Simple Summary
The attraction of moths to light sources has long been used to reduce the damage of agricultural pests, starting in antiquity with controlled fires in the vicinity of crops. Coinciding with their development in the 18th century, light traps have been used to control pest populations by culling densities of adults lured to death at light sources (light trap-based mass trapping, or LTmt). Historically, the most extensive large-scale LTmt trials were conducted in the U.S.A. starting in the 1920s, validating in the process the hypothesis that crop damage can be reduced by removing egg-carrying females from the pest reproductive pool. Light-based mass trapping programs were phased out in the 1970s, coinciding with the implementation of pheromone-based pest management. With the advent of LEDs, solar power sources, and intelligent designs, recent years have seen an uptick of interest in LTmt, with the majority of contemporary studies conducted in Asia. As a rule, LTmt trials have been conducted exclusively in agricultural landscapes. A novel approach is proposed here to control epidemic populations of a tortricid forest pest, spruce budworm, in geographically isolated forests of balsam firs at a high risk of intense defoliation and mortality.
Abstract
The management of Lepidopteran pests with light traps (LTs) is often achieved by luring adults to death at light sources (light trap-based mass trapping, or LTmt). Large-scale LTmt programs against agricultural pests initiated in the late 1920s in the United States were phased out in the 1970s, coinciding with the rise of pheromone-based management research. The interest in LTmt has surged in recent years with the advent of light emitting diodes, solar power sources, and intelligent design. The first step in implementing LTmt is to identify a trapping design that maximizes the capture of target pests and minimizes the capture of non-target beneficial insects—with a cautionary note that high captures in LTs are not equivalent to the feasibility of mass trapping: the ultimate objective of LTmt is to protect crop plants from pest damage, not to trap adults. The captures of egg-carrying females in light traps have a greater impact on the efficiency of LTmt than the captures of males. When LTmt is defined as a harvesting procedure, the biomass of females in LTs may be viewed as the best estimator of the mass trapping yield; biomass proxy has universal application in LTmt as every living organism can be defined on a per weight basis. While research has largely focused on agricultural pests, an attempt is made here to conceptualize LTmt as a pest management strategy in forest ecosystems, using spruce budworm as a case study. The mass trapping of female budworms is impossible to achieve in endemic populations due to the large spatial scale of forest landscapes (implying the deployment of a prohibitively large number of LTs); in addition, ovipositing female budworms do not respond to light sources at a low density of conspecifics. The light-based mass trapping of female budworms may provide a realistic management option for geographically isolated forest stands heavily infested with budworms, as a tool to prevent tree mortality. Somehow unexpectedly, however, one factor obscuring the feasibility of LTmt is as follows: the complex (‘unknowable’) economic valuation of forest stands as opposed to agricultural landscapes.
1. Introduction
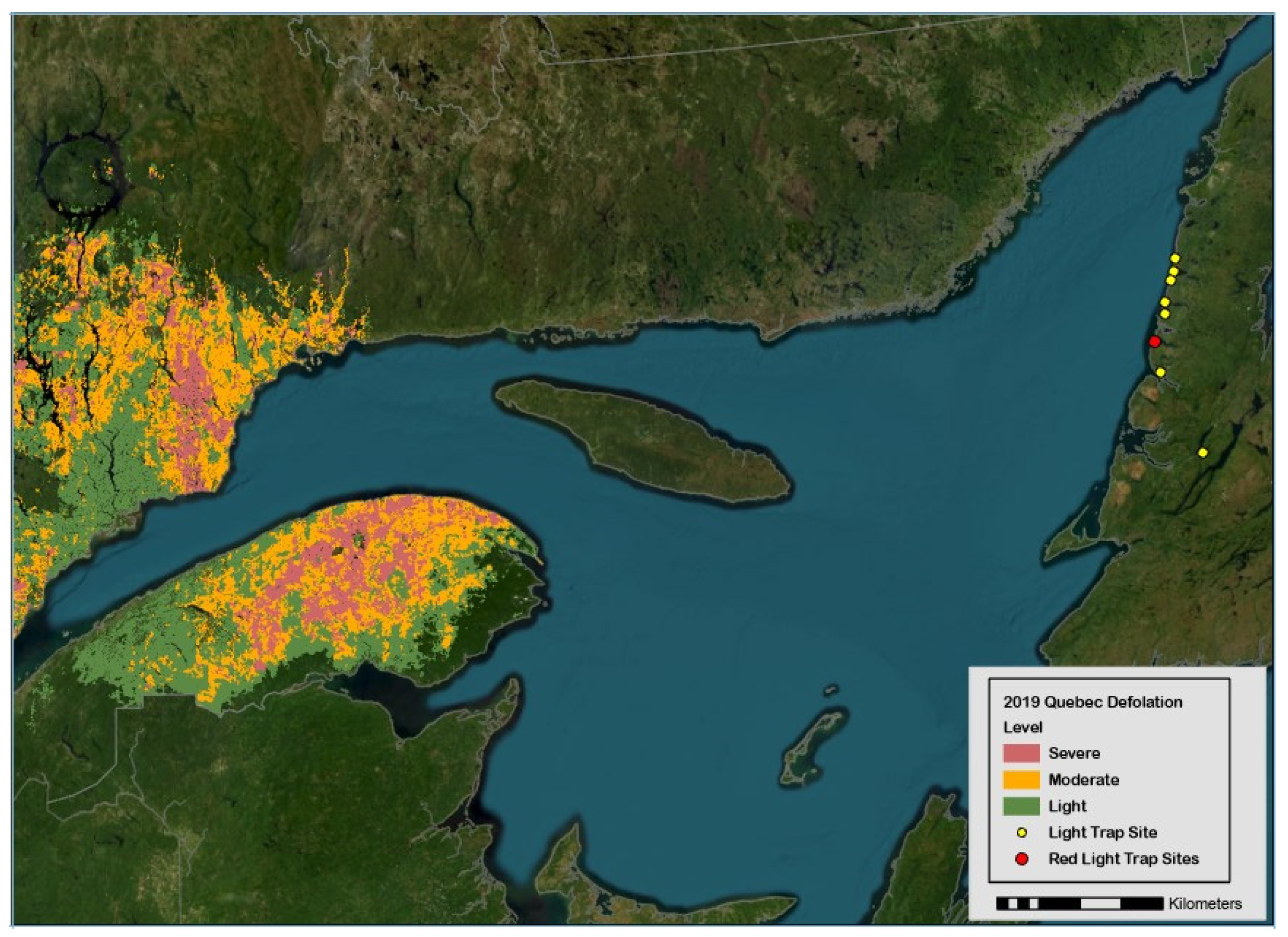
The spruce budworm, Choristoneura fumiferana Clem. (Lepidoptera: Tortricidae), is a severe epidemic defoliator of conifers [balsam fir, Abies balsamea, and spruce trees, Picea sp.] in eastern Canada and the northeast states in the U.S.A. [1,2,3]. Concomitant with the large-scale outbreak of budworms in the province of Québec, two massive immigrations of females were detected in light traps (LTs) deployed in Sally’s Cove in 2017 and 2018 (red dot in Figure 1), which led to a fortyfold two-year increase in the abundance of overwintering larvae from 0.2 to 8.6 individuals per branch [2] and nearly one million adults in 16 LTs in 2019 (Section 3). The initial research question at hand (the recognition of immigration patterns in LTs) was defeated by the a posteriori obvious reality that immigration is hard to discern in a high local density of adults. On the other hand, the high numerical abundance (106) in 2019 suggests that budworm populations could in theory be managed by luring females to death with LTs—noting that studies explicitly designed to validate mass trapping in agricultural settings rarely attain 106 individuals in LTs for any given year, even when hundreds of traps are deployed (Section 2). With some leap of faith, the rationale for mass trapping spruce budworms is inferred numerically.
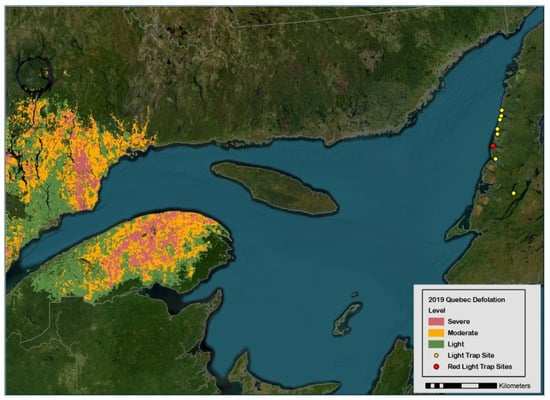
Figure 1.
Geographic location of forest sites on west coast of Newfoundland where adult spruce budworms were collected in light traps in 2019 (yellow dots, with red dot representing Sally’s Cove). Defoliated forest stands on the south–north shores of Saint Lawrence River are represented to highlight emigration pressures originating from budworm populations in province of Québec.
Unbeknown to many entomologists, the successful control of Lepidopteran agricultural pests is often achieved with LTs, either to suppress/eradicate isolated populations (the sustained deployment of LTs over multiple generations) [4,5,6] or reduce short-term pest damage by culling populations of reproductive adults (LT-based mass trapping per se, or LTmt). The commonality of LTmt is highlighted by the large number of field trials testing the approach [7,8,9,10,11], pest management guidelines in multiple crops [12,13,14,15,16,17], and recent deployment of 170 million LT units to control agricultural pests in China [18].
The research on LTmt was pioneered by American entomologists who tested (and often validated) the concept that plant damage caused by insect pests can be mitigated by mass trapping adults in LTs: starting in the late 1920s, field experiments carried out over tens of thousands ha led to the capture of several millions adult pests, yielding in the process a trove of quality data (Table 1; see [9] for a detailed review) [6,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68]. Large-scale LTmt trials were phased out in the early 1970s, coinciding with the rise of pheromone-based management research. The research output was low worldwide between 1975 and 2010 but steadily increased since; the geographic pole of research has shifted from the U.S.A. to Asia (Table 1).

Table 1.
Summary of field studies evaluating feasibility of light trap-based mass trapping in Lepidoptera, as determined by literature search in Google Scholar using combinations of following keywords (V: and/or): “light trap” V; “mass trap” V control, V pest, V Lepidoptera. Values of multi-year studies are averaged on per year basis: surface area, in ha; number of traps, or T; and number of adults captured, or np.
The main objective of this study is to explore the plausibility (what if?) of LTmt as a management tool for spruce budworm using two steps. (1) Based on a large (but not exhaustive) review of the literature on Lepidoptera, identify the parameters influencing the efficacy of LTmt (Ē) in multiple contexts. (2) Parameterize Ē in budworm, in particular a ‘novel’ yield proxy (biomass of trapped females) with universal application.
2. Parameters Influencing E
Based on the literature review including 51 relevant studies (Table 1), Ē was defined relative to the light trapping protocols, demography of pests, and economic impact of LTmt, as summarized in Table 2. Equations are provided to iterate processes involved in the implementation of LTmt; equation parameters are described in Table 3.

Table 2.
Parameters related to efficiency of LTmt. (1) Trap attributes: ratio of target pests versus beneficial insects (np/nb); height (hi) of LTs above ground. Demography of target pests: sex ratios of adults in LTs (n♀/n♂).

Table 3.
Parameters used to model efficacy of light trap-based mass trapping.
2.1. Light Trapping Protocols
The efficacy of LTmt relies on trap attributes that simultaneously maximize captures of target pests and minimize captures of beneficial insects. Notwithstanding monetary constraints, LTs should be deployed at a density (traps/ha) and height above ground that optimize Ē.
2.1.1. Trapping Design
One objective of LTmt is to identify a specific design (di, overall trap configuration including light attributes, power source, funnel, and collecting device) that maximizes the number of target pests in LTs (np, including ♂ and ♀):
Ēi = di for {np} (max)
In principle, trap i corresponding to {np} (max) is used in large-scale mass trapping trials.
The recent literature is pervaded by a circular assumption as to the identification of the design i being near equivalent to the efficacy of LTmt [19,25,26,29,31,36,37,38,41,69,70,71]. In reality, a high np does not imply a high Ē without accounting for the time-delayed effects of mass trapping on crop damage (Section 2.3).
The attraction/retention of adults in LTs often increase with trap size, i.e., the surface of the light source and volume of receptacles where trapped moths are collected [9,31,33,36,43,51,72,73]. Recent trends toward the miniaturization of LTs may thus impede the development of LTmt because small light sources attract relatively few adults and small receptacles rapidly saturate.
The development of novel LTs has blossomed in recent years (Table 1) with the advent of light emitting diodes, solar-powered energy sources, and intelligent designs [13,18,26,70,71,73,74,75], leading to a renewed interest in LTmt. The high rate of innovation in designing LTs for insect control is not problematic per se; after all, improvements in trap design have been an intrinsic part of LT development since early inception [9]. On the other hand, the high diversity of LT designs hampers comparisons between pests/studies hence the need for standardized protocols in plant protection programs [12,76,77,78].
The intractable issue of ever-increasing trap innovation is resolved here with a simple assumption as to LTs in different mass trapping studies being near equivalent between studies (‘a trap is a trap’) but obviously not within studies (Section 2.1.4). In any event, LT design may be largely irrelevant when captures at the trap level are more strongly impacted by environmental effects than trap attributes [79,80,81].
The mechanisms influencing the response of insects to light sources [circadian rhythms and photoperiod; the physiology of flight-to-light, including moon/abiotic/environmental effects; and interspecific variation in attraction to light attributes] have already been thoroughly reviewed [8,9,10,11,12,13] and deemed outside the scope of this study. In this context, variable captures in LTs with different light sources are not accounted for in Table 1 and Table 2, i.e., ‘a trap is a trap’.
2.1.2. Trap Specificity
Because many insect species across all major taxonomic orders are attracted to LTs [12,13,14,31,32,35,41,53,73,82,83], mass trapping programs are designed with light sources that predominantly capture key target pests and reduce the attraction of beneficial insects. The problem can be approximated with a redefined optimal trap design dj associated with the highest ratios of target pests to beneficial insects (np/nb):
Ēj = dj for {np/nb} (max)
Upper boundaries of Ēj exceeding three orders of magnitude have been reported (1000 individual pests for every beneficial insect) [8,18]. As a general trend among beneficial insects, predatory beetles and lacewings are most often captured in LTs [23,29,31,82]. While the notion of ‘friendly’ traps has long been on the mind of applied entomologists [49,53,63], the ratios of pests to beneficial insects were rarely recorded before 1980 (11% of studies) but have since become a mainstay of LTmt (48% of studies) (Table 2).
For simplicity, the underlying assumption below is that the optimal design ij is used in LTmt.
2.1.3. Density of Light Traps
Because the attraction of adult insects to LTs is usually short-range, the performance of LTmt is strongly dependent on the number of traps per unit area [7,8,9,15,47,49,84,85,86]. Parameter Ē, approximated as the cumulative number of pests in all LTs (Ʃnp, relative to the number of pests in individual traps np), is dependent on the density of traps (T, on a per ha basis), behavioral/functional response of adults to multiple traps (k), and monetary constraints ($) as to the realistic number of traps to be deployed.
ĒT = T × k for {Ʃnp/$} (max)
Coefficient k is either 1 when np is independent of T, <1 when np declines with T, or >1 when np increases with T—with a cautionary note that [to the author’s knowledge] rigorous estimates of k are not available for any species targeted by LTmt. In a figurative sense, the deployment of multiple ‘illumination’ traps high above the plant canopy may be viewed as a single large source of light interfering with the reproductive activities of females [20,49,63,84,85] rather than mass trapping per se. In the absence of monetary constraints (the number of trapped pests per $), the optimal resolution of Equation (3) might involve a near infinite number of traps to be deployed.
Indirect approaches to infer k include variation in the number of light sources per trap [21,33,78,85] and a variable distance between the traps/number of traps per hectare, in order to evaluate the attraction range of individual traps [15,21,23,30,45,50,86]. The issue is further complexified when males and females exhibit a distinct response to trap density [87].
2.1.4. Position of Traps
The spatial position of LTs influences the level of captures. For example, large-scale LTmt (>104 ha in some instances; Table 1) often includes traps deployed outside treated areas (control traps) to assess Ē (Section 2.3).
The height deployment of traps (hi, ranging between the ground level and tower-mounted traps tens of m high) also influences captures in LTs. Overall, nine studies investigated the effects of hi on LTmt (six before 1980 and three after) (Table 2). In theory, traps should be deployed at a height that maximizes np [10,11,15,51]. In practice, however, the magnitude and directionality of height effects are difficult to generalize as they often vary with the plant growth stage [20,23,31,44].
2.2. Demography of Target Pests
The feasibility of LTmt has been investigated in 13 families of Lepidoptera, most notably Noctuidae (12 species), Crambidae (5 species), and Gelechiidae–Geometridae–Tortricidae (3 species each). In total, >30 million adult pests were killed in LTs in these studies (including references in [9]), ranging four orders of magnitude (102 to 106 individuals) between studies (Table 1).
2.2.1. Numerical Estimates of Source Populations
From a strict perspective, mass trapping efficiency can be rigorously evaluated if, and only if, an estimate of the local pest abundance is known—so that the proportion of insects successfully mass trapped can be inferred.
Ω = Ʃnp/Np
Parameter Ω, defined as the ratio between Ʃnp and the local density of adults (Np), is positively correlated with Ē [5,8,88,89]. Estimates of Ω may reach 98% indoors (apple storage rooms [9]), but precise field values are usually not available in LTmt studies.
Tools available to approximate Ω include estimates of pest abundance before the emergence of adults, modeling seasonal flight patterns in LTs, and the mark–release of adults [4,8,9,37,47,48,51,70].
2.2.2. Conspecific Density Effects
It is often assumed that LTmt is most effective at a low population density, in part based on the following statement: “trapping 80% of a population of 10,000 would have much greater effect than trapping 80% of a population of 1,000,000” [8]. While the argument is intuitively sound, it assumes that captures in LTs are proportional to the local population density, i.e., a constant proportion c of adults is captured in traps independently of density:
np = Np/c for Ω ┴ Np
In natural conditions, however, Ω is expected to exhibit strong density dependence: attraction to LTs reflects both the abundance and dispersal movements of adults, the latter of which often increases with population density [90,91,92]. Assuming positive correlations between Ω and Np (as opposed to a density-independent scenario Ω ┴ Np), the original statement above becomes the following: “trapping 80% of a population of 10,000 may have much lower effect than trapping >>> 80% of a population of 1,000,000”—implying that LTmt is, in some circumstances, more effective at controlling epidemic populations than endemic populations (Section 3.2.2).
2.2.3. Sex Ratio
Light traps capture adults of both sexes, and ‘female removal’ is often mentioned as the primary objective of LTmt [4,8,9,15,28,32,39,45,52,59,60,89]. The mass trapping of males per se is unlikely to suppress female mating success to a near-zero level because a single male can inseminate several female partners during its lifetime [93,94,95].
Captures of egg-carrying females in LTs are thus expected to increase the efficacy of LTmt by factor S relative to captures of sperm-carrying males:
Ē♀ = Ē♂/S
Assuming that S is large enough, the abundance of males in LTs becomes largely inconsequential. If valid, long-held views that LTmt prioritizes female targets may imply the recalibration of all equations above based on n♀ (as opposed to np including both sexes).
2.2.4. Average and Residual Fecundity of Females in LTs
The feasibility of LTmt can be assessed by dissecting females in LTs to assess the mating status (the presence/absence of spermatophore) and residual fecundity (eggs in the abdomen of females relative to a full complement at emergence) [22,39,45,52]:
Ēegg = n♀ × (F − L)
For any given species, F and L represent the average fecundity and proportion of eggs laid by individual females at capture. Virgin/young gravid females with a near-full egg complement are the prime targets of LTmt; in contrast, captures of old spent females with few eggs in the abdomen are largely irrelevant.
Residual fecundity was commonly reported during the early phase of LTmt (29% of studies) but not once since 1980 (references in Table 2); these measurements are useful to infer whether mass trapping is a viable option for any pest/crop association. Integrating residual fecundity into Equation (7) remains challenging, however, both logistically (the time-consuming nature of female dissection measurements) and analytically (the intraspecific variation in female body size positively correlates with fecundity in many Lepidopteran species, an effect never accounted for in LTmt studies).
2.2.5. Body Mass of Females in LTs
The issue above can be simplified in Lepidopteran pest species with non-feeding females by noting that initial weight is set at emergence and monotonically declines over time as eggs are laid [96,97,98]. The cumulative weights of n females in LTs (W♀) ‘recapitulate’ the effects of body size and age on the reproductive condition at capture, e.g., large/young females are heavier than small/old females.
ĒW = W♀
Estimates of mass trapping efficiency are deemed more accurate when expressed in terms of the biomass of females than other proxies (ĒW > Ēegg > Ē♀ > Ē♀,♂). The widespread adoption of ĒW may be constrained by the prevailing mindset focusing on the numerical abundance of adults in LTs; only three studies in Table 1 report the weight of adults in LTs, none of which differentiated between males and females [23,36,46].
2.3. Beneficial Impact of LTmt on Crop Plants
Major pests of economically important crop plants have always been prioritized in LTmt studies. Before the 1980s, American pioneers of LTmt focused on Lepidopteran pests of apple, cotton, corn, lettuce, and tobacco. Crops targeted by LTmt have diversified since then, now including cabbage, eggplant, eucalyptus, legumes, nuts, olive, onion, palm fruits, sugarcane, tea, and tomato (Table 1).
Based on budget itemizations available in some mass trapping studies [18,24,26,39,41,73,99], a simple approach is proposed whereby the incremental yield of crop plants in plots treated with LTs (Y = YLTmt – Ycontrol, in $) is assumed to be dependent on a single factor:
Y ≈ $/W♀
In principle, LTmt can be recommended when the cost ($/W♀) is low.
Parameters related to Y were assessed in the majority of studies in Table 2 (>60% before and after 1980), including the density of eggs/larvae in offspring generations, proportion of damaged plants, yield estimates, and reduction in the number of insecticide applications (references in Table 2). The efficacy of LTmt is often distance-dependent: crop damage is the highest further away from mass trapping plots [21,24,26,41,59,63]. Some mass trapping studies did not record adult abundance, focusing instead on measurable consequences of LTmt on Y [21,24,53]. The numbers of mass-trapped females do not need to be recorded to assess the benefit of mass trapping, provided that the strong attraction of target pests to traps deployed in LTmt is demonstrated.
Somehow surprisingly, the comparison of plant damage in the control versus light-treated plots suggests near-universal positive effects of LTmt on Y (YLTmt > Ycontrol) (references in Table 2; see also [8,9,39]) with two caveats. (1) Publication bias may lead to an artificially high rate of LTmt success if studies failing to document reduced plant damage are unlikely to be published or to report said measurements. (2) The monetary benefit associated with the yield increment is often dwarfed by the cost of mass trapping [8,20,21,60], in which case LTmt does not provide effective pest control.
3. Spruce Budworms at Light Traps: Experimental Data
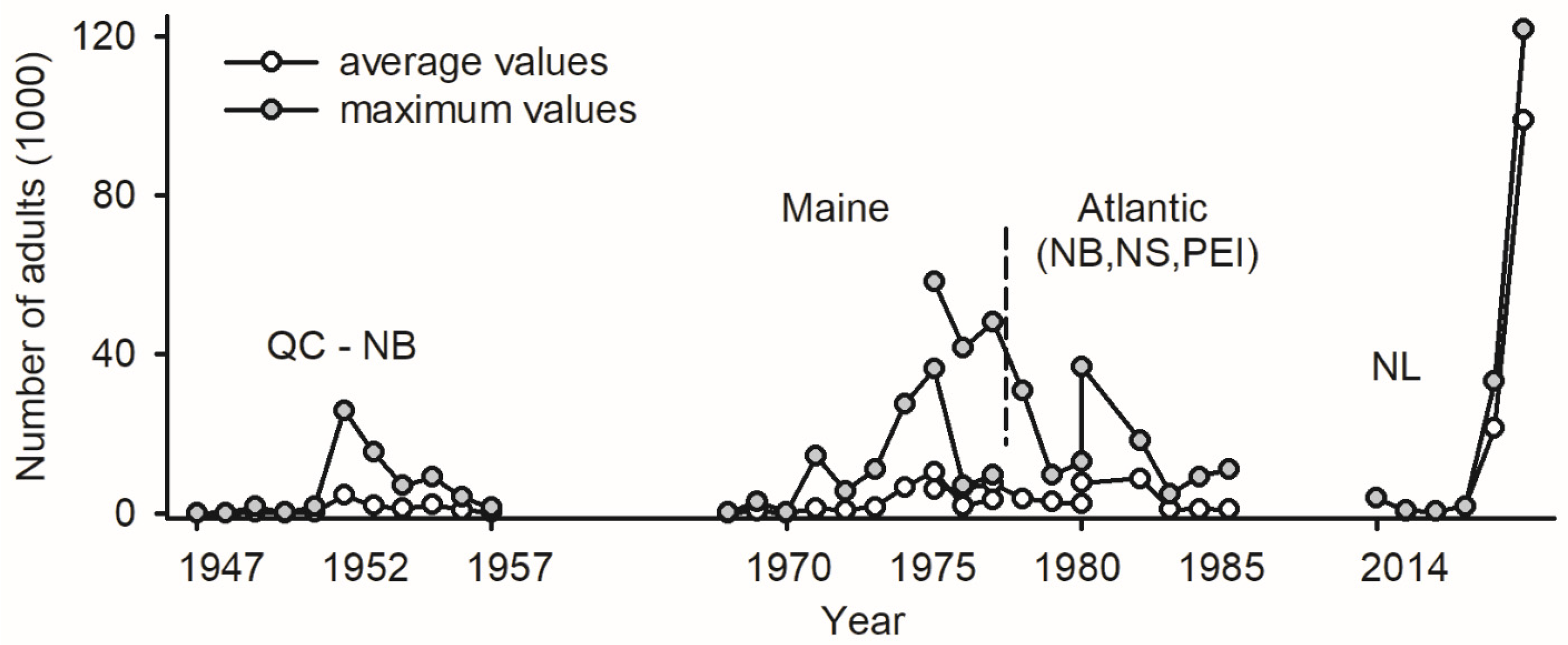
The deployment of LTs as a monitoring tool for budworms nearly coincided with the first report of a positive phototactic reaction in adults [100]: traps were deployed in multiple locations in Québec and New Brunswick between 1945 and 1957 (4–26 sites each year) to record the abundance of unsexed specimens [101]. The data for each site/year included short time series of abundance with at least ten consecutive days; in total, >250,000 adult budworms were captured in LTs (Figure 2). Due to the age of the data, the attributes of LTs are unknown.
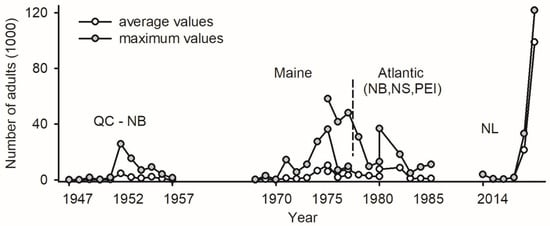
Figure 2.
Abundance of adult spruce budworms in light traps in Canadian provinces of Québec and New Brunswick between 1947 and 1957 [101], in state of Maine between 1968 and 1977 [102,103], in Atlantic Canada between 1976 and 1986 (provinces of New Brunswick, Nova Scotia, and Prince Edward Island) [104], and on west coast of Newfoundland (Sally’s Cove) between 2014 and 2019 [2].
The monitoring of adult budworms with LTs was implemented in Maine (1968–1977) and Atlantic Canada (1976–1986), with 6 to 19 sites sampled each year for the two jurisdictions (the trap design, location of sites, and raw data in [102,103,104]). The abundance of unsexed budworms in LTs was recorded every day for the entire flight season in each site/year, providing reference material for future phenology studies. In total, >350,000 budworms were captured in Maine and >750,000 in Atlantic Canada (Figure 2).
Light trapping conducted at Sally’s Cove on the west coast of Newfoundland (NL) between 2014 and 2019 yielded > 1,200,000 sexed budworms, the majority of which (>80%) occurred in 2019 (Figure 2). A historical increase in the abundance of budworms in LTs on a per trap basis over 70 years (maximal values of 26,000 budworms in 1952, 36,000 in Maine in 1975, 58,000 in Atlantic Canada in 1976, and 120,000 in NL in 2019) likely reflects increasingly efficient trap designs (Figure 2). To the author’s knowledge, the number of adults in LTs represents the only proxy of budworm abundance available for the last three outbreaks.
3.1. Light Trapping Protocols
Sixteen stainless steel vane LTs (Leptraps, Georgetown, KY, USA) with a 15 W white neon tube as a light source and powered by marine batteries were used to capture adult budworms in forest stands dominated by balsam fir at eight locations in central Western Newfoundland in 2019 (Figure 1, Annex I, Supplementary Materials; Ref. [2,96,105]). A summary of trap captures in the 16 LTs in 2019 is provided in Table 4.

Table 4.
Demographic parameters of adult spruce budworms captured in light traps deployed in west coast of Newfoundland in 2019 (Figure 1). One trap was deployed inland in Pasadena (PAS), 40 km from coast. Other 15 traps were deployed within 3 km from coast, either within Gros Morne National Park [Rocky Harbor (RH), Sally’s Cove (SC), and Cowhead (CH) or north of Gros Morne [Three Mile Road (TMR), Arches (ARC), Portland Creek (PC), and Daniel Harbor (DH)] (Figure 1). Average date of capture is expressed as number of days after June 30th. Average parameter values (Table 3) are as follows on per trap basis. I. Abundance: number and fresh biomass of budworms (np and wp), weight of bycatch (wb: all non-budworms in light traps). II. Seasonality: average date of capture for n♂, n♀. III. Sex ratio {n♀/(n♂ + n♀)}. IV. Protandry: average date of flight of ♀—average date of flight of ♂.
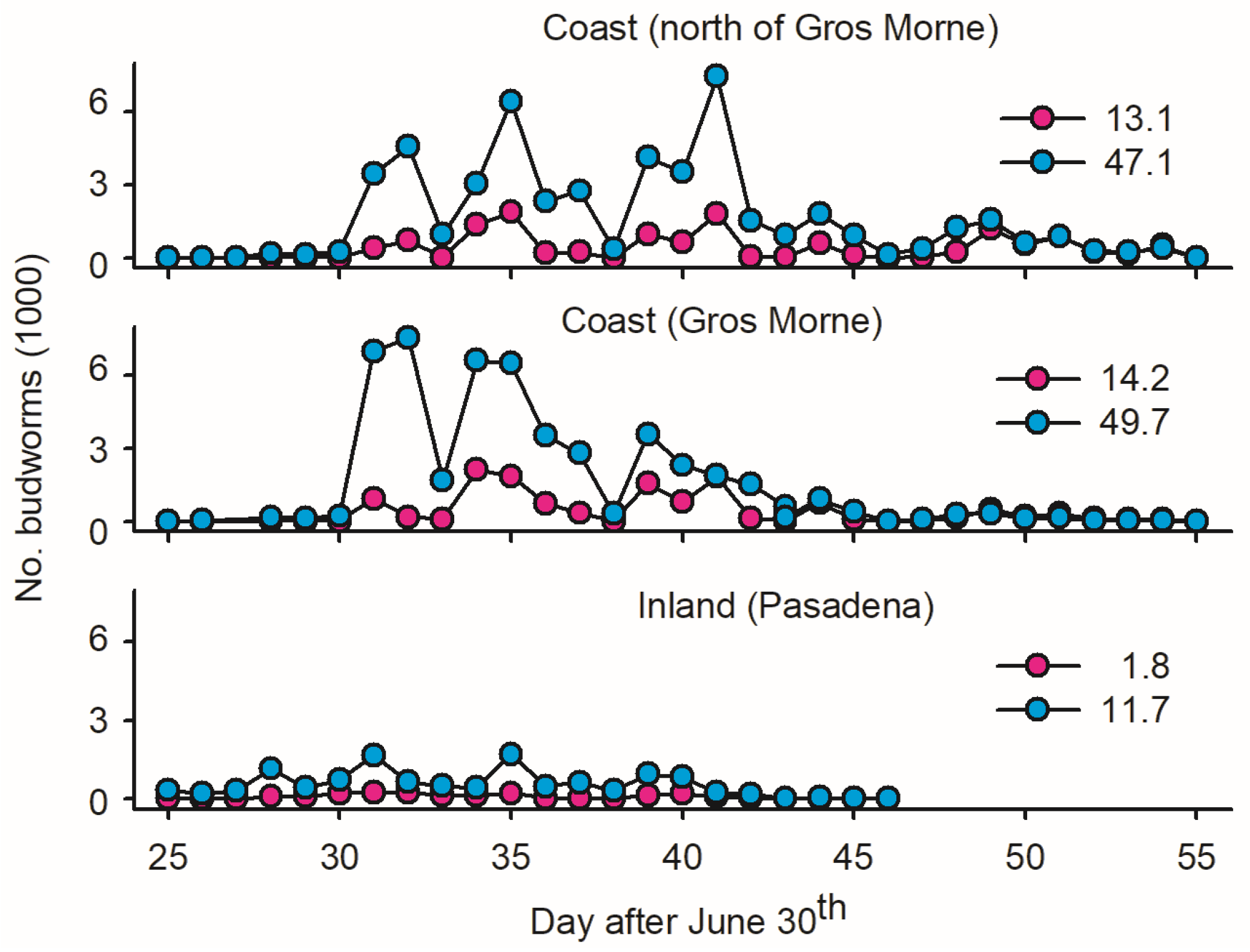
One ‘inland trap’ in Pasadena, 40 km from the coast, served as the control to evaluate the geographic variation in mass trapping feasibility. The data suggest a low Ē inland in terms of females per trap (eight-times-lower estimates than on the coast; Figure 3) and a high incidence of non-target insects (Section 2.1.2).
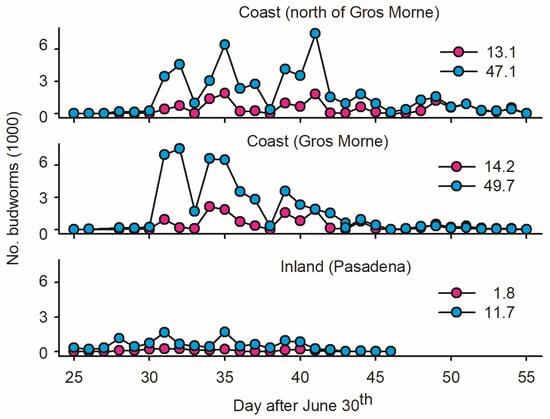
Figure 3.
Average number of male and female spruce budworms (pink and blue dots, respectively, averaged on per light trap basis) at three locations on west coast of Newfoundland: (1) one inland light trap in Pasadena; (2) twelve light traps deployed in coastal forests within Gros Morne National Park; and (3) five light traps deployed in coastal forest north of Gros Morne. Location of sites as depicted in Figure 1.
3.1.1. Trapping Design
The traps used in this study performed well relative to LTs deployed in previous outbreaks (Figure 2). Of all trap attributes i corresponding to the design di used here (light source li, funnel fi, volume vi; Table 3), the latter (the small volume of the collecting bucket below the funnel; v = 10.5 l) may have constrained the captures of budworms to the largest extent: LTs appeared to saturate when the abundance/biomass of budworms exceeded 16,000 individuals/160 g on a fresh weight basis (Annex I, Supplementary Materials). The effect of v on the efficiency of LTmt can be assessed as follows:
Ēi = vi for {np} (max)
Sampling all traps every day, as conducted in 2019, would be prohibitively costly in an operational LTmt program. In particular, the problem of trap saturation becomes more stringent if traps are emptied every nth day as opposed to every day. Trap capacity vi may be increased by retrofitting LT on 200 l barrel drums (Figure 2 in [39]).
3.1.2. Trap Specificity: Bygone Ghost of Bycatch
Resolving the issue of bycatch (the sum of non-target insects captured in LTs) is critical to implement LTmt: separating targets from non-targets is labor-intensive and hazardous (exposure to insect allergens and decomposition volatiles, mostly if LTs are left uncollected for several days).
Surprisingly, the LTmt literature rarely mentions the term ‘bycatch’. Issues of bycatch may have become irrelevant with the development of ‘friendly’ traps or with the detailed records of abundance for multiple taxonomic groups/species in LTs (references in Table 2); without explicit statements by the authors, it is hard to conclude.
Discarding bycatch in LTs, either physically or conceptually, is unfortunate. The parameterization of the LTmt efficiency in terms of biomass provides a simple direct comparison of target (p) versus non-target insects (←p):
Ēj = dj for {wp/w¬p } (max)
The ratio of target/non-target biomass is highly relevant to LTmt in budworms, with broadly similar bycatch constraints to the fishing industry [106,107,108].
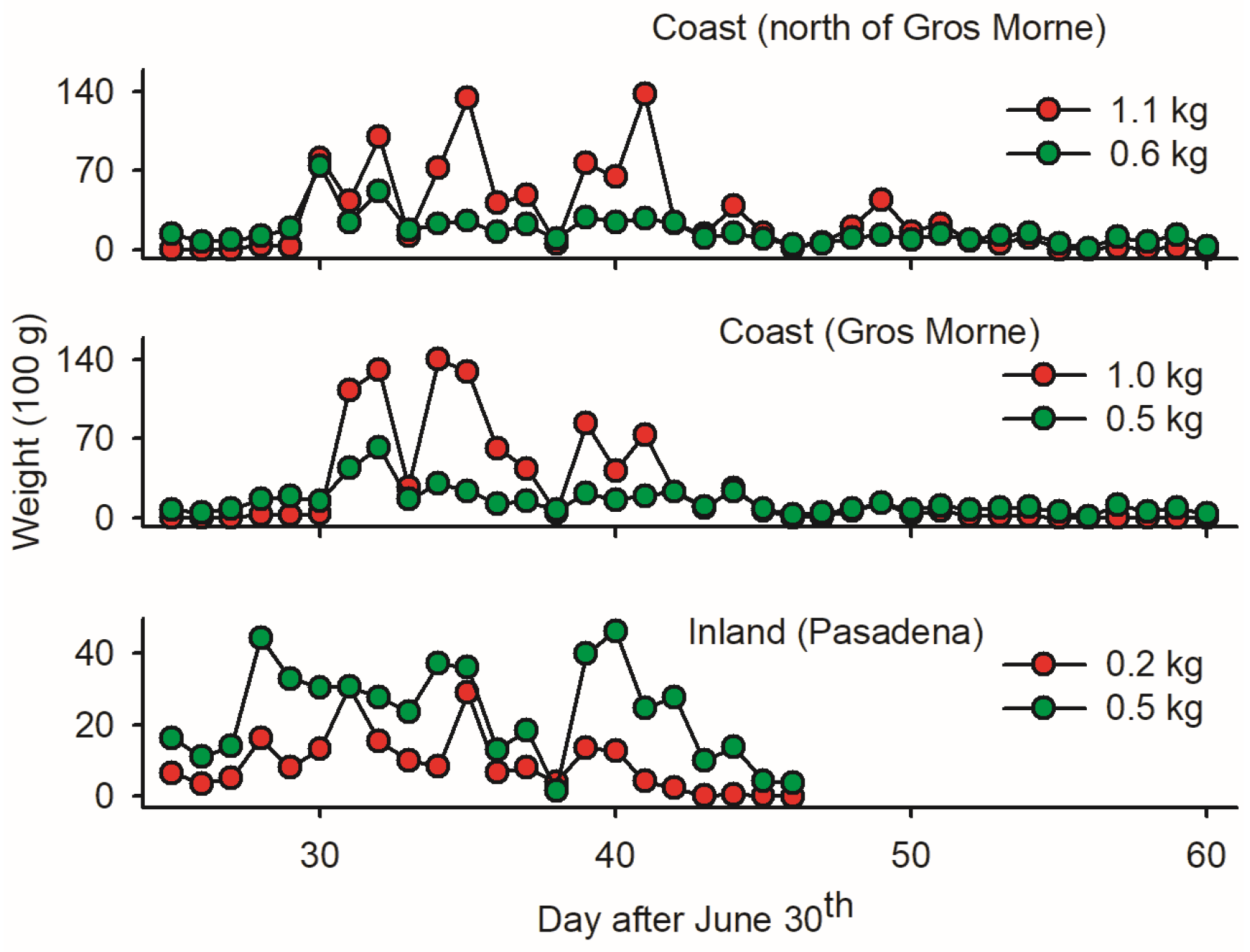
The low ratio of budworm biomass relative to non-targets in LTs in Pasadena (0.2 versus 0.5 kg; Figure 4) implies that inland mass trapping is not sound due to the substantial removal of beneficial insects. In coastal sites, in contrast, the mass of budworms was two times larger than the mass of non-targets (Figure 4)—suggesting a ‘limited’ non-target cost of LTmt in budworms. Based on wp/wb ratios, the first ten days of August provided optimal timing for mass trapping budworms.
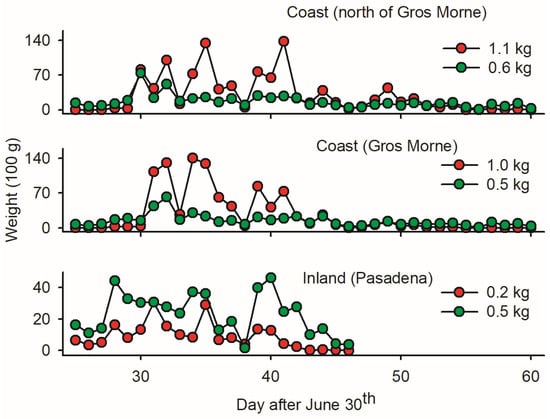
Figure 4.
Average cumulative fresh biomass of adult spruce budworms and bycatch (red and green dots, respectively, averaged on per light trap basis) at three locations on west coast of Newfoundland: (1) one inland light trap in Pasadena; (2) twelve light traps deployed in coastal forests within Gros Morne National Park; and (3) five light traps deployed in coastal forest north of Gros Morne. Location of sites as depicted in Figure 1.
3.1.3. Density of Light Traps
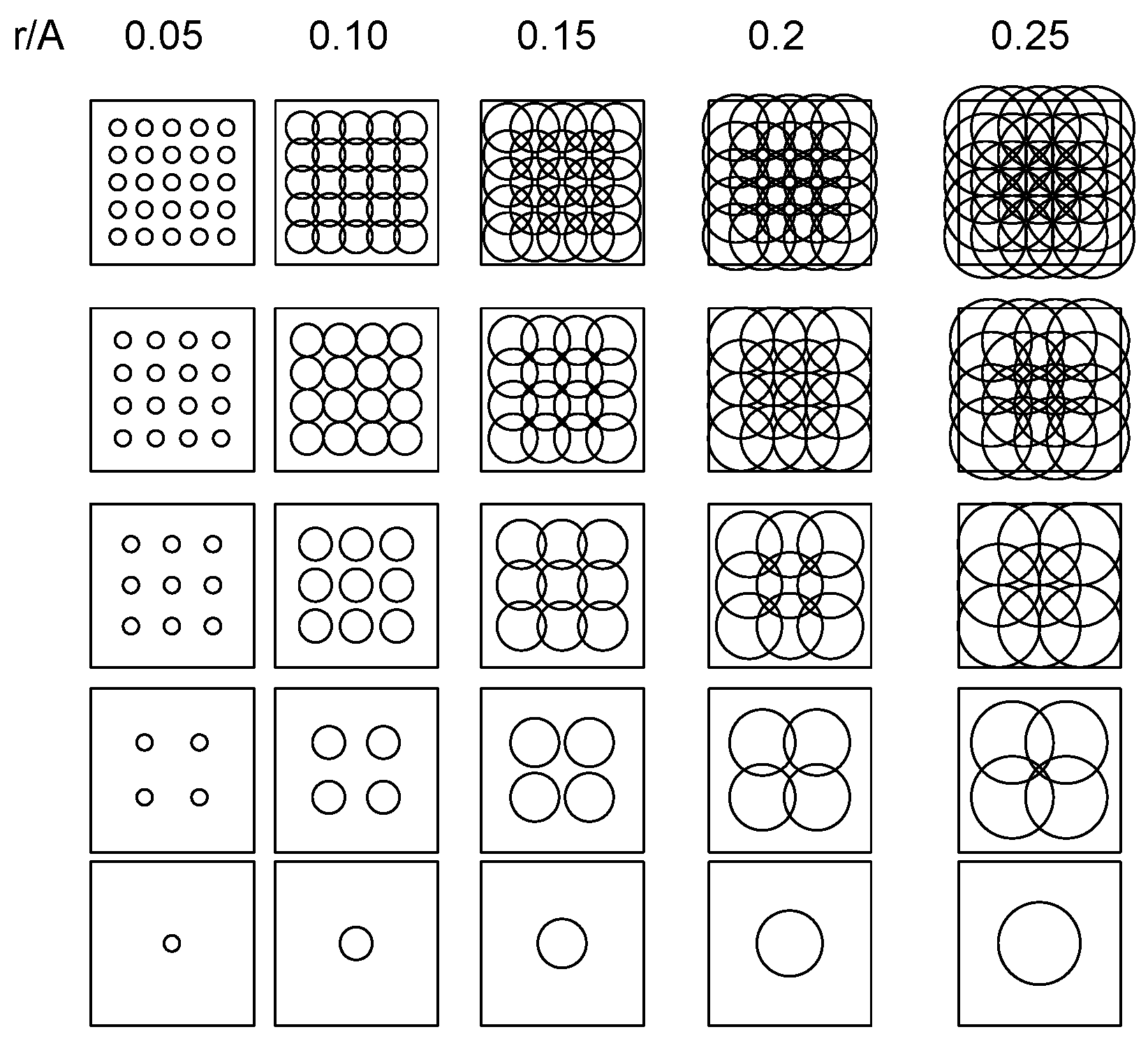
The estimates of k are expected to fluctuate non-linearly with T, i.e., differential responses of adults exposed to 1–5 traps/ha versus 5 to 25 traps/ha. The problem is illustrated using relationships between the range of the attraction (r, in m) of omnidirectional LTs (360° light irradiation) and the number of resident adults available for trapping (Np):
Np = Π (r/2) 2
The range of attraction to LTs in Lepidoptera generally varies between a few meters and about 150 m [109,110,111], although estimates as high as 3 km have been reported [47].
For any grid of T traps evenly deployed within a square area A2 (T = 1, 4, 9, 16, and 25 traps in the example here), np is expected to strongly increase with T when r <<< A (left plots in Figure 5); as r increases relative to A, np becomes independent of T due to the overlapping attraction between traps (right plots in Figure 5).
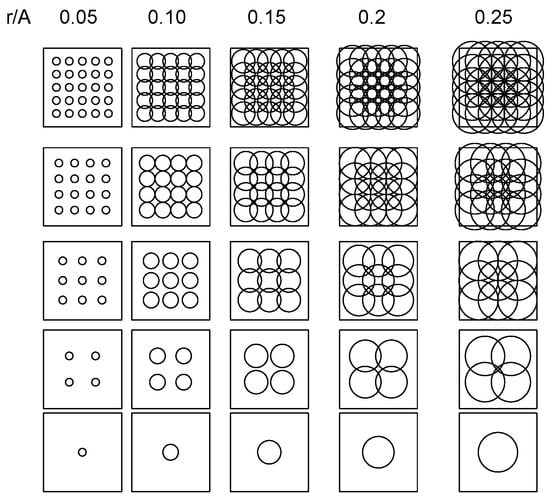
Figure 5.
Relationships between surface area illuminated by variable number of light traps (T = 1, 4, 9, 16, and 25) deployed in evenly spaced grids within square plot with dimension A2, as resolved for different attraction ranges of light sources (r in m, ranging between r/A = 0.05 and 0.25).
3.1.4. Position of Traps
The vertical distribution of flight in insects usually ranges between 0.2 and 3.8 m above ground [112]. Light traps used to attract budworms are either deployed in the upper canopy of host trees > 5 m high [113,114] or (as in this study) on low branches 2.5 m above ground [2,105,115]. Oftentimes, the vertical position of traps is not reported [101,102,103,104], and no direct comparison of budworm abundance in relation to the height of LTs is available.
3.2. Demography of Spruce Budworms in Light Traps
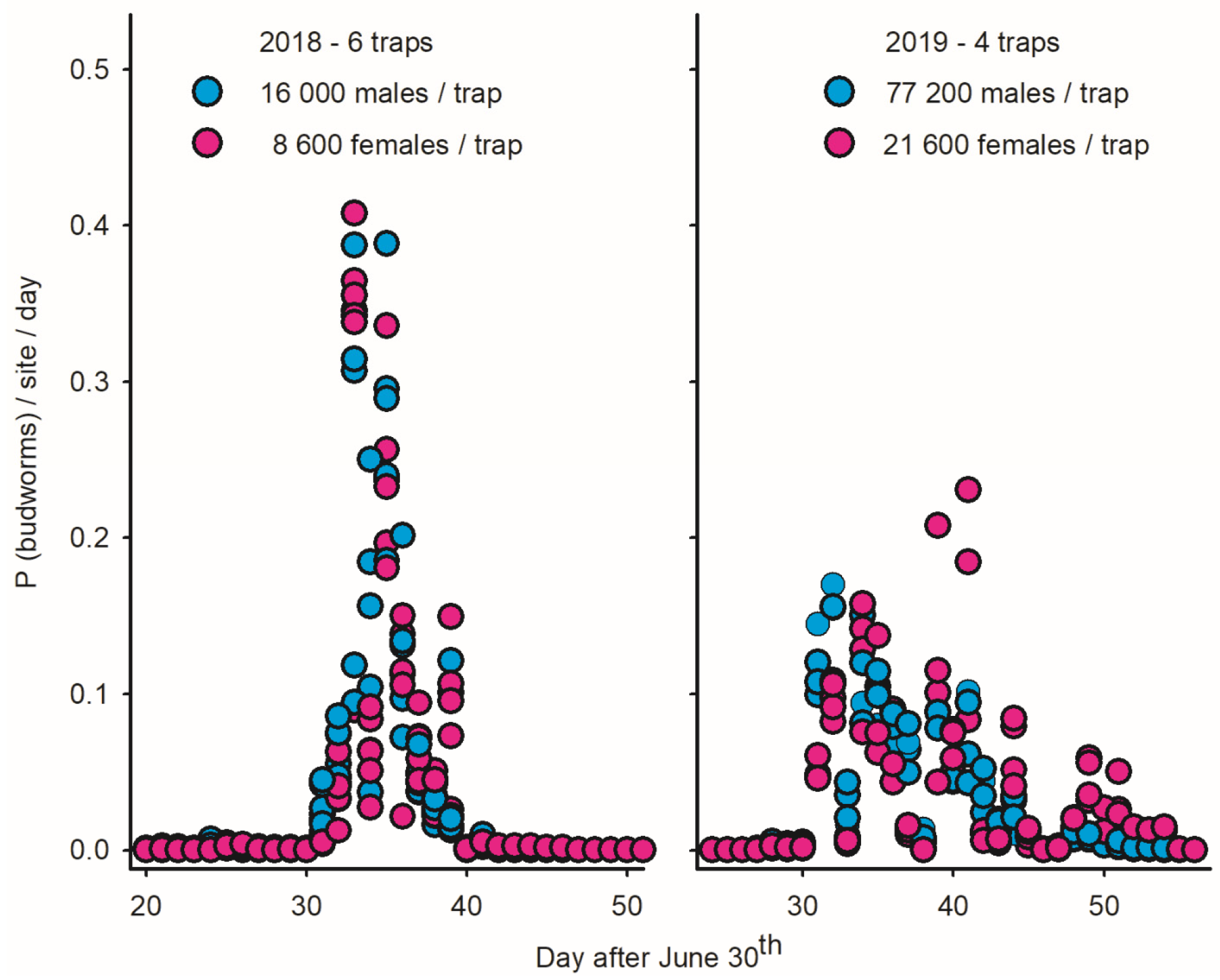
Because immigrations are pulsed in time and include both males and females, they are characterized by specific patterns in LTs: (1) a large increase in the abundance of males and females from one night to the next; (2) high numbers of budworms following immigration due to the post-migration longevity of adults; (3) strong phenological synchrony among traps when the inter-trap distance is small relative to the spatial scale of the immigration event; and (4) a similar seasonal timing of flight for males and females when immigrants >>> residents (as opposed to protandrous flight in closed populations with residents >>> immigrants) [2]. The signature traits of immigration in LTs are illustrated for Sally’s Cove in 2018 (left plot in Figure 6).
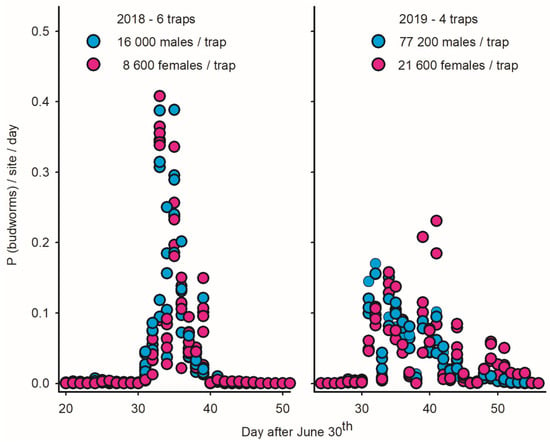
Figure 6.
Proportion of male/female spruce budworms captured on daily basis in light traps deployed in Sally’s Cove on west coast of Newfoundland in 2018 (N = 6 traps) and 2019 (N = 4 traps). (Left) Immigration in SC in late July–early August 2018 is inferred based on strong synchrony of captures in light traps for both males and females [2]. (Right) Strong variation in phenology between traps/sexes in 2019 suggests that immigration was indiscernible due to high population density of resident adult budworms.
While the abundance of budworms in SC increased fourfold between 2018 and 2019 (25,000 to 100,000 individuals per trap), the flight patterns observed in LTs in SC in 2019 (as well as other coastal sites) were inconsistent with the scenarios of immigration (right plot in Figure 6), in particular a lack of phenological synchrony between traps and significant protandry in 15 of the 16 LTs (right plot of Figure 6; Table 4). These observations do not imply that immigration did not take place in 2019 but rather that they were indiscernible due to the high local density of residents.
3.2.1. Numerical Estimates of Source Populations
The intensity of immigration in budworms (the number of females per ha) can reach 105 [116] and possibly >107 when accounting for three-dimensional swarms of budworms in the troposphere (Figure 5 in [117]).
In closed populations with limited immigration, estimates of Np range between 103 and 105 adults per ha in endemic and epidemic populations [118]. Considering that budworms emerge over a 2–3-week period in July–August [2,96,105], the number of budworms available for trapping on any given night is assumed to be < 104 adults/ha when computing the efficiency of LTmt (Ω):
Ω = np/104 ha−1
By definition, values of np exceeding 104 adults/ha (corresponding to Ω > 1) are assumed de facto indicative of immigration.
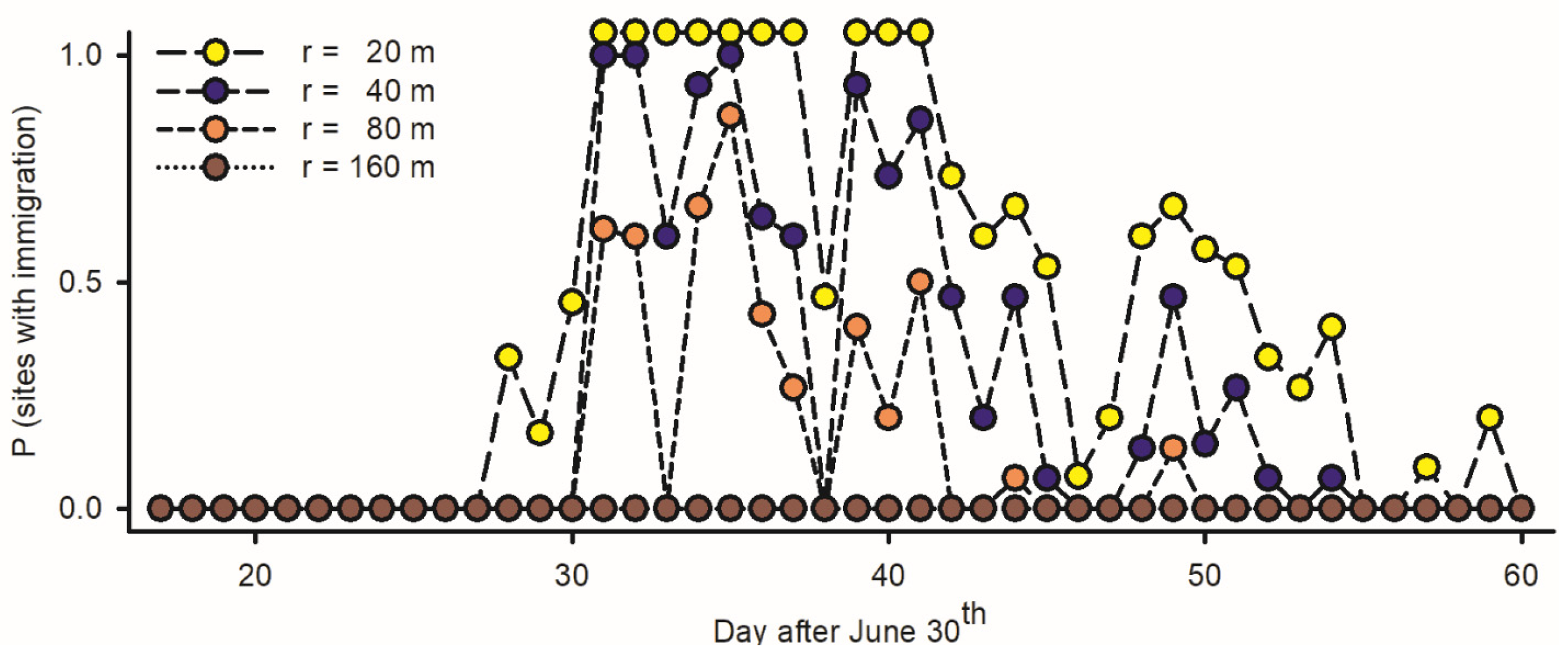
An inference as to the incidence/non-incidence of immigration is dependent on the range of the attraction of LTs (r, in m): the numbers of budworms available for light trapping per night (Equation (6)) range between Np = 315 adults/ha if r = 20 m, Np = 1258 if r = 40 m, Np = 5034 if r = 80 m, and Np = 22,650 if r = 160 m. The incidence of immigration in different traps (the number of nights with np > Np) is inversely proportional to the range of attraction: (1) if r is low (20 m), immigrations are assumed to have taken place in all traps during ten nights between 31 July and 10 August and in most traps thereafter; (2) if r is intermediate (40–80 m), immigrations took place at all traps during a few nights and at some traps most nights; and (3) if r is large (160 m), immigration may not have taken place at all during the interval of study (Figure 7). Observations at night suggest r > 20 m in budworms.
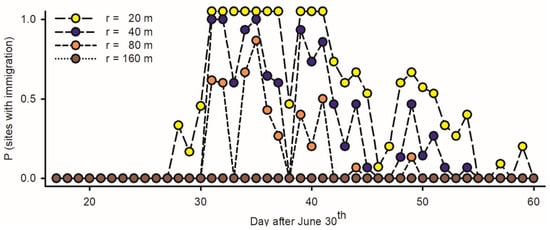
Figure 7.
Estimated rate of immigration among adult spruce budworms at fifteen light traps deployed in west coast of Newfoundland in 2019, based on number of specimens at individual traps (np) relative to maximal density of inflight resident moths on any given night (Np = 104 adults per ha) [incidence of immigration (II) for any given night at each light trap defined as P (np > Np)]. Because captures of budworms are dependent on range of attraction of light traps (Equation (6): r, in m), rates of immigration were computed with different values of r each corresponding to specific number of resident adults available for trapping each night.
3.2.2. Conspecific Density Effects
The sex-specific density responses of adult budworms in LTs [119] can be summarized as follows: (1) captures of males are more or less proportional to the local population density with constant proportion c captured in traps (linear response) and (2) females are rarely captured in LTs when the density is low, as approximated with exponential parameter e:
n♂ = N♂/c, with Ω ┴ N♂
n♀= N♀ e, with Ω ┬ N♀
While neither c nor e is known, Equations (14) and (15) are biologically sound when contrasting high (density-independent) dispersal activity among promiscuous mate-seeking males resulting in high captures in LTs (Ω ┴ N♂) relative to coy egg-laying females not responding to LTs unless foliage resources become depleted (Ω ┬ N♀) [90,91,92,120,121,122].
3.2.3. Sex Ratio
The sex ratio in resident budworm populations is approximately 1:1 as determined by field collections of pupae or adults sampled on host trees (either with sweep nets or by fogging trees with insecticides) [113,119,123]. As is generally the rule in moths [124,125,126], the sex ratios of budworms in LTs are most often male-biased [2,96,105,117,119].
The early emergence of males relative to females (protandry) is ubiquitous in moths [127,128] including budworms: protandrous flight patterns were detected at 15 of the 16 LTs, with males flying 2.3 days before females on average (Table 3).
Considering that the vast majority of females in feral populations of budworm are mated, as referenced in [129], parameter S in Equation (6) is assumed high enough that only the capture of females influences Ē. In principle, early season trapping consisting of mostly males does not contribute to LTmt.
3.2.4. Average and Residual Fecundity of Female Spruce Budworms in LTs
Female budworms attract males for mating with sex pheromones released shortly after emergence and thereafter lay eggs in a batch on the foliage of host trees; fecundity averages 200 eggs per female [118,123,130,131]. Female budworms exhibit an unusual reproductive strategy (inter-reproductive migrations) with sequential sedentary–dispersive phases [113,117,132]. I. Young gravid females are in principle incapable to sustain flight/migrate due to a heavy abdomen full of eggs and thus assumed not available for light trapping. II. After having laid approximately 50% of their eggs, partly spent females readily disperse and fly to LTs, especially so at a high density of conspecifics [133,134,135].
The prime targets of LTmt are young females with a near-full egg complement, as opposed to nearly spent females with <50% eggs remaining in the abdomen. Inasmuch, a physical impossibility to capture near-gravid female budworms in LTs (immutable physiological flight constraint related to wing load) would be extremely detrimental to LTmt. Virgin females are observed in LTs in some conditions, however, as in populations with extreme female-biased sex ratios following the extermination of early season males with DDT [136]. Egg dumping, a common behavior in virgin female budworms, may have evolved as a strategy to facilitate flight take-off [137,138]. In addition, a dispersing morph of females in depleted forest stands (large wings and low body mass) readily fly to LTs after having laid ca. 25% of their egg complement [133,139,140].
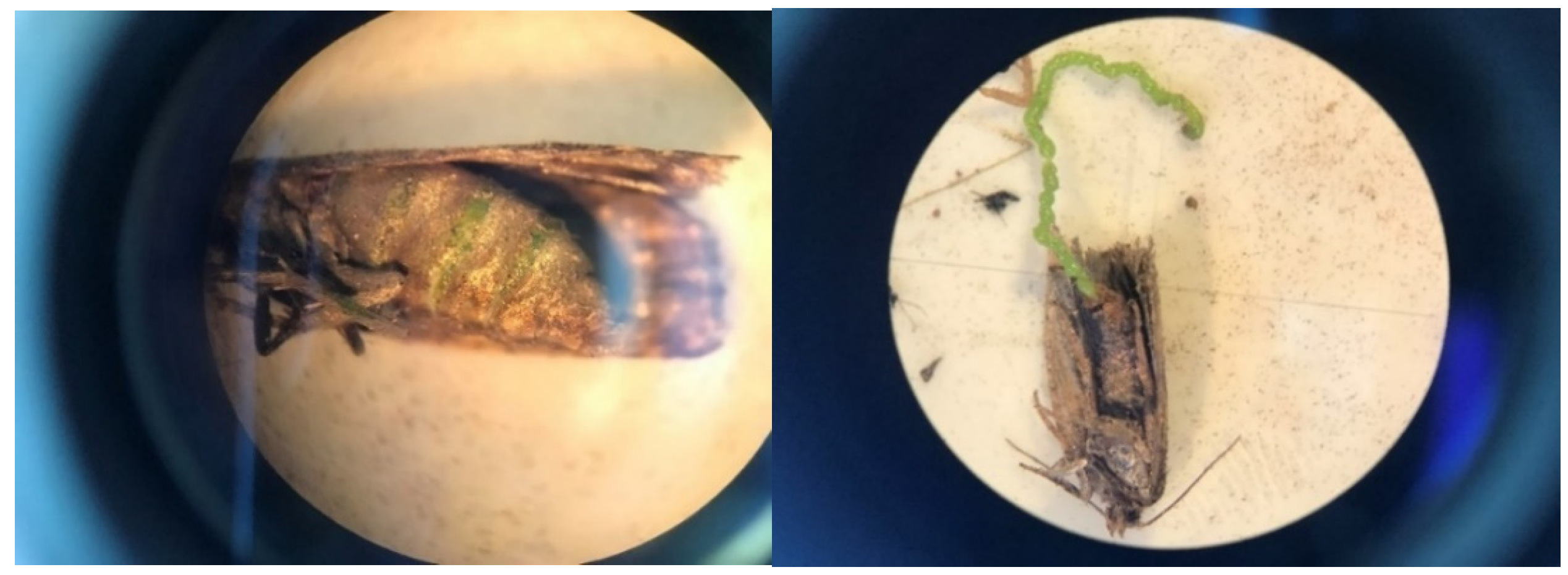
Gravid females may be more common in LTs than generally assumed due to a methodological flaw when processing adults. During a 5 to 7 d interval in early August 2019, 2 to 5% of female budworms in many LTs were gravid (a plump abdomen filled with green eggs often visible through the integument; Figure 8). No formal records were made at the time; instead, LT samples were stored in paper bags labeled by sites/dates and frozen for 48 h for subsequent data assessments. After 48 h freezing exposure, unfortunately, the ‘obvious’ plump abdomen of fresh (unfrozen) gravid females had become unrecognizable. It is disconcerting that a procedure routinely used over the years (freezing specimens before processing) leads to systematically biased observations.
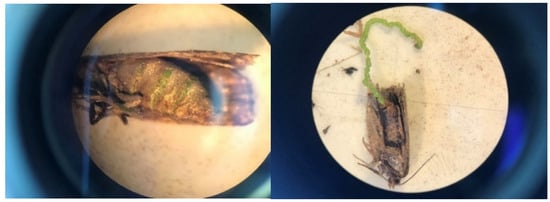
Figure 8.
Gravid female spruce budworm captured in light trap on west coast of Newfoundland in early August 2019. Left: plump abdomen filled with green eggs visible through integument. Right: eggs laid by gravid females while being processed for demographic assessments.
3.2.5. Body Mass of Females Spruce Budworms in Light Traps
Biomass is universal in nature: all living organisms can be defined (and compared) on a per weight basis. When LTmt is viewed through the lens of harvesting [99,141], the biomass of females in LTs (w♀) is hypothesized, not only to provide the best approximation of Ē but also to have universal application for any other pest targeted by LTmt.
The widespread adoption of biomass parameterization may be confronted with the objective of LTmt generally conceived as to maximize the number of adults in LTs, sometimes with a focus on females. When pests are strictly defined on a numerical basis, however, adults become dimensionless points with no physical size.
Female budworms captured in LTs are heavier than males: 420 of 424 (99.1%) daily LT samples in 2019 including at least two adults of each sex yielded w♀/n♀ ≥ w♂/n♂. Assuming that only captures of females in LTs contribute to Ē, the intraspecific measure of LTmt performance can be approximated with either the numerical or biomass sex ratio:
Ē(n) = n♀/(n♂ + n♀)
Ē(w) = w♀/(w♂ + w♀)
Consistent with the higher weight of females than males, the estimates Ē(w) are 1.23 times higher than Ē(n), i.e., the number of females represented 21.8% of budworms in LTs relative to 26.7% female biomass (Table 5).

Table 5.
Numerical (n) and biomass (w) abundance of male and female spruce budworms captured in light traps deployed along west coast of Newfoundland in 2019, including sex ratio assessments [♀/(♀ + ♂)]. Index w♀/n♀ for each trap corresponds to [♀/(♀ + ♂)w]/[♀/(♀ + ♂)n].
3.3. Management of Budworm Infestations in Gros Morne National Park with LTmt?
The management objective of LTmt in budworms is not to prevent outbreaks, first as it is impractical due to the vast spatial scale of forest landscapes and second due to females ignoring LTs at low conspecific density. Instead, LTmt aims at mass trapping females in geographically isolated outbreaks to prevent tree mortality. Starting in 2021, on the west coast of NL, populations of late instar budworms were treated with Bacillus thurigiensis kurstaki; Btk treatments were not authorized at Gros Morne National Park to avoid the mortality of native caterpillars.
With hindsight, sites within Gros Morne (Rocky Harbor: RH; Sally’s Cove: SC; Cow Head: CH) should have been prioritized for research over sites north of the park (Table 3). The potential of LTmt is likely the highest in SC than other sites in the park for three reasons: the (1) small area and geographical isolation of forest stands surrounded by wetland; (2) high captures of budworms and target/non-target ratios relative to other sites (Table 3); and (3) proximity to the coast in SC (<100 m, relative to >1 km in RH–CH) imply an enhanced likelihood to mass trap immigrant female budworms before they oviposit in the park.
The quantitative pest management objectives of LTmt are (in ascending order of importance) to reduce larval density in offspring generations, limit defoliation, and prevent the mortality of balsam fir. The biodiversity loss associated with bycatch in LTs is not accounted for here, with the caveat that such loss may be deemed unacceptable to park management or potentially worse than the Btk-induced mortality of native caterpillars.
In the worst-case scenario, severe budworm defoliation and the associated massive mortality of balsam fir, combined with a limited prospect for natural regeneration due to high moose density and the consequent browsing of seedling trees [142,143], imply that the coastal forest stand in SC is at a high risk (Ȓ = 1) of transiting to a wetland ‘ghost forest’ with predominantly dead trees [144,145]. In the best-case scenario, the forest stand in SC may be at low risk (Ȓ ≈ 0)—noting that centuries-old coastal balsam firs have so far survived a large number of budworm immigrations.
In theory, the objective of LTmt in SC can be quantified by computing the yield of mass trapping (ca. 4 kg fresh female biomass/$ 10,000), economic valuation of the forest stand in SC (V), and risk of conversion to a swamp ghost forest (Ȓ):
ĒSC: ƒ (w♀/$, V, Ȓ)
The plausibility of LTmt (defined as a tool to reduce defoliation/tree mortality caused by budworms) is ultimately determined by the economic valuation of coastal firs in SC relative to the wetland (including ecosystem services, biodiversity value, touristic attributes, etc.). Somehow unexpectedly, a complex monetary assessment of forest value [146,147,148] seems to be the major factor obscuring the application of LTmt in forestry versus agriculture.
4. Conclusions
With the future assumed here to be unknowable, the full-fledged potential of LTmt against Lepidopteran agricultural pests cannot be ascertained. That said, cautionary optimism is warranted when considering the contemporary uptick in research interest, low cost of light traps, and environmentally friendly nature of LTmt relative to insecticide applications. The outlook of LTmt against forest defoliators is more uncertain than in agriculture because the approach is explored here for the first time in spruce budworms.
As a general conclusion, six maxims are proposed as major take-home messages for future light-based mass trapping studies:
- (1)
- The ultimate objective of mass trapping is to reduce crop damage, not to increase captures in traps per se.
- (2)
- Imperative for standardized trapping protocols in pest management programs, the hyper-inflationary rate of trap innovation has become counter-productive.
- (3)
- The weight of the bycatch in traps (non-pests) provides a simple proxy of the biodiversity loss in traps.
- (4)
- In general, females captured in traps matter, males do not.
- (5)
- Gravid females with a near-full egg complement are the primary targets of mass trapping.
- (6)
- The weight of female pests at traps provides a universal proxy to quantify the yield of mass trapping, i.e., biomass is universal in nature.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects15040267/s1, ANNEX I: Light trapping protocols.
Funding
This research was funded by SERG-I, grant number 2015/13-2019-912.
Acknowledgments
The 2019 field study was supported by the Department of Natural Resources in Newfoundland–Labrador, in particular D. Lavigne, J. Motty, T. Rideout, and R. Carroll. I am grateful to my colleague M. Stastny for careful reviews/edits on early versions of the manuscript. Figure 1 was constructed by R. Jennings. M. Beats kindly provided photographs of gravid females in light traps. Comments by two anonymous reviewers were useful in improving the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Boulanger, Y.; Arseneault, D. Spruce Budworm Outbreaks in Eastern Quebec over the Last 450 Years. Can. J. For. Res. 2004, 34, 1035–1043. [Google Scholar] [CrossRef]
- Rhainds, M.; Lavigne, D.; Boulanger, Y.; DeMerchant, I.; Delisle, J.; Motty, J.; Rideout, T.; Labrecque, A. I Know It When I See It: Incidence, Timing and Intensity of Immigration in Spruce Budworm. Agric. Forest Entomol. 2021, 24, 152–166. [Google Scholar] [CrossRef]
- Cooke, B.J.; Robert, L.E.; Stuertevant, B.R.; Kneeshaw, D. Confronting the Cycle Synchronisation Paradigm of Defoliator Outbreaks in Space and Time: Evidence for Two Systems in a Mixed-Species Forest Landscape. J. Ecol. 2023, 112, 152–173. [Google Scholar] [CrossRef]
- Snow, J.W.; Cantelo, W.W.; Bowman, M.C. Distribution of the Corn Earworm on St. Croix, U.S. Virgin Islands, and Its Relation to Suppression Programs. J. Econ. Entomol. 1969, 62, 606–611. [Google Scholar] [CrossRef]
- Cantelo, W.W.; Goodenough, J.L.; Baumhover, A.H.; Smith, J.S.; Stanley, J.M.; Henneberry, T.J. Mass Trapping with Blacklight: Effects on Isolated Populations of Insects. Environ. Entomol. 1974, 3, 389–395. [Google Scholar] [CrossRef]
- Hegazi, E.; Khafagi, W.E.; Konstantopoulour, M.; Raptopoulos, D.; Tawfik, H.; Abd El-Azis, S.M.; Atwa, A.; Aggamy, E.; Showeil, S. Efficient Mass-Trapping Method as an Alternative Tactic for Suppressing Populations of Leopard Moth (Lepidoptera: Cossidae). Ann. Entomol. Soc. Am. 2009, 102, 809–818. [Google Scholar] [CrossRef]
- Hartsock, J.G.; Deay, H.D.; Barrett, J.R. Practical Application of Insect Attraction in the use of Light Traps. Bull. Entomol. Soc. Am. 1966, 12, 375–377. [Google Scholar] [CrossRef]
- Cantelo, W.W. Blacklight Traps as Control Agents: An Appraisal. Bull. Ent. Soc. Am. 1974, 20, 279–282. [Google Scholar] [CrossRef]
- Hienton, T.E. Summary of Investigations of Electric Inset Traps; Technical Bulletin; United States Department of Agriculture: Washington, DC, USA, 1974; Volume 1498, p. 136. Available online: https://books.google.ca/books?id=04F1mIxXW4AC&printsec=frontcover#v=onepage&q&f=false (accessed on 15 February 2024).
- Shimoda, M.; Honda, K. Insect Reactions to Light and Its Applications to Pest Management. Appl. Entomol. Zool. 2013, 48, 413–421. [Google Scholar] [CrossRef]
- Kim, K.N.; Huang, Q.Y.; Lei, C.L. Advances in Insect Phototaxis and Applications to Pest Management: A Review. Pest Manag. Sci. 2019, 75, 3135–3143. [Google Scholar] [CrossRef]
- Kammar, V.; Rani, A.; Krishna, K.R.; Chakravarthy, A.K. Light Trap: A Dynamic Tool for Data Analysis, Documenting, and Monitoring Insect Populations and Diversity. In Innovative Pest Management Approaches for the 21st Century; Chakravarthy, A., Ed.; Springer: Singapore, 2020; pp. 137–163. ISBN 978-981-15-0794-6. [Google Scholar]
- Frolov, A.N. Controlling the Behavior of Harmful Insects: Light and Chemical Signals and Their Combined Action. Entomol. Rev. 2022, 102, 782–819. [Google Scholar] [CrossRef]
- Redondo, G.O.; Launio, C.C.; Manalili, R. Farmers’ Knowledge, Perceptions, and Management Practices on Rice Black Bug. In Rice Black Bugs: Taxonomy, Ecology and Management of Invasive Species; Joshi, R.C., Barrion, A.T., Sebastian, L.S., Eds.; Philippine Rice Research Institute: Maligaya, Philippines, 2007; pp. 287–305. [Google Scholar]
- Ardeh, M.J.; Farhangi, S.V.; Seyahooei, M.A. Light Trap Density for Capturing the Tomato Leafminer Moth Tuta absoluta in Greenhouses. J. Plant Prot. Res. 2019, 33, 187–192. [Google Scholar] [CrossRef]
- Ashik-E-Rabban, M.; Basir, M.S.; Aliuzzaman, M.; Rahman, A. Optimization of a Solar Light Trap for Controlling the Pest in Rice Field. Agric. Eng. Int. CIGR J. Open Access 2022, 24, 43–50. [Google Scholar]
- Desikan, R.; Chandrasekaran, M.; Soundararajan, R.P.; Subramanian, P.; Palled, V.; Kumar, D.P. Solar-Powered Plant Protection Equipment: Perspective and Prospects. Energies 2022, 15, 7379. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Zhang, J.C.; Zhao, S.Y. Discussion on Applicability of the Technology of Using Light to Trap in the Field of Pests and Diseases Control in Tea Plantation of China. Key Eng. Mater. 2013, 575–576, 487–493. [Google Scholar] [CrossRef]
- Saedi, Z. Integration of Pheromone and Light Traps for Mass Trapping of Leopard Moth, Zeuzera pyrina (Lepidoptera: Cossidae) in Walnut Orchards. J. Entomol. Soc. Iran 2021, 41, 123–133. [Google Scholar] [CrossRef]
- Ficht, G.A.; Hienton, T.E. Studies on the Flight of European Corn Borer Moths to Light Traps: A Progress Report. J. Econ. Entomol. 1939, 32, 520–526. [Google Scholar] [CrossRef]
- Barrett, J.R.; Deay, H.O.; Hartsock, J.G. Reduction in Insect Damage to Cucumbers, Tomatoes, and Sweet Corn Through use of Electric Light Traps. J. Econ. Entomol. 1971, 64, 1241–1249. [Google Scholar] [CrossRef]
- Pérez Pérez, R.; Hensley, S.D. A Comparison of Pheromone and Blacklight Traps for Attracting Sugarcane Borer (Diatraea saccharalis (F.)) Adults from a Natural Population. J. Agric. Univ. P. R. 1973, 57, 320–329. [Google Scholar] [CrossRef]
- Youm, O.; Beevor, P.S.; McVeigh, L.J.; Diop, A. Effect of Trap Height and Spacing in Relation to Crop Height on Catches of the Millet Stemborer, Coniesta ignefusalis Males. Int. J. Trop. Insect Sci. 1997, 17, 235–240. [Google Scholar] [CrossRef]
- Prabaningrum, L.; Moekasan, T.K. Use of Light Trap for Controlling Cabbage Pests. IOP Conf. Ser. Earth Environ. Sci. 2021, 752, 012027. [Google Scholar] [CrossRef]
- Sajid, Z.; Hassan, H.; Ashraf, N.; Saeed, M.T.; Sarwar, S.; Ali, H.; Tayyab, U.B.; Murtaza, G. Impact of Infested Shoot Removal and Light Trap on Leucinodes orbonalis Infestation on Eggplant Fruit. Indian J. Pure Appl. Biosci. 2021, 9, 176–182. [Google Scholar] [CrossRef]
- Rakibuzzaman, M.; Rahman, M.M.; Hossain, M.S.; Hossain, M.E. Effect of Organic Insect Pest Management Strategies on Brinjal Production. Int. J. Entomol. Res. 2023, 8, 78–83. [Google Scholar]
- Gentry, C.R.; Smith, J.S.; Blythe, J.L.; Edwards, G.W. Black-Light Traps to Control Hickory Shuckworms on Pecans. Agric. Res. Serv. 1976, 105, 4. [Google Scholar]
- Glick, P.A.; Hollingsworth, J.P. Further Studies on the Attraction of Pink Bollworm Moths to Ultraviolet and Visible Radiation. J. Econ. Entomol. 1956, 49, 158–161. [Google Scholar] [CrossRef]
- Bhute, N.K.; Pathan, Y.K.; Gaikwad, S.A. Evaluation of Solar Light Traps Against Pink Bollworm, Pectinophora gossypiella (Saunders) in Bt Cotton. Pharma Innov. J. SP 2021, 10, 297–300. [Google Scholar]
- Kirisik, M.; Erler, F.; Kahraman, T. A New-Designed Light Trap for the Control of Potato Tuber Moth, Phthorimaea operculella (Zeller) (Lepidoptera: Gelechiidae), in Stored Potatoes. Potato Res. 2022, 66, 245–254. [Google Scholar] [CrossRef]
- Pezhman, H.; Saeidi, K. Effectiveness of Various Solar Light Traps With and Without Sex Pheromone for Mass Trapping of Tomato Leaf Miner (Tuta absoluta) in a Tomato Field. Not. Sci. Biol. 2018, 10, 475–484. [Google Scholar] [CrossRef]
- Erler, F.; Bayram, Y. Efficacy of mass trapping of tomato moth, Tuta absoluta (Meyrick, 1917) (Lepidoptera: Gelechiidae), using a new-designed light trap in reducing leaf and fruit damages in greenhouse-grown tomatoes. J. Plant Dis. Prot. 2021, 128, 1177–1185. [Google Scholar] [CrossRef]
- Nunez, E.; Barbosa, L.S.; Avelino-Capistrano, F. Efficiency of Capture of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) with Mosquito Killer Light Traps. Agron. Colomb. 2023, 41, 1–5. [Google Scholar] [CrossRef]
- Mangrio, G.Q.; Gilal, A.A.; Rajput, L.B.; Hajano, J.; Gabol, A.H. Performance of Pheromone and Light Traps in Monitoring and Management of Tomato Leafminer, Tuta absoluta (Lepidoptera: Gelechiidae). J. Saudi Soc. Agric. Sci. 2023, 22, 288–297. [Google Scholar] [CrossRef]
- Bian, L.; Cai, X.M.; Luo, Z.X.; Li, Z.Q.; Chen, Z.M. Decreased Capture of Natural Enemies of Pests in Light Traps with Light-Emitting Diode Technology. Ann. Appl. Biol. 2018, 173, 251–260. [Google Scholar] [CrossRef]
- Mafia, R.G.; Loureiro, E.B.; Silva, J.B.; Simoes, J.A.C.; Zarpelon, T.G.; Bezerra, N.S.; Damacena, M.B. A New Light Trap Model as an Alternative for Controlling Pests in Eucalyptus Plantations. Neotrop. Entomol. 2018, 47, 326–328. [Google Scholar] [CrossRef]
- Sujatha, A.; Emmanuel, N.; Raj, S.A. Light Trap—Induced Suppression of Coconut Slug Caterpillar, Macroplectra nararia Moore Menace in East Coast of India. J. Plant. Crops 2011, 39, 390–395. [Google Scholar]
- Ahmad, N.M.; Faraj, F.H. Effect of Bait and Light Intensity of Color Traps of the Population Ocnerogyla amanda Staudinger 1891 (Lymantridae: Lepidoptera) Kane Kawai CHBUX. Tikrit J. Agric. Sci. 2023, 23, 72–86. [Google Scholar] [CrossRef]
- Debolt, J.W.; Wolf, W.W.; Henneberry, T.J.; Vail, V. Evaluation of Light Traps and Sex Pheromones for Control of Cabbage Looper and Other Lepidopterous Insect Pests of Lettuce; Technical Bulletin; U.S. Department of Agriculture: Washington, DC, USA, 1979; Volume 1606, p. 39. [Google Scholar]
- Ullah, F.; Ali, M.; Ahmad, S.; Badshah, H. Impact of Light Traps on Population Density of Gram Pod Borer, Helicoverpa armigera (Hub) (Lepidoptera: Noctuidae) and Its Larval Parasitoid (Campoletis chloridea Uchida) in Rod Kohi Area of Dera Ismail Khan, Pakistan. J. Entomol. Zool. Stud. 2015, 3, 203–207. [Google Scholar]
- Abbas, M.; Ramzan, M.; Hussain, N.; Ghaffar, A.; Hussain, K.; Abbas, S.; Raza, A. Roles of Light Traps in Attracting, Killing, and Biodiversity Studies of Insect Pests in Thal. Pak. J. Agric. Res. 2019, 32, 684–690. [Google Scholar] [CrossRef]
- Pan, H.; Xu, Y.; Liang, G.; Wyckhuys, K.A.G.; Yang, Y.; Lu, Y. Field Evaluation of Light-Emitting Diodes to Trap the Cotton Bollworm, Helicoverpa armigera. Crop Prot. 2020, 137, 105267. [Google Scholar] [CrossRef]
- Balamurugan, R.; Kandasami, P. Effectiveness of Portable Solar-Powered Light-Emitting Diodes Insect Trap: Experimental Investigation in a Groundnut Field. J. Asia-Pacific Entomol. 2021, 24, 1024–1032. [Google Scholar] [CrossRef]
- Gentry, C.R.; Thomas, W.W.; Stanley, J.M. Integrated Control as an Improved Means of Reducing Populations of Tobacco Pests. J. Econ. Entomol. 1969, 62, 1274–1277. [Google Scholar] [CrossRef]
- Gentry, C.R.; Dickerson, W.A.; Stanley, J.M. Populations and Mating of Adult Tobacco Budworms and Corn Earworms in Northwest Florida Indicated by Traps. J. Econ. Entomol. 1971, 64, 335–338. [Google Scholar] [CrossRef]
- Hendricks, D.E. Use of Virgin Females Tobacco Budworms to Increase Catch of Males in Blacklight Traps and Evidence that Trap Location and Wind Influence Catch. J. Econ. Entomol. 1968, 61, 1581–1585. [Google Scholar] [CrossRef]
- Hartstack, A.W.; Hollingsworth, J.P.; Ridgway, R.L.; Hunt, H.H. Determination of Trap Spacings Required to Control an Insect Population. J. Econ. Entomol. 1971, 64, 1090–1100. [Google Scholar] [CrossRef]
- Hartstack, A.W.; Hollingsworth, J.P.; Ridgway, R.L.; Coppedge, J.R. A Population Dynamics Study of the Bollworm and the Tobacco Budworm with Light Traps. Environ. Entomol. 1973, 2, 244–252. [Google Scholar] [CrossRef]
- Herms, W.B. Some Problems in the use of Artificial Light in Crop Protection. Hilgardia 1947, 17, 359–375. [Google Scholar] [CrossRef]
- Lam, J.J.; Stewart, P.A. Modified Traps Using Blacklight Lamps to Capture Nocturnal Tobacco Insects. J. Econ. Entomol. 1969, 62, 1378–1381. [Google Scholar] [CrossRef]
- Stewart, P.A.; Lam, J.J. Catch of Insects at Different Heights in Traps Equipped with Blacklight Lamps. J. Econ. Entomol. 1968, 61, 1227–1230. [Google Scholar] [CrossRef]
- Graham, H.M.; Holingsworth, J.P.; Lukefahr, M.J.; Llannes, J.R. Effects of a High Density of Blacklight Traps on Corn Earworm Populations in Corn. Prod. Res. Rep. 1971, 127, 24. [Google Scholar]
- Taft, H.M.; Agee, H.R.; Hopkins, A.R.; James, W. Field Evaluation of Artificial Light for Control of Bollworms on Cotton. Environ. Entomol. 1972, 1, 295–300. [Google Scholar] [CrossRef]
- López, J.D.; Witz, J.A.; Hartstack, A.W.; Hollingsworth, J.P. Reproductive Condition of Bollworm Moths Caught in Blacklight Traps in Corn, Sorghum, and Cotton. J. Econ. Entomol. 1978, 71, 961–966. [Google Scholar] [CrossRef]
- Carnegie, A.J.M.; Leslie, G.W. Trends Shown by Light Trap Catches of Some Sugarcane Pests. In Proceedings of the Annual Congress—South African Sugar Technologists’ Association, Durban, South Africa, 10–12 June 1991; Volume 65, pp. 87–91. [Google Scholar]
- Wulandari, R.; Santoso, R.E.; Prasetiyo, D.; Lestari, A.S.; Mizar, A.; Puspitasari, P.D. Increasing the Weight of Onion Bulbs Due to the Reduction of Spodoptera exigua Using a Portable Light Trap. IOP Conf. Ser. Mater. Sci. Eng. 2019, 694, 012010. [Google Scholar] [CrossRef]
- Gebreziher, H.G.; Gebreazgaabher, F.G. Night-Time Light-Traps and Push-Pull Integrated System Enhanced the Control of Different Life Stages of Fall Armyworm, Spodoptera frugiperda, (Lepidoptera: Noctuidae). Res. Sq. 2023. [Google Scholar] [CrossRef]
- Herms, W.B.; Ellsworth, J.K. Field Tests of the Efficacy of Colored Light in Trapping Insect Pests. J. Econ. Entomol. 1934, 28, 1055–1067. [Google Scholar] [CrossRef]
- Gentry, C.R.; Lawson, F.R.; Knott, C.M.; Stanley, J.; Lam, J.J. Control of Hornworms by Trapping with Blacklight and Stalk Cutting in North Carolina. J. Econ. Entomol. 1967, 60, 1437–1442. [Google Scholar] [CrossRef]
- Stanley, J.M.; Dominick, C.B. Response of Tobacco- and Tomato-Hornworm Moths to Black Light. J. Econ. Entomol. 1958, 51, 78–80. [Google Scholar] [CrossRef]
- Scott, L.B.; Milam, J.R. Isoamyl Salicylate as an Attractant for Hornworm Moths. J. Econ. Entomol. 1943, 36, 712–715. [Google Scholar] [CrossRef]
- Jones, G.A.; Thurston, R. Effect of an Area Program Using Blacklight Traps to Control Populations of Tobacco Hornworms in Kentucky. J. Econ. Entomol. 1970, 63, 1187–1194. [Google Scholar] [CrossRef]
- H–H. Bulletin No. 583. Available online: https://ecommons.cornell.edu/server/api/core/bitstreams/6b231853-1dc2-4335-89be-8919107617b1/content (accessed on 15 February 2024).
- Collins, D.L.; Nixon, M.W. Responses to Light of the Bud Moth and Leaf Roller; New York State Agricultural Experiment Station: New York, NY, USA, 1930. [Google Scholar]
- Collins, D.L.; Machado, W.M. Effects of Light Traps on a Codling Moth Infestation. J. Econ. Entomol. 1937, 30, 422–427. [Google Scholar] [CrossRef]
- Hamilton, D.W.; Steiner, L.F. Light Trap and Codling Moth Control. J. Econ. Entomol. 1939, 32, 867–872. [Google Scholar] [CrossRef]
- Erler, F.; Tosun, H.S. Mass trapping the codling moth, Cydia pomonella (L.) (Lepidoptera: Tortricidae), using newly designed light trap reduces fruit damage in apple orchards. J. Plant Dis. Prot. 2023, 130, 795–807. [Google Scholar] [CrossRef]
- Muralimohan, K.; Sreekanth, P.N.; Srinivasa, Y.B. Light traps for managing the coconut black headed caterpillar. Pest Manag. Hortic. Ecsyst. 2007, 13, 159–164. [Google Scholar]
- El-Saadany, G. A New Light Trap for Controlling Lepidopterous Insect Pests in Egypt. Z. Ang. Entomol. 1974, 77, 137–141. [Google Scholar] [CrossRef]
- Telaumbanua, M.; Haryanto, A.; Wisnu, F.K.; Lanya, B.; Wiratama, W. Design of Insect Trap Automatic Control System for Cacao Plants. Procedia Environ. Sci. Eng. Manag. 2021, 8, 167–175. [Google Scholar]
- Al Mamun, M.R.; Keya, A.C.; Alim, M.S.; Hossen, M.A.; Mondal, M.F.; Soeb, M.J.A. Potentiality Assessment of Solar Based LED Light Trap as Pest Management Tool in Tea (Camellia sinensis L.). Smart Agric. Entomol. 2023, 5, 100304. [Google Scholar] [CrossRef]
- Moayeri, H.S.; Azadi, M. Evaluation and Comparison of Light, Pheromone, and Light-Pheromone Traps for Collecting Cydia pomonella. Appl. Plant Prot. 2018, 7, 1. [Google Scholar]
- Rashid, M.; Ridoy, M.K.; Rahman, M.M.; Rahman, M.M.; Mondal, M.F. Does Solar Light Trap Reduce the Cost of Pesticides Used in Rice Field? SAARC J. Agric. 2022, 20, 171–183. [Google Scholar] [CrossRef]
- Yao, Q.; Lv, J.; Liu, Q.; Diao, G.; Yang, B.; Chen, H.; Tang, J. An Insect Imaging System to Automate Rice Light-Trap Pest Identification. J. Integr. Agric. 2012, 11, 978–985. [Google Scholar] [CrossRef]
- Meshram, S.A.; Kapade, S.A.; Chaudhari, A.D.; Nagane, K.B. Design of a Solar Light Trap for Control of Field Crop Insects. Int. Res. J. Eng. Tech. 2018, 5, 1252–1254. [Google Scholar]
- Harding, W.C.; Hartsock, J.G.; Rohwer, G.G. Blacklight Trap Standards for General Insect Surveys. Bull. Entomol. Soc. Am. 1966, 12, 31–32. [Google Scholar] [CrossRef]
- Montgomery, G.A.; Belitz, M.W.; Guralnick, R.; Tingley, M.W. Standards and Best Practices for Monitoring and Benchmarking Insects. Front. Ecol. Evol. 2021, 8, 579193. [Google Scholar] [CrossRef]
- Hollingsworth, J.P.; Hartstack, A.W.; Lindquist, D.A. Influence of Near-Ultraviolet Output of Attractant Lamps on Catches of Insects by Light Traps. J. Econ. Entomol. 1968, 61, 515–521. [Google Scholar] [CrossRef]
- Zemel, R.S.; Houghton, D.C. The Ability of Specific-Wavelength LED Lights to Attract Night-Flying Insects. Gt. Lakes Entomol. 2017, 50, 79–85. [Google Scholar] [CrossRef]
- Niermann, J.; Brehm, G. The Number of Moths Caught by Light Traps Is Affected More by Microhabitat than the Type of UV Lamp Used in a Grassland Habitat. Eur. J. Entomol. 2022, 119, 36–42. [Google Scholar] [CrossRef]
- Gopalakrishnan, R.; Elango, K.; Arunkumar, P. Insect reactions to light and its applications for pest management. In Advances in Agricultural and Horticultural Sciences; Shinde, Y.A., Ed.; Advances in Agricultural and Horticultural Sciences; p. 29. Available online: https://www.researchgate.net/publication/371761999_Advances_in_Agricultural_and_Horticultural_Sciences (accessed on 15 February 2024).
- Ma, G.; Ma, C. Differences in the Nocturnal Flight Activity of Insect Pests and Beneficial Predatory Insects Recorded by Light Traps: Possible Use of a Beneficial-Friendly Trapping Strategy for Controlling Insect Pests. Eur. J. Entomol. 2012, 109, 395–401. [Google Scholar] [CrossRef]
- Herms, W.B. A Field Test of the Effect of Artificial Light on the Behavior of the Codling Moth Carpocapsa pomonella Linn. J. Econ. Entomol. 1929, 22, 78–88. [Google Scholar] [CrossRef]
- Headlee, T.J. Further Studies on the Effects of Electromagnetic Waves on Insects. J. Econ. Entomol. 1932, 25, 276–288. [Google Scholar] [CrossRef]
- Carbajal-Morán, H.; Camarena, J.F.M.; Maldonado, C.A.G.; Quiñones, R.H.Z.; Maldonado, A.C.G. Low Energy Trap with Light Emitting Diode for Increased Attraction of Phthorimeaea operculella Zeller. Ecol. Eng. Environ. Technol. 2023, 24, 270–275. [Google Scholar] [CrossRef]
- McFadden, M.W.; Lam, J.J. Influence of Population Level and Trap Spacing on Capture of Tobacco Hornworm Moths in Blacklight Traps with Virgin Females. J. Econ. Entomol. 1968, 61, 1150–1152. [Google Scholar] [CrossRef]
- Smith, J.S.; Cantelo, W.W. Single vs. Multilamp Blacklight Insect Trap Collections of Tobacco Hornworm Moths. J. Econ. Entomol. 1971, 64, 19–20. [Google Scholar] [CrossRef]
- Hartstack, A.W.; Hollingsworth, J.P.; Lindquist, D.A. A Technique for Measuring Trapping Efficiency of Electric Insect Traps. J. Econ. Entomol. 1968, 61, 546–552. [Google Scholar] [CrossRef]
- Vaishampayan, S.M. Utility of Light Trap in Integrated Pest Management. In Entomology: Novel Approaches; Jain, P.C., Bhargave, M.C., Eds.; New India Publishing Agency: New Delhi, India, 2007; pp. 193–210. [Google Scholar]
- Poethke, H.J.; Hovestadt, T. Evolution of Density–and Patch–Size–Dependent Dispersal Rates. Proc. R. Soc. B Biol. Sci. 2002, 269, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Parvinen, K.; Seppänen, A.; Nagy, J.D. Evolution of Complex Density-Dependent Dispersal Strategies. Bull. Math. Biol. 2012, 74, 2622–2649. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.A.J. Spatial Distribution, Sampling Efficiency and Taylor’s Power Law. 2. Interpreting Density-Dependent Sampling Efficiency. Agric. For. Entomol. 2020, 23, 173–188. [Google Scholar] [CrossRef]
- Knight, E.A.; Preti, M.; Basoalto, E.; Fuentes-Contreras, E. Increasing Captures of Adult Moth Pests (Lepidoptera: Tortricidae) in Pome Fruit with Low-Intensity LED Lights Added to Sex Pheromone/Kairomone Lure-Baited Traps. J. Appl. Entomol. 2023, 147, 843–856. [Google Scholar] [CrossRef]
- Quiring, D.T.; Timmins, P.R. Influence of reproductive ecology on feasibility of mass trapping Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae). J. Appl. Ecol. 1990, 27, 965–982. [Google Scholar] [CrossRef]
- Witzgall, P.; Kirsch, P.; Cork, A. Sex Pheromones and Their Impact on Pest Management. J. Chem. Ecol. 2010, 36, 80–100. [Google Scholar] [CrossRef] [PubMed]
- Rhainds, M. Wing Wear and Body Size Measurements of Adult Spruce Budworms Captured at Light Traps: Inference on Seasonal Patterns Related to Reproduction. Appl. Entomol. Zool. 2015, 50, 477–485. [Google Scholar] [CrossRef]
- Delisle, J.; West, R.; Bowers, W.W. The Relative Performance of Pheromone and Light Traps in Monitoring the Seasonal Activity of Both Sexes of the Eastern Hemlock Looper, Lambdina fiscellaria fiscellaria. Entomol. Exp. Appl. 1998, 89, 87–98. [Google Scholar] [CrossRef]
- Karlsson, B. Feeding habits and change of body composition with age in three nymphalid butterfly species. Oikos 1994, 69, 224–230. [Google Scholar] [CrossRef]
- Sengendo, F.; Subramanian, S.; Kidoido, M.; Chemurot, M.; Tanga, C.; Egonyu, J.P. Cost–Benefit Analysis of Improved Light Trap for Harvesting the Edible Grasshopper, Ruspolia differens (Orthoptera: Tettigoniidae): Evidence from Uganda. Int. J. Trop. Insect Sci. 2021, 41, 1913–1921. [Google Scholar] [CrossRef]
- Wellington, W.G. The Light Reactions of the Spruce Budworm, Choristoneura fumiferana Clemens (Lepidoptera Tortricidae). Can. Entomol. 1948, 80, 56–82. [Google Scholar] [CrossRef]
- Simpsons, L.J. Spruce Budworm Flights in New Brunswick, as Indicated by Light Traps; Interim Report 1957-5; Forest Biology Laboratory: Fredericton, NB, USA, 1958. [Google Scholar]
- Weed, D. Spruce Budworm in Maine, 1910–1976 Infestations and Control; Maine Department of Convervartion, Maine Forest Service: Augusta, ME, USA, 1977. [Google Scholar]
- Simmons, G.A.; Elliott, N.C. Use of Moths Caught in Light Traps for Predicting Outbreaks of the Spruce Budworm (Lepidoptera: Tortricidae) in Maine. J. Econ. Entomol. 1985, 78, 362–365. [Google Scholar] [CrossRef]
- Hurley, J.E.; Titus, F.A. Summary of light trap catches for the Maritimes 1976–1986, Information Report M-X-163; Natural Resources Canada, Canadian Forest Services, Atlantic Forestry Centre: Fredericton, NB, Canada, 1987. [Google Scholar]
- Rhainds, M.; Lavigne, D.; Rideout, D.; Candau, J.N. Temporal Variation in Abundance of Male and Female Spruce Budworms at Combinatory Associations of Light Traps and Pheromone Traps. Entomol. Exp. Appl. 2019, 167, 526–533. [Google Scholar] [CrossRef]
- Gilman, E.; Hall, M.; Booth, H.; Gupta, T.; Chaloupka, M.; Fennell, H.; Kaiser, M.J.; Karnad, D.; Milner-Gulland, E.J. A Decision Support Tool for Integrated Fisheries Bycatch Management. Rev. Fish Biol. Fish. 2022, 32, 441–472. [Google Scholar] [CrossRef]
- Santos, C.C.; Rosa, D.; Gonçalves, J.M.S.; Coelho, R. A Review of Reported Effects of Pelagic Longline Fishing Gear Configurations on Target, Bycatch and Vulnerable Species. Aquatic Conserv. Mar. Freshw. Ecosyst. 2023, 34, 1–17. [Google Scholar] [CrossRef]
- Elliott, B.; Tarzia, M.; Read, A.J. Cetacean Bycatch Management in Regional Fisheries Management Organizations: Current Progress, Gaps, and Looking Ahead. Front. Mar. Sci. 2023, 9, 1006894. [Google Scholar] [CrossRef]
- Truxa, C.; Fiedler, K. Attraction to Light—From How Far Do Moths (Lepidoptera) Return to Weak Artificial Sources of Light? Eur. J. Entomol. 2012, 109, 77–84. [Google Scholar] [CrossRef]
- Van Grunsven, R.H.A.; Lham, D.; Van Geffen, K.G.; Veenendaal, E.M. Range of Attraction of a 6-W Moth Light Trap. Entomol. Exp. Appl. 2014, 152, 87–90. [Google Scholar] [CrossRef]
- Adams, C.G.; Schenker, J.H.; McGhee, P.; Gut, L.J.; Brunner, J.F.; Miller, J.R. Maximizing Information Yield from Pheromone-Baited Monitoring Traps: Estimating Plume Reach, Trapping Radius, and Absolute Density of Cydia pomonella (Lepidoptera: Tortricidae) in Michigan Apple. J. Econ. Entomol. 2017, 110, 305–318. [Google Scholar] [CrossRef]
- Byers, J.A. Analysis of Vertical Distributions and Effective Flight Layers of Insects: Three-Dimensional Simulation of Flying Insects and Catch at Trap Heights. Environ. Entomol. 2011, 40, 1210–1222. [Google Scholar] [CrossRef]
- Rhainds, M.; Kettela, E.G. Oviposition Threshold for Flight in an Inter-Reproductive Migrant Moth (Lepidoptera: Tortricidae). J. Insect. Behav. 2013, 26, 850–859. [Google Scholar] [CrossRef]
- Westwood, R.; Saunders, D.; Westwood, A.R.; Holliday, N.J. Effects of Tebufenozide on the Assemblage of Moths (Lepidoptera) in an Operational Spruce Budworm (Lepidoptera: Tortricidae) suppression programme. Can. Entomol. 2019, 151, 651–676. [Google Scholar] [CrossRef]
- Greenbank, D.O. The Role of Climate and Dispersal in the Initiation of Outbreaks of the Spruce Budworm in New Brunswick. II. The role of dispersal. Can. J. Zool. 1957, 35, 385–403. [Google Scholar] [CrossRef]
- Miller, C.A.; Greenbank, D.O.; Kettela, E.G. Estimated Egg Deposition by Invading Spruce Budworm Moths (Lepidoptera: Tortricidae). Can. Entomol. 1978, 110, 609–615. [Google Scholar] [CrossRef]
- Greenbank, D.O.; Schaefer, G.W.; Rainey, R.C. Spruce Budworm (Lepidoptera: Tortricidae) Moth Flight and Dispersal: New Understanding from Canopy Observations, Radar, and Aircraft. Mem. Ent. Soc. Can. 1980, 112, 1–49. [Google Scholar] [CrossRef]
- Sanders, C.J. Evaluation of Sex Attract Ant Traps for Monitoring Spruce Budworm Populations (Lepidoptera: Tortricidae). Can. Entomol. 1978, 110, 43–50. [Google Scholar] [CrossRef]
- Rhainds, M.; Heard, S.B. Sampling Procedures and Adult Sex Ratios in Spruce Budworm. Entomol. Exp. Appl. 2014, 154, 91–101. [Google Scholar] [CrossRef]
- Gros, A.; Hovestadt, T.; Poethke, J. Evolution of sex-biased dispersal: The role of sex-specific dispersal costs, demographic stochasticity, and inbreeding. Ecol. Model. 2008, 219, 226–233. [Google Scholar] [CrossRef]
- Nowicky, P.; Vrabec, V. Evidence for positive density-dependent emigration in butterfly metapopulations. Oecologia 2011, 167, 657–665. [Google Scholar] [CrossRef]
- Plazio, E.; Margol, T.; Nowicky, P. Intersexual differences in density-dependent dispersal and their evolutionary drivers. J. Evol. Biol. 2020, 33, 1495–1506. [Google Scholar] [CrossRef]
- Outram, I. Aspects of Mating in the Spruce Budworm, Choristoneura fumiferana (Lepidoptera: Tortricidae). Can. Entomol. 1971, 103, 1121–1128. [Google Scholar] [CrossRef]
- Turner, W.B. Female Lepidoptera at Light Traps. J. Agric. Res. 1918, 18, 475–481. [Google Scholar]
- Williams, C.B. An Analysis of Four Years Captures of Insects in a Light Trap. Part I. General Survey; Sex Proportion; Phenology; and Time of Flight. Proc. R. Entomol. Soc. 1939, 89, 79–131. [Google Scholar] [CrossRef]
- Garris, H.W.; Snyder, J.A. Sex-specific Attraction of Moth Species to Ultraviolet Light Traps. Southeast. Nat. 2010, 9, 427–434. [Google Scholar] [CrossRef]
- Teder, T.; Kaasik, A.; Taits, K.; Tammaru, T. Why Do Males Emerge before Females? Sexual Size Dimorphism Drives Sexual Bimaturism in Insects. Biol. Rev. 2021, 96, 2461–2475. [Google Scholar] [CrossRef] [PubMed]
- Ekrem, R.K.; Kokko, H. Sexual Conflict over Phenological Traits: Selection for Protandry Can Lock Populations into Temporally Mismatched Reproduction. Evolution 2022, 77, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Rhainds, M. Field Assessment of Female Mating Success Based on the Presence—Absence of Spermatophore: A Case Study with Spruce Budworm, Choristoneura fumiferana. Ann. Zool. Fenn. 2013, 50, 377–384. [Google Scholar] [CrossRef]
- Régnière, J. An oviposition model for the spruce budworms, Choristoneura fumiferana (Lepidoptera: Tortricidae). Can. Entomol. 1983, 115, 1371–1382. [Google Scholar] [CrossRef]
- Roscoe, L.E.; MacKinnon, W.; Régnière, J.; Forbes, G.; Brophy, M.; Lamb, R. Use of sprayable sex pheromone formulation in landscape-level control of Choristoneura fumiferana populations. Insects 2022, 13, 1175. [Google Scholar] [CrossRef]
- Gatehouse, A.G. Behavior and Ecological Genetics of Wind-Borne Migration by Insects. Annu. Rev. Entomol. 1997, 42, 475–502. [Google Scholar] [CrossRef]
- Rhainds, M. Variation in Wing Load of Female Spruce Budworms (Lepidoptera: Tortricidae) During the Course of an Outbreak: Evidence for Phenotypic Response to Habitat Deterioration in Collapsing Populations. Environ. Entomol. 2019, 49, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Régnière, J.; Nealis, V.G. Density Dependence of Egg Recruitment and Moth Dispersal in Spruce Budworms. Forests 2019, 10, 706. [Google Scholar] [CrossRef]
- Garcia, M.; Sturtevant, B.R.; Saint-Amant, R.; Charney, J.J.; Delisle, J.; Boulanger, Y.; Townsend, P.A.; Régnière, J. Modeling Weather-Driven Long-Distance Dispersal of Spruce Budworm Moths (Choristoneura fumiferana). Part 1. Model Description. Agric. Forest. Meteorol. 2022, 315, 108815. [Google Scholar] [CrossRef]
- Thomas, A.W. Relationship Between Oviposition History, Current Fecundity, and the Susceptibility of Spruce Budworm Moths (Lepidoptera: Tortricidae) to ULV Aerial Sprays of Insecticides. Can. Entomol. 1978, 110, 337–344. [Google Scholar] [CrossRef]
- Rivet, M.P.; Albert, P.J. Oviposition Behavior in Spruce budworm Choristoneura Fumiferana (Clem.) (Lepidoptera: Tortricidae). J. Insect. Behav. 1990, 3, 395–400. [Google Scholar] [CrossRef]
- Wallace, E.K.; Albert, P.; McNeil, J.N. Oviposition Behavior of the Eastern Spruce Budworm Choristoneura fumiferana (Clemens) (Lepidoptera: Tortricidae). J. Insect. Behav. 2004, 17, 145–154. [Google Scholar] [CrossRef]
- Blais, J.R. Effects of the Destruction of the Current Year’s Foliage of Balsam Fir on the Fecundity and Habits of Flight of the Spruce Budworm. Can. Entomol. 1953, 85, 446–448. [Google Scholar] [CrossRef]
- Miller, C.A. A Technique for Estimating the Fecundity of Natural Populations of the Spruce Budworm. Can. J. Zool. 1957, 35, 1–13. [Google Scholar] [CrossRef]
- Van Nest, K.; Swistek, S.E.; Olmstead, M.L.; Mota-Peynado, A.; Ewing, R.D.; Brabec, D.; Mitzel, D.; Oppert, B.; Cohnstaedt, L.W.; Shults, P. Assessing the Feasibility, Safety, and Nutritional Quality of using Wild-Caught Pest Flies in Animal Feed. J. Econ. Entomol. 2024, in press. [Google Scholar] [CrossRef]
- Nosko, P.; Roberts, K.; Knight, T.; Marcellus, A. Growth and Chemical Responses of Balsmal Fir Saplings released from intense browsing pressure in the boreal forests of western Newfoundland, Canada. Forest Ecol. Manag. 2020, 460, 117839. [Google Scholar] [CrossRef]
- Buchkowski, R.W.; Leroux, S.J.; Yan, Y.; Entem, A.; Schmelzer, I.; Fenichel, E.P. The effects of moose browsing on balsam fir and forest recovery vary with bioclimatic and human use across the island of Newfoundland. Can. J. Forest Res. 2023, 53, 688–699. [Google Scholar] [CrossRef]
- Amani, M.; Salehi, B.; Mahdavi, S.; Granger, J.E.; Hisco, B.; Hansson, A. Wetland classification using multi-source and multi-temporal optical remote sensing data in Newfoundland and Labrador, Canada. Can. J. Remote Sens. 2017, 43, 360–373. [Google Scholar] [CrossRef]
- Mahdianpari, M.; Jafarzadeh, J.; Granger, J.E.; Mohammadimanesh, F.; Brisco, B.; Salehi, B.; Homayouni, S.; Weng, Q. A large-scale change monitoring of wetlands using time series Landsat imagery on Google Earth Engine: A case study in Newfoundland. GIScience Remote Sens. 2020, 57, 1102–1124. [Google Scholar] [CrossRef]
- Fox, G.; Beke, J.; Hopkin, T.; McKenney. A framework for the use of economic thresholds in forest pest management. Forestry Chronicle 1997, 73, 331–339. [Google Scholar] [CrossRef]
- Pearce, D.W. The economic value of forest ecosystems. Ecosyst. Health 2001, 7, 284–296. [Google Scholar] [CrossRef]
- Raihan, A. A review on the integrative approach for economic valuation of forest ecosystem services. J. Environ. Sci. Econ. 2023, 2, 1–18. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).