Simple Summary
The queen bee specializes in colony reproduction and undergoes behavioral and physical changes following egg-laying. Metabolomics is a high-throughput technique that can unveil the intricate connection between biological phenotypic states and an organism’s small molecules. We are intrigued to determine the characteristic substances present in the hemolymph and ovary, as well as to understand how their rapid metabolism contributes to the process of egg-laying by queens. In this study, we reared Apis mellifera queens from three distinct time periods: newly emerged queen (NEQ), newly laying queen (NLQ), and old laying queen (OLQ). Using widely targeted metabolomics, we found that NLQ and OLQ exhibited significant metabolite alterations compared to NEQ, such as up-regulated expression of carnitines, fatty acids, and some antioxidants, and significantly down-regulated expression of amino acids, etc. The results of this study provide a novel perspective for analyzing the oviposition mechanism of queens.
Abstract
The queen bee is a central and pivotal figure within the colony, serving as the sole fertile female responsible for its reproduction. The queen possesses an open circulatory system, with her ovaries immersed in hemolymph. A continuous and intricate transportation and interchange of substances exist between the ovaries and hemolymph of queen bees. To determine the characteristic metabolites in the hemolymph and ovary, as well as understand how their rapid metabolism contributes to the process of egg-laying by queens, we reared Apis mellifera queens from three different age groups: newly emerged queen (NEQ), newly laying queen (NLQ), and old laying queen (OLQ). Using widely targeted metabolomics, our study revealed that the laying queen (NLQ and OLQ) exhibited faster fatty acid metabolism, up-regulated expression of antioxidants, and significant depletion of amino acids compared to the NEQ. This study revealed that the levels of carnitine and antioxidants (GSH, 2-O-α-D-glucopyranosyl-L-ascorbic acid, L-ascorbic acid 2-phosphate, etc.) in the NLQ and OLQ were significantly higher compared to NEQ. However, most of the differentially expressed amino acids, such as L-tryptophan, L-tyrosine, L-aspartic acid, etc., detected in NLQ and OLQ were down-regulated compared to the NEQ. Following egg-laying, pathways in the queens change significantly, e.g., Tryptophan metabolism, Tyrosine metabolism, cAMP signaling pathway, etc. Our results suggest that carnitine and antioxidants work together to maintain the redox balance of the queen. Additionally, various amino acids are responsible for maintaining the queen’s egg production.
1. Introduction
The colony’s reproduction and growth depend on the queen, who must maintain high rates of egg production. Apis mellifera queens have the capacity to lay 1500–2000 eggs daily [1,2,3]. The queens’ ovaries have a rapid metabolism, allowing them to lay more eggs in 24 h than their own body weight. However, in the practice of beekeeping, the queen’s egg-laying ability is influenced by various factors, including rearing methods [4,5], rearing season [6], and comb age [7,8], with the primary factor being the queens themselves, their genome [9,10], age [11], health [11,12], etc.
The queen bee possesses a highly developed reproductive system. The fully developed ovaries of the queen consist of a pair of large, pear-shaped ovarioles. The estimated number of ovarioles per ovary in Apis mellifera (A. mellifera) is generally between 100 and 180 [1,13,14]. The ovary is essential for the queen; the weight of the ovaries and the number of ovarioles are common indicators of the reproductive capacity of honeybee queens [15,16]. Additionally, the developmental and reproductive performance of queens is usually measured by the use of genes highly expressed in the ovaries, such as Hexamerin (Hex) 110 [17,18,19], Hex 70a [20,21], and Vitellogenin (Vg) [22,23].
The honeybee has an open circulatory system with a fluid similar to blood, called hemolymph, and its ovary is immersed in it [24,25]. The hemolymph is essential to honeybee immunity and is widely used as a research medium for investigating viral, bacterial, and parasitic infections in honeybees [26,27,28,29,30]. It is also used to transfer micro- and macro-elements and has been employed in the assessment of metal pollution [31]. The main components of honeybee hemolymph are proteins, lipids, carbohydrates, and amino acids, as well as water [32]. Therefore, the hemolymph facilitates the transportation of various nutrients to the queen’s ovaries. For example, vitellogenin (Vg), synthesized by the honeybee’s fat body, plays a role in oocyte growth and embryo energy supply [33,34] and is taken up by oocytes in the honeybee ovary [35,36].
Metabolomics is a high-throughput technique that can unveil the intricate connection between biological phenotypic states and an organism’s small molecules. In honeybee metabolomics research, commonly employed experimental samples include the brain, head part, gut, and hemolymph [37]. These studies have primarily focused on investigating the impacts of insecticide and herbicide exposure [38,39,40,41], infections [42,43,44], dietary changes [45,46], and other factors on honeybees. Previous studies have also analyzed the metabolic profiles of the spermatheca [47] and ovaries [48] in the laying queen (LQ). However, a lack of research exists into the metabolite alterations in ovaries and hemolymph between newly emerged queen (NEQ) and LQ (A. mellifera).
There is a continuous and intricate transportation and interchange of substances between the ovaries and hemolymph of queens, which is intimately linked with the process of oviposition. Therefore, we are intrigued to determine the characteristic substances present in the hemolymph and ovary, as well as to understand how their rapid metabolism contributes to the process of egg-laying by queens. In this study, we reared A. mellifera queens from three distinct time periods. We aimed to identify differentially expressed metabolites (DEMs) and important pathways between NEQ and LQ through metabolomic analysis. This provides a novel perspective for analyzing the oviposition mechanism of queens.
2. Materials and Methods
2.1. Acquisition of Experimental Samples
The honeybees (A. mellifera ligustica) were maintained at the Honeybee Research Institute of Jiangxi Agricultural University (28.46° N, 115.49° E). The queens were collected from three different age groups: newly emerged queen (NEQ, zero days old), newly laying queen (NLQ, approximately one month old), and old laying queen (OLQ, approximately one year old). The NEQ was obtained through the standard artificial queen rearing method [2]. Each age group had six replicates. The NLQ and OLQ were natural mating. Six NEQs, three NLQs, or three OLQs were chosen for each ovary sample. Six queens were selected for every hemolymph sample.
2.2. Gathering the Ovary and Hemolymph of the Queen Bee
The queens were attached to a beeswax dish with insect pins after the wings and legs were removed. Subsequently, their ovaries were then promptly preserved in liquid nitrogen with freezing tubes after being stripped using sterile scissors and tweezers. For hemolymph, after comparing the various methods [49,50,51], we chose the most effective method, i.e., dorsal sinus with a capillary hemolymph sampling (DCHS). Firstly, the queens were anesthetized at −20 °C for 10 min. Then, the connecting membrane between the third and fourth dorsal segments was pierced using a capillary. The hemolymph was introduced into the capillary and subsequently extruded into a freezing tube before being stored in liquid nitrogen. All processes are carried out on an ultra-clean table.
2.3. Widely Targeted Metabolomics Profiling Methods and Conditions
T3 UPLC Conditions: The LC-ESI-MS/MS system (UPLC, ExionLC AD, https://sciex.com.cn/, accessed on 17 July 2022; MS, QTRAP® System, https://sciex.com/, accessed on 17 July 2022) was utilized for the analysis of sample extracts. The experimental parameters were set as follows: UPLC included a Waters ACQUITY UPLC HSS T3 C18 column (1.8 μm, 2.1 mm × 100 mm) maintained at a temperature of 40 °C with a flow rate of 0.4 mL/min. The sample was injected either at a volume of 2 μL or 5 μL using a solvent system consisting of water with 0.1% formic acid and acetonitrile with 0.1% formic acid. The gradient program included the following steps: 95:5 v/v at 0 min, 10:90 v/v at 10.0 min, 10:90 v/v at 11.0 min, 95:5 v/v at 11.1 min, 95:5 v/v at 14.0 min.
Considering it could acquire MS/MS spectra in an information-dependent manner (IDA) during an LC/MS experiment, the Triple TOF mass spectrometer was employed. In this mode, the MS/MS spectra acquisition is triggered by certain conditions, and the acquisition software (TripleTOF 6600, AB SCIEX) continually assesses the full scan survey MS data as it is being collected. In this mode, the acquisition software (TripleTOF 6600, AB SCIEX) continuously evaluates the full scan survey MS data while collecting it and triggers the acquisition of MS/MS spectra based on preselected criteria. Twelve precursor ions with intensities higher than 100 were chosen for fragmentation at a collision energy (CE) of 30 V during each cycle. This produced 12 MS/MS events with product ion accumulation times of 50 msec each. The following parameters were configured for the ESI source: Ion source gas 1 at 50 Psi, Ion source gas 2 at 50 Psi, Curtain gas at 25 Psi, source temperature at 500 °C, and Ion Spray Voltage Floating (ISVF) set to either 5500 V or −4500 V in positive or negative modes, respectively.
The QTRAP® LC-MS/MS System, functioning as a triple quadrupole-linear ion trap mass spectrometer with an ESI Turbo Ion-Spray interface, was used to acquire LIT and triple quadrupole (QQQ) scans. Under the software control of Analyst 1.6.3 (Sciex), the apparatus functioned in both positive and negative ion modes. The 500 °C source temperature, 5500 V (positive) and −4500 V (negative) ion spray voltage (IS), 50, 50, and 25.0 psi for ion source gas I (GSI), gas II (GSII), and curtain gas (CUR), respectively, and high collision gas (CAD) were the settings for the ESI source. In QQQ and LIT modes, 10 and 100 μmol/L polypropylene glycol solutions were used for instrument tuning and mass calibration, respectively. Specific MRM transitions were monitored for each period based on the eluted metabolites.
2.4. Data Analysis and Statistics
DEMs were identified based on criteria, including |Log2FC| > 1.0 and VIP ≥ 1, with VIP values obtained from the OPLS-DA results utilizing the R package MetaboAnalystR. Analyzing data and plotting ring and PCA plots using the Metware Cloud platform (https://cloud.metware.cn/, accessed on 21 December 2023).The Kyoto Encyclopedia of Genes and Genomes (KEGG) Compound database (http://www.kegg.jp/kegg/compound/, accessed on 7 January 2024) was used to annotate the identified metabolites. Pathways containing significantly regulated metabolites were subjected to metabolite set enrichment analysis (MSEA), with significance assessed using the p-values derived from the hypergeometric test.
3. Results
3.1. Identification of Metabolites and Widely Targeted Metabolomic Analysis
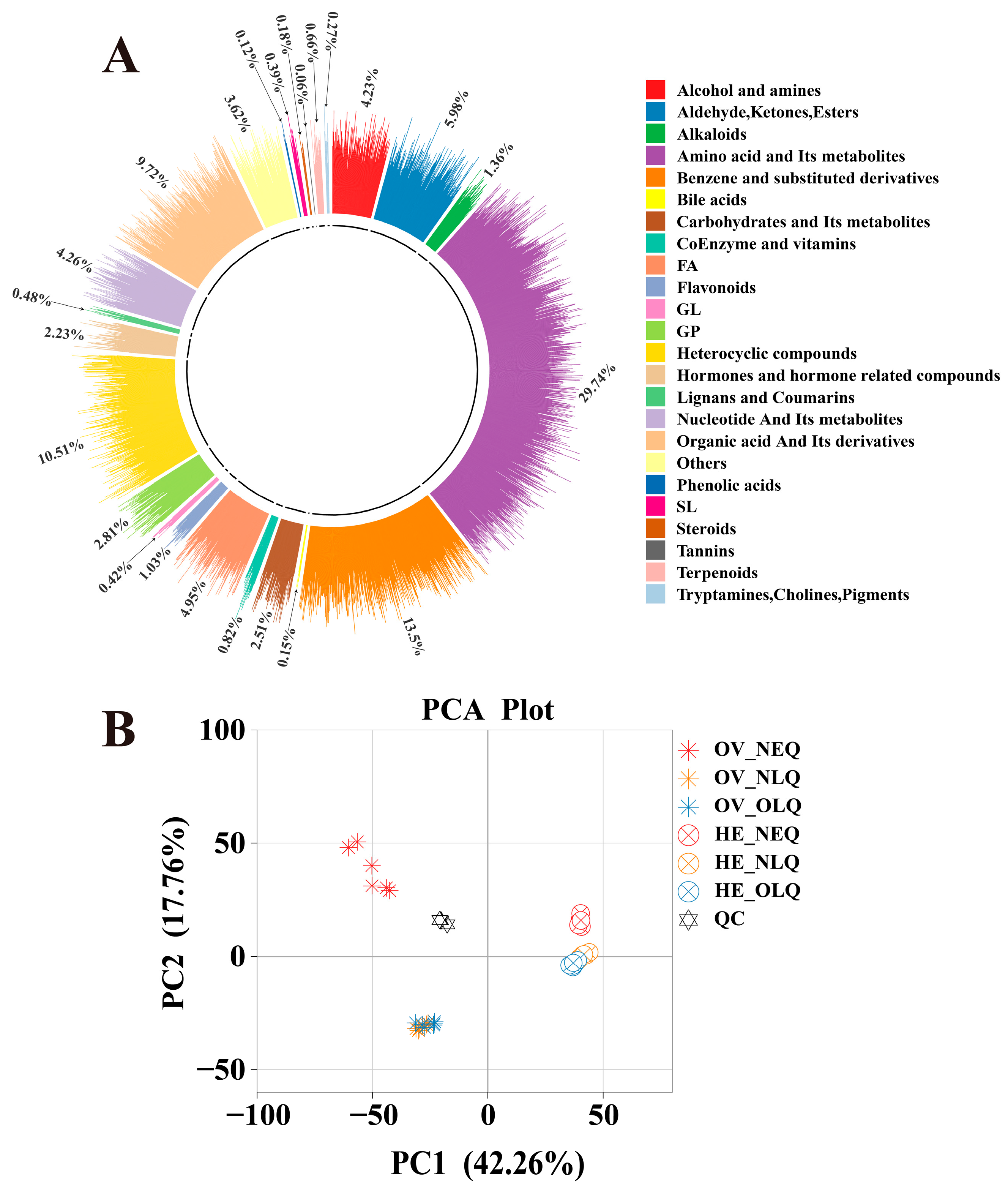
The widely targeted metabolomics analysis of the 36 samples from the ovary and hemolymph revealed a total of 3312 metabolites. These included 24 different classes of metabolites (Figure 1A), such as “Amino acid and its metabolites”, “Benzene and substituted derivatives”, “Heterocyclic compounds”, “Organic acid and its derivatives”, “Aldehyde, Ketones, and Esters”, etc. After performing principal component analysis (PCA) on ovary and hemolymph, we found that there was a clear difference between the metabolites of LQ and NEQ, but the difference between NLQ and OLQ is relatively small (Figure 1B). We conducted an orthogonal partial least squares discriminant analysis (OPLS-DA) concurrently (Figure S1). These findings imply that the OPLS-DA models are dependable (Q2 ≥ 0.9810) and can be utilized for additional research on differentially expressed metabolites (DEMs).
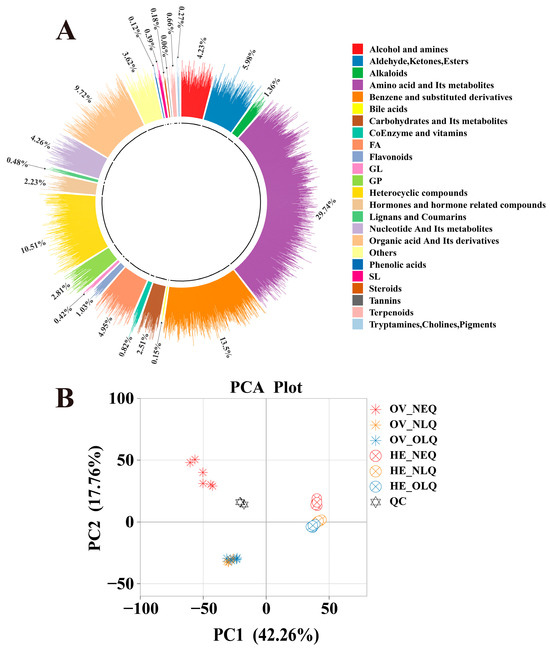
Figure 1.
Ring plot and PCA plots in all groups. (A) Ring plot of metabolites in queens’ ovary and hemolymph. (B) PCA plots in all groups of queens’ ovary and hemolymph. “OV”: Ovary; “HE”: Hemolymph.
3.2. DEMs in Ovary and Hemolymph
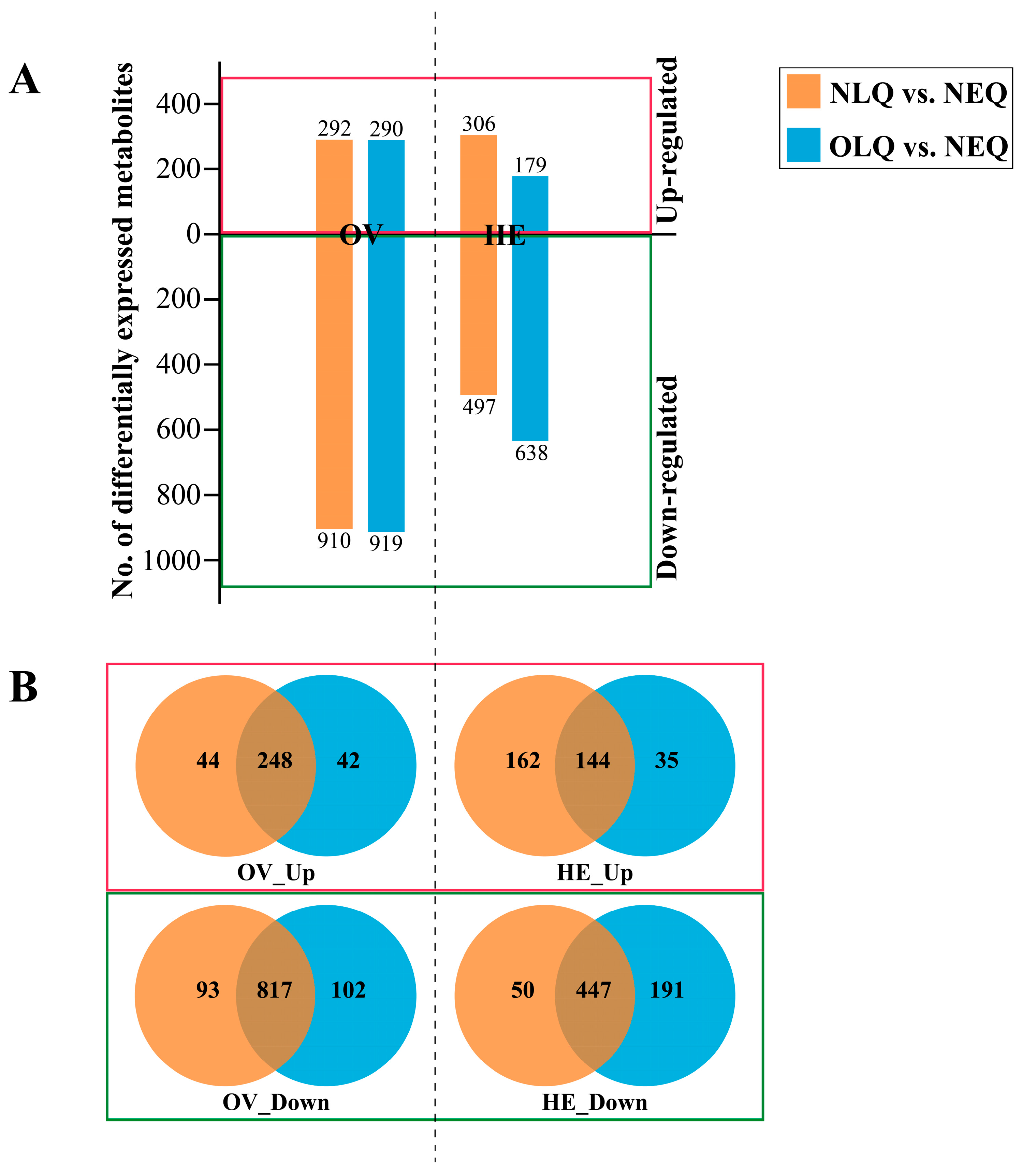
The number of DEMs in the A. mellifera ovary (OV) in the NLQ vs. NEQ and OLQ vs. NEQ groups was 1202 and 1209, respectively. In the A. mellifera hemolymph (HE), the number of DEMs in the NLQ vs. NEQ and OLQ vs. NEQ groups was 803 and 817, respectively (Figure 2A). To identify the common key metabolites in NLQ vs. NEQ and OLQ vs. NEQ, we utilized Venn diagrams to classify the up-regulated and down-regulated metabolites. In comparison to NEQ, a total of 248 metabolites were found to be up-regulated in OV from NLQ and OLQ (Figure 2B, OV_Up). However, a total of 817 metabolites were down-regulated in OV from NLQ and OLQ compared to NEQ (Figure 2B, OV_Down). Furthermore, the results showed an upregulation of 144 metabolites in HE from NLQ and OLQ when compared to NEQ (Figure 2B, HE_Up). In addition, 447 down-regulated metabolites were found in HE from NLQ and OLQ compared to NEQ (Figure 2B, HE_Down).
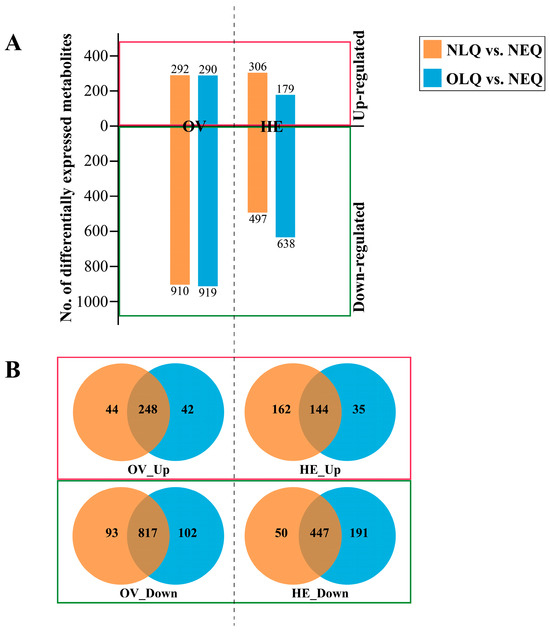
Figure 2.
Analysis of DEMs. (A) Number of DEMs in all groups. (B) The Venn diagram shows the common DEMs between NLQ vs. NEQ and OLQ vs. NEQ. “OV_Up”: The metabolites that expressed higher in the OV of NLQ and OLQ than in the OV of NEQ; “HE_Up”: The metabolites that expressed higher in the HE of NLQ and OLQ than in the MH of NEQ; “OV_Down” and “HE_Down”: The down-regulated metabolites that showed a lower expression level in the OV or HE of NLQ and OLQ compared to those in NEQ.
3.3. Analysis of Key Metabolites with Consistent Trends
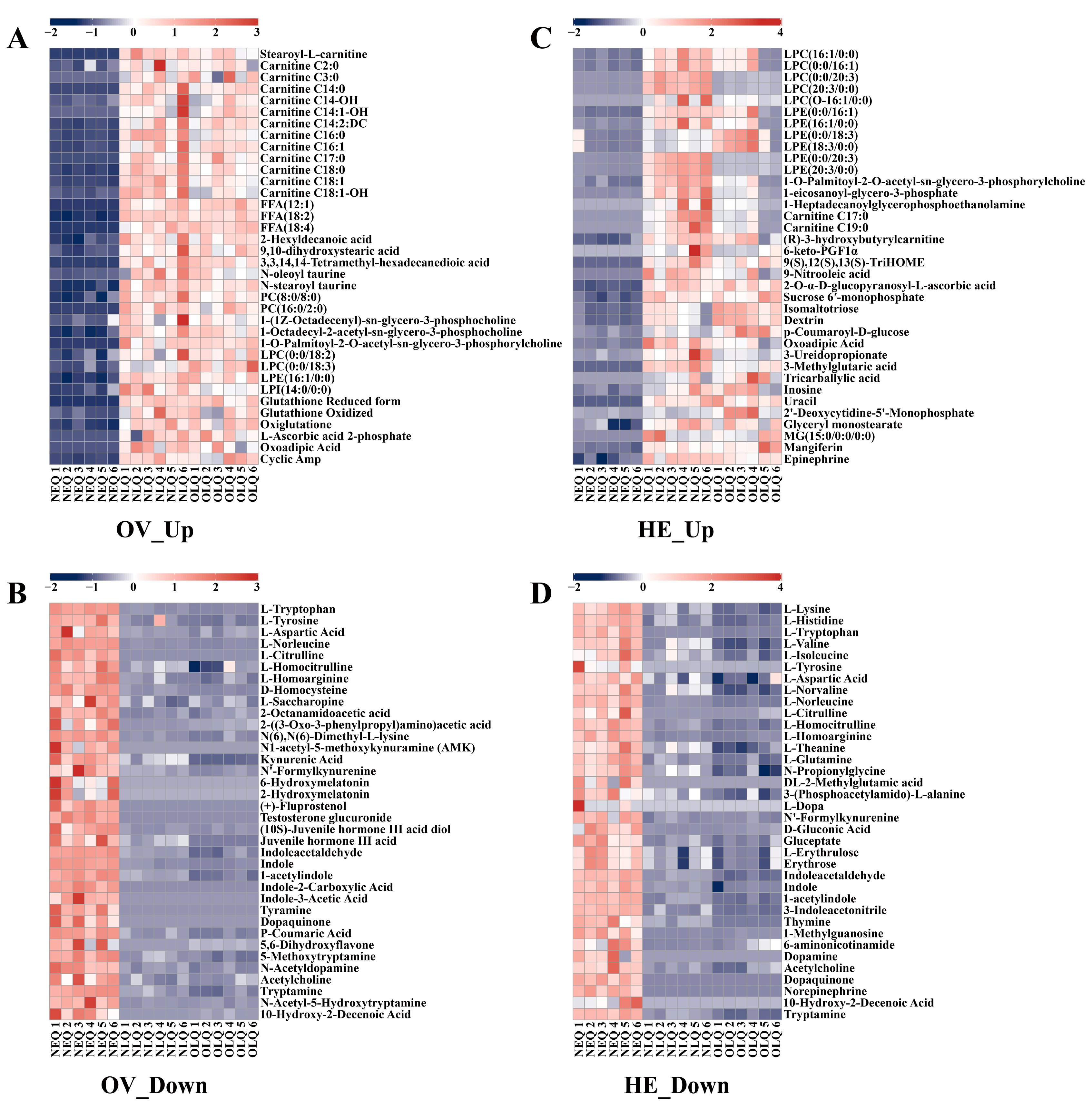
To better understand the trends in the expression of honeybee queen ovary and hemolymph metabolites, we selected some of the critical DEMs to create heatmaps. In the ovaries of NLQ and OLQ, the expression of the fatty acyls (FA) and glycerophospholipid (GP) classes was much higher than NEQ (Table S1), including stearoyl-L-carnitine, carnitine C2:0, carnitine C3:0, FFA (12:1), PC (8:0/8:0), PC (16:0/2:0), LPC (0:0/18:2), LPE (16:1/0:0), LPI (14:0/0:0), etc. Moreover, the levels of glutathione reduced form, glutathione oxidized, oxiglutatione, and L-ascorbic acid 2-phosphate were significantly increased in the ovaries of LQ compared to NEQ (Figure 3A). The metabolites highly expressed in the NEQ ovary are mainly concentrated in amino acid classes. In particular, DEMs such as N1-acetyl-5-methoxykynuramine (AMK), 6-hydroxymelatonin, 2-hydroxymelatonin, (+)-fluprostenol, and testosterone glucuronide were exclusively detected in the ovaries of NEQ (Figure 3B).
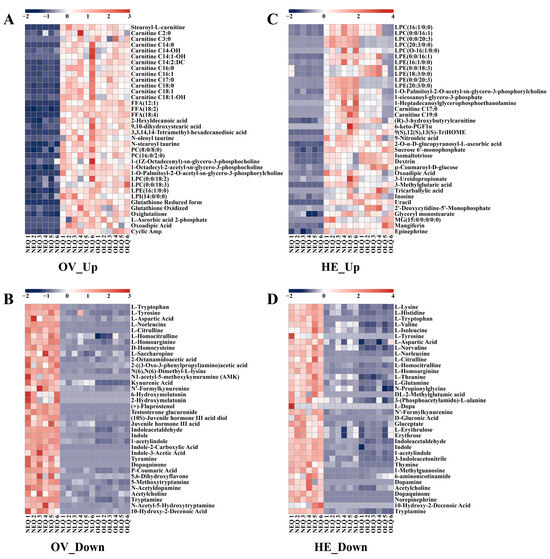
Figure 3.
The heatmaps show the representatively metabolites in the ovary and hemolymph. (A) The DEMs that showed higher expression levels in the ovary of NLQ and OLQ compared to that of NEQ. (B) The DEMs that showed lower expression levels in the ovary of NLQ and OLQ compared to that of NEQ. (C) The DEMs that showed higher expression levels in the hemolymph of NLQ and OLQ compared to that of NEQ. (D) The DEMs that showed lower expression levels in the hemolymph of NLQ and OLQ compared to that of NEQ.
In hemolymph, the up-regulated metabolites were primarily FA and GP (Table S2), in addition to the usual nucleotides, carbohydrates, and their metabolites. The metabolites highly expressed in the NEQ hemolymph are also mainly concentrated in amino acid classes. Furthermore, dopamine, L-dopa, DL-2-methylglutamic acid, and norepinephrine were only found in the hemolymph of NEQ (Figure 3D).
3.4. Important Metabolic Pathways Affected by Metabolite Variations
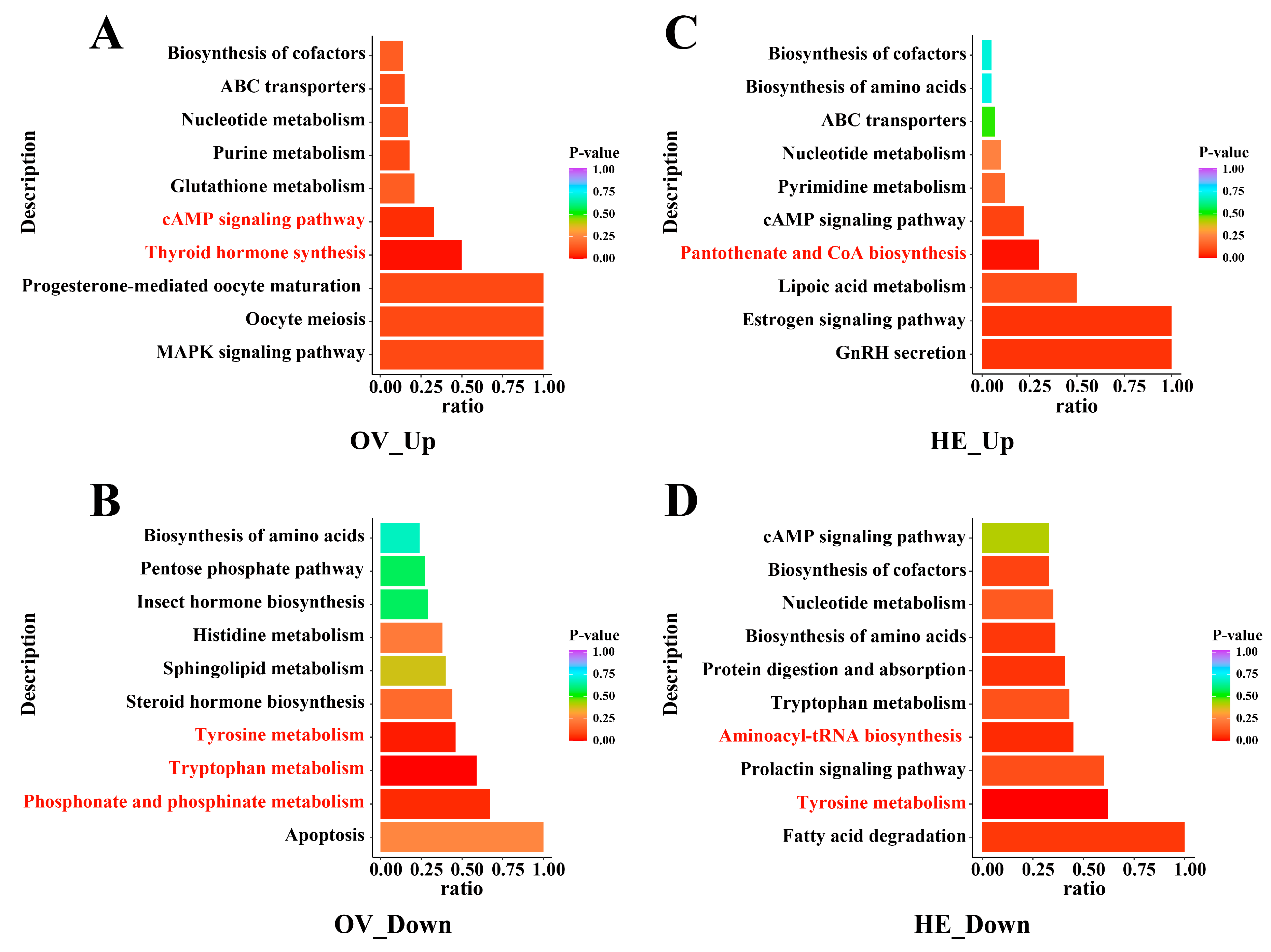
We employed two sets of intersecting metabolites in the Venn diagram, NLQ vs. NEQ and OLQ vs. NEQ (Table S3), to perform KEGG analysis. The results demonstrated that, following egg-laying, pathways in the ovary and hemolymph of queens change significantly (p < 0.05), such as Tryptophan metabolism, Tyrosine metabolism, cAMP signaling pathway, Thyroid hormone synthesis, Phosphonate and phosphinate metabolism, Pantothenate and CoA biosynthesis, and Aminoacyl-tRNA biosynthesis (Figure 4).
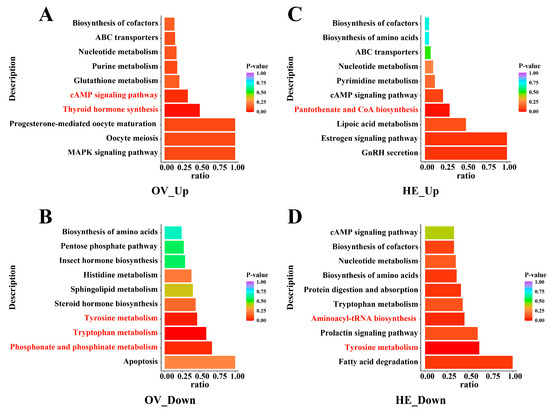
Figure 4.
The bar plot of KEGG metabolic pathways commonly shared by NLQ vs. NEQ and OLQ vs. NEQ. (A) Up-regulated metabolic pathways in the ovary. (B) Down-regulated metabolic pathways in the ovary. (C) Up-regulated metabolic pathways in the hemolymph. (D) Down-regulated metabolic pathways in the hemolymph. The redder colors of the bar indicate pathways that are more important. Pathways marked in red font indicate significant differences. p < 0.05 was considered statistically significant.
4. Discussion
In this study, it was observed that the levels of carnitine in the ovaries of LQ were significantly higher compared to NEQ. The trend of free fatty acids, such as FFA (18:2), FFA (18:4), FFA (12:1), etc., in queen bee ovaries was consistent with carnitine. Carnitine acts as a carrier molecule for activated fatty acids, promoting fatty acid oxidation within the mitochondria [52,53]. L-carnitine has been extensively studied compared to other carnitines, with numerous investigations demonstrating its efficacy in increasing oocyte quality and expediting blastocyst formation during in vitro oocyte maturation across various species, including pigs [54,55], cattle [56,57], mice [58,59], etc. Notably, Li et al. conducted single-cell metabolomic sequencing on mouse oocytes at three different developmental stages and confirmed the heightened levels of carnitines during oocyte meiosis [60]. Furthermore, DL-carnitine has been identified as a stimulant for oviposition in Drosophila melanogaster (D. melanogaster) by Geer and Dolph [61]. Therefore, it is postulated that carnitine plays a crucial role in promoting fatty acid metabolism during queen oviposition, ensuring a continuous supply of fatty acids for rapid oocyte development. Additionally, owing to its antioxidant properties [62,63], carnitine may protect against oxidative stress within the ovary.
Oxidative stress occurs when the body produces an excessive amount of reactive oxygen species (ROS) or fails to effectively eliminate the excess ROS. Antioxidants can regulate ROS levels and maintain redox balance in the body. In mammals, antioxidants are regarded as pivotal elements in ovarian physiological metabolism. Enzymatic antioxidants, including catalase (CAT) [64,65], superoxide dismutase (SOD) [66,67], glutathione-S-transferase (GST) [68,69], etc., have been demonstrated to contribute significantly to the development of oocytes. Glutathione (GSH) [70,71], ascorbic acid (AA) [72,73], melatonin (MLT) [74,75,76], etc., which are non-enzymatic antioxidants, safeguard oocytes against damage caused by ROS and enhance the quality of oocytes. In honeybees, the activities of the queen ovary CAT, SOD, and GST transcripts increase with the reproductive maturity of the queen [77]. Weirich et al. conducted assays and found no significant differences in the activities of CAT, SOD, and GST in the hemolymph between NEQ and LQ [78]. Non-enzymatic antioxidants in honeybees have received limited attention, despite their significance. Notably, GSH has been exclusively identified in the ovaries of LQ. Consequently, we propose a hypothesis that LQ produces substantial amounts of ROS and necessitates elevated levels of GSH to maintain homeostasis. Honeybees have the ability to produce AA [79]. Supplementation of honeybee colonies with AA during the winter and early spring has been shown to enhance honeybees’ resistance to oxidative stress, resulting in a reduced infestation rate by Varroa destructor [80,81]. We only detected L-AA in the hemolymph of queens, and there was a significant difference between NLQ vs. NEQ groups, but not between OLQ vs. NEQ groups. 2-O-α-D-glucopyranosyl-L-ascorbic acid (AA-2G), the stable AA derivative, exhibits increased levels in LQ hemolymph compared to NEQ. AA-2G possesses inherent resistance against oxidative stress and can also be enzymatically converted into the highly potent antioxidant AA [82,83]. In the LQ ovary, we found another AA derivative, L-ascorbic acid 2-phosphate (AA-2P). AA-2P is highly stable under normal cell culture conditions and exhibits a longer duration of vitamin C activity compared to AA, thereby effectively preventing oxidative DNA damage [84,85,86]. MLT is mostly found in the head of honeybees. This study revealed the absence of MLT in the ovaries and hemolymph of queens. Interestingly, three distinct MLT metabolites, namely AMK, 6-hydroxymelatonin, and 2-hydroxymelatonin, were exclusively identified within the ovaries of NEQ. This phenomenon is likely to be attributed to the LQ’s uninterrupted oviposition and their absence of diel rhythmicity, while the NEQ demonstrates free-running circadian rhythms [87]. MLT can enhance honeybees’ ability to resist cold tolerance [88] and imidacloprid [89]. Further study is needed to determine whether MLT also affects egg production in queens, similar to its effects on D. melanogaster [90].
The antioxidant capacity is closely associated with the queen’s high egg-laying performance, while the amino acid requirement also reflects the speed of the queen’s high egg-laying. Amino acids play a crucial role in facilitating ovarian functions through their contribution to protein synthesis, hormone production, immune regulation, and other essential processes [91,92,93,94,95]. Honeybees, similar to other animals, have 10 essential amino acids (EAAs) that need to be supplemented in order to maintain normal growth, development, and reproduction: lysine (Lys), histidine (His), tryptophan (Try), valine (Val), arginine (Arg), isoleucine (Ile), leucine (Leu), methionine (Met), phenylalanine (Phe), and threonine (Thr) [96,97]. In our study, we found that most of the differentially expressed amino acids detected in LQ were down-regulated compared to NEQ. The EAAs that were down-regulated in hemolymph were L-Lys, L-His, L-Try, L-Val, and L-Ile. The down-regulated amino acids shared in the ovary and hemolymph include L-Try, L-tyrosine (L-Tyr), L-aspartic acid (L-Asp), L-citrulline (L-Cit), L-homocitrulline (L-HC), L-homoarginine (L-HA), and L-norleucine (L-NLE). Hrassnigg et al. also found that free amino acids without proline and some free EAAs in hemolymph were decreased in LQ compared to NEQ, e.g., Tyr, Arg, Ile, Leu, Lys, and His [98]. Egg-laying by queens can require substantial amounts of amino acids, resulting in a down-regulation of most amino acids compared to NEQ. Sang and King discovered that egg production in D. melanogaster requires all 10 EAAs and that non-essential amino acids also maintain egg production [99]. Alves et al. showed that a diet deficient in a single amino acid (Arg, Ile, Leu, Lys, Phe, Thr, and Try) resulted in a sharp drop in egg production in D. melanogaster, whereas deficiencies in Met, His, and Val declined more gradually in egg production [100]. The queen can acquire EAAs from royal jelly [101,102], enabling her to sustain her egg-laying capacity for an extended period of time.
The metabolism of Try and Tyr shows a great impact after egg-laying in queens. Metabolites in the tryptophan and tyrosine metabolic pathways are predominantly down-regulated in LQ compared to NEQ. This is most likely to be due to the sustained consumption of Try, Tyr, and their metabolites by the queen in her reproductive role. Try is an EAA for all insects, playing crucial roles in various physiological processes, including reproduction. Try can promote yolk formation [103], induce and promote ovarian development [104,105], and regulate queen ovary metabolism [48]. Tyr is a non-EAA that is synthesized via Phe. Tyr is the key precursor for tanning Aedes aegypti eggs [106]. Tyr deficiency significantly reduces insect egg production and affects egg hatchability [107,108,109]. Tyr in royal jelly may promote workers’ ovarian development in queenless colonies [110]. It is also likely that queens laying eggs require large amounts of Tyr from royal jelly. L-Lys, L-His, L-Val, L-Ile, L-aspartic acid, etc., may also have a close correlation with queens laying eggs, but additional verification is required.
5. Conclusions
The high rate of fatty acid metabolism and the high expression of antioxidants in the ovary and hemolymph provide material security and stress resistance for laying queens. Various amino acids are responsible for maintaining the queen’s egg production; oviposition significantly depletes amino acid reserves in queens. Following egg-laying, tryptophan and tyrosine metabolic pathways are significantly affected in queens’ ovary and hemolymph, underscoring the pivotal role of tryptophan and tyrosine in facilitating the queen bee’s reproductive capacity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects15040263/s1; Figure S1: The PCA plots, the OPLS-DA score plots, and the OPLS-DA permutation test plots are in each group. (A) The group NLQ vs. NEQ in the ovary. (B) The group OLQ vs. NEQ in the ovary. (C) The group NLQ vs. NEQ in the hemolymph. (D) The group OLQ vs. NEQ in the hemolymph; Table S1: The up-regulated differential metabolites FA and GP in the ovary of NLQ and OLQ compared to that of NEQ; Table S2: The up-regulated differential metabolites FA and GP in the hemolymph of NLQ and OLQ compared to that of NEQ; Table S3: Table S3.1 The metabolites expressed higher in the ovary of NLQ and OLQ than in the ovary of NEQ; Table S3.2 The down-regulated metabolites showed a lower expression level in the ovary of NLQ and OLQ compared to those in NEQ; Table S3.3 The metabolites expressed higher in the hemolymph of NLQ and OLQ than in the hemolymph of NEQ; Table S3.4 The down-regulated metabolites showed a lower expression level in the hemolymph of NLQ and OLQ compared to those in NEQ.
Author Contributions
Conceptualization, S.Z., L.P., Z.W. and Z.Z.; methodology, S.Z.; software, S.Z.; validation, Z.W. and Z.Z.; formal analysis, S.Z. and L.P.; investigation, Z.Z.; resources, Z.Z.; data curation, S.Z. and L.P.; writing—original draft preparation, Z.Z.; writing—review and editing, S.Z. and L.P.; visualization, S.Z. and L.P.; supervision, Z.Z.; project administration, Z.Z.; funding acquisition, Z.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key R&D Program Project of China (2022YFD1600202), the National Natural Science Foundation of China (32172790) and the Earmarked Fund for China Agriculture Research System (CARS-44-KXJ15).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
The authors thank Qiang Huang for helping us to improve our English language. The authors are thankful to the National Key R&D Program Project of China (2022YFD1600202), the National Natural Science Foundation of China (32172790), and the Earmarked Fund for China Agriculture Research System (CARS-44-KXJ15). Quantification of metabolite content in ovary and hemolymph was performed by the widely targeted metabolomics technique at Wuhan Metware Biotechnology Co. Ltd., Wuhan, China. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Snodgrass, R.E. Anatomy of the Honey Bee; Cornell University Press: Ithaca, NY, USA, 1956. [Google Scholar]
- Büchler, R.; Andonov, S.; Bienefeld, K.; Costa, C.; Hatjina, F.; Kezic, N.; Kryger, P.; Spivak, M.; Uzunov, A.; Wilde, J. Standard methods for rearing and selection of Apis mellifera queens. J. Apic. Res. 2013, 52, 1–30. [Google Scholar] [CrossRef]
- Kumar, N. Evaluation of larval grafted queen and natural reared queen of Italian honey bees (Apis mellifera L.). J. Pharmacogn. Phytochem. 2018, 7, 3181–3183. [Google Scholar]
- Woyke, J. Correlations between the age at which honeybee brood was grafted, characteristics of the resultant queens, and results of insemination. J. Apic. Res. 1971, 10, 45–55. [Google Scholar] [CrossRef]
- Yi, Y.; Liu, Y.-B.; Barron, A.B.; Zeng, Z.-J. Effects of commercial queen rearing methods on queen fecundity and genome methylation. Apidologie 2021, 52, 282–291. [Google Scholar] [CrossRef]
- Al-Ghzawi, A.A.M.; Zaitoun, S. Origin and rearing season of honeybee queens affect some of their physiological and reproductive characteristics. Entomol. Res. 2008, 38, 139–148. [Google Scholar] [CrossRef]
- Taha, E.-K.A.; Al-Kahtani, S.N. The relationship between comb age and performance of honey bee (Apis mellifera) colonies. Saudi J. Biol. Sci. 2020, 27, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Taha, E.-K.A.; Shawer, M.B.; Taha, R.; Elashmawy, A.; Gaber, S.; Mousa, K. Comb age significantly influences the emergency queen rearing, morphometric and reproductive characteristics of the queens. J. Apic. Res. 2024. [Google Scholar] [CrossRef]
- Tarpy, D.R. Genetic diversity within honeybee colonies prevents severe infections and promotes colony growth. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Mattila, H.R.; Seeley, T.D. Genetic diversity in honey bee colonies enhances productivity and fitness. Science 2007, 317, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Simeunovic, P.; Stevanovic, J.; Cirkovic, D.; Radojicic, S.; Lakic, N.; Stanisic, L.; Stanimirovic, Z. Nosema ceranae and queen age influence the reproduction and productivity of the honey bee colony. J. Apic. Res. 2014, 53, 545–554. [Google Scholar] [CrossRef]
- Amiri, E.; Strand, M.K.; Rueppell, O.; Tarpy, D.R. Queen quality and the impact of honey bee diseases on queen health: Potential for interactions between two major threats to colony health. Insects 2017, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.T.; Tarpy, D.R.; Fahrbach, S.E. Histological estimates of ovariole number in honey bee queens, Apis mellifera, reveal lack of correlation with other queen quality measures. J. Insect Sci. 2011, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Alqarni, A.S.; Balhareth, H.M.; Owayss, A.A. Queen morphometric and reproductive characters of Apis mellifera jemenitica, a native honey bee to Saudi Arabia. Bull. Insectol. 2013, 66, 239–244. [Google Scholar]
- Gilley, D.C.; Tarpy, D.R.; Land, B.B. Effect of queen quality on interactions between workers and dueling queens in honeybee (Apis mellifera L.) colonies. Behav. Ecol. Sociobiol. 2003, 55, 190–196. [Google Scholar] [CrossRef]
- Gregorc, A.; Škerl, M.I.S. Characteristics of honey bee (Apis mellifera carnica, Pollman 1879) queens reared in Slovenian commercial breeding stations. J. Apic. Sci. 2015, 59, 5–12. [Google Scholar] [CrossRef]
- Martins, J.R.; Nunes, F.M.; Cristino, A.S.; Simões, Z.L.; Bitondi, M.M. The four hexamerin genes in the honey bee: Structure, molecular evolution and function deduced from expression patterns in queens, workers and drones. BMC Mol. Biol. 2010, 11, 23. [Google Scholar] [CrossRef]
- Martins, J.R.; Bitondi, M.M.G. The HEX 110 hexamerin is a cytoplasmic and nucleolar protein in the ovaries of Apis mellifera. PLoS ONE 2016, 11, e0151035. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Qu, Y.; Dong, K.; He, S.; Jie, W.; Huang, J. Characterization and developmental expression patterns of four hexamerin genes in the bumble bee, Bombus terrestris (Hymenoptera: Apidae). J. Insect Sci. 2021, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.R.; Nunes, F.M.F.; Simões, Z.L.P.; Bitondi, M.M.G. A honeybee storage protein gene, hex 70a, expressed in developing gonads and nutritionally regulated in adult fat body. J. Insect Physiol. 2008, 54, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.R.; Anhezini, L.; Dallacqua, R.P.; Simoes, Z.L.; Bitondi, M.M. A honey bee hexamerin, HEX 70a, is likely to play an intranuclear role in developing and mature ovarioles and testioles. PLoS ONE 2011, 6, e29006. [Google Scholar] [CrossRef] [PubMed]
- Amdam, G.V.; Simões, Z.L.; Hagen, A.; Norberg, K.; Schrøder, K.; Mikkelsen, Ø.; Kirkwood, T.B.; Omholt, S.W. Hormonal control of the yolk precursor vitellogenin regulates immune function and longevity in honeybees. Exp. Gerontol. 2004, 39, 767–773. [Google Scholar] [CrossRef]
- Corona, M.; Velarde, R.A.; Remolina, S.; Moran-Lauter, A.; Wang, Y.; Hughes, K.A.; Robinson, G.E. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc. Natl. Acad. Sci. USA 2007, 104, 7128–7133. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.C. The Circulatory System of Insects; Charles C. Thomas: Springfield, IL, USA, 1977. [Google Scholar]
- Hillyer, J.F.; Pass, G. The insect circulatory system: Structure, function, and evolution. Annu. Rev. Entomol. 2020, 65, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-I.; Moeller, F. Comparison of the free amino acid composition in the hemolymph of healthy and Nosema-infected female honey bees. J. Invertebr. Pathol. 1970, 15, 202–206. [Google Scholar] [CrossRef]
- Mayack, C.; Naug, D. Parasitic infection leads to decline in hemolymph sugar levels in honeybee foragers. J. Insect Physiol. 2010, 56, 1572–1575. [Google Scholar] [CrossRef] [PubMed]
- Aliferis, K.A.; Copley, T.; Jabaji, S. Gas chromatography–mass spectrometry metabolite profiling of worker honey bee (Apis mellifera L.) hemolymph for the study of Nosema ceranae infection. J. Insect Physiol. 2012, 58, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Burritt, N.L.; Foss, N.J.; Neeno-Eckwall, E.C.; Church, J.O.; Hilger, A.M.; Hildebrand, J.A.; Warshauer, D.M.; Perna, N.T.; Burritt, J.B. Sepsis and hemocyte loss in honey bees (Apis mellifera) infected with Serratia marcescens strain sicaria. PLoS ONE 2016, 11, e0167752. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, J.; Zhang, Y.; Li, Z.; Evans, J.D.; Rose, R.; Gilligan, T.M.; LeBrun, A.; He, N.; Zheng, T. A novel method for the detection and diagnosis of virus infections in honey bees. J. Virol. Methods 2021, 293, 114163. [Google Scholar] [CrossRef] [PubMed]
- Ilijević, K.; Vujanović, D.; Orčić, S.; Purać, J.; Kojić, D.; Zarić, N.; Gržetić, I.; Blagojević, D.P.; Čelić, T.V. Anthropogenic influence on seasonal and spatial variation in bioelements and non-essential elements in honeybees and their hemolymph. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 239, 108852. [Google Scholar] [CrossRef]
- Horvatinec, J.; Svečnjak, L. Infrared (FTIR) spectral features of honey bee (Apis mellifera L.) hemolymph. J. Cent. Eur. Agric. 2020, 21, 37–41. [Google Scholar] [CrossRef]
- Sappington, T.W.; Raikhel, A.S. Molecular characteristics of insect vitellogenins and vitellogenin receptors. Insect Biochem. Mol. Biol. 1998, 28, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Guidugli, K.R.; Piulachs, M.D.; Bellés, X.; Lourenço, A.P.; Simões, Z.L. Vitellogenin expression in queen ovaries and in larvae of both sexes of Apis mellifera. Arch. Insect Biochem. Physiol. Publ. Collab. Entomol. Soc. Am. 2005, 59, 211–218. [Google Scholar] [CrossRef]
- Excels, W. Occurrence and significance of vitellogenins in female castes of social Hymenoptera. Am. Zool. 1974, 14, 1229–1237. [Google Scholar] [CrossRef]
- Fleig, R. Role of the follicle cells for yolk uptake in ovarian follicles of the honey bee Apis mellifera L. (Hymenoptera: Apidae). Int. J. Insect Morphol. Embryol. 1995, 24, 427–433. [Google Scholar] [CrossRef]
- Jung, J. Metabolomic Studies in Apis mellifera. J. Apic. 2023, 38, 151–162. [Google Scholar] [CrossRef]
- Shi, T.; Burton, S.; Wang, Y.; Xu, S.; Zhang, W.; Yu, L. Metabolomic analysis of honey bee, Apis mellifera L. response to thiacloprid. Pestic. Biochem. Physiol. 2018, 152, 17–23. [Google Scholar] [CrossRef]
- Gao, J.; Yang, Y.; Ma, S.; Liu, F.; Wang, Q.; Wang, X.; Wu, Y.; Zhang, L.; Liu, Y.; Diao, Q. Combined transcriptome and metabolite profiling analyses provide insights into the chronic toxicity of carbaryl and acetamiprid to Apis mellifera larvae. Sci. Rep. 2022, 12, 16898. [Google Scholar] [CrossRef]
- Wang, B.; Habermehl, C.; Jiang, L. Metabolomic analysis of honey bee (Apis mellifera L.) response to glyphosate exposure. Mol. Omics 2022, 18, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Shi, J.; Yu, L.; Wu, X. Metabolic profiling of Apis mellifera larvae treated with sublethal acetamiprid doses. Ecotoxicol. Environ. Saf. 2023, 254, 114716. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-L.; Zhou, C.-X.; Wu, P.-J.; Xu, J.; Guo, Y.-Q.; Xue, F.; Getachew, A.; Xu, S.-F. Brain metabolomic profiling of eastern honey bee (Apis cerana) infested with the mite Varroa destructor. PLoS ONE 2017, 12, e0175573. [Google Scholar] [CrossRef]
- Jousse, C.; Dalle, C.; Abila, A.; Traïkia, M.; Diogon, M.; Lyan, B.; El Alaoui, H.; Vidau, C.; Delbac, F. A combined LC-MS and NMR approach to reveal metabolic changes in the hemolymph of honeybees infected by the gut parasite Nosema ceranae. J. Invertebr. Pathol. 2020, 176, 107478. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hou, M.; Qiu, Y.; Zhao, B.; Nie, H.; Su, S. Changes in antioxidant enzymes activity and metabolomic profiles in the guts of honey bee (Apis mellifera) larvae infected with Ascosphaera apis. Insects 2020, 11, 419. [Google Scholar] [CrossRef] [PubMed]
- Ricigliano, V.A.; Cank, K.B.; Todd, D.A.; Knowles, S.L.; Oberlies, N.H. Metabolomics-guided comparison of pollen and microalgae-based artificial diets in honey bees. J. Agric. Food Chem. 2022, 70, 9790–9801. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Ding, G.; Jia, G.; Feng, M.; Huang, J. Hemolymph Metabolism Analysis of Honey Bee (Apis mellifera L.) Response to Different Bee Pollens. Insects 2023, 14, 37. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, F.; Li, G.; Chi, X.; Wang, Y.; Wang, H.; Ma, L.; Han, K.; Zhao, G.; Guo, X. Metabolite support of long-term storage of sperm in the spermatheca of honeybee (Apis mellifera) queens. Front. Physiol. 2020, 11, 574856. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-L.; Huang, Q.; Li, J.-L.; Wu, P.; Wei, B.; Li, X.-J.; Tang, Q.-H.; Dong, Z.-X.; Xiong, J.; Tang, H. Gut microbiota-driven regulation of queen bee ovarian metabolism. Microbiol. Spectr. 2023, 11, e02145-23. [Google Scholar] [CrossRef]
- Kostecki, R. Investigation on the Haemocytes and Haemolymph of Honeybees. J. Apic. Res. 1965, 4, 49–54. [Google Scholar] [CrossRef]
- Garrido, P.M.; Martin, M.L.; Negri, P.; Eguaras, M.J. A standardized method to extract and store haemolymph from Apis mellifera and the ectoparasite Varroa destructor for protein analysis. J. Apic. Res. 2013, 52, 67–68. [Google Scholar] [CrossRef]
- Borsuk, G.; Ptaszyńska, A.A.; Olszewski, K.; Domaciuk, M.; Krutmuang, P.; Paleolog, J. A new method for quick and easy hemolymph collection from apidae adults. PLoS ONE 2017, 12, e0170487. [Google Scholar] [CrossRef] [PubMed]
- Fritz, I.B. Carnitine and its role in fatty acid metabolism. Adv. Lipid Res. 1963, 1, 285–334. [Google Scholar] [PubMed]
- Bremer, J. Carnitine--metabolism and functions. Physiol. Rev. 1983, 63, 1420–1480. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.-Q.; Jia, B.-Y.; Li, J.-J.; Fu, X.-W.; Zhou, G.-B.; Hou, Y.-P.; Zhu, S.-E. L-carnitine enhances oocyte maturation and development of parthenogenetic embryos in pigs. Theriogenology 2011, 76, 785–793. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Lee, J.; Hyun, S.-H.; Lee, E. L-carnitine treatment during oocyte maturation improves in vitro development of cloned pig embryos by influencing intracellular glutathione synthesis and embryonic gene expression. Theriogenology 2012, 78, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Phongnimitr, T.; Liang, Y.; Srirattana, K.; Panyawai, K.; Sripunya, N.; Treetampinich, C.; Parnpai, R. Effect of L-carnitine on maturation, cryo-tolerance and embryo developmental competence of bovine oocytes. Anim. Sci. J. 2013, 84, 719–725. [Google Scholar] [CrossRef]
- Knitlova, D.; Hulinska, P.; Jeseta, M.; Hanzalova, K.; Kempisty, B.; Machatkova, M. Supplementation of l-carnitine during in vitro maturation improves embryo development from less competent bovine oocytes. Theriogenology 2017, 102, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Dunning, K.R.; Akison, L.K.; Russell, D.L.; Norman, R.J.; Robker, R.L. Increased beta-oxidation and improved oocyte developmental competence in response to l-carnitine during ovarian in vitro follicle development in mice. Biol. Reprod. 2011, 85, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Moawad, A.R.; Xu, B.; Tan, S.L.; Taketo, T. L-carnitine supplementation during vitrification of mouse germinal vesicle stage–oocytes and their subsequent in vitro maturation improves meiotic spindle configuration and mitochondrial distribution in metaphase II oocytes. Hum. Reprod. 2014, 29, 2256–2268. [Google Scholar] [CrossRef]
- Li, L.; Zhu, S.; Shu, W.; Guo, Y.; Guan, Y.; Zeng, J.; Wang, H.; Han, L.; Zhang, J.; Liu, X. Characterization of metabolic patterns in mouse oocytes during meiotic maturation. Mol. Cell 2020, 80, 525–540.e529. [Google Scholar] [CrossRef]
- Geer, B.; Dolph, W. A dietary choline requirement for egg production in Drosophila melanogaster. Reproduction 1970, 21, 9–15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gülçin, I. Antioxidant and antiradical activities of L-carnitine. Life Sci. 2006, 78, 803–811. [Google Scholar] [CrossRef]
- Solarska, K.; Lewińska, A.; Karowicz-Bilińska, A.; Bartosz, G. The antioxidant properties of carnitine in vitro. Cell. Mol. Biol. Lett. 2010, 15, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Choi, A.; Hope, Y.Y.; Czerniak, S.M.; Holick, E.A.; Paolella, L.J.; Agarwal, A.; Combelles, C.M. Fluctuations in total antioxidant capacity, catalase activity and hydrogen peroxide levels of follicular fluid during bovine folliculogenesis. Reprod. Fertil. Dev. 2011, 23, 673–680. [Google Scholar] [CrossRef]
- Park, Y.S.; You, S.Y.; Cho, S.; Jeon, H.-J.; Lee, S.; Cho, D.-H.; Kim, J.-S.; Oh, J.S. Eccentric localization of catalase to protect chromosomes from oxidative damages during meiotic maturation in mouse oocytes. Histochem. Cell Biol. 2016, 146, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Corona de Lau, C. Correlation between follicle levels of superoxide dismutase and oocyte quality, fertilization rates and embryo development. Ginecol. Obstet. Mex. 2004, 72, 335–344. [Google Scholar]
- Tatemoto, H.; Muto, N.; Sunagawa, I.; Shinjo, A.; Nakada, T. Protection of porcine oocytes against cell damage caused by oxidative stress during in vitro maturation: Role of superoxide dismutase activity in porcine follicular fluid. Biol. Reprod. 2004, 71, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Meijide, S.; Hernández, M.L.; Navarro, R.; Larreategui, Z.; Ferrando, M.; Ruiz-Sanz, J.I.; Ruiz-Larrea, M.B. Glutathione S-transferase activity in follicular fluid from women undergoing ovarian stimulation: Role in maturation. Free Radic. Biol. Med. 2014, 75, S41. [Google Scholar] [CrossRef] [PubMed]
- Thaker, R.; Mishra, V.; Gor, M.; Agarwal, R.; Sheth, H.; Kapadia, P.; Kumar, S. The role of stimulation protocol, number of oocytes retrieved with respect to follicular fluid oxidative stress and IVF outcome. Hum. Fertil. 2020, 23, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Barros, F.D.d.A.; Adona, P.R.; Guemra, S.; Damião, B.C.M. Oxidative homeostasis in oocyte competence for in vitro embryo development. Anim. Sci. J. 2019, 90, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Hao, Y.; Liu, Z.; Li, S.; Wang, C.; Wang, B.; Liu, Y.; Liu, G.; Dai, Y. Effect of exogenous glutathione supplementation on the in vitro developmental competence of ovine oocytes. Theriogenology 2021, 173, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Kere, M.; Siriboon, C.; Lo, N.-W.; Nguyen, N.T.; Ju, J.-C. Ascorbic acid improves the developmental competence of porcine oocytes after parthenogenetic activation and somatic cell nuclear transplantation. J. Reprod. Dev. 2013, 59, 78–84. [Google Scholar] [CrossRef]
- Yu, X.-X.; Liu, Y.-H.; Liu, X.-M.; Wang, P.-C.; Liu, S.; Miao, J.-K.; Du, Z.-Q.; Yang, C.-X. Ascorbic acid induces global epigenetic reprogramming to promote meiotic maturation and developmental competence of porcine oocytes. Sci. Rep. 2018, 8, 6132. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Takasaki, A.; Miwa, I.; Taniguchi, K.; Maekawa, R.; Asada, H.; Taketani, T.; Matsuoka, A.; Yamagata, Y.; Shimamura, K. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J. Pineal Res. 2008, 44, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Takasaki, A.; Taketani, T.; Tanabe, M.; Kizuka, F.; Lee, L.; Tamura, I.; Maekawa, R.; Aasada, H.; Yamagata, Y. The role of melatonin as an antioxidant in the follicle. J. Ovarian Res. 2012, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, K.; Itoh, M.T.; Takahashi, N.; Tarumi, W.; Ishizuka, B. The rat oocyte synthesises melatonin. Reprod. Fertil. Dev. 2013, 25, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.; Williams, V.; Evans, J. Sperm storage and antioxidative enzyme expression in the honey bee, Apis mellifera. Insect Mol. Biol. 2004, 13, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Weirich, G.F.; Collins, A.M.; Williams, V.P. Antioxidant enzymes in the honey bee, Apis mellifera. Apidologie 2002, 33, 3–14. [Google Scholar] [CrossRef]
- Herbert, E., Jr.; Vanderslice, J.; Higgs, D. Effect of dietary vitamin C levels on the rate of brood production of free-flying and confined colonies of honey bees. Apidologie 1985, 16, 385–394. [Google Scholar] [CrossRef]
- Farjan, M.; Dmitryjuk, M.; Lipiński, Z.; Biernat-Łopieńska, E.; Żółtowska, K. Supplementation of the honey bee diet with vitamin C: The effect on the antioxidative system of Apis mellifera carnica brood at different stages. J. Apic. Res. 2012, 51, 263–270. [Google Scholar] [CrossRef]
- Farjan, M.; Łopieńska-Biernat, E.; Lipiński, Z.; Dmitryjuk, M.; Żółtowska, K. Supplementing with vitamin C the diet of honeybees (Apis mellifera carnica) parasitized with Varroa destructor: Effects on antioxidative status. Parasitology 2014, 141, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Arai, N.; Kohno, K.; Ushio, S.; Fukuda, S. Anti-oxidative and anti-aging activities of 2-O-α-glucopyranosyl-L-ascorbic acid on human dermal fibroblasts. Eur. J. Pharmacol. 2012, 674, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Hanada, Y.; Iomori, A.; Ishii, R.; Gohda, E.; Tai, A. Protection of free radical-induced cytotoxicity by 2-O-α-d-glucopyranosyl-l-ascorbic acid in human dermal fibroblasts. Biosci. Biotechnol. Biochem. 2014, 78, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Hata, R.I.; Senoo, H. L-ascorbic acid 2-phosphate stimulates collagen accumulation, cell proliferation, and formation of a three-dimensional tissuelike substance by skin fibroblasts. J. Cell. Physiol. 1989, 138, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Saika, S.; Kanagawa, R.; Uenoyama, K.; Hiroi, K.; Hiraoka, J.-I. L-ascorbic acid 2-phosphate, a phosphate derivative of L-ascorbic acid, enhances the growth of cultured rabbit keratocytes. Graefe’s Arch. Clin. Exp. Ophthalmol. 1991, 229, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Shima, N.; Kimoto, M.; Yamaguchi, M.; Yamagami, S. Increased proliferation and replicative lifespan of isolated human corneal endothelial cells with L-ascorbic acid 2-phosphate. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8711–8717. [Google Scholar] [CrossRef]
- Johnson, J.N.; Hardgrave, E.; Gill, C.; Moore, D. Absence of consistent diel rhythmicity in mated honey bee queen behavior. J. Insect Physiol. 2010, 56, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Li, G.; Zhang, X.; Wang, Y.; Wang, C.; Xu, B.; Guo, X.; Li, H. The role of melatonin and Tryptophan-5-hydroxylase-1 in different abiotic stressors in Apis cerana cerana. J. Insect Physiol. 2021, 128, 104180. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Duan, J.; Chen, L.; Wang, Y.; Qin, Q.; Dang, X.; Zhou, Z. Melatonin enhances the antioxidant capacity to rescue the honey bee Apis mellifera from the ecotoxicological effects caused by environmental imidacloprid. Ecotoxicol. Environ. Saf. 2022, 239, 113622. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, L.; Callebert, J.; Launay, J.; Jallon, J. Melatonin biosynthesis in Drosophila: Its nature and its effects. J. Neurochem. 1988, 50, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Estienne, M.; Barb, C. The control of adenohypophysial hormone secretion by amino acids and peptides in swine. Domest. Anim. Endocrinol. 2005, 29, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yin, Y.-L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Grohmann, U.; Bronte, V. Control of immune response by amino acid metabolism. Immunol. Rev. 2010, 236, 243–264. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Dai, Z.; Li, D.; Wang, J.; Wu, Z. Amino acid nutrition in animals: Protein synthesis and beyond. Annu. Rev. Anim. Biosci. 2014, 2, 387–417. [Google Scholar] [CrossRef] [PubMed]
- De Groot, A. Amino acid requirements for growth of the honeybee (Apis mellifica L.). Experientia 1952, 8, 192–194. [Google Scholar] [CrossRef]
- De Groot, A.P. Protein and amino acid requirements of the honeybee (Apis mellifica L.). Physiol. Comp. Oecol. 1953, 3, 197–285. [Google Scholar]
- Hrassnigg, N.; Leonhard, B.; Crailsheim, K. Free amino acids in the haemolymph of honey bee queens (Apis mellifera L.). Amino Acids 2003, 24, 205–212. [Google Scholar] [CrossRef]
- Sang, J.H.; King, R.C. Nutritional requirements of axenically cultured Drosophila melanogaster adults. J. Exp. Biol. 1961, 38, 793–809. [Google Scholar] [CrossRef]
- Alves, A.N.; Sgrò, C.M.; Piper, M.D.; Mirth, C.K. Target of Rapamycin Drives Unequal Responses to Essential Amino Acid Depletion for Egg Laying in Drosophila Melanogaster. Front. Cell Dev. Biol. 2022, 10, 822685. [Google Scholar] [CrossRef] [PubMed]
- Boselli, E.; Caboni, M.F.; Sabatini, A.G.; Marcazzan, G.L.; Lercker, G. Determination and changes of free amino acids in royal jelly during storage. Apidologie 2003, 34, 129–137. [Google Scholar] [CrossRef]
- Xue, X.; Wu, L.; Wang, K. Chemical Composition of Royal Jelly. In Bee Products—Chemical and Biological Properties; Springer: Cham, Switzerland, 2017; pp. 181–190. [Google Scholar]
- Harlow, P.M. A study of ovarial development and its relation to adult nutrition in the blowfly Protophormia terrae-novae (RD). J. Exp. Biol. 1956, 33, 777–797. [Google Scholar] [CrossRef]
- Kobayashi, K.; Maezawa, T.; Tanaka, H.; Onuki, H.; Horiguchi, Y.; Hirota, H.; Ishida, T.; Horiike, K.; Agata, Y.; Aoki, M. The identification of d-tryptophan as a bioactive substance for postembryonic ovarian development in the planarian Dugesia ryukyuensis. Sci. Rep. 2017, 7, 45175. [Google Scholar] [CrossRef] [PubMed]
- Maezawa, T.; Ishikawa, M.; Sekii, K.; Nagamatsu, G.; Furukawa, R.; Kobayashi, K. D-Tryptophan enhances the reproductive organ-specific expression of the amino acid transporter homolog Dr-SLC38A9 involved in the sexual induction of planarian Dugesia ryukyuensis. Zool. Lett. 2021, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Christensen, B.M. Involvement of l-tyrosine and phenol oxidase in the tanning of Aedes aegypti eggs. Insect Biochem. Mol. Biol. 1993, 23, 739–748. [Google Scholar] [CrossRef]
- Zografou, E.; Tsiropoulos, G.; Margaritis, L. Effect of phenylalanine and tyrosine analogues on Bactrocera oleae Gmelin (Dipt., Tephritidae) reproduction. J. Appl. Entomol. 2001, 125, 365–369. [Google Scholar] [CrossRef]
- Fuchs, S.; Behrends, V.; Bundy, J.G.; Crisanti, A.; Nolan, T. Phenylalanine metabolism regulates reproduction and parasite melanization in the malaria mosquito. PLoS ONE 2014, 9, e84865. [Google Scholar] [CrossRef] [PubMed]
- Sterkel, M.; Oliveira, P.L. Developmental roles of tyrosine metabolism enzymes in the blood-sucking insect Rhodnius prolixus. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162607. [Google Scholar] [CrossRef]
- Matsuyama, S.; Nagao, T.; Sasaki, K. Consumption of tyrosine in royal jelly increases brain levels of dopamine and tyramine and promotes transition from normal to reproductive workers in queenless honey bee colonies. Gen. Comp. Endocrinol. 2015, 211, 1–8. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).