Ethylene: A Modulator of the Phytohormone-Mediated Insect Herbivory Network in Plants
Abstract
:Simple Summary
Abstract
1. Introduction
2. Plant Defence against Insect Herbivory
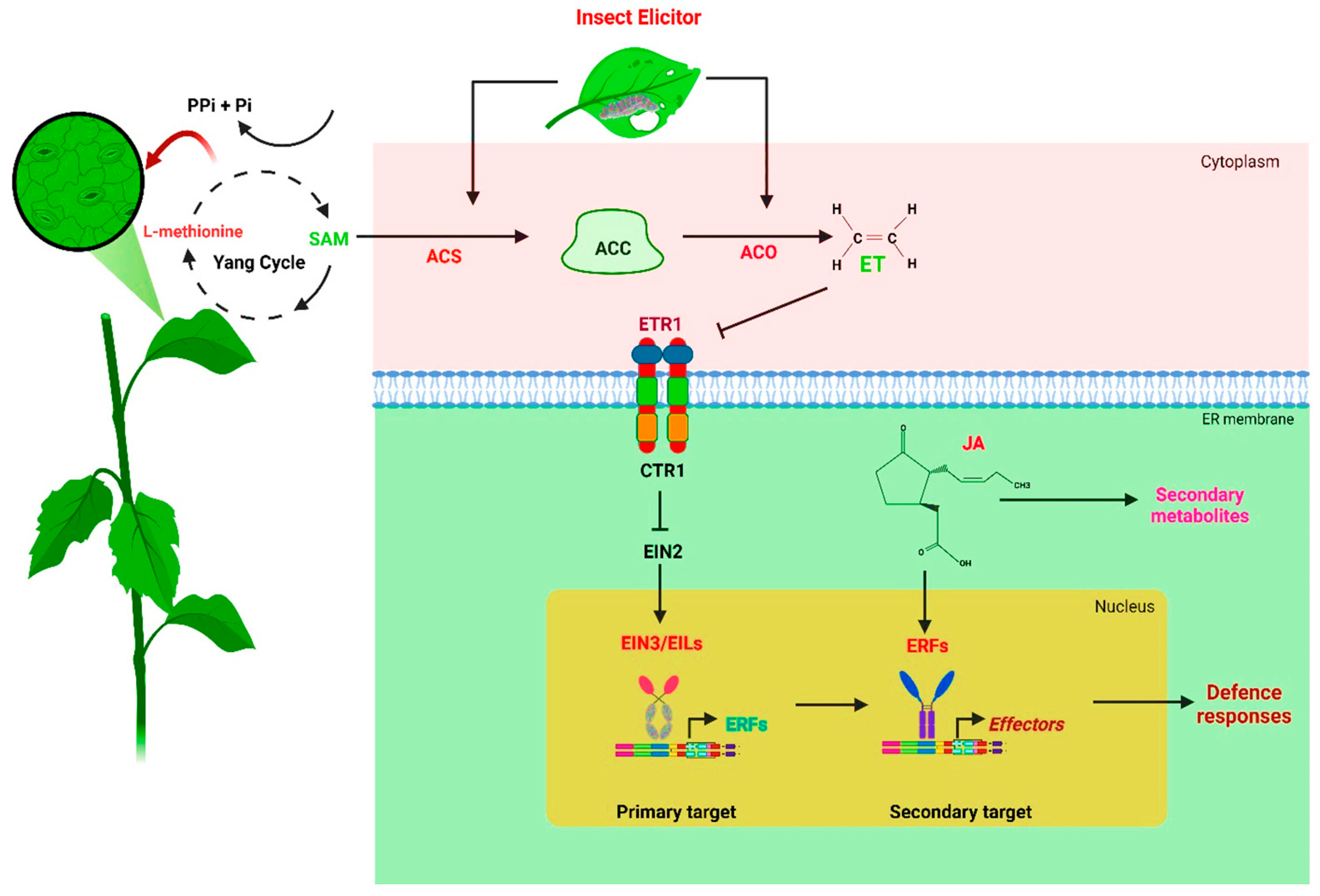
3. ET Biosynthesis, Signal Transduction, and the Response to Herbivory
4. Crosstalk between ET and Other Phytohormones in Insect Herbivory
4.1. JA and ET Synergically Help Plants Resistant to Herbivory
4.2. SA Suppresses JA/ET during Herbivory
4.3. Prospects and Potential of BRs in ET-Mediated Insect Herbivory
5. Ethephon Applications in Herbivory Studies
6. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santamaria, M.E.; Martinez, M.; Cambra, I.; Grbic, V.; Diaz, I. Understanding plant defence responses against herbivore attacks: An essential first step towards the development of sustainable resistance against pests. Transgenic Res. 2013, 22, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Mertens, D.; Fernández de Bobadilla, M.; Rusman, Q.; Bloem, J.; Douma, J.C.; Poelman, E.H. Plant defence to sequential attack is adapted to prevalent herbivores. Nat. Plants 2021, 7, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Sewelam, N.A.; Kazan, K.; Schenk, P.M. Global Plant Stress Signaling: Reactive Oxygen Species at the Cross-Road. Front. Plant Sci. 2016, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Poelman, E.H.; Bourne, M.E.; Croijmans, L.; Cuny, M.A.C.; Delamore, Z.; Joachim, G.; Kalisvaart, S.N.; Kamps, B.B.J.; Longuemare, M.; Suijkerbuijk, H.A.C.; et al. Bringing Fundamental Insights of Induced Resistance to Agricultural Management of Herbivore Pests. J. Chem. Ecol. 2023, 49, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Martinez, M.; Diaz, I.; Santamaria, M.E. The Price of the Induced Defense Against Pests: A Meta-Analysis. Front. Plant Sci. 2020, 11, 615122. [Google Scholar] [CrossRef] [PubMed]
- El Sabagh, A.; Islam, M.S.; Hossain, A.; Iqbal, M.A.; Mubeen, M.; Waleed, M.; Reginato, M.; Battaglia, M.L.; Ahmed, S.; Rehman, A.; et al. Phytohormones as Growth Regulators During Abiotic Stress Tolerance in Plants. Front. Agron. 2022, 4, 765068. [Google Scholar] [CrossRef]
- Chen, H.; Bullock, D.A.; Alonso, J.M.; Stepanova, A.N. To Fight or to Grow: The Balancing Role of Ethylene in Plant Abiotic Stress Responses. Plants 2022, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhao, X.; Bürger, M.; Chory, J.; Wang, X. The role of ethylene in plant temperature stress response. Trends Plant Sci. 2023, 28, 808–824. [Google Scholar] [CrossRef]
- von Dahl, C.C.; Baldwin, I.T. Deciphering the Role of Ethylene in Plant–Herbivore Interactions. J. Plant Growth Regul. 2007, 26, 201–209. [Google Scholar] [CrossRef]
- Tlili, A.; Altinay, F.; Huang, R.; Altinay, Z.; Olivier, J.; Mishra, S.; Jemni, M.; Burgos, D. Are we there yet? A systematic literature review of Open Educational Resources in Africa: A combined content and bibliometric analysis. PLoS ONE 2022, 17, e0262615. [Google Scholar] [CrossRef] [PubMed]
- Bertocci, F.; Mannino, G. Pearls before Swine: Plant-Derived Wastes to Produce Low-Cholesterol Meat from Farmed Pigs—A Bibliometric Analysis Combined to Meta-Analytic Studies. Foods 2023, 12, 571. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.A. Interplay between insects and plants: Dynamic and complex interactions that have coevolved over millions of years but act in milliseconds. J. Exp. Bot. 2015, 66, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Tooker, J.F.; Giron, D. The Evolution of Endophagy in Herbivorous Insects. Front. Plant Sci. 2020, 11, 581816. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Reymond, P. Molecular Interactions Between Plants and Insect Herbivores. Annu. Rev. Plant Biol. 2019, 70, 527–557. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Malukani, K.K.; Chandan, R.K.; Sonti, R.V.; Jha, G. How Plants Respond to Pathogen Attack: Interaction and Communication. In Sensory Biology of Plants; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Duan, C.; Yu, J.; Bai, J.; Zhu, Z.; Wang, X. Induced defense responses in rice plants against small brown planthopper infestation. Crop J. 2014, 2, 55–62. [Google Scholar] [CrossRef]
- Zebelo, S.A.; Maffei, M.E. Role of early signalling events in plant-insect interactions. J. Exp. Bot. 2015, 66, 435–448. [Google Scholar] [CrossRef] [PubMed]
- War, A.R.; Taggar, G.K.; Hussain, B.; Taggar, M.S.; Nair, R.M.; Sharma, H.C. Plant Defense Against Herbivory and Insect Adaptations. AoB Plants 2018, 10, ply037. [Google Scholar] [CrossRef]
- Croijmans, L.; Valstar, R.T.; Schuur, L.; Jacobs, I.; van Apeldoorn, D.F.; Poelman, E.H. Intraspecific plant variation and nonhost herbivores affect parasitoid host location behaviour. Anim. Behav. 2022, 194, 169–184. [Google Scholar] [CrossRef]
- Gasmi, L.; Martínez-Solís, M.; Frattini, A.; Ye, M.; Collado, M.C.; Turlings, T.C.J.; Erb, M.; Herrero, S. Can Herbivore-Induced Volatiles Protect Plants by Increasing the Herbivores’ Susceptibility to Natural Pathogens? Appl. Environ. Microbiol. 2018, 85, e01468-18. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Maioli, A.; Gianoglio, S.; Moglia, A.; Acquadro, A.; Valentino, D.; Milani, A.M.; Prohens, J.; Orzaez, D.; Granell, A.; Lanteri, S.; et al. Simultaneous CRISPR/Cas9 Editing of Three PPO Genes Reduces Fruit Flesh Browning in Solanum melongena L. Front. Plant Sci. 2020, 11, 607161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shen, Y.; Li, Z.-h.; Xie, X.; Gong, E.; Tian, J.; Si, X.; Wang, Y.; Gao, N.; Shu, C.; et al. Effects of high hydrostatic pressure and thermal processing on anthocyanin content, polyphenol oxidase and β-glucosidase activities, color, and antioxidant activities of blueberry (Vaccinium Spp.) puree. Food Chem. 2020, 342, 128564. [Google Scholar] [CrossRef] [PubMed]
- Tetreault, H.M.; Grover, S.; Scully, E.D.; Gries, T.; Palmer, N.A.; Sarath, G.; Louis, J.; Sattler, S.E. Global Responses of Resistant and Susceptible Sorghum (Sorghum bicolor) to Sugarcane Aphid (Melanaphis sacchari). Front. Plant Sci. 2019, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Kiani, M.; Szczepaniec, A. Effects of sugarcane aphid herbivory on transcriptional responses of resistant and susceptible sorghum. BMC Genom. 2018, 19, 774. [Google Scholar] [CrossRef] [PubMed]
- Rehrig, E.M.; Appel, H.M.; Jones, A.D.; Schultz, J.C.; Bostock, R. Roles for jasmonate- and ethylene-induced transcription factors in the ability of Arabidopsis to respond differentially to damage caused by two insect herbivores. Front. Plant Sci. 2014, 5, 407. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, S.; Wang, Y.; Zeng, W.; Jin, B. Plant Responses to Herbivory, Wounding, and Infection. Int. J. Mol. Sci. 2022, 23, 7031. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene Role in Plant Growth, Development and Senescence: Interaction with Other Phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S.; Simancas, B.; Müller, M. Ethylene signaling cross-talk with other hormones in Arabidopsis thaliana exposed to contrasting phosphate availability: Differential effects in roots, leaves and fruits. J. Plant Physiol. 2018, 226, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Riyazuddin, R.; Verma, R.; Singh, K.; Nisha, N.; Keisham, M.; Bhati, K.K.; Kim, S.T.; Gupta, R. Ethylene: A Master Regulator of Salinity Stress Tolerance in Plants. Biomolecules 2020, 10, 959. [Google Scholar] [CrossRef] [PubMed]
- Fatma, M.; Asgher, M.; Iqbal, N.; Rasheed, F.; Sehar, Z.; Sofo, A.; Khan, N.A. Ethylene Signaling under Stressful Environments: Analyzing Collaborative Knowledge. Plants 2022, 11, 2211. [Google Scholar] [CrossRef] [PubMed]
- Keunen, E.; Schellingen, K.; Vangronsveld, J.; Cuypers, A. Ethylene and Metal Stress: Small Molecule, Big Impact. Front. Plant Sci. 2016, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Cao, X.; Qin, C.; Chen, S.; Cai, J.; Sun, C.; Kong, L.; Tao, J. Effects of plant growth regulators and sucrose on proliferation and quality of embryogenic tissue in Picea pungens. Sci. Rep. 2023, 13, 13194. [Google Scholar] [CrossRef] [PubMed]
- Poel, B.V.d.; Smet, D.; Straeten, D.V.D. Update on Ethylene and Hormonal Cross Talk Ethylene and Hormonal Cross Talk in Vegetative Growth and Development. Plant Physiol. 2015, 169, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Binder, B.M. Ethylene signaling in plants. J. Biol. Chem. 2020, 295, 7710–7725. [Google Scholar] [CrossRef] [PubMed]
- Ravanbakhsh, M.; Sasidharan, R.; Voesenek, L.A.C.J.; Kowalchuk, G.A.; Jousset, A. Microbial modulation of plant ethylene signaling: Ecological and evolutionary consequences. Microbiome 2018, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Houben, M.; Van de Poel, B. 1-Aminocyclopropane-1-Carboxylic Acid Oxidase (ACO): The Enzyme That Makes the Plant Hormone Ethylene. Front. Plant Sci. 2019, 10, 695. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Yue, P.; Bu, H.; Han, D.; Wang, A. Genome-wide analysis of ACO and ACS genes in pear (Pyrus ussuriensis). Vitr. Cell. Dev. Biol.-Plant 2019, 56, 193–199. [Google Scholar] [CrossRef]
- Schellingen, K.; Van Der Straeten, D.; Remans, T.; Vangronsveld, J.; Keunen, E.; Cuypers, A. Ethylene signalling is mediating the early cadmium-induced oxidative challenge in Arabidopsis thaliana. Plant Sci. Int. J. Exp. Plant Biol. 2015, 239, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Pattyn, J.; Vaughan-Hirsch, J.; Van de Poel, B. The regulation of ethylene biosynthesis: A complex multilevel control circuitry. New Phytol. 2020, 229, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Yoon, G.M.; Shemansky, J.M.; Lin, D.Y.; Ying, Z.I.; Chang, J.; Garrett, W.M.; Kessenbrock, M.; Groth, G.; Tucker, M.L.; et al. CTR1 phosphorylates the central regulator EIN2 to control ethylene hormone signaling from the ER membrane to the nucleus in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 19486–19491. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Shen, Z.; Huang, S.-s.C.; Schmitz, R.J.; Urich, M.A.; Briggs, S.P.; Ecker, J.R. Processing and Subcellular Trafficking of ER-Tethered EIN2 Control Response to Ethylene Gas. Science 2012, 338, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Zhang, C.; Ji, Y.; Zhao, Q.; He, W.; An, F.; Jiang, L.; Guo, H. Activation of ethylene signaling is mediated by nuclear translocation of the cleaved EIN2 carboxyl terminus. Cell Res. 2012, 22, 1613–1616. [Google Scholar] [CrossRef] [PubMed]
- Groen, S.C.; Whiteman, N.K.; Bahrami, A.K.; Wilczek, A.M.; Cui, J.; Russell, J.A.; Cibrián-Jaramillo, A.; Butler, I.A.; Rana, J.D.; Huang, G.H.; et al. Pathogen-Triggered Ethylene Signaling Mediates Systemic-Induced Susceptibility to Herbivory in Arabidopsis. Plant Cell 2013, 25, 4755–4766. [Google Scholar] [CrossRef] [PubMed]
- Debbarma, J.; Sarki, Y.N.; Saikia, B.; Boruah, H.P.D.; Singha, D.L.; Chikkaputtaiah, C. Ethylene Response Factor (ERF) Family Proteins in Abiotic Stresses and CRISPR–Cas9 Genome Editing of ERFs for Multiple Abiotic Stress Tolerance in Crop Plants: A Review. Mol. Biotechnol. 2019, 61, 153–172. [Google Scholar] [CrossRef] [PubMed]
- Dolgikh, V.; Pukhovaya, E.M.; Zemlyanskaya, E.V. Shaping Ethylene Response: The Role of EIN3/EIL1 Transcription Factors. Front. Plant Sci. 2019, 10, 1030. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.-Y.; Catinot, J.r.m.; Zimmerli, L. Ethylene response factors in Arabidopsis immunity. J. Exp. Bot. 2016, 67, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, X.; Zhang, J.; Zhao, H.; Tan, S.; Xu, W.-L.; Pan, J.; Yang, F.; Pi, E. ERF subfamily transcription factors and their function in plant responses to abiotic stresses. Front. Plant Sci. 2022, 13, 1042084. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, R.; Voesenek, L.A.C.J. Update: Ethylene-Mediated Traits Ethylene-Mediated Acclimations to Flooding Stress. Plant Physiol. 2015, 169, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.-J.; Chen, H.-W.; Ma, B.; Zhang, W.-K.; Chen, S.-Y.; Zhang, J.-S. The Role of Ethylene in Plants Under Salinity Stress. Front. Plant Sci. 2015, 6, 1059. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.H.; Campol, J.R.; Kang, H.; Xu, J.; Chung, M.-Y.; Kim, C.K. Role of Ethylene Biosynthesis Genes in the Regulation of Salt Stress and Drought Stress Tolerance in Petunia. Front. Plant Sci. 2022, 13, 844449. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhang, Y.K.; Kremling, K.A.G.; Ding, Y.; Bennett, J.S.; Bae, J.S.; Kim, D.K.; Kolomiets, M.V.; Schmelz, E.A.; Schroeder, F.C.; et al. Ethylene signaling regulates natural variation in the abundance of antifungal acetylated diferuloylsucroses and Fusarium graminearum resistance in maize seedling roots. New Phytol. 2018, 221, 2096–2111. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wei, C.; Ma, Q.; Dong, H.; Shi, K.; Zhou, Y.; Foyer, C.H.; Yu, J. Ethylene response factors 15 and 16 trigger jasmonate biosynthesis in tomato during herbivore resistance. Plant Physiol. 2021, 185, 1182–1197. [Google Scholar] [CrossRef] [PubMed]
- Figon, F.; Baldwin, I.T.; Gaquerel, E. Ethylene is a local modulator of jasmonate-dependent phenolamide accumulation during Manduca sexta herbivory in Nicotiana attenuata. Plant Cell Environ. 2021, 44, 964–981. [Google Scholar] [CrossRef] [PubMed]
- Harfouche, A.L.; Shivaji, R.; Stocker, R.; Williams, P.W.; Luthe, D.S. Ethylene signaling mediates a maize defense response to insect herbivory. Mol. Plant-Microbe Interact. 2006, 19, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Casteel, C.L.; De Alwis, M.; Bak, A.; Dong, H.; Whitham, S.A.; Jander, G. Disruption of Ethylene Responses by Turnip mosaic virus Mediates Suppression of Plant Defense against the Green Peach Aphid Vector. Plant Physiol. 2015, 169, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Bak, A.; Patton, M.F.; Perilla-Henao, L.M.; Aegerter, B.J.; Casteel, C.L. Ethylene signaling mediates potyvirus spread by aphid vectors. Oecologia 2019, 190, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kamphuis, L.G.; Guo, Y.-Q.; Jacques, S.; Singh, K.B.; Gao, L. Ethylene Is Not Essential for R-Gene Mediated Resistance but Negatively Regulates Moderate Resistance to Some Aphids in Medicago truncatula. Int. J. Mol. Sci. 2020, 21, 4657. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Yang, X.; Shi, Z.; Miao, X. Novel crosstalk between ethylene- and jasmonic acid-pathway responses to a piercing-sucking insect in rice. New Phytol. 2020, 225, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, J.; Ju, H.; Liu, X.; Erb, M.; Wang, X.; Lou, Y.-g. Contrasting effects of ethylene biosynthesis on induced plant resistance against a chewing and a piercing-sucking herbivore in rice. Mol. Plant 2014, 7, 1670–1682. [Google Scholar] [CrossRef] [PubMed]
- Altmann, M.; Altmann, S.; Rodríguez, P.A.L.; Weller, B.; Elorduy Vergara, L.; Palme, J.; Marín-de la Rosa, N.; Sauer, M.; Wenig, M.; Villaécija-Aguilar, J.A.; et al. Extensive signal integration by the phytohormone protein network. Nature 2020, 583, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Dillon, F.M.; Tejedor, M.D.; Ilina, N.; Chludil, H.D.; Mithöfer, A.; Pagano, E.A.; Zavala, J.A. Solar UV-B radiation and ethylene play a key role in modulating effective defenses against Anticarsia gemmatalis larvae in field-grown soybean. Plant Cell Environ. 2018, 41, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Dillon, F.M.; Chludil, H.D.; Mithöfer, A.; Zavala, J.A. Solar UVB-inducible ethylene alone induced isoflavonoids in pods of field-grown soybean, an important defense against stink bugs. Environ. Exp. Bot. 2020, 178, 104167. [Google Scholar] [CrossRef]
- Louis, J.; Basu, S.; Varsani, S.; Castano-Duque, L.; Jiang, V.; Williams, W.P.; Felton, G.W.; Luthe, D.S. Ethylene Contributes to maize insect resistance1-Mediated Maize Defense against the Phloem Sap-Sucking Corn Leaf Aphid. Plant Physiol. 2015, 169, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Collum, T.D.; Culver, J.N. The impact of phytohormones on virus infection and disease. Curr. Opin. Virol. 2016, 17, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, Y. Current understanding of the interplays between host hormones and plant viral infections. PLoS Pathog. 2021, 17, e1009242. [Google Scholar] [CrossRef] [PubMed]
- Berens, M.L.; Berry, H.M.; Mine, A.; Argueso, C.T.; Tsuda, K. Evolution of Hormone Signaling Networks in Plant Defense. Annu. Rev. Phytopathol. 2017, 55, 401–425. [Google Scholar] [CrossRef] [PubMed]
- Klessig, D.F.; Choi, H.W.; Dempsey, D.M.A. Systemic Acquired Resistance and Salicylic Acid: Past, Present, and Future. Mol. Plant-Microbe Interact. 2018, 31, 871–888. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z. Molecular basis for jasmonate and ethylene signal interactions in Arabidopsis. J. Exp. Bot. 2014, 65, 5743–5748. [Google Scholar] [CrossRef] [PubMed]
- Venkategowda, R.; Maria Vera, J.; Sapna, H.; Nataraja, N.K.; Sheshshayee, M.S. Chapter 17—Abiotic and biotic stress interactions in plants: A cross-tolerance perspective. In Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants; Mohammad Anwar, H., Fulai, L., David, J.B., Masayuki, F., Bingru, H., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 267–302. [Google Scholar]
- Song, S.; Qi, T.; Wasternack, C.; Xie, D. Jasmonate signaling and crosstalk with gibberellin and ethylene. Curr. Opin. Plant Biol. 2014, 21, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Lee, B.M. Friends or foes: New insights in jasmonate and ethylene co-actions. Plant Cell Physiol. 2015, 56, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-C.; Liao, P.-M.; Kuo, W.-W.; Lin, T.-p. The Arabidopsis ETHYLENE RESPONSE FACTOR1 Regulates Abiotic Stress-Responsive Gene Expression by Binding to Different cis-Acting Elements in Response to Different Stress Signals. Plant Physiol. 2013, 162, 1566–1582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, Z.; An, F.; Hao, D.; Li, P.; Song, J.; Yi, C.; Guo, H. Jasmonate-Activated MYC2 Represses ETHYLENE INSENSITIVE3 Activity to Antagonize Ethylene-Promoted Apical Hook Formation in Arabidopsis. Plant Cell 2014, 26, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; An, F.; Feng, Y.; Li, P.; Xue, L.; Jiang, Z.; Kim, J.-M.; To, T.K.; Li, W.; Zhang, X.; et al. Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 12539–12544. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C.M. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed]
- Nishad, R.; Ahmed, T.A.; Rahman, V.j.; Kareem, A. Modulation of Plant Defense System in Response to Microbial Interactions. Front. Microbiol. 2020, 11, 1298. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Huang, H.; Gao, H.; Wang, J.; Wu, D.; Liu, X.; Yang, S.; Zhai, Q.; Li, C.; Qi, T.; et al. Interaction between MYC2 and ETHYLENE INSENSITIVE3 Modulates Antagonism between Jasmonate and Ethylene Signaling in Arabidopsis. Plant Cell 2014, 26, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Paudel, J.R.; Bede, J.C. Ethylene Signaling Modulates Herbivore-Induced Defense Responses in the Model Legume Medicago truncatula. Mol. Plant-Microbe Interact. 2015, 28, 569–579. [Google Scholar] [CrossRef]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.S.; Singh, V.K.; Singh, A.K.; et al. Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef] [PubMed]
- Walling, L.L. Avoiding Effective Defenses: Strategies Employed by Phloem-Feeding Insects. Plant Physiol. 2008, 146, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Vos, I.A.; Pieterse, C.M.J.; Wees, S.C.M.V. Costs and benefits of hormone-regulated plant defences. Plant Pathol. 2013, 62, 43–55. [Google Scholar] [CrossRef]
- Van der Does, D.; Leon-Reyes, A.; Koornneef, A.; Van Verk, M.C.; Rodenburg, N.; Pauwels, L.; Goossens, A.; Körbes, A.P.; Memelink, J.; Ritsema, T.; et al. Salicylic Acid Suppresses Jasmonic Acid Signaling Downstream of SCFCOI1-JAZ by Targeting GCC Promoter Motifs via Transcription Factor ORA59. Plant Cell 2013, 25, 744–761. [Google Scholar] [CrossRef] [PubMed]
- Zander, M.; Thurow, C.; Gatz, C. TGA Transcription Factors Activate the Salicylic Acid-Suppressible Branch of the Ethylene-Induced Defense Program by Regulating ORA59 Expression. Plant Physiol. 2014, 165, 1671–1683. [Google Scholar] [CrossRef] [PubMed]
- Caarls, L.; Pieterse, C.M.J.; Van Wees, S.C.M. How salicylic acid takes transcriptional control over jasmonic acid signaling. Front. Plant Sci. 2015, 6, 170. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Jiang, J.; Wang, C.-Q.; Dehesh, K. ORA59 and EIN3 interaction couples jasmonate-ethylene synergistic action to antagonistic salicylic acid regulation of PDF expression. J. Integr. Plant Biol. 2017, 59, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sonbol, F.-M.; Huot, B.; Gu, Y.; Withers, J.; Mwimba, M.; Yao, J.; He, S.Y.; Dong, X. Salicylic acid receptors activate jasmonic acid signalling through a non-canonical pathway to promote effector-triggered immunity. Nat. Commun. 2016, 7, 13099. [Google Scholar] [CrossRef] [PubMed]
- Leon-Reyes, A.; Du, Y.; Koornneef, A.; Proietti, S.; Körbes, A.P.; Memelink, J.; Pieterse, C.M.J.; Ritsema, T. Ethylene signaling renders the jasmonate response of Arabidopsis insensitive to future suppression by salicylic Acid. Mol. Plant-Microbe Interact. 2010, 23, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Alon, M.; Malka, O.; Eakteiman, G.; Elbaz, M.; Moyal Ben Zvi, M.; Vainstein, A.; Morin, S. Activation of the Phenylpropanoid Pathway in Nicotiana tabacum Improves the Performance of the Whitefly Bemisia tabaci via Reduced Jasmonate Signaling. PLoS ONE 2013, 8, e76619. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-X.; Qian, L.-X.; Wang, X.-W.; Shao, R.X.; Hong, Y.; Liu, S.-S.; Wang, X. A salivary effector enables whitefly to feed on host plants by eliciting salicylic acid-signaling pathway. Proc. Natl. Acad. Sci. USA 2018, 116, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, M.; Abd-El-Haliem, A.; Bleeker, P.; Dicke, M.; Escobar-Bravo, R.; Cheng, G.; Haring, M.A.; Kant, M.R.; Kappers, I.F.; Klinkhamer, P.G.L.; et al. Thrips advisor: Exploiting thrips-induced defences to combat pests on crops. J. Exp. Bot. 2018, 69, 1837–1848. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Liu, Y.; Dong, R.; Bai, L.; Yu, X.; Li, Y. The physiological and molecular mechanism of brassinosteroid in response to stress: A review. Biol. Res. 2018, 51, 46. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.L.; de Almeida, M.; Rossi, M.L.; Martinelli, A.P.; Litholdo Junior, C.G.; Figueira, A.; Rampelotti-Ferreira, F.T.; Vendramim, J.D.; Benedito, V.A.; Peres, L.E.P. Brassinosteroids interact negatively with jasmonates in the formation of anti-herbivory traits in tomato. J. Exp. Bot. 2009, 60, 4347–4361. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.-H.; Baldwin, I.T.; Wu, J. Silencing brassinosteroid receptor BRI1 impairs herbivory-elicited accumulation of jasmonic acid-isoleucine and diterpene glycosides, but not jasmonic acid and trypsin proteinase inhibitors in Nicotiana attenuata. J. Integr. Plant Biol. 2013, 55, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Chae, H.S.; Kieber, J.J. Regulation of ACS protein stability by cytokinin and brassinosteroid. Plant J. Cell Mol. Biol. 2009, 57, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Chen, Y.-C.; Kieber, J.J.; Yoon, G.M. Regulation of the turnover of ACC synthases by phytohormones and heterodimerization in Arabidopsis. Plant J. 2017, 91, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Park, H.L.; Park, C.; Chen, Y.-C.; Yoon, G.M. Reciprocal antagonistic regulation of E3 ligases controls ACC synthase stability and responses to stress. Proc. Natl. Acad. Sci. USA 2021, 118, e2011900118. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Li, J.; Xia, F.-N.; Chen, J.-Y.; Lei, X.; Han, M.-Q.; Xie, L.-J.; Zhou, Q.-M.; Xiao, S. Arabidopsis SINAT Proteins Control Autophagy by Mediating Ubiquitylation and Degradation of ATG13. Plant Cell 2019, 32, 263–284. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.M.; Brennan, B.; Yang, M.; Chen, J.; Zhang, M.; Li, Z.; Wang, X.; Bassham, D.C.; Walley, J.W.; Yin, Y. Selective Autophagy of BES1 Mediated by DSK2 Balances Plant Growth and Survival. Dev. Cell 2017, 41, 33–46.e37. [Google Scholar] [CrossRef]
- Zhao, N.; Zhao, M.; Tian, Y.; Wang, Y.; Han, C.; Fan, M.; Guo, H.; Bai, M.-y. Interaction between BZR1 and EIN3 mediates signaling crosstalk between brassinosteroids and ethylene. New Phytol. 2021, 232, 2308–2323. [Google Scholar] [CrossRef] [PubMed]
- Chang, C. Q&A: How do plants respond to ethylene and what is its importance? BMC Biol. 2016, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, Y.-P.; Yang, H.-B. Effects of ethephon on physiological characteristics and gene expression of Tartary buckwheat under salt stress. Chil. J. Agric. Res. 2022, 82, 234–243. [Google Scholar] [CrossRef]
- Islam, M.N.; Mursalat, M.; Khan, M.S. A review on the legislative aspect of artificial fruit ripening. Agric. Food Secur. 2016, 5, 8. [Google Scholar] [CrossRef]
- Shakar, M.; Yaseen, M.; Mahmood, R.; Ahmad, I. Calcium carbide induced ethylene modulate biochemical profile of Cucumis sativus at seed germination stage to alleviate salt stress. Sci. Hortic. 2016, 213, 179–185. [Google Scholar] [CrossRef]
- Zhang, W.; Wen, C.-K. Preparation of ethylene gas and comparison of ethylene responses induced by ethylene, ACC, and ethephon. Plant Physiol. 2010, 48, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-y.; Zhang, T.-J.; Su, J.; Soon Chow, W.; Liu, J.-Q.; Chen, L.-L.; Li, W.; Peng, S.; Peng, C. A novel role of ethephon in controlling the noxious weed Ipomoea cairica (Linn.) Sweet. Sci. Rep. 2015, 5, 11372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, W.; Sun, G.-m.; Liu, S.-h.; Li, Y.; Wu, Q.; Wei, Y. Phenological growth stages of pineapple (Ananas comosus) according to the extended Biologische Bundesantalt, Bundessortenamt and Chemische Industrie scale. Ann. Appl. Biol. 2016, 169, 311–318. [Google Scholar] [CrossRef]
- Cocco, C.; Silvestre, W.P.; Schildt, G.W.; Tessaro, F.A. Effect of Ethephon Application on Fruit Quality at Harvest and Post-harvest Storage of Japanese Plum (Prunus salicina) cv. Fortune. Braz. Arch. Biol. Technol. 2022, 65, e20210183. [Google Scholar] [CrossRef]
- Gautam, H.; Fatma, M.; Sehar, Z.; Iqbal, N.; Albaqami, M.; Khan, N.A. Exogenously-Sourced Ethylene Positively Modulates Photosynthesis, Carbohydrate Metabolism, and Antioxidant Defense to Enhance Heat Tolerance in Rice. Int. J. Mol. Sci. 2022, 23, 1031. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Peiffer, M.; Moraes, C.M.D.; Felton, G.W. Roles of ethylene and jasmonic acid in systemic induced defense in tomato (Solanum lycopersicum) against Helicoverpa zea. Planta 2014, 239, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Abdelsamad, N.A.; Macintosh, G.C.; Leandro, L.F.S. Induction of ethylene inhibits development of soybean sudden death syndrome by inducing defense-related genes and reducing Fusarium virguliforme growth. PLoS ONE 2019, 14, e0215653. [Google Scholar] [CrossRef] [PubMed]
- Faurie, B.; Cluzet, S.; Corio-Costet, M.-F.; Mérillon, J.-M. Methyl jasmonate/ethephon cotreatment synergistically induces stilbene production in Vitis vinifera cell suspensions but fails to trigger resistance to Erysiphe necator. OENO One 2009, 43, 99–110. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bungala, L.T.d.C.; Park, C.; Dique, J.E.L.; Sathasivam, R.; Shin, S.Y.; Park, S.U. Ethylene: A Modulator of the Phytohormone-Mediated Insect Herbivory Network in Plants. Insects 2024, 15, 404. https://doi.org/10.3390/insects15060404
Bungala LTdC, Park C, Dique JEL, Sathasivam R, Shin SY, Park SU. Ethylene: A Modulator of the Phytohormone-Mediated Insect Herbivory Network in Plants. Insects. 2024; 15(6):404. https://doi.org/10.3390/insects15060404
Chicago/Turabian StyleBungala, Leonel Tarcisio da Cristina, Chanung Park, José Eulário Lampi Dique, Ramaraj Sathasivam, Su Young Shin, and Sang Un Park. 2024. "Ethylene: A Modulator of the Phytohormone-Mediated Insect Herbivory Network in Plants" Insects 15, no. 6: 404. https://doi.org/10.3390/insects15060404





