Host Volatiles Potentially Drive Two Evolutionarily Related Weevils to Select Different Grains
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Cereals
2.2. Olfactory Bioassays
Four-Arm Olfactometer
2.3. Volatile Profiles Analyses
2.3.1. Isolation and Concentration of Volatiles
2.3.2. Gas Chromatography–Mass Spectrometry Conditions
2.4. Y-Tube Olfactory Bioassay
2.5. Data Analyses
3. Results
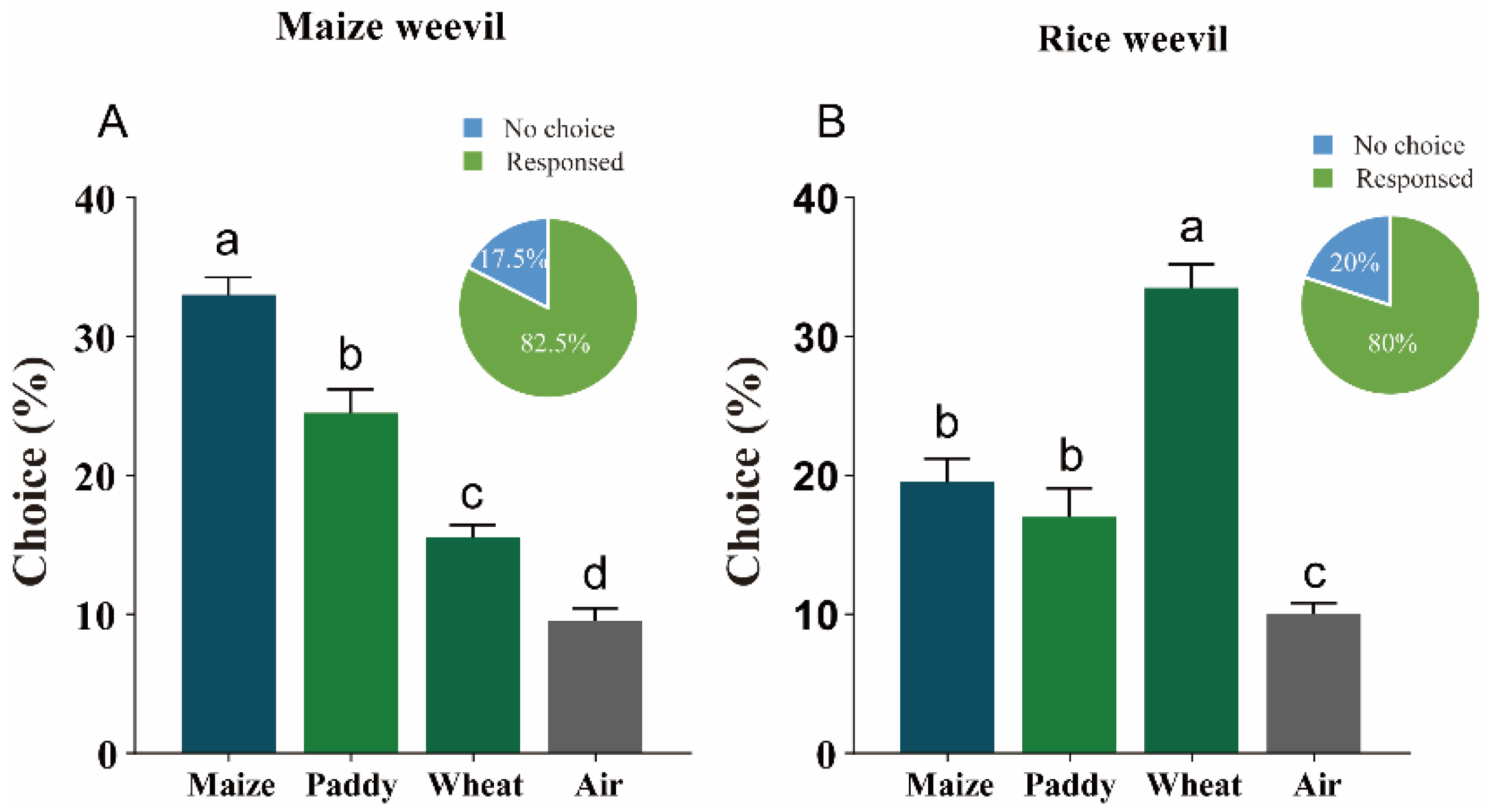
3.1. Olfactometer Bioassays
3.2. Volatile Profiles among Different Grains
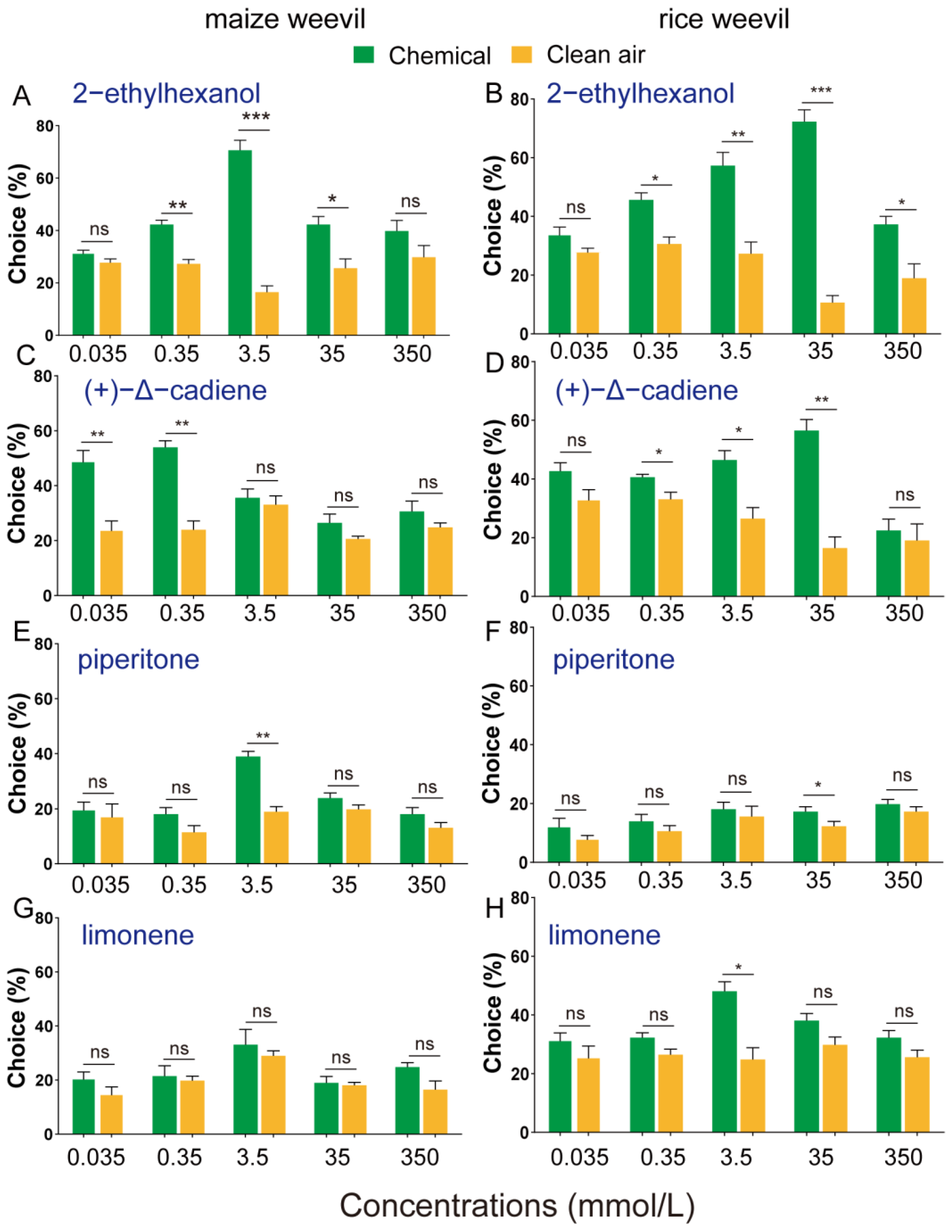
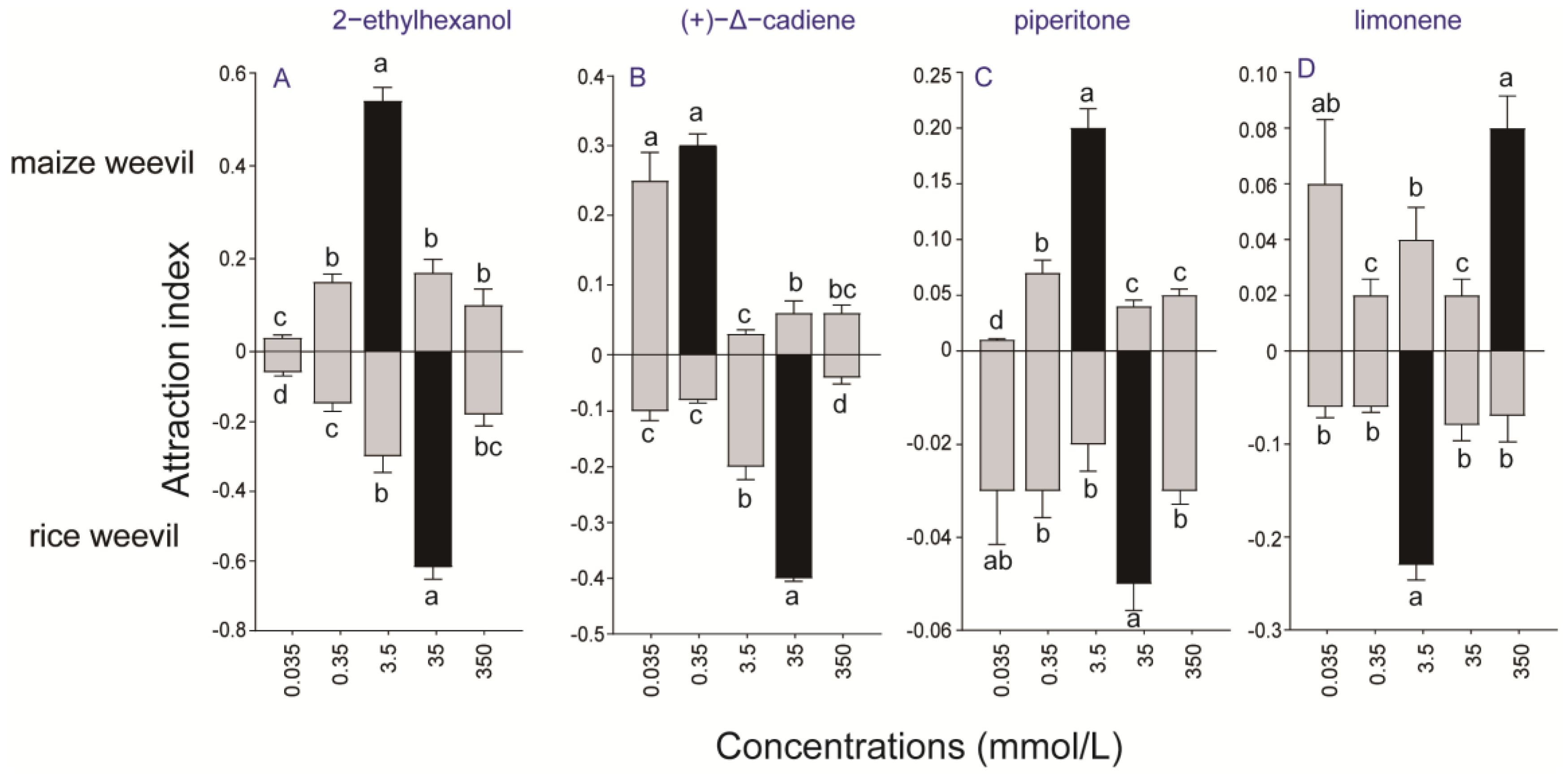
3.3. Olfactory Attraction of Selected Volatiles to Maize and Rice Weevils
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peng, W.K.; Lin, H.C.; Chen, C.N.; Wang, C.H. DNA identification of two laboratory colonies of the weevils, Sitophilus oryzae (L.) and S. zeamais Motschulsky (Coleoptera: Curculionidae) in Taiwan. J. Stored Prod. Res. 2003, 39, 225–235. [Google Scholar] [CrossRef]
- Corrêa, A.S.; Vinson, C.C.; Braga, L.S.; Guedes, R.N.C.; de Oliveira, L.O. Ancient origin and recent range expansion of the maize weevil, and its genealogical relationship to the rice weevil. Bull. Entomol. Res. 2017, 107, 9–20. [Google Scholar] [CrossRef]
- Corrêa, A.S.; de Oliveira, L.O.; Braga, L.S.; Guedes, R.N.C. Distribution of the related weevil species Sitophilus oryzae and S. zeamais in Brazil. Insect Sci. 2013, 20, 763–770. [Google Scholar] [CrossRef]
- Devi, S.R.; Thomas, A.; Rebijith, K.B.; Ramamurthy, V.V. Biology, morphology and molecular characterization of Sitophilus oryzae and Sitophilus zeamais (Coleoptera: Curculionidae). J. Stored Prod. Res. 2017, 73, 135–141. [Google Scholar] [CrossRef]
- Corrêa, A.S.; Pereira, E.J.G.; Cordeiro, E.M.G.; Braga, L.S.; Guedes, R.N.C. Insecticide resistance, mixture potentiation and fitness in populations of the maize weevil (Sitophilus zeamais). Crop Prot. 2011, 30, 1655–1666. [Google Scholar] [CrossRef]
- Pimentel, C.S.D.; Albuquerque, B.N.D.; da Rocha, S.K.L.; da Silva, A.S.; da Silva, A.B.V.; Bellon, R.; Agra-Neto, A.C.; de Aguiar, J.C.R.D.F.; Paiva, P.M.G.; Princival, J.L.; et al. Insecticidal activity of the essential oil of leaves and its major compound (1-butyl-3,4-methylenedioxybenzene) against the maize weevil, Sitophilus zeamais. Pest Manag. Sci. 2022, 78, 1008–1017. [Google Scholar] [CrossRef]
- Liu, Y.B.; Yang, X.B.; Masuda, T. Procedures of laboratory fumigation for pest control with nitric oxide gas. JOVE—J. Vis. Exp. 2017, e56309. [Google Scholar] [CrossRef]
- Chiluwal, K.; Lee, B.H.; Kwon, T.H.; Kim, J.; Park, C.G. Post-fumigation sub-lethal activities of phosphine and ethyl formate on survivorship, fertility and female sex pheromone production of Callosobruchus chinensis (L.). Sci. Rep. 2023, 13, 4333. [Google Scholar] [CrossRef]
- Golden, G.; Quinn, E.; Shaaya, E.; Kostyukovsky, M.; Poverenov, E. Coarse and nano emulsions for effective delivery of the natural pest control agent pulegone for stored grain protection. Pest Manag. Sci. 2018, 74, 820–827. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Lima, J.O.G.; Santos, J.P.; Cruz, C.D. Resistance to ddt and pyrethroids in Brazilian populations of Sitophilus zeamais Motsch (Coleoptera, Curculionidae). J. Stored Prod. Res. 1995, 31, 145–150. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Collins, P.J.; Duong, T.M.; Schlipalius, D.I.; Ebert, P.R. Genetic conservation of phosphine resistance in the rice Weevil Sitophilus oryzae (L.). J. Hered. 2016, 107, 228–237. [Google Scholar] [CrossRef]
- Nayak, M.K.; Daglish, G.J.; Phillips, T.W.; Ebert, P.R. Resistance to the fumigant phosphine and its management in insect pests of stored products: A global perspective. Annu. Rev. Entomol. 2020, 65, 333–350. [Google Scholar] [CrossRef]
- Byers, J.A.; Levi-Zada, A. Modelling push-pull management of pest insects using repellents and attractive traps in fruit tree orchards. Pest Manag. Sci. 2022, 78, 3630–3637. [Google Scholar] [CrossRef]
- Pickett, J.A.; Woodcock, C.M.; Midega, C.A.O.; Khan, Z.R. Push-pull farming systems. Curr. Opin. Biotechnol. 2014, 26, 125–132. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Erb, M. Tritrophic interactions mediated by herbivore-induced plant volatiles: Mechanisms, ecological relevance, and application potential. Annu. Rev. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef]
- Mitra, P.; Das, S.; Debnath, R.; Mobarak, S.H.; Barik, A. Identification of Lathyrus sativus plant volatiles causing behavioral preference of Aphis craccivora. Pest Manag. Sci. 2021, 77, 285–299. [Google Scholar] [CrossRef]
- Klassen, D.; Lennox, M.D.; Dumont, M.J.; Chouinard, G.; Tavares, J.R. Dispensers for pheromonal pest control. J. Environ. Manag. 2023, 325, 116590. [Google Scholar] [CrossRef]
- Stevens, C.V.; Smagghe, G.; Rammeloo, T.; De Kimpe, N. Insect repellent/antifeedant activity of 2,4-methanoproline and derivatives against a leaf- and seed-feeding pest insect. J. Agric. Food Chem. 2005, 53, 1945–1948. [Google Scholar] [CrossRef]
- Guarino, S.; Basile, S.; Arif, M.A.; Manachini, B.; Peri, E. Odorants of Capsicum spp. dried fruits as candidate attractants for Lasioderma serricorne F. (Coleoptera: Anobiidae). Insects 2021, 12, 61. [Google Scholar] [CrossRef]
- Basile, S.; Badalamenti, N.; Riccobono, O.; Guarino, S.; Ilardi, V.; Bruno, M.; Peri, E. Chemical composition and evaluation of insecticidal activity of Calendula incana subsp. maritima and Laserpitium siler subsp. siculum essential oils against stored products pests. Molecules 2022, 27, 588. [Google Scholar] [CrossRef]
- Tiwari, S.; Bhandari, G.; Kc, G.B.; Thapa, R.B.; Sharma, S.D. Studies on food preferences of maize weevil, Sitophilus zeamais Mots. to different crops in Chitwan, Nepal. J. Maize Res. Dev. 2016, 2, 58–65. [Google Scholar] [CrossRef]
- Trematerra, P. Preferences of Sitophilus zeamais to different types of Italian commercial rice and cereal pasta. Bull. Insectol. 2009, 62, 103–106. [Google Scholar]
- Mwando, N.L.; Tamiru, A.; Nyasani, J.O.; Obonyo, M.A.O.; Caulfield, J.C.; Bruce, T.J.A.; Subramanian, S. Maize chlorotic mottle virus induces changes in host plant volatiles that attract vector thrips species. J. Chem. Ecol. 2018, 44, 681–689. [Google Scholar] [CrossRef]
- Sanchez, G.; Besada, C.; Badenes, M.L.; Monforte, A.J.; Granell, A. A non-targeted approach unravels the volatile network in peach fruit. PLoS ONE 2012, 7, e38992. [Google Scholar] [CrossRef]
- Wei, G.; Tian, P.; Zhang, F.X.; Qin, H.; Miao, H.; Chen, Q.W.; Hu, Z.Y.; Cao, L.; Wang, M.J.; Gu, X.F.; et al. Integrative analyses of nontargeted volatile profiling and transcriptome data provide molecular insight into VOC diversity in cucumber plants (Cucumis sativus). Plant Physiol. 2016, 172, 603–618. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, B.; He, H.; Yan, M.; Li, J.; Yan, F. Changes in visual and olfactory cues in virus-infected host plants alter the behavior of Bemisia tabaci. Front. Ecol. Evol. 2022, 10, 766570. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, M.; Wang, X.; Gou, J.; Li, T.; Zhao, T.; Zhou, L.; Zhang, F.; Cheng, F.; Wang, L. Plant volatiles mediated the orientation preference of slugs to different plant species. Pest Manag. Sci. 2023, 80, 267–274. [Google Scholar] [CrossRef]
- van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Ukeh, D.A.; Birkett, M.A.; Bruce, T.J.A.; Allan, E.J.; Pickett, J.A.; Mordue, A.J. Behavioural responses of the maize weevil, Sitophilus zeamais, to host (stored-grain) and non-host plant volatiles. Pest Manag. Sci. 2010, 66, 44–50. [Google Scholar] [CrossRef]
- Missbach, C.; Dweck, H.K.M.; Vogel, H.; Vilcinskas, A.; Stensmyr, M.C.; Hansson, B.S.; Grosse-Wilde, E. Evolution of insect olfactory receptors. eLife 2014, 3, e02115. [Google Scholar] [CrossRef]
- Bi, Y.L.; Zhang, X.; Chang, X.F.; Li, J.X.; Xiao, S.; Zhang, B.; Dang, C.; Sun, L.L.; Yao, H.W.; Fang, Q.; et al. Olfactory behavioral responses of two Drosophila species and their pupal parasitoid to volatiles from bananas. Pest Manag. Sci. 2023, 79, 4309–4318. [Google Scholar] [CrossRef]
- Hanks, L.M.; Millar, J.G. Sex and aggregation-sex pheromones of cerambycid beetles: Basic science and practical applications. J. Chem. Ecol. 2016, 42, 631–654. [Google Scholar] [CrossRef]
- Miller, S.E.; Legan, A.W.; Flores, Z.A.; Ng, H.Y.; Sheehan, M.J. Strong, but incomplete, mate choice discrimination between two closely related species of paper wasp. Biol. J. Linn. Soc. 2019, 126, 614–622. [Google Scholar] [CrossRef]
- Hori, M.; Aoki, Y.; Shinoda, K.; Chiba, M.; Sasaki, R. Wood volatiles as attractants of the confused flour beetle, Tribolium confusum (Coleoptera: Tenebrionidae). Sci. Rep. 2019, 9, 11544. [Google Scholar] [CrossRef]
- Wei, S.; Marton, I.; Dekel, M.; Shalitin, D.; Lewinsohn, E.; Bravdo, B.A.; Shoseyov, O. Manipulating volatile emission in tobacco leaves by expressing Aspergillus nigerbeta-glucosidase in different subcellular compartments. Plant Biotechnol. J. 2004, 2, 341–350. [Google Scholar] [CrossRef]
- Zu, P.J.; Boege, K.; del-Val, E.; Schuman, M.C.; Stevenson, P.C.; Zaldivar-Riverón, A.; Saavedra, S. Information arms race explains plant-herbivore chemical communication in ecological communities. Science 2020, 368, 1377–1381. [Google Scholar] [CrossRef]
- Cai, L.; Macfadyen, S.; Hua, B.; Zhang, H.; Xu, W.; Ren, Y. Identification of biomarker volatile organic compounds released by three stored-grain insect pests in wheat. Molecules 2022, 27, 1963. [Google Scholar] [CrossRef]
- Jia, M.; Wang, X.; Liu, J.; Wang, R.; Wang, A.; Strappe, P.; Shang, W.; Zhou, Z. Physicochemical and volatile characteristics present in different grain layers of various rice cultivars. Food Chem. 2022, 371, 131119. [Google Scholar] [CrossRef]
- Ashraf, U.; Hussain, S.; Naveed Shahid, M.; Anjum, S.A.; Kondo, M.; Mo, Z.; Tang, X. Alternate wetting and drying modulated physio-biochemical attributes, grain yield, quality, and aroma volatile in fragrant rice. Physiol. Plant. 2022, 174, e13833. [Google Scholar] [CrossRef]
- Liao, M.; Li, S.; Wu, H.; Gao, Q.; Shi, S.; Huang, Y.; Cao, H. Transcriptomic analysis of Sitophilus zeamais in response to limonene fumigation. Pest Manag. Sci. 2022, 78, 4774–4782. [Google Scholar] [CrossRef]
- Del Mármol, J.; Yedlin, M.A.; Ruta, V. The structural basis of odorant recognition in insect olfactory receptors. Nature 2021, 597, 126–131. [Google Scholar] [CrossRef]
- Tang, Q.F.; Shen, C.; Zhang, Y.; Yang, Z.P.; Han, R.R.; Wang, J. Antennal transcriptome analysis of the maize weevil Sitophilus zeamais: Identification and tissue expression profiling of candidate odorant-binding protein genes. Arch. Insect Biochem. Physiol. 2019, 101, e21542. [Google Scholar] [CrossRef]
- Sun, L.L.; Hou, W.H.; Zhang, J.J.; Dang, Y.L.; Yang, Q.Y.; Zhao, X.C.; Ma, Y.; Tang, Q.B. Plant metabolites drive different responses in caterpillars of two closely related Helicoverpa species. Front. Physiol. 2021, 12, 662978. [Google Scholar] [CrossRef]
- Orsucci, M.; Audiot, P.; Nidelet, S.; Dorkeld, F.; Pommier, A.; Vabre, M.; Severac, D.; Rohmer, M.; Gschloessl, B.; Streiff, R. Transcriptomic response of female adult moths to host and non-host plants in two closely related species. BMC Evol. Biol. 2018, 18, 145. [Google Scholar] [CrossRef]
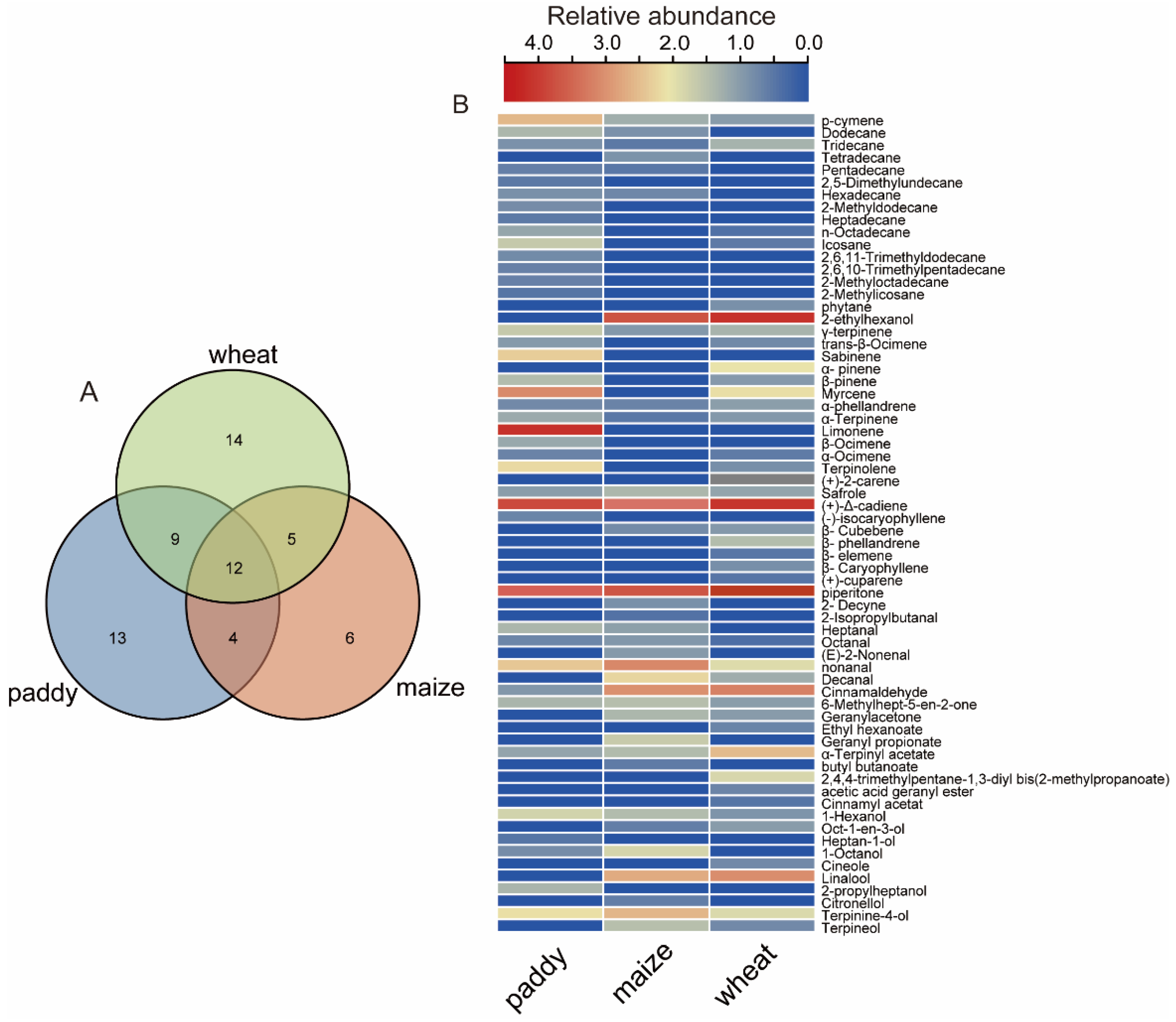
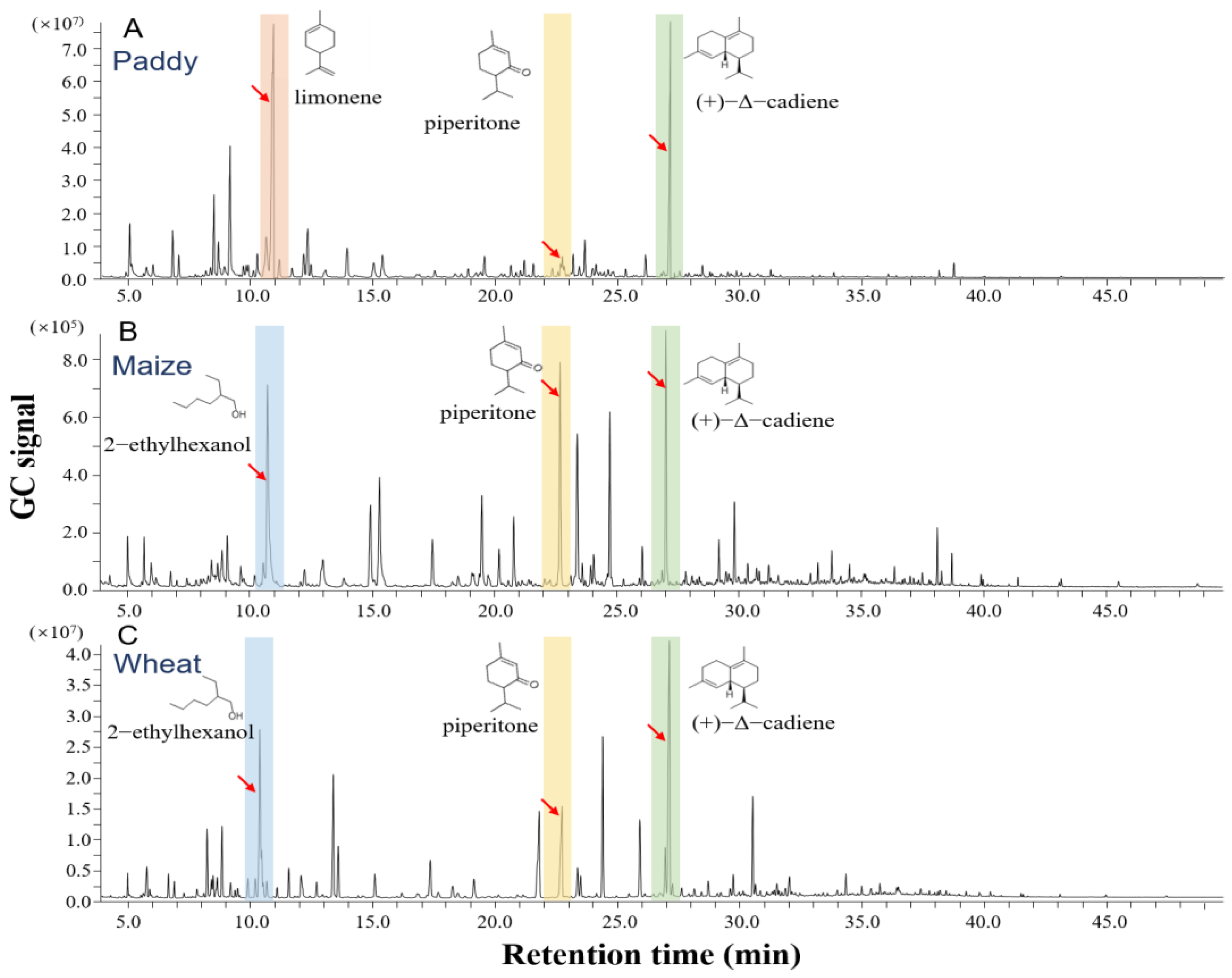
| Chemicals | Paddy | Maize | Wheat | |||
|---|---|---|---|---|---|---|
| Intensity | RI | Intensity | RI | Intensity | RI | |
| 2-ethylhexanol | - | - | 5.88 ± 0.23 *** | 1055 | 7.20 ± 0.17 | 1062 |
| limonene | 16.71 ± 3.84 | 1068 | - | - | - | - |
| (+)-Δ-cadiene | 7.86 ± 0.33 a | 1841 | 5.91 ± 0.17 b | 1824 | 7.62 ± 0.21 a | 1923 |
| piperitone | 2.94 ± 0.15 c | 1565 | 5.24 ± 0.29 b | 1467 | 7.17 ± 0.26 a | 1571 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, S.; Zhang, L.; Lu, Y.; Chen, M.; Wang, Z. Host Volatiles Potentially Drive Two Evolutionarily Related Weevils to Select Different Grains. Insects 2024, 15, 300. https://doi.org/10.3390/insects15050300
Lu S, Zhang L, Lu Y, Chen M, Wang Z. Host Volatiles Potentially Drive Two Evolutionarily Related Weevils to Select Different Grains. Insects. 2024; 15(5):300. https://doi.org/10.3390/insects15050300
Chicago/Turabian StyleLu, Shaohua, Lingfang Zhang, Yujie Lu, Mingshun Chen, and Zhengyan Wang. 2024. "Host Volatiles Potentially Drive Two Evolutionarily Related Weevils to Select Different Grains" Insects 15, no. 5: 300. https://doi.org/10.3390/insects15050300
APA StyleLu, S., Zhang, L., Lu, Y., Chen, M., & Wang, Z. (2024). Host Volatiles Potentially Drive Two Evolutionarily Related Weevils to Select Different Grains. Insects, 15(5), 300. https://doi.org/10.3390/insects15050300






