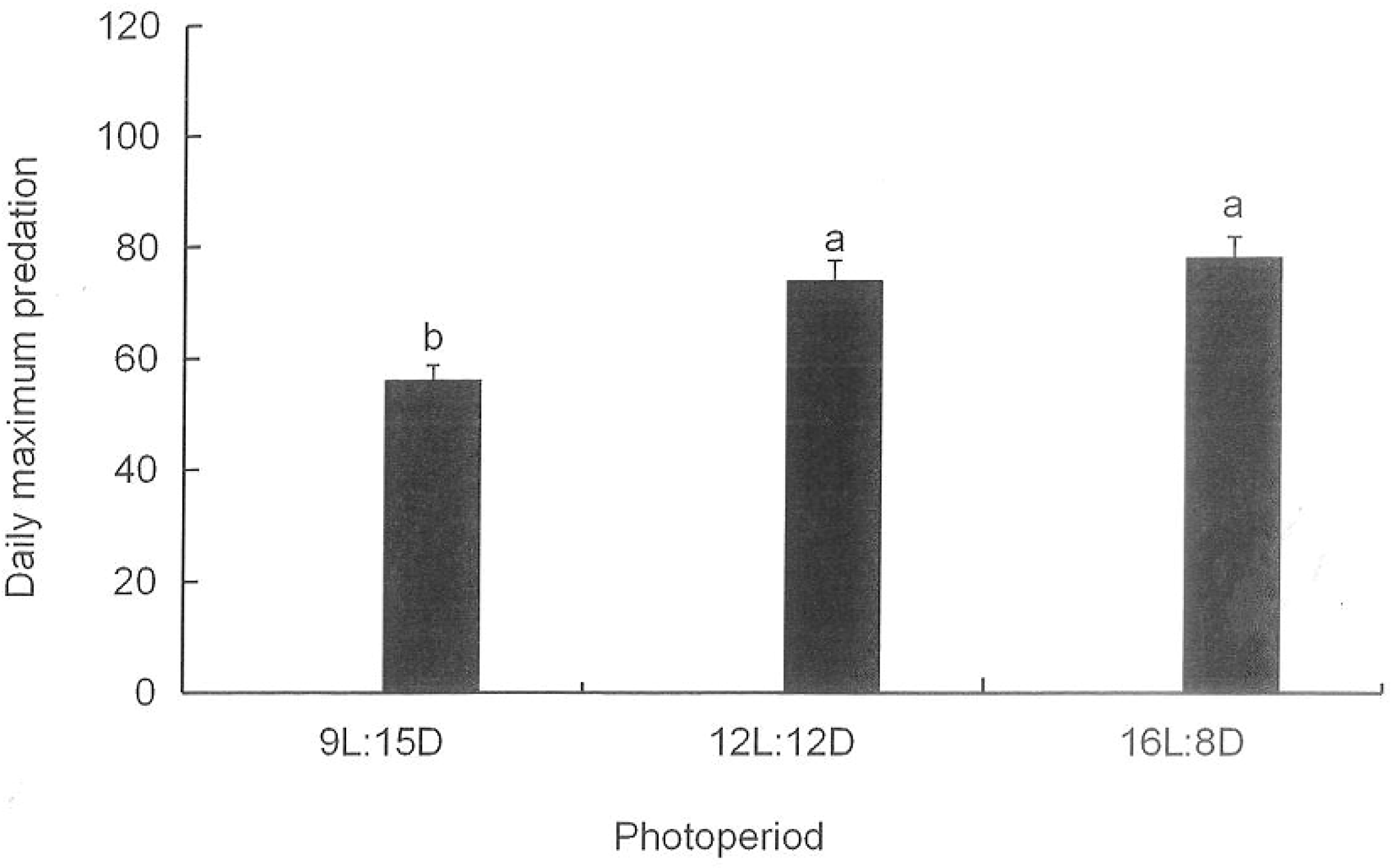
3.7. Maximum Daily Predation Amounts by H. axyridis on M. persicae in the Greenhouse under Different Photoperiods
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hodek, I. Biology of Coccinellidae, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1973; pp. 92–93. [Google Scholar]
- Hodek, I.; Honek, A. Ecology of Coccinellidae, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 276–277. [Google Scholar]
- Omkar; Pathak, S. Effects of different photoperiods and wavelengths of light on the life-history traits of an aphidophagous ladybird, Coelophora saucia (Mulsant). J. Appl. Èntomol. 2006, 130, 45–50. [Google Scholar] [CrossRef]
- Berkvens, N.; Bonte, J.; Berkvens, D.; Tirry, L.; De Clercq, P. Influence of diet and photoperiod on development and reproduction of European populations of Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). BioControl 2008, 53, 211–221. [Google Scholar] [CrossRef]
- Reznik, S.Y. On the effects of diet and photoperiod on Harmonia axyridis (Pallas) (Coleoptera, Coccinellidae) larval development. Èntomol. Rev. 2010, 90, 411–414. [Google Scholar] [CrossRef]
- Reznik, S.Y.; Vaghina, N.P. Photoperiodic control of development and reproduction in Harmonia axyridis (Coleoptera: Coccinellidae). Eur. J. Èntomol. 2011, 108, 385–390. [Google Scholar] [CrossRef]
- Majumder, J.; Agarwala, B.K. Biology and population dynamics of giant ladybird predator Anisolemnia dilatata (F.) (Coleoptera: Coccinellidae): A specialized predator of wooly aphids of bamboo plants in northeast India. World J. Zool. 2013, 8, 55–61. [Google Scholar] [CrossRef]
- Kumar, B.; Omkar, O. Temperature and photoperiod influence prey consumption and utilization by two sympatric Coccinella species (Coleoptera: Coccinellidae) in conspecific and heterospecific combinations. Acta Entomol. Sin. 2015, 58, 297–307. [Google Scholar]
- Omkar, O.; Kumar, B. Effects of temperature and photoperiod on predation attributes and development of two Coccinella species (Coleoptera: Coccinellidae). Acta Entomol. Sin. 2016, 59, 64–76. [Google Scholar]
- Reznik, S.Y.; Dolgovskaya, M.Y.; Ovchinnikov, A.N.; Belyakova, N.A. Weak photoperiodic response facilitates the biological invasion of the harlequin ladybird Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). J. Appl. Èntomol. 2015, 139, 241–249. [Google Scholar] [CrossRef]
- Hodek, I.; Ruzicka, Z. Photoperiodic response in relation to diapause in Coccinella septempunctata (Coleoptera). Acta Entomol. Bohemoslov. 1979, 76, 209–218. [Google Scholar]
- Hodek, I.; Honek, A.; Van Emden, H.F. Ecology and Behaviour of the Ladybird Beetles (Coccinellidae), 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 276–281. [Google Scholar]
- Wang, S.; Tan, X.-L.; Guo, X.-J.; Zhang, F. Effect of Temperature and Photoperiod on the Development, Reproduction, and Predation of the Predatory Ladybird Cheilomenes sexmaculata (Coleoptera: Coccinellidae). J. Econ. Èntomol. 2013, 106, 2621–2629. [Google Scholar] [CrossRef]
- Wang, D.C.; Yuan, Z.L.; Luo, L.; Zhu, M.L. Predation of Harmonia axyridis (Pallas) on Hyalopterus arundimis. Plant Prot. 2001, 27, 29–31. [Google Scholar]
- Zhao, T.X.; Yuan, M.L. Biological and ecological characteristics of Harmonia axyridis in China. Pratacultural Sci. 2017, 34, 614–629. [Google Scholar] [CrossRef]
- Qin, Q.; Liu, S.; Li, S.; Zhang, W.; He, Y. Role of photoperiod in the development and reproduction of Harmonia axyridis (Coleoptera, Coccinellidae). Biocontrol Sci. Technol. 2016, 26, 116–124. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-Table Analysis Incorporating Both Sexes and Variable Development Rates Among Individuals. Environ. Èntomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H. TIMING-MS Chart: A Computer Program for the Population Projection Based on age-Stage, Two-Sex Life Table; National Chung Hsing University: Taichung, Taiwan, 2017; Available online: http://140.120.197.173/Ecology/Download/Timing-MSChart (accessed on 3 September 2021).
- Wei, M.; Chi, H.; Guo, Y.; Li, X.; Zhao, L.; Ma, R. Demography of Cacopsylla chinensis (Hemiptera: Psyllidae) Reared on Four Cultivars of Pyrus bretschneideri (Rosales: Rosaceae) and P. communis Pears with Estimations of Confidence Intervals of Specific Life Table Statistics. J. Econ. Èntomol. 2020, 113, 2343–2353. [Google Scholar] [CrossRef]
- Chi, H.; Kara, H.; Özgökçe, M.S.; Atlihan, R.; Güncan, A.; Rişvanlı, M.R. Innovative application of set theory, Cartesian product, and multinomial theorem in demographic research. Èntomol. Gen. 2022, 42, 863–874. [Google Scholar] [CrossRef]
- Holling, C.S. The Components of Predation as Revealed by a Study of Small-Mammal Predation of the European Pine Sawfly. Can. Èntomol. 1959, 91, 293–320. [Google Scholar] [CrossRef]
- Malaquias, J.B.; Ramalho, F.S.; Fernandes, F.S.; Souza, J.V.S.; Azeredo, T.L. Effects of photoperiod on the development and growth of Podisus nigrispinus, a predator of cotton leafworm. Phytoparasitica 2009, 37, 241–248. [Google Scholar] [CrossRef]
- Kutcherov, D.A.; Lopatina, E.B.; Kipyatkov, V.E. Photoperiod modifies thermal reaction norms for growth and development in the red poplar leaf beetle Chrysomela populi (Coleoptera: Chrysomelidae). J. Insect Physiol. 2011, 57, 892–898. [Google Scholar] [CrossRef]
- Li, Y.Y.; Zhang, L.S.; Chen, H.Y.; Wang, W.; Zhang, J. Temperature and photoperiodic response of diapause induction in Aphidius gifuensis. Chin. J. Appl. Entomol. 2013, 50, 718–726. [Google Scholar]
- Zhang, W.; Liu, S.; Li, N.; Chen, J.; He, Y.Z.; Qiu, Q.J. Effects of photoperiods on adult diapause of Harmonia axyridis (Pallas). Acta Phytophylacica Sin. 2014, 41, 495–500. [Google Scholar]
- Danks, H.V. Insect Dormancy: An Ecological Perspective; Biological Survey of Canada: Calgary, AB, Canada, 1987; pp. 17–18. [Google Scholar]
- Doležal, P.; Habuštová, O.; Sehnal, F. Effects of photoperiod and temperature on the rate of larval development, food conversion efficiency, and imaginal diapause in Leptinotarsa decemlineata. J. Insect Physiol. 2007, 53, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Hahn, D.A.; Denlinger, D.L. Energetics of Insect Diapause. Annu. Rev. Èntomol. 2011, 56, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Fukuda, T.; Hayakawa, H.; Arakawa, R.; Saito, Y. Relationship between body colour, feeding, and reproductive arrest under short-day development in Tetranychus pueraricola (Acari: Tetranychidae). Exp. Appl. Acarol. 2013, 60, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wei, B.-X.; Liu, W.; Wang, J.-L.; Zhou, X.-M.; Wang, X.-P. Differences in the Development of Internal Reproductive Organs, Feeding Amount and Nutrient Storage between Pre-Diapause and Pre-Reproductive Harmonia axyridis Adults. Insects 2019, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Nakamuta, K. Diel rhythmicity of prey-search activity and its predominance over starvation in the ladybeetle, Coccinella septempunctata brucki Mulsant (Coleoptera: Coccinellidae): Releasing stimuli and decision of giving-up time. Physiol. Entomol. 1987, 12, 91–98. [Google Scholar] [CrossRef]
- Velez-Ramirez, A.I.; van Leperen, W.; Vreugdenhil, D.; Millenaar, F.F. Plants under continuous light. Trends Plant Sci. 2011, 16, 310–318. [Google Scholar] [CrossRef]
- Proietti, S.; Scariot, V.; De Pascale, S.; Paradiso, R. Flowering mechanisms and environmental stimuli for flower transition: Bases for production scheduling in greenhouse floriculture. Plants 2022, 11, 432. [Google Scholar] [CrossRef]


| Parameters | 9L:15D | 12L:12D | 16L:8D |
|---|---|---|---|
| Duration of egg/d | 9.31 ± 0.06 a | 9.29 ± 0.08 a | 9.34 ± 0.08 a |
| Duration of 1st instar/d | 5.36 ± 0.17 a | 5.31 ± 0.18 a | 5.71 ± 0.24 a |
| Duration of 2nd instar/d | 4.97 ± 0.14 b | 5.33 ± 0.17 a | 4.39 ± 0.13 c |
| Duration of 3rd instar/d | 5.10 ± 0.17 b | 5.57 ± 0.24 a | 4.80 ± 0.12 c |
| Duration of 4th instar/d | 10.81 ± 0.20 a | 8.68 ± 0.20 b | 9.00 ± 0.17 b |
| Duration of larva/d | 26.24 ± 0.26 a | 24.77 ± 0.26 b | 23.73 ± 0.29 c |
| Duration of pupa/d | 15.61 ± 0.17 a | 15.65 ± 0.27 a | 15.62 ± 0.21 a |
| Pre-adult period/d | 52.63 ± 0.26 a | 49.02 ± 0.33 b | 48.29 ± 0.21 c |
| Longevity of female/d | 162.83 ± 4.57 a | 146.62 ± 6.89 b | 136.03 ± 7.42 c |
| Longevity of male/d | 169.33 ± 4.18 a | 148.84 ± 3.69 b | 138.17 ± 3.32 c |
| Proportion of ovipositing females/% | 96.30 ± 2.14 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a |
| Fecundity of ovipositing females (Fr) | 229.0 ± 6.2 c | 380.4 ± 12.6 b | 513.1 ± 28.4 a |
| Fecundity of all females (F) | 213.2 ± 22.4 c | 380.4 ± 12.6 b | 513.1 ± 28.4 a |
| Pre-oviposition period (APOP)/d | 53.37 ± 5.53 a | 38.86 ± 1.87 b | 23.79 ± 0.64 c |
| Total pre-oviposition period (TPOP)/d | 104.96 ± 5.20 a | 87.27 ± 1.94 b | 73.03 ± 4.10 c |
| Population Parameter | 9L:15D | 12L:12D | 16L:8D |
|---|---|---|---|
| Intrinsic rate of increase (r, per day) | 0.0329 ± 0.0013 c | 0.0452 ± 0.0016 b | 0.0556 ± 0.0018 a |
| Finite rate of increase (λ, per day) | 1.0335 ± 0.0014 c | 1.0463 ± 0.0017 b | 1.0571 ± 0.0019 a |
| Net reproduction rate (R0) | 76.37 ± 12.21 c | 137.89 ± 20.91 b | 185.89 ± 26.65 a |
| Mean generation time T (days) | 131.40 ± 1.36 a | 108.69 ± 1.37 b | 93.88 ± 0.85 c |
| Photoperiod | Replicates | Functional Response Equation | R2 | χ2 |
|---|---|---|---|---|
| 9L:15D | 1 | Na = 1.1175 × No/(1 + 1.1175 × 0.0162No) | 0.9948 | 0.13 |
| 2 | Na = 1.1056 × No/(1 + 1.1056 × 0.0200No) | 0.9866 | 0.98 | |
| 3 | Na = 1.0564 × No/(1 + 1.0564 × 0.0161No) | 0.9965 | 0.63 | |
| 12L:12D | 1 | Na = 1.0438 × No/(1 + 1.0438 × 0.0114No) | 0.9980 | 0.43 |
| 2 | Na = 1.0455 × No/(1 + 1.0455 × 0.0136No) | 0.9906 | 0.81 | |
| 3 | Na = 1.0884 × No/(1 + 1.0884 × 0.0134No) | 0.9975 | 0.08 | |
| 16L:8D | 1 | Na = 0.9743 × No/(1 + 0.9743 × 0.0099No) | 0.9924 | 0.70 |
| 2 | Na = 1.0064 × No/(1 + 1.0064 × 0.0099No) | 0.9926 | 0.71 | |
| 3 | Na = 0.9968 × No/(1 + 0.9968 × 0.0092No) | 0.9955 | 0.65 |
| Prey Density (Aphids/Plant) | 9L:15D | 12L:12D | 16L:8D |
|---|---|---|---|
| 5 | 0.9980 ± 0.0257 ab | 1.0090 ± 0.0216 a | 0.9456 ± 0.0140 b |
| 10 | 0.9183 ± 0.0244 ab | 0.9477 ± 0.0138 a | 0.9030 ± 0.0129 b |
| 20 | 0.7920 ± 0.0273 b | 0.8451 ± 0.0036 a | 0.8284 ± 0.0132 a |
| 50 | 0.5612 ± 0.0323 b | 0.6383 ± 0.0143 a | 0.6639 ± 0.0177 a |
| 80 | 0.4348 ± 0.0311 c | 0.5130 ± 0.0191 b | 0.5541 ± 0.0198 a |
| 110 | 0.3549 ± 0.0286 c | 0.4289 ± 0.0204 b | 0.4754 ± 0.0202 a |
| Photoperiod | Instantaneous Attack Rate (A) | Handling Time (Th/d) |
|---|---|---|
| 9L:15D | 1.0931 ± 0.0324 a | 0.0174 ± 0.0022 a |
| 12L:12D | 1.0592 ± 0.0252 a | 0.0128 ± 0.0012 b |
| 16L:8D | 0.9925 ± 0.0165 b | 0.0097 ± 0.0004 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Yuan, X.; Xie, Z.; Zhang, Q.; Zheng, C.; Sun, L. A Long Photoperiod Promoted the Development, Reproduction, and Predation of Harmonia axyridis Pallas (Coleoptera: Coccinellidae) at an Average Greenhouse Temperature during the Winter. Insects 2024, 15, 214. https://doi.org/10.3390/insects15040214
Yu H, Yuan X, Xie Z, Zhang Q, Zheng C, Sun L. A Long Photoperiod Promoted the Development, Reproduction, and Predation of Harmonia axyridis Pallas (Coleoptera: Coccinellidae) at an Average Greenhouse Temperature during the Winter. Insects. 2024; 15(4):214. https://doi.org/10.3390/insects15040214
Chicago/Turabian StyleYu, Haixia, Xinjuan Yuan, Zhiqiang Xie, Qiqi Zhang, Changying Zheng, and Lijuan Sun. 2024. "A Long Photoperiod Promoted the Development, Reproduction, and Predation of Harmonia axyridis Pallas (Coleoptera: Coccinellidae) at an Average Greenhouse Temperature during the Winter" Insects 15, no. 4: 214. https://doi.org/10.3390/insects15040214





