The Bombyx mori singed Gene Is Involved in the High-Temperature Resistance of Silkworms
Abstract
:Simple Summary
Abstract
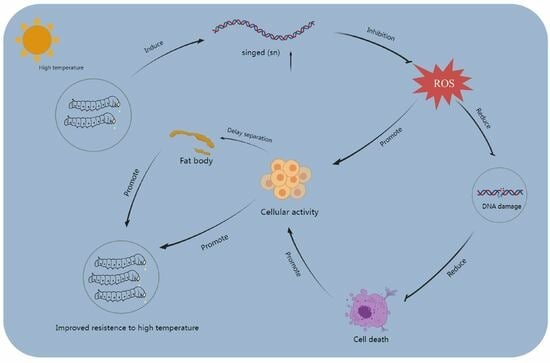
1. Introduction
2. Materials and Methods
2.1. Gene Analysis and Vector Construction
2.2. Cell Culture and Transfection
2.3. Treatment of Silkworms
2.4. Total RNA Extraction
2.5. Quantitative Real-Time PCR (qRT-PCR)
2.6. Detection of ROS
2.7. Cell Proliferation Activity Detection
2.8. Single Cell Gel Electrophoresis Assay
2.9. CalCEIIN-AM/PI Fluorescent Double Staining
2.10. Construction of Transgenic Vector of Silkworm
2.11. Statistical Analysis
3. Results
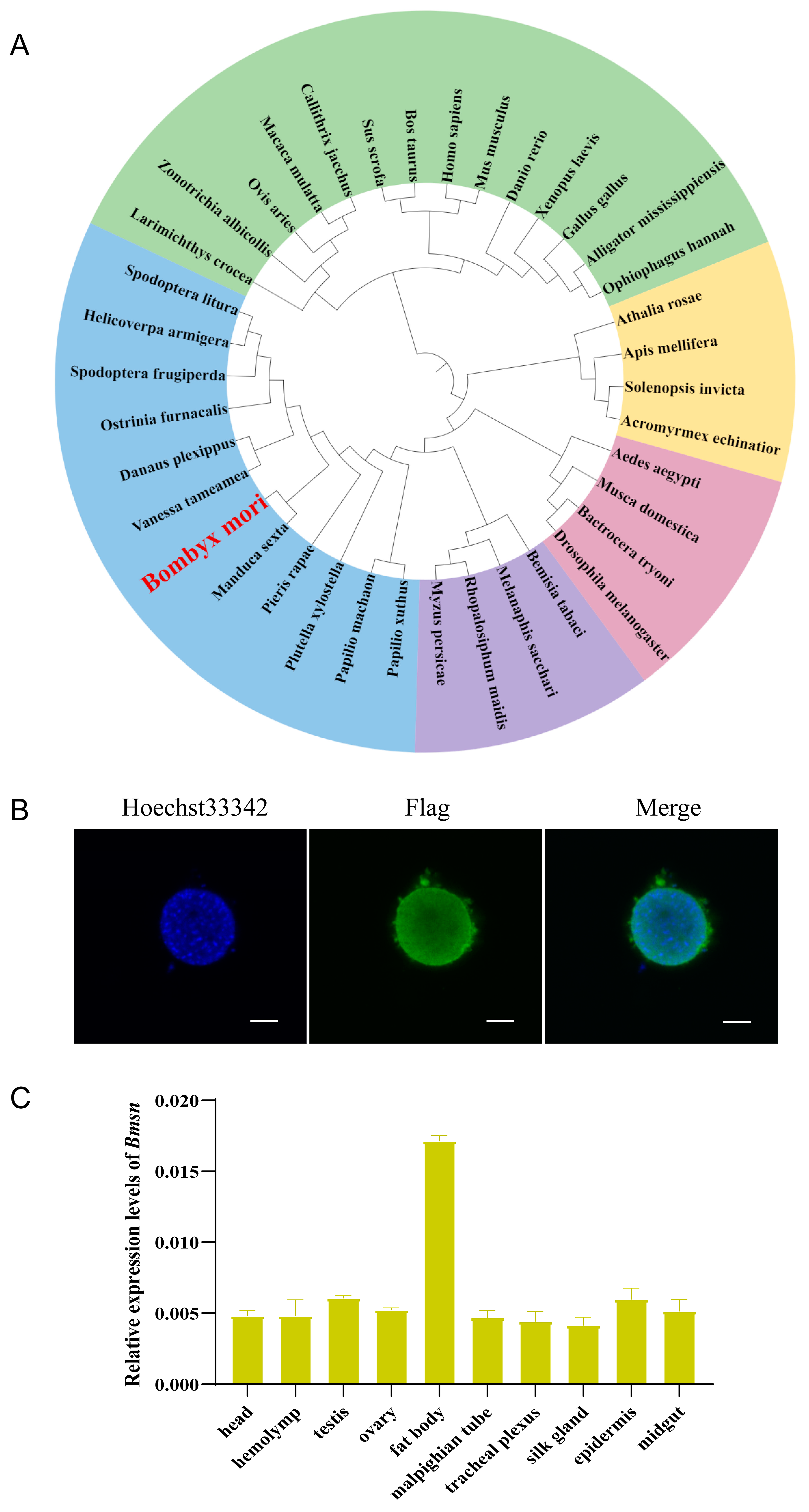
3.1. Identification and Characteristic Analysis of the Bmsn Gene
3.2. Effect of Bmsn on the Proliferation Activity of Silkworm Cells
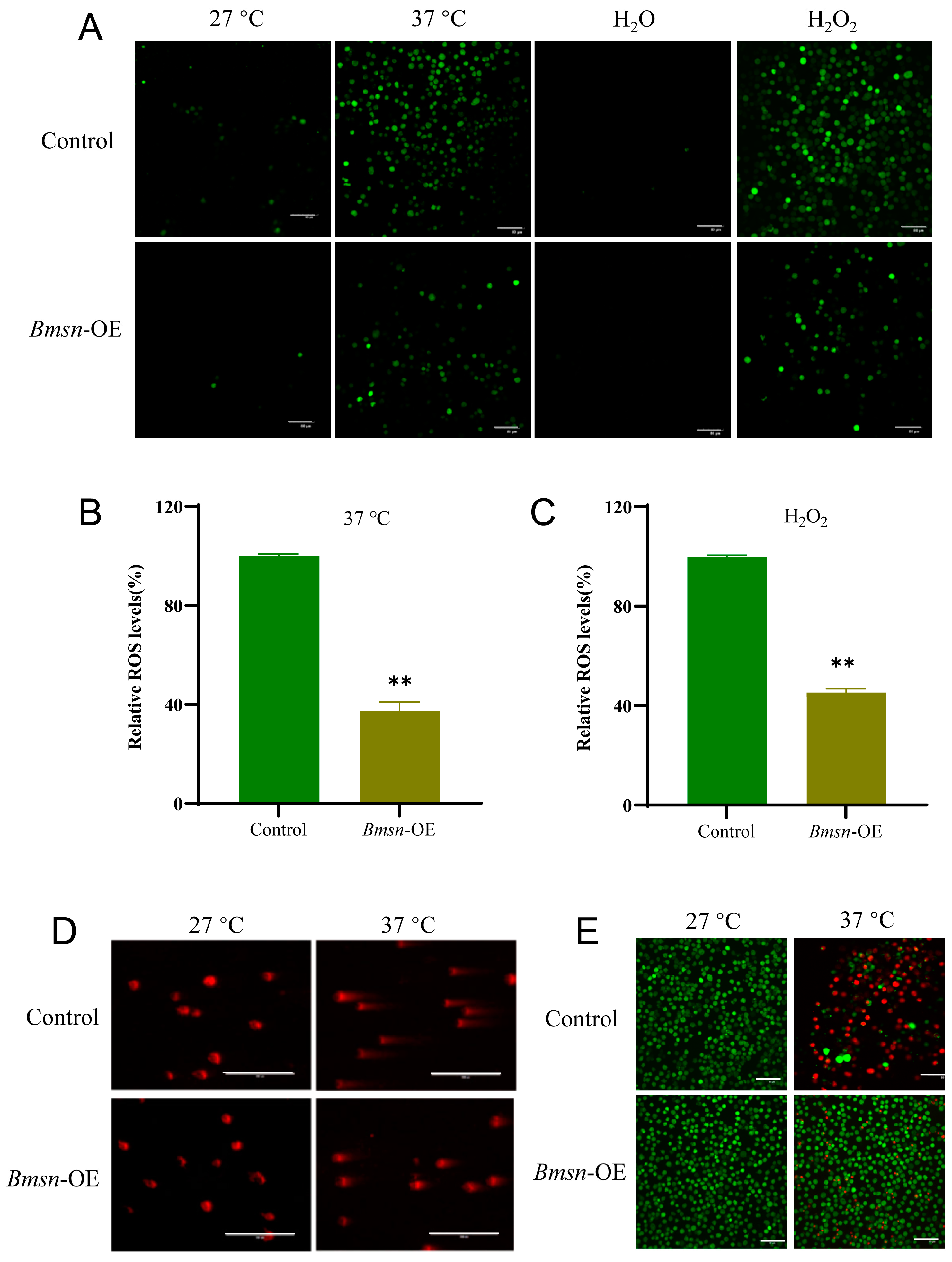
3.3. Effects of the Bmsn Gene on ROS Levels and Cell Survival in BmN Cells
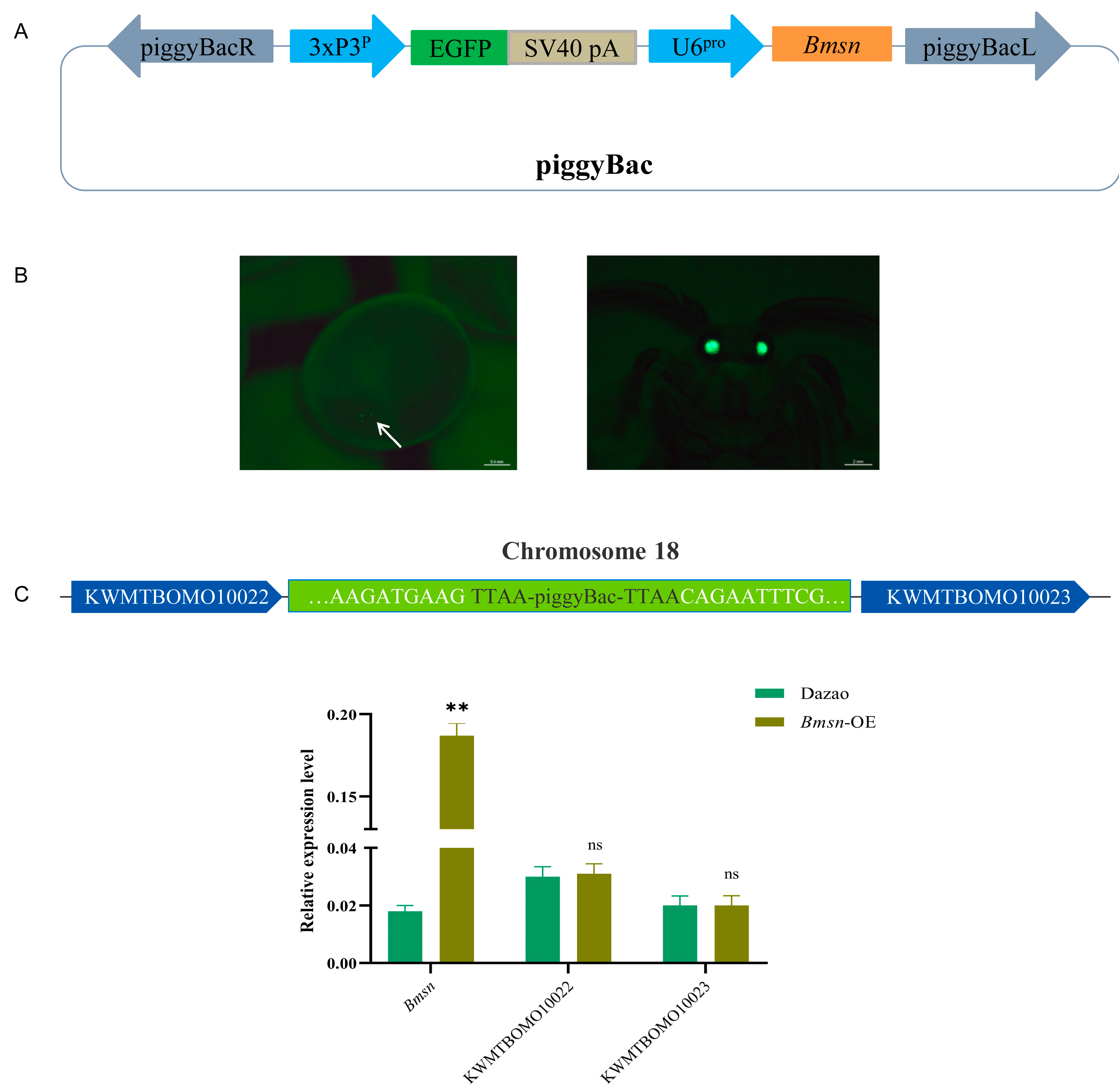
3.4. Construction and Characteristic Analysis of Transgenic Silkworms Overexpressing the Bmsn Gene
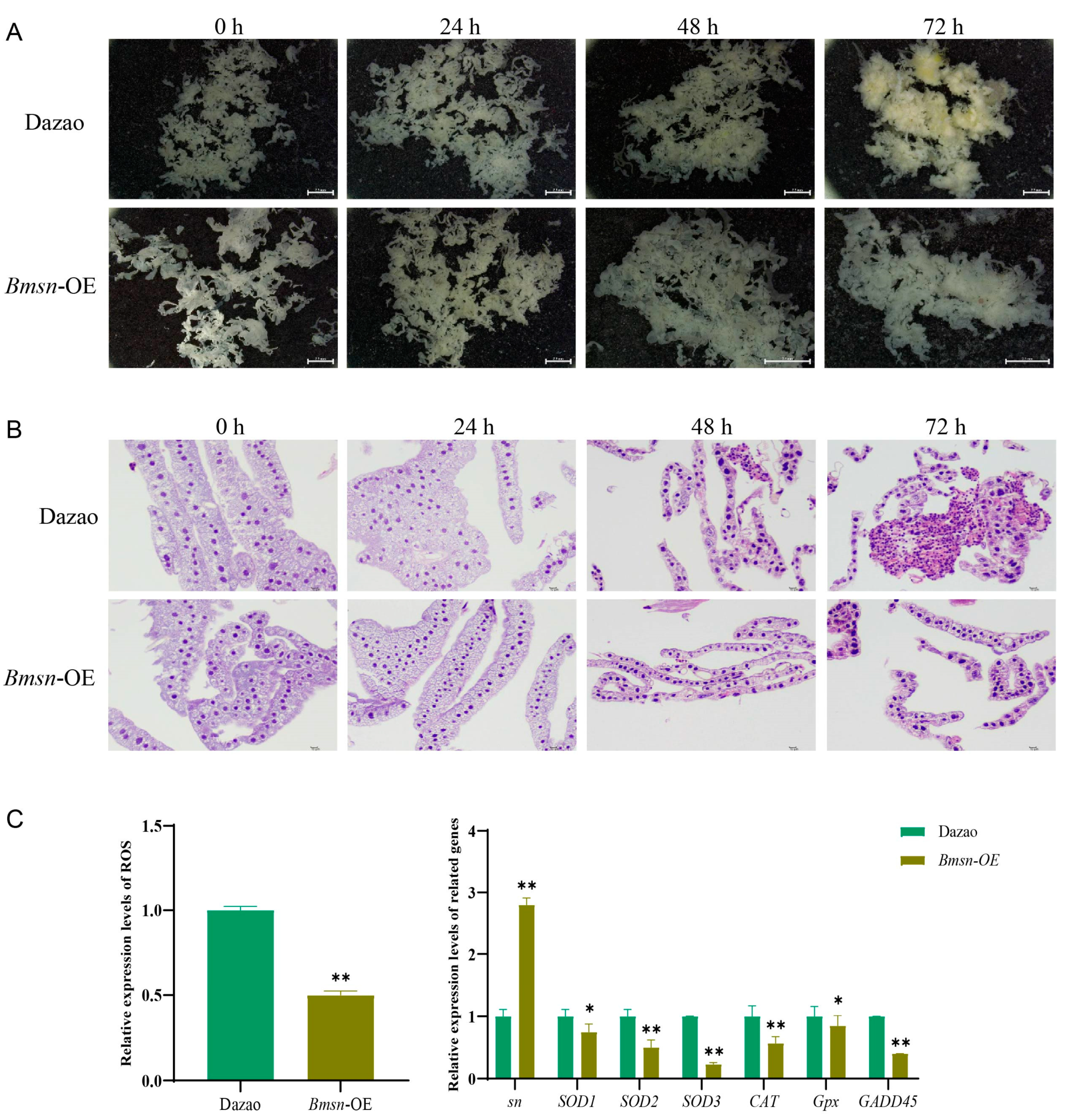
3.5. Effects of High Temperature on Transgenic Silkworms Overexpressing Bmsn Gene
3.6. Effects of Overexpression of the Bmsn Gene on the Silkworm Fat Body
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rozsypal, J. Estimating the potential of insects from warmer regions to overwinter in colder regions under a warming winter scenario using simulation experiments: A case study in Sesamia nonagrioides. Insects 2023, 14, 957. [Google Scholar] [CrossRef] [PubMed]
- Kryukov, V.Y.; Yaroslavtseva, O.N.; Whitten, M.M.A.; Tyurin, M.V.; Ficken, K.J.; Greig, C.; Melo, N.R.; Glupov, V.V.; Dubovskiy, I.M.; Butt, T.M. Fungal infection dynamics in response to temperature in the lepidopteran insect Galleria mellonella. Insect Sci. 2018, 25, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Kausar, S.; Abbas, M.N.; Yang, L.; Cui, H. Biotic and abiotic stress induces the expression of Hsp70/90 organizing protein gene in silkworm, Bombyx mori. Int. J. Biol. Macromol. 2020, 143, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Hrncir, M.; Maia-Silva, C.; da Silva Teixeira-Souza, V.H.; Imperatriz-Fonseca, V.L. Stingless bees and their adaptations to extreme environments. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2019, 205, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Bubliy, O.A.; Riihimaa, A.; Norry, F.M.; Loeschcke, V. Variation in resistance and acclimation to low-temperature stress among three geographical strains of Drosophila melanogaster. J. Therm. Biol. 2002, 27, 337–344. [Google Scholar] [CrossRef]
- Terlau, J.F.; Brose, U.; Eisenhauer, N.; Amyntas, A.; Boy, T.; Dyer, A.; Gebler, A.; Hof, C.; Liu, T.; Scherber, C.; et al. Microhabitat conditions remedy heat stress effects on insect activity. Glob. Chang. Biol. 2023, 29, 3747–3758. [Google Scholar] [CrossRef]
- Li, X.; Ma, W.; Jiang, Y. Honeybees (Hymenoptera: Apidae) Adapt to the Shock of High Temperature and High Humidity Through Changes in Sugars and Polyols and Free Amino Acids. J. Insect. Sci. 2023, 23, 4. [Google Scholar] [CrossRef]
- Bryan, J.; Edwards, R.; Matsudaira, P.; Otto, J.; Wulfkuhle, J. Fascin, an echinoid Actin-bundling protein, is a homolog of the Drosophila singed gene product. Proc. Natl. Acad. Sci. USA 1993, 90, 9115–9119. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, M.; Hou, X.; Meng, S.; Zhang, F.; Ma, J. Adaptation of the egg of the desert beetle, Microdera punctipennis (Coleoptera: Tenebrionidae), to arid environment. J. Insect Sci. 2014, 14, 246. [Google Scholar] [CrossRef]
- Marada, A.; Karri, S.; Singh, S.; Allu, P.K.; Boggula, Y.; Krishnamoorthy, T. A single point mutation in mitochondrial Hsp70 cochaperone Mge1 gains thermal stability and resistance. Biochemistry 2016, 55, 7065–7072. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Guo, H.; Wang, Y.; Xu, B.; Guo, X.; Wang, C. Activating transcription factor 2 (AccATF2) regulates tolerance to oxidative stress in Apis cerana cerana. Pestic. Biochem. Physiol. 2022, 186, 105179. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, N.N.; Thorshauge, P.M.; Kristensen, T.N.; de Jonge, N.; Bahrndorff, S.; Kjeldal, H.; Nielsen, J.L. Strong responses of Drosophila melanogaster microbiota to developmental temperature. Fly 2018, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pagabeleguem, S.; Ravel, S.; Dicko, A.H.; Vreysen, M.J.B.; Parker, A.; Takac, P.; Huber, K.; Sidibe, I.; Gimonneau, G.; Bouyer, J. Influence of temperature and relative humidity on survival and fecundity of three tsetse strains. Parasits Vectors 2016, 9, 520. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, D.D.; Ranga, P.; Kalra, B.; Parkash, R.; Rashkovetsky, E.; Bantis, L.E. Rapid effects of humidity acclimation on stress resistance in Drosophila melanogaster. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 166, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.J.; Liu, Y.J.; Cheng, J.; Li, Z.; Michaud, J.; Liu, X.X. High temperature exposure reduces the susceptibility of Helicoverpa armigera to its nucleopolyhedrovirus (HearNPV) by enhancing expression of heat shock proteins. Pest. Manag. Sci. 2022, 78, 2378–2389. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, M.R.; Shimada, T.; Abe, H. The genetics and genomics of the silkworm, Bombyx mori. Annu. Rev. Entomol. 2005, 50, 71–100. [Google Scholar] [CrossRef]
- Kelley, J.L.; Peyton, J.T.; Fiston-Lavier, A.S.; Teets, N.M.; Yee, M.C.; Johnston, J.S.; Bustamante, C.D.; Lee, R.E.; Denlinger, D.L. Compact genome of the Antarctic midge is likely an adaptation to an extreme environment. Nat. Commun. 2014, 5, 4611. [Google Scholar] [CrossRef]
- Li, J.; Lu, Z.; Mao, T.; Li, M.; Wang, H.; Qu, J.; Chen, J.; Fang, Y.; Li, F.; Li, B. Identification of the nucleotide exchange factor BmGrpE and its role under high-temperature stress in silkworm, Bombyx mori. Arch. Insect Biochem. Physiol. 2020, 104, e21664. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, P.; Xiao, J.; Wang, L.; Liu, T.; Wu, Y.; Dong, F.; Jiang, Y.; Pan, M.; Zhang, Y.; et al. Comparative transcriptome profiling of a thermal resistant vs. sensitive silkworm strain in response to high temperature under stressful humidity condition. PLoS ONE 2017, 12, e0177641. [Google Scholar] [CrossRef]
- Pan, M.H.; Cai, X.J.; Liu, M.; Lv, J.; Tang, H.; Tan, J.; Lu, C. Establishment and characterization of an ovarian cell line of the silkworm, Bombyx mori. Tissue Cell 2010, 42, 42–46. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye Res. 2020, 79, 100858. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Bai, D.; Wei, Z.; Zhang, Y.; Huang, Y.; Deng, H.; Huang, X. Curcumin attenuates oxidative stress in RAW264.7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS ONE 2019, 14, e0216711. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Li, X.; Shen, J.; Du, Y.; Xu, K.; Jiang, Y. Transcriptomic analysis reveals Apis mellifera adaptations to high temperature and high humidity. Ecotoxicol. Environ. Saf. 2019, 84, 109599. [Google Scholar] [CrossRef] [PubMed]
- Bjørge, J.D.; Overgaard, J.; Malte, H.; Gianotten, N.; Heckmann, L.H. Role of temperature on growth and metabolic rate in the tenebrionid beetles Alphitobius diaperinus and Tenebrio molitor. J. Insect Sci. 2018, 107, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, B.J.; Marshall, K.E. The many roles of fats in overwintering insects. J. Exp. Biol. 2018, 221, jeb161836. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Chen, P.; Liu, P.; Bu, C.; Zhang, D. High-Temperature-Responsive poplar lncRNAs modulate target gene expression via RNA interference and act as RNA scaffolds to enhance heat tolerance. Int. J. Mol. Sci. 2020, 21, 6808. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Dong, Z.; Pan, M. Common strategies in silkworm disease resistance breeding research. Pest. Manag. Sci. 2023, 79, 2287–2298. [Google Scholar] [CrossRef] [PubMed]
- Rusch, T.W.; Adutwumwaah, A.; Beebe, L.E.; Tomberlin, J.K.; Tarone, A.M. The upper thermal tolerance of the secondary screwworm, Cochliomyia macellaria Fabricius (Diptera: Calliphoridae). J. Therm. Biol. 2019, 85, 102405. [Google Scholar] [CrossRef]
- Ma, J.; Ma, L.; Zhang, Z.; Li, K.; Wang, Y.; Chen, X.; Zhang, H. In vivo evaluation of insect wax for hair growth potential. PLoS ONE 2018, 13, e0192612. [Google Scholar] [CrossRef]
- Kureishy, N.; Sapountzi, V.; Prag, S.; Anilkumar, N.; Adams, J.C. Fascins, and their roles in cell structure and function. Bioessays 2002, 24, 350–361. [Google Scholar] [CrossRef]
- Ardelan, A.; Tsai, A.; Will, S.; McGuire, R.; Amarasekare, P. Increase in heat tolerance following a period of heat stress in a naturally occurring insect species. J. Anim. Ecol. 2023, 92, 2039–2051. [Google Scholar] [CrossRef] [PubMed]
- Dimauro, I.; Mercatelli, N.; Caporossi, D. Exercise-induced ROS in heat shock proteins response. Free Radic. Biol. Med. 2016, 98, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zhang-Akiyama, Q.M. Deficiency of Grx1 leads to high sensitivity of HeLaS3 cells to oxidative stress via excessive accumulation of intracellular oxidants including ROS. Free Radic. Res. 2020, 54, 585–605. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Moghaddam, S.H.H.; Du, X.; Zhong, B.X.; Chen, Y.Y. Comparative analysis on the expression of inducible HSPs in the silkworm, Bombyx mori. Mol. Biol. Rep. 2012, 39, 3915–3923. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yu, X.; Feng, Q. Fat body biology in the last decade. Annu. Rev. Entomol. 2019, 64, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhou, Z.; Zhang, H.; Chen, M.; Li, J.; Ma, Y.; Zhong, B. Shotgun proteomic analysis of the fat body during metamorphosis of domesticated silkworm (Bombyx mori). Amino Acids 2010, 38, 1333–1342. [Google Scholar] [CrossRef]
- Des Marteaux, L.E.; Štětina, T.; Koštál, V. Insect fat body cell morphology and response to cold stress is modulated by acclimation. J. Exp. Biol. 2018, 221, jeb189647. [Google Scholar] [CrossRef]




| Group | Tail Length (µm + SD) | Head DNA (%) | Tail DNA (%) | Olive Tail Moments |
|---|---|---|---|---|
| Control (37 °C) | 56.94 ± 11.49 a | 65.98 ± 8.78 a | 34.02 ± 8.78 a | 24.32 ± 6.63 a |
| Bmsn-OE (37 °C) | 34.42 ± 8.48 bbb | 75.64 ± 7.59 bbb | 24.36 ± 7.59 bbb | 12.64 ± 4.58 bbb |
| Control (27 °C) | 6.34 ± 5.51 ccc | 97.96 ± 3.42 ccc | 2.04 ± 3.42 ccc | 1.03 ± 1.49 ccc |
| Bmsn-OE (27 °C) | 5.26 ± 5.62 ccc | 98.36 ± 2.65 ccc | 1.64 ± 2.65 ccc | 0.82 ± 1.23 ccc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Li, C.; Yang, W.; Wu, Q.; Xiao, W.; Zhu, Y.; Wei, Q.; Dong, Z.; Zhang, G.; Lu, C.; et al. The Bombyx mori singed Gene Is Involved in the High-Temperature Resistance of Silkworms. Insects 2024, 15, 264. https://doi.org/10.3390/insects15040264
Liu Z, Li C, Yang W, Wu Q, Xiao W, Zhu Y, Wei Q, Dong Z, Zhang G, Lu C, et al. The Bombyx mori singed Gene Is Involved in the High-Temperature Resistance of Silkworms. Insects. 2024; 15(4):264. https://doi.org/10.3390/insects15040264
Chicago/Turabian StyleLiu, Zhenye, Cong Li, Wenyu Yang, Qiao Wu, Wenfu Xiao, Yan Zhu, Qiongqiong Wei, Zhanqi Dong, Guizheng Zhang, Cheng Lu, and et al. 2024. "The Bombyx mori singed Gene Is Involved in the High-Temperature Resistance of Silkworms" Insects 15, no. 4: 264. https://doi.org/10.3390/insects15040264
APA StyleLiu, Z., Li, C., Yang, W., Wu, Q., Xiao, W., Zhu, Y., Wei, Q., Dong, Z., Zhang, G., Lu, C., Pan, M., & Chen, P. (2024). The Bombyx mori singed Gene Is Involved in the High-Temperature Resistance of Silkworms. Insects, 15(4), 264. https://doi.org/10.3390/insects15040264