Chilean Darwin Wasps (Ichneumonidae): Biogeographic Relationships and Distribution Patterns
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
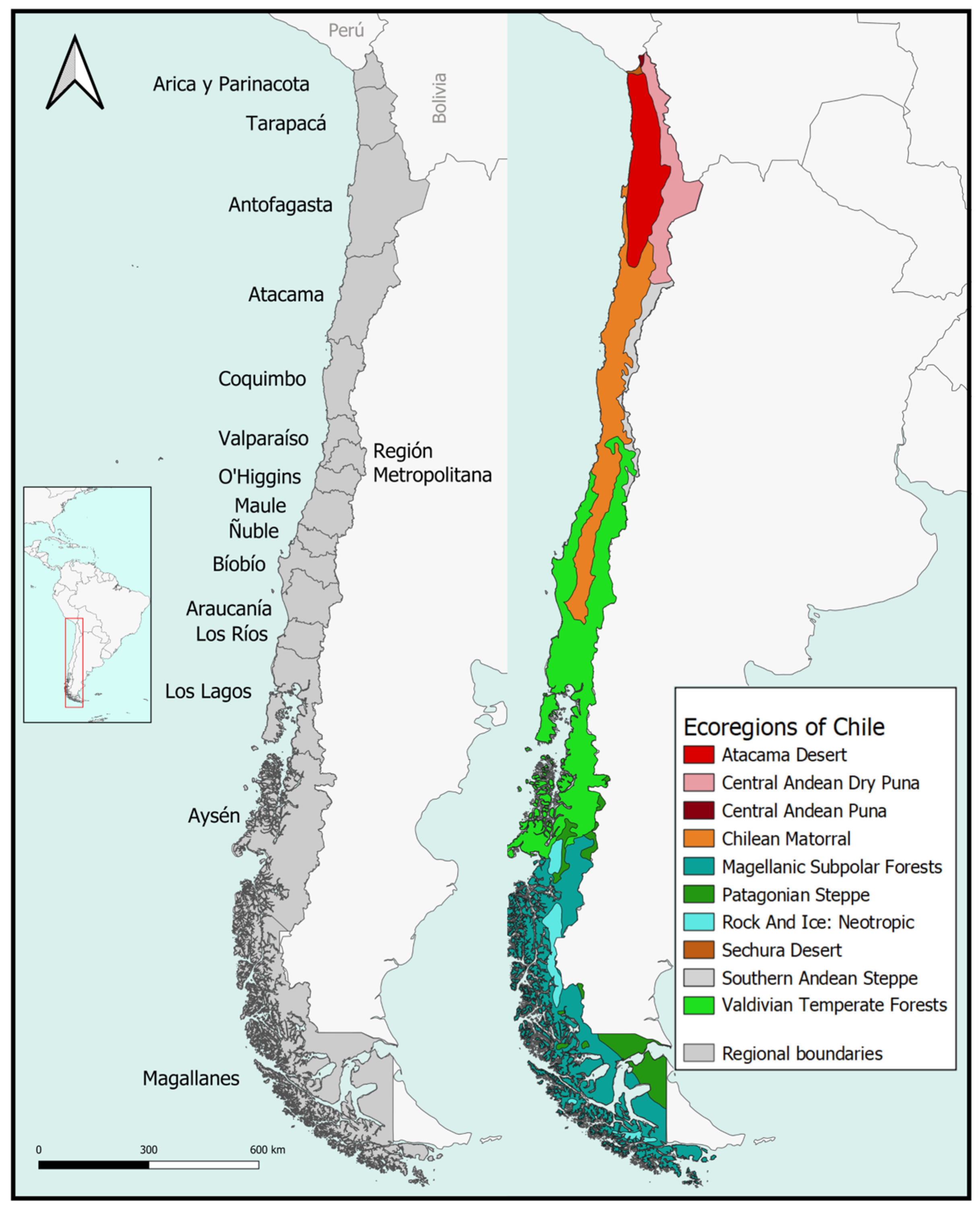
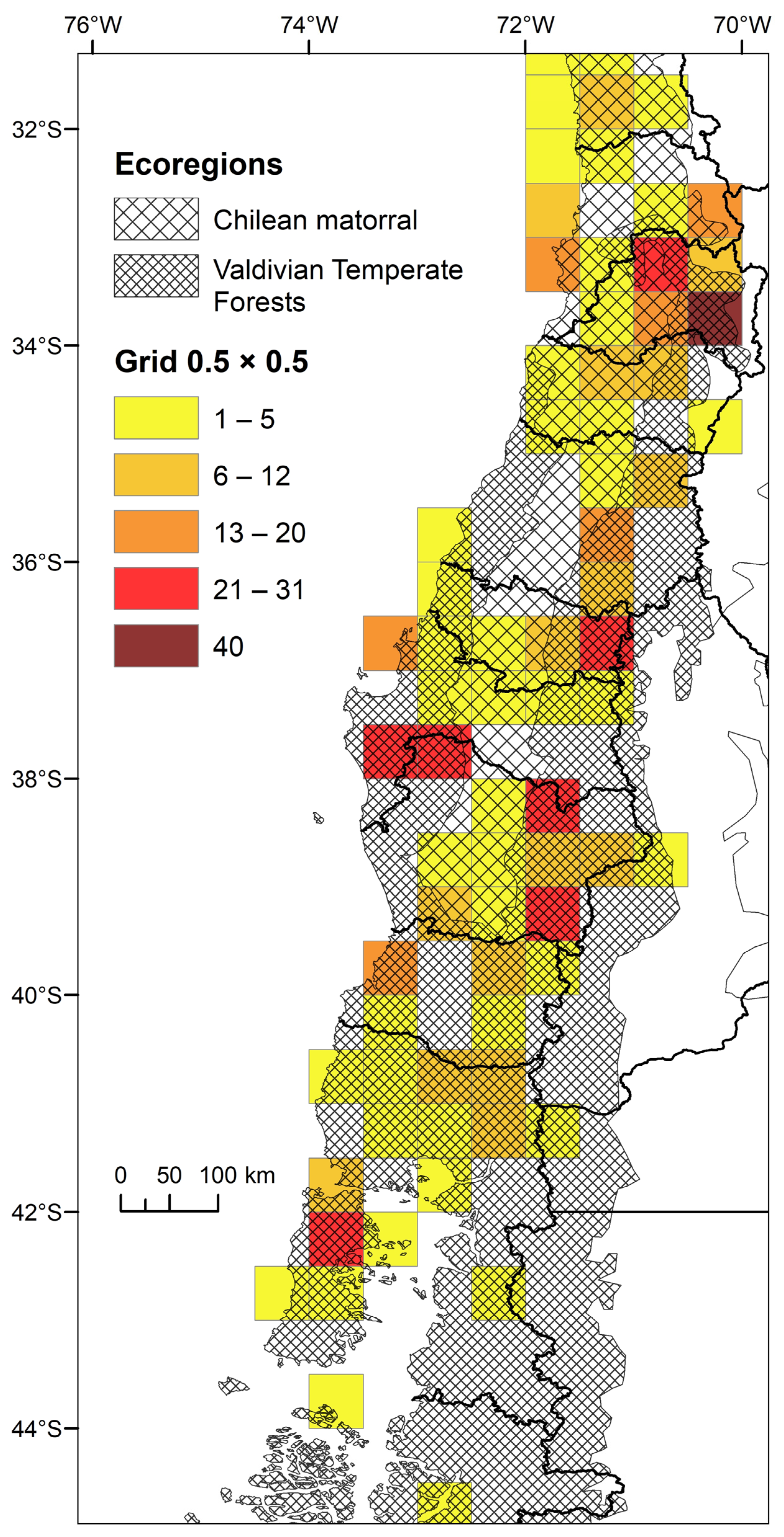
2.1. Study Area
2.2. Biogeographic Relationships
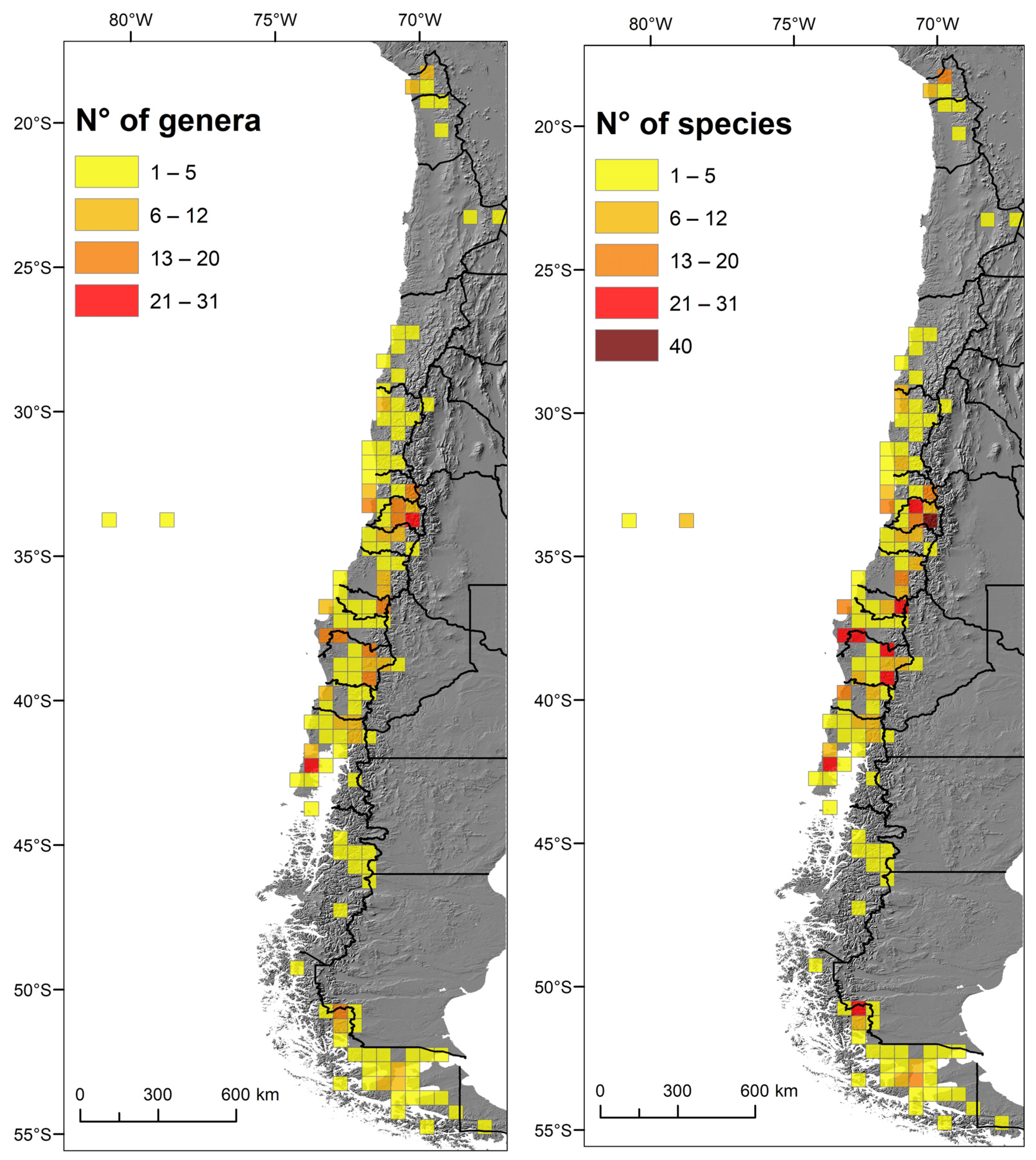
2.3. Biodiversity Spatial Patterns
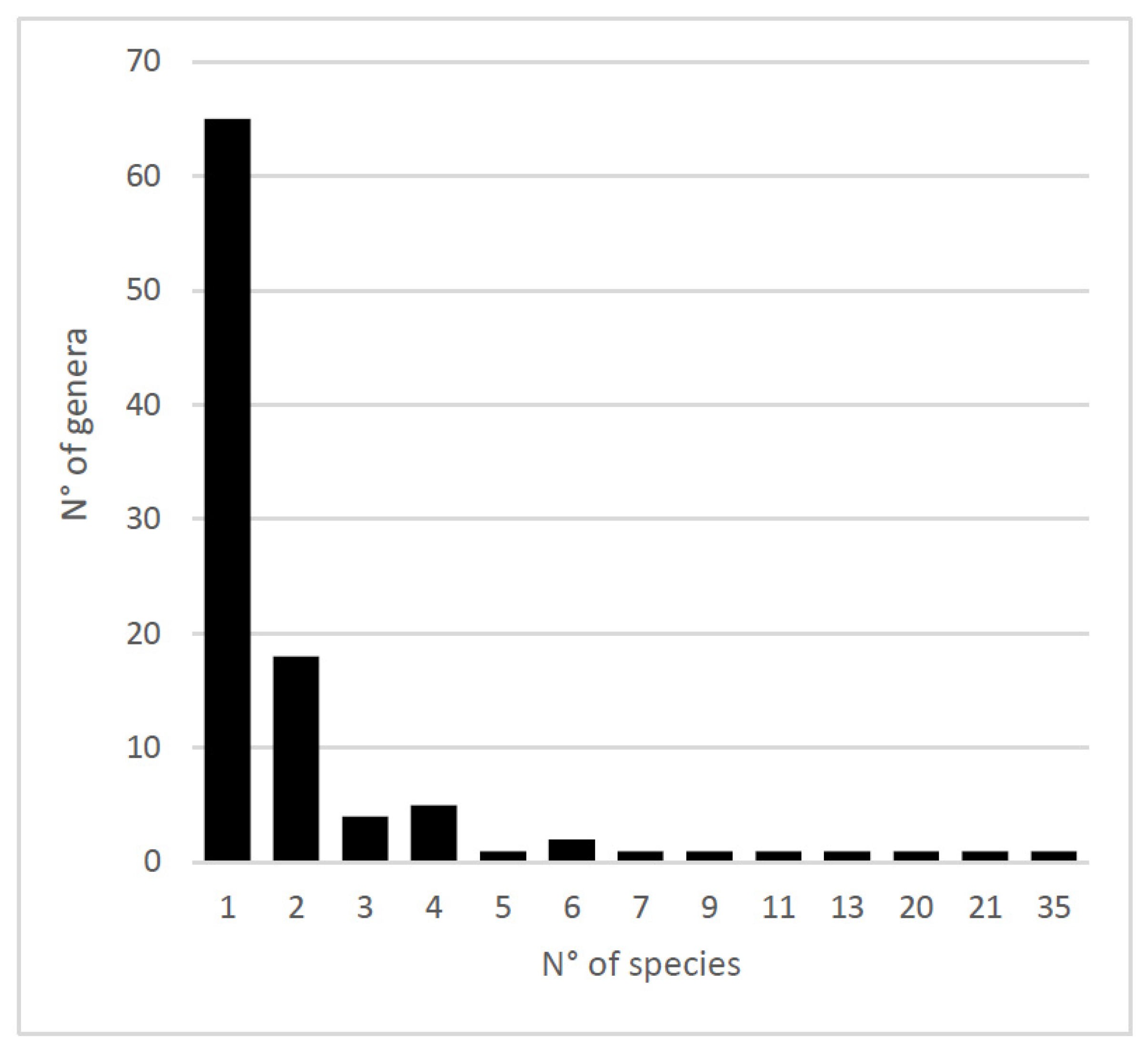
3. Results
3.1. Biogeographic Relationships
3.1.1. Cosmopolitan Element
3.1.2. Endemic Element
3.1.3. Neotropical Element
3.1.4. Holarctic–Oriental Element
3.1.5. South-Temperate Element
3.1.6. Australasian Element
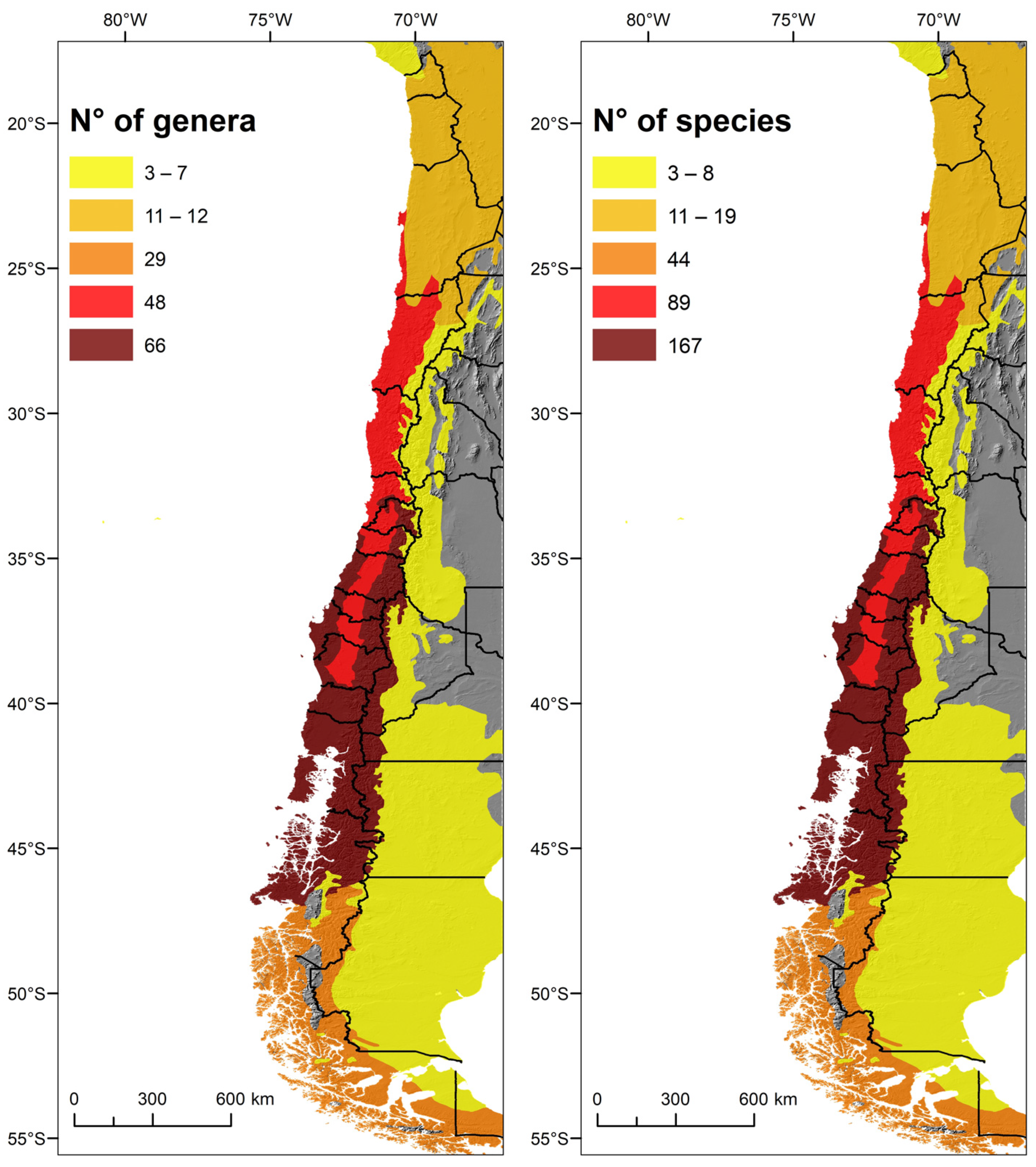
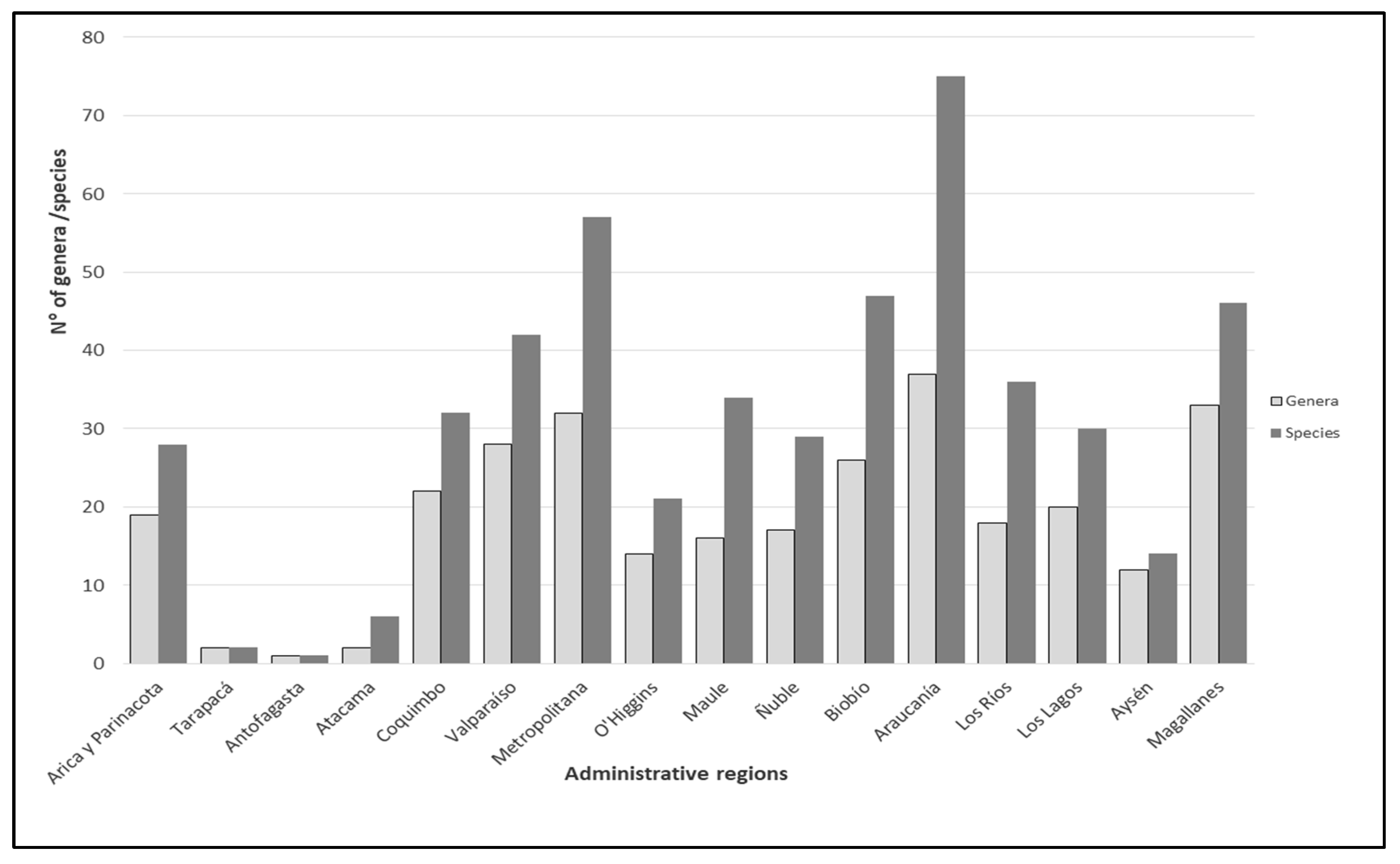
3.2. Spatial Pattern of Biodiversity along the Latitudinal Gradient
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klopfstein, S.; Santos, B.F.; Shaw, M.R.; Alvarado, M.; Bennett, A.M.R.; Dal Pos, D.; Giannotta, M.; Herrera Florez, A.F.; Karlsson, D.; Khalaim, K.I.; et al. Darwin wasps: A new name heralds renewed efforts to unravel the evolutionary history of Ichneumonidae. Entomol. Commun. 2019, 1, ec01006. [Google Scholar] [CrossRef]
- Saunders, T.E.; Ward, D.F. Variation in the diversity and richness of parasitoid wasps based on sampling effort. Peerj 2018, 6, e4642. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, P.; Erwin, T.L.; Borges, P.A.V.; New, T.R. The seven impediments in invertebrate conservation and how to overcome them. Biol. Conserv. 2011, 144, 2647–2655. [Google Scholar] [CrossRef]
- New, T.R. Invertebrate Surveys for Conservation; Oxford University Press: Oxford, UK, 1998; pp. 1–240. [Google Scholar]
- Sanchéz-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Milić, D.; Radenković, S.; Ačanski, J.; Vujić, A. The importance of hidden diversity for insect conservantion: A case study in hoverflies (the Merodon atratus complex, Syrphidae, Diptera). J. Insect Conserv. 2019, 23, 29–44. [Google Scholar] [CrossRef]
- Veijalainen, A.; Wahlberg, N.; Broad, G.R.; Erwin, T.L.; Longino, J.T.; Sääksjärvi, I.E. Unprecedented ichneumonid parasitoid wasp diversity in tropical forests. Proc. R. Soc. B Biol. Sci. 2012, 279, 4694–4698. [Google Scholar] [CrossRef] [PubMed]
- Fernández, F.; Sharkey, M.J. Introducción a los Hymenoptera de la Región Neotropical; Sociedad Colombiana de Entomología y Universidad Nacional de Colombia: Bogotá, Colombia, 2006; pp. 1–894. [Google Scholar]
- Bennett, A.M.R.; Cardinal, S.; Gauld, I.D.; Wahl, D.B. Phylogeny of the subfamilies of Ichneumonidae (Hymenoptera). J. Hymenopt. Res. 2019, 71, 1–156. [Google Scholar] [CrossRef]
- Yu, D.S.K.; Achterberg, C.; Horstmann, K. (2016) Taxapad 2016, Ichneumonoidea 2015. Taxapad, Ottawa, Database on flashdrive. Available online: http://www.taxapad.com (accessed on 30 January 2023).
- Moreira-Muñoz, A. Plant and Vegetation 5: Plant Geography of Chile; Werger, M.J.A., Ed.; Springer: New York, NY, USA, 2011; pp. 1–320. [Google Scholar]
- Santos, A.M.C.; Quicke, D.L.J. Large-scale diversity patterns of parasitoid insects. Entomol. Sci. 2011, 14, 371–382. [Google Scholar] [CrossRef]
- Kishineysky, M.; Keasar, T. Trait-based characterisation of parasitoid wasp communities in natural and agricultural areas. Ecol. Entomol. 2022, 47, 657–667. [Google Scholar] [CrossRef]
- Porter, C.C. Zoogeografia de las Ichneumonidae Latino-Americanas (Hymenoptera). Acta Zool. Lilloana 1980, 36, 5–52. [Google Scholar]
- Porter, C.C. Biogeographic of Chilean Ichneumonid Flies (Hymenoptera: Ichneumonidae). Acta Entom. Chil. 1991, 16, 37–68. [Google Scholar]
- Porter, C.C. Guía de los Géneros de Ichneumonidae en la Región Neantática del Sur de Surdamérica. Opera Lillo. 1998, 42, 1–229. [Google Scholar]
- Elgueta, M.; Rojas, F. Hymenoptera de Chile. In Hacia un Proyecto CYTED para el Inventario y Estimación de la Diversidad Entomológica en Iberoamérica: PrIBES-2000; Martín-Piera, F., Morrone, J.J., Melic, A., Eds.; m3m: Monografías Tercer Milenio: Zaragoza, Spain, 2000; Volume 1, pp. 245–251. [Google Scholar]
- Dinerstein, E.; Olson, D.; Joshi, A.; Vynne, C.; Burgess, N.D.; Wikramanayake, E.; Hahn, N.; Palminteri, S.; Hedao, P.; Noss, R.; et al. An Ecoregion-Based Approach to Protecting Half the Terrestrial Realm. BioScience 2017, 67, 534–545. [Google Scholar] [CrossRef]
- Araujo, R.O.; Dal Pos, D.; Fernandes, D.R.R.; Moreira-Muñoz, A.; Pádua, D.G. Unveiling the secrets of South American Darwin Wasps: A comprehensive Catalog of the Chilean Ichneumonidae (Hymenoptera: Ichneumonidae). Cent. Investig. Estud. Av. Del Maule Univ. Católica Del Maule Talca Chile, 2024; manuscript in preparation. [Google Scholar]
- Darlington, P.J. Biogeography of the Southern End of the World. Distribution and History of Far-Southern Life and Land, with an Assessment of Continental Drift; Harvard University Press: Cambridge, MA, USA, 1965; pp. 1–246. [Google Scholar]
- Holloway, J.D.; Jardine, N. Two approaches to zoogeography: A study based on the distributions of butterflies, birds and bats in the Indo-Australian area. Proc. Linn. Soc. 1968, 179, 153–188. [Google Scholar] [CrossRef]
- Gauld, I.D. An Introduction to the Ichneumonidae of Australia; British Museum: London, UK, 1984; pp. 1–413. [Google Scholar]
- Hausdorf, B.; Hennig, C. Biotic Element Analysis in Biogeography. Syst. Biol. 2003, 52, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Crisci, J.V.; Cigliano, M.M.; Morrone, J.J.; Roig-Junent, S. Historical Biogeography of Southern South America. Syst. Biolo. 1991, 40, 152–171. [Google Scholar] [CrossRef]
- Crisci, J.; Katinas, L.; Posadas, P. Historical Biogeography: An Introduction; Harvard University Press: Cambridge, MA, USA, 2003; pp. 1–264. [Google Scholar]
- Fuentes-Castillo, T.; Hernández, H.J.; Pliscoff, P. Hotspots and ecoregion vulnerability driven by climate change velocity in Southern South America. Reg. Environ. Chang. 2020, 20, 1–15. [Google Scholar] [CrossRef]
- Moreira-Muñoz, A.; Scherson, R.; Luebert, F.; Román, M.J.; Monge, M.; Diazgranados, M.; Silva, H. Biogeography, phylogenetic relationships and morphological analyses of the South American genus Mutisia L.f. (Asteraceae) shows early connections of two disjunct biodiversity hotspots. Org. Div. Evol. 2020, 20, 639–656. [Google Scholar] [CrossRef]
- Forbes, A.A.; Bagley, R.K.; Beer, M.A.; Hippee, A.C.; Widmayer, H.A. Quantifying the unquantifiable: Why Hymenoptera, not Coleoptera, is the most speciose animal order. BMC Ecol. 2018, 18, 21. [Google Scholar] [CrossRef]
- Lanfranco, L.D. Contribución al conocimiento de los icneumónidos de Chile. Rev. Chil. Entomol. 1980, 10, 77–84. [Google Scholar]
- Cox, C.B. The biogeographic regions reconsidered. J. Biogeogr. 2001, 28, 511–523. [Google Scholar]
- Morrone, J.J. Biogeographical regions under track and cladistic scrutiny. A comment on C. B. Cox (2001). J. Biogeogr. 2002, 29, 149–152. [Google Scholar] [CrossRef]
- Skottsberg, C. Remarks on the plant geography of the southern cold temperate zone. Proc. R. Soc. B Biol. Sci. 1960, 152, 447–457. [Google Scholar]
- Moreira-Muñoz, A. The Austral floristic realm revisited. J. Biogeogr. 2007, 34, 1649–1660. [Google Scholar] [CrossRef]
- Villagrán, C.; Hinojosa, L.F. Historia de los bosques del sur de Sudamérica, II: Análisis fitogeográfico. Rev. Chil. Hist. Nat. 1997, 70, 241–267. [Google Scholar]
- Fernandes, D.R.R.; Santos, B.F.; Pádua, D.G.; Araujo, R.O. Ichneumonidae in Catálogo Taxonômico da Fauna do Brasil; PNUD, 2024. Available online: http://fauna.jbrj.gov.br/fauna/faunadobrasil/18131 (accessed on 7 May 2024).
- Gauld, I.D.; Wahl, D.B. The Labeninae (Hymenoptera: Ichneumonidae): A study in phylogenetic reconstruction and evolutionary biology. Zool. J. Linn. Soc. 2000, 129, 271–347. [Google Scholar] [CrossRef]
- Santos, B.F.; Sandoval, M.; Spasojevic, T.; Giannotta, M.M.; Brady, S.G. A Parasitoid Puzzle: Phylogenomics, Total-evidence Dating, and the Role of Gondwanan Vicariance in the Diversification of Labeninae (Hymenoptera, Ichneumonidae). Insect Syst. Divers. 2022, 6, 3. [Google Scholar] [CrossRef]
- Grimaldi, D.; Engel, M.S. Evolution of the Insects; Cambridge University Press: New York, NY, USA, 2005; pp. 1–755. [Google Scholar]
- Kuschel, G. Biogeography and Ecology of South American Coleoptera. In Biogeography and Ecology in South America; Fittkau, E.J., Illies, J., Klinge, H., Schwabe, G.H., Sioli, H., Eds.; Monographiae Bioloficae; Springer: Dordrecht, The Netherlands; Berlin/Heidelberg, Germany, 2012; Volume 2, pp. 709–722. [Google Scholar]
- Ruíz-Cancino, E.; Kasparyan, D.R.; González-Moreno, A.; Khalaim, A.I.; Coronado-Blanco, J.M. Biodiversidad de Ichneumonidae (Hymenoptera) en México. Rev. Mex. Biodivers. 2014, 85, 385–391. [Google Scholar] [CrossRef]
- Peña, L.E. A Preliminary Attempt to Divide Chile into Entomofaunal Regions, Based on the Tenebrionidae (Coleptera). Postilla 1966, 97, 1–18. [Google Scholar]
- Morrone, J.J. Biogeographical regionalization of the Andean region. Zootaxa 2015, 3936, 207–236. [Google Scholar] [CrossRef] [PubMed]
- Marshall, L.; Ascher, J.S.; Villagra, C.; Beaugendre, A.; Herrera, V.; Henríquez-Piskulich, P.; Vera, A.; Vereecken, N.J. Chilean bee diversity: Contrasting patterns of species and phylogenetic turnover along a large-scale ecological gradient. Ecosphere 2023, 14, e4535. [Google Scholar] [CrossRef]
- Samaniego, H.; Marquet, P.A. Mammal and butterfly species richness in Chile: Taxonomic covariation and history. Rev. Chil. Hist. Nat. 2009, 82, 135–151. [Google Scholar] [CrossRef]
- Moreira-Muñoz, A.; Monge, M.; Grossi, M.A.; Avila, F.A.; Morales-Fierro, V.; Heiden, G.; Britto, B.; Beck, S.; Nakajima, J.; Salgado, V.G.; et al. South America holds the greatest diversity of native daisies (Asteraceae) in the world: An updated catalogue supporting continental-scale conservation. Front. Plant Sci. 2024, 15, 1393241. [Google Scholar] [CrossRef]
- Moreira-Muñoz, A.; Palchetti, M.V.; Morales-Fierro, V.; Duval, V.S.; Allesch-Villalobos, R.; Gonzalez-Orozco, C.E. Diversity and Conservation Gap Analysis of the Solanaceae of Southern South America. Front. Plant Sci. 2022, 13, 854372. [Google Scholar] [CrossRef] [PubMed]
- Segovia, R.A.; Armesto, J.J. The Gondwanan legacy in South American biogeography. J. Biogeogr. 2015, 42, 209–217. [Google Scholar] [CrossRef]
- Bauerle, P.; Rutherford, P.; Lanfranco, D. Defoliadores de roble (Nothofagus obliqua), raulí (N. alpina), coigüe (N. dombeyi) y lenga (N. pumilio). Bosque 1997, 18, 97–107. [Google Scholar] [CrossRef]
- Pizarro-Araya, J.; Villalobos, E.V.; Alfaro, F.M.; Moreira-Muñoz, A. Conservation efforts in need of survey improvement in epigean beetles from the Atacama coast, Chile. J. Arid Environ. 2023, 214, 104995. [Google Scholar] [CrossRef]
- Flinte, V.; Pádua, D.G.; Durand, E.M.; Hodgin, C.; Khattar, G.; da Silveira, L.F.L.; Fernandes, D.R.R.; Sääksjärvi, I.E.; Monteiro, R.F.; Macedo, M.V.; et al. Variation in a Darwin Wasp (Hymenoptera: Ichneumonidae) Community along an Elevation Gradient in a Tropical Biodiversity Hotspot: Implications for Ecology and Conservation. Insects 2023, 14, 861. [Google Scholar] [CrossRef]
- Townes, H.K. The genera of Ichneumonidae, Part 4. Mem. Amer. Ent. Inst. 1971, 17, 1–372. [Google Scholar]
- Gauld, I.D. The Ichneumonidae of Costa Rica, I. Mem. Amer. Ent. Inst. 1991, 47, 1–589. [Google Scholar]
- Quicke, D.L.J. We Know Too Little about Parasitoid Wasp Distributions to Draw Any Conclusions about Latitudinal Trends in Species Richness, Body Size and Biology. PLoS ONE 2012, 7, e32101. [Google Scholar] [CrossRef] [PubMed]
- Jerez, V.; Zúñiga-Reinoso, A.; Muñoz-Escobar, C.; Pizarro-Araya, J. Acciones y avances sobre la conservación de insectos en Chile. Gayana 2003, 79, 1–3. [Google Scholar] [CrossRef]
- Jervis, M.A.; Ferns, P.N.; Heimpel, G.E. Body size and the timing of egg production in parasitoid wasps: A comparative analysis. Funct. Ecol. 2003, 17, 375–383. [Google Scholar] [CrossRef]
- Townes, H.K. The genera of Ichneumonidae, Part 2. Mem. Amer. Ent. Inst. 1970, 12, 1–537. [Google Scholar]
- Townes, H.K.; Townes, M. A catalogue and reclassification of the Neotropic Ichneumonidae. Mem. Amer. Ent. Inst. 1966, 8, 1–367. [Google Scholar]
- Lanfranco, L.D. Contribución al conocimiento de la icneumonofauna de la región de Magallanes (Hymenoptera - Ichneumonidae). An. Inst. Patagon. 1974, 5, 199–208. [Google Scholar]
- Porter, C.C. A revision of the south American species of Trachysphyrus. Mem. Amer. Ent. Inst. 1967, 10, 1–368. [Google Scholar]
- Alvarado, M. Revision of the South American wasp genus Alophophion Cushman, 1947 (Hymenoptera: Ichneumonidae: Ophioninae). Rev. Peru. Biol. 2014, 21, 3–60. [Google Scholar] [CrossRef]
- Jerez, V.; Lanfranco, L.D.; Andrade, B. Aspectos ecologicos de los ichneumonidos del Bosque de Quintero. An. Mus. Hist. Nat. Valpso. 1977, 10, 161–168. [Google Scholar]
- Porter, C.C. A review of the Chilean genera of the tribe Mesostenini (Hym. Ichneumonidae). Studia Ent. 1967, 10, 369–418. [Google Scholar]
- Porter, C.C. New species and records of Anacis (Hymenoptera: Ichneumonidae: Cryptini) from tropical and temperate Andean South America. Insecta Mundi 2004, 17, 119–127. [Google Scholar]
- Porter, C.C. A revision of the Chilean Mesostenini (Hymenoptera: Ichneumonidae). Contrib. Am. Entomol. Inst. 1987, 23, 1–164. [Google Scholar]
- Bordera, S.; Mazón, M.; Sääksjärvi, I.E. The Neotropical species of Atractodes (Hymenoptera, Ichneumonidae, Cryptinae), II: The A. pleuripunctatus species-group. Zootaxa 2016, 4161, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Gauld, I.D.; Wahl, D.B. The Eucerotinae: A Gondwanan origin for a cosmopolitan group of Ichneumonidae? J. Nat. Hist. 2002, 36, 2229–2248. [Google Scholar] [CrossRef]
- Townes, H.K. The genera of Ichneumonidae, Part 3. Mem. Amer. Ent. Inst. 1970, 13, 1–307. [Google Scholar]
- Spinola, M. Icneumonitos. Zoologia. 6: "Historia física y politica de Chile."; Gay, C., Ed.; Paris, France, 1851; pp. 471–550. Available online: https://www.biodiversitylibrary.org/item/130101#page/7/mode/1up (accessed on 30 January 2023).
- Porter, C.C. Ichneumonidae de Tarapacá. 1. Subfamily Ephialtinae (Hymenoptera). Idesia 1979, 5, 157–187. [Google Scholar]
- Broad, G.R.; Sääksjärvi, I.E.; Veijalainen, A.; Notton, D.G. Three new genera of Banchinae (Hymenoptera: Ichneumonidae) from Central and South America. J. Nat. Hist. 2011, 45, 1311–1329. [Google Scholar] [CrossRef]
- Porter, C.C. Joppini (Hymenoptera: Ichneumonidae) of Tarapacá. Fla. Entomol. 1980, 63, 226–242. [Google Scholar] [CrossRef]
- Townes, H.K. Revisions of twenty genera of Gelini (Ichneumonidae). Mem. Amer. Ent. Inst. 1983, 35, 1–281. [Google Scholar]
- Wahl, D.B. Cladistics of the genera of Mesochorinae (Hymenoptera: Ichneumonidae). Syst. Entomol. 1993, 18, 371–387. [Google Scholar] [CrossRef]
- Dasch, C.E. Neotropic Mesochorinae (Hymenoptera: Ichneumonidae). Mem. Amer. Ent. Inst. 1974, 22, 1–509. [Google Scholar]
- Lee, J.W. A revision of the genus Cidaphus (Hymenoptera: Ichneumonidae: Mesochorinae). Contrib. Am. Entomol. Inst. 1991, 5, 1–48. [Google Scholar]
- Roman, A. Ichneumoniden von Juan Fernandez, 3: "The Natural History of Juan Fernandez and Easter Island"; Skottsberg, C., Ed.; New York Botanical Garden: New York, NY, USA, 1920; pp. 289–295. [Google Scholar]
- Bordera, S.; Palacio, E.; Martinez, J.J. The Neotropical species of Clistopyga (Hymenoptera, Ichneumonidae, Pimplinae). Part V: The C. diazi species group, with the description of three new species. Zootaxa 2019, 4661, 545–565. [Google Scholar] [CrossRef]
- Porter, C.C. A revision of Cosmiocryptus in the coastal desert of Peru and north Chile (Hymenoptera: Ichneumonidae). Psyche 1985, 92, 463–492. [Google Scholar] [CrossRef]
- Porter, C.C. Cyclaulus in the Peruvian coastal desert (Hymenoptera: Ichneumonidae). Fla. Entomol. 1976, 59, 353–360. [Google Scholar] [CrossRef]
- Haliday, A.H. Descriptions of the Hymenoptera collected by Captain P.P. King, R.N., F.R.S., in the survey of the Straits of Magellan. Trans. Linn. Soc. Lond. 1836, 17, 316–331. [Google Scholar]
- Guerrero, S.M.A.; Lamborot, C.L.; Arretz, V.P. Parasitic action of three Hymenopteran species on the larvae and pupae of Plutella xylostella in a cabbage field. Rev. Chil. Entomol. 1986, 13, 17–20. [Google Scholar]
- Muriel, S.B.; Grez, A.A. Abundancia y parasitismo de Plutella xylostella L. (Lepidoptera: Plutellidae) en parches de Brassica oleracea con diferente forma y vegetacion circundante. Actu. Biol. 2003, 25, 99–103. [Google Scholar] [CrossRef]
- Brèthes, J. Quelques Ichneumonidae Nouveaux. Bol. Mus. Nac. Hist. Nat. 1913, 5, 310–312. [Google Scholar] [CrossRef]
- Diller, E.; Schoenitzer, K. Verbreitung neotropischer Phaeogenini der Gattung Dicaelotus Wesmael (1845), mit Beschreibungen neuer Taxa (Insecta, Hymenoptera, Ichneumonidae, Ichneumoninae, Phaeogenini). Linz. Biol. Beitr. 2009, 41, 1089–1102. [Google Scholar]
- Dasch, C.E. The neotropic Diplazontinae. Contrib. Am. Entomol. Inst. 1964, 1, 1–77. [Google Scholar]
- Ruiz, P.F. Himenopteros de la Provincia de Coquimbo. Rev. Ch. Hist. Nat. 1936, 40, 159–169. [Google Scholar]
- Brèthes, J. Cueillette d'insectes au Rio Blanco. Rev. Ch. Hist. Nat. 1919, 22, 161–171. [Google Scholar]
- Townes, H.K. The genera of Ichneumonidae, Part 1. Mem. Am. Ent. Inst. 1969, 11, 1–300. [Google Scholar] [CrossRef]
- Broad, G.R. A review of the genus Geraldus Fitton (Hymenoptera: Ichneumonidae: Banchinae), with description of a new species. J. Nat. Hist. 2010, 44, 1419–1425. [Google Scholar] [CrossRef]
- Quicke, D.L.J.; Laurenne, N.M.; Fitton, M.G.; Broad, G.R. A thousand and one wasps: A 28S rDNA and morphological phylogeny of the Ichneumonidae (Insecta: Hymenoptera) with an investigation into alignment parameter space and elision. J. Nat. Hist. 2009, 43, 1305–1421. [Google Scholar] [CrossRef]
- Porter, C.C. A Taxonomic Revision of the Chilean Groteini. Acta Entomol. Ch. 1989, 15, 143–162. [Google Scholar]
- Brèthes, J. Quelques Hyménopteres du Chili. Rev. Ch. Hist. Nat. 1916, 20, 83–89. [Google Scholar]
- Porter, C.C. Habronyx Foerster (Hymenoptera: Ichneumonidae: Anomaloninae) in Andean and Neantarctic South America with description of new species from Bolivia and Chile. Insecta Mundi 2007, 20, 1–8. [Google Scholar]
- Porter, C.C. Ichneumoninae of the genera Hoplismenus and Platylabus in Tarapacá (Chile) (Hymenoptera: Ichneumonidae). Idesia 1986, 10, 19–28. [Google Scholar]
- Lanfranco, L.D. Ichneumonidos (Hymenoptera-Ichneumonidae) del Parque Nacional “Vicente Perez Rosales”. An. Mus. Hist. Nat. 1974, 7, 261–267. [Google Scholar]
- Porter, C.C. New Chilean Itamuton (Hymenoptera: Ichneumonidae: Mesostenini) reared from Elicura litigator (Neuroptera: Myrmeleontidae). Fla. Entomol. 1989, 72, 660–664. [Google Scholar] [CrossRef]
- Porter, C.C. A revision of the South American species of Itoplectis (Hymenoptera, Ichneumonidae). Acta Zool. Lill. 1970, 26, 63–104. [Google Scholar]
- Porter, C.C. The Transantarctic genus Labena (Hymenoptera: Ichneumonidae: Labenini) in Chile. Insecta Mundi 2005, 19, 177–185. [Google Scholar]
- Araujo, R.O.; Vivallo, F. Taxonomic revision of Lepidura Townes, 1971 (Hymenoptera: Ichneumonidae: Mesochorinae) with the description of three new species, new distribution records and a key to the all known species. Zootaxa 2018, 4514, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K. A revision of the genus Microcharops (Hymenoptera: Ichneumonidae). Cont. Am. Ent. Inst. 1987, 23, 1–42. [Google Scholar]
- Araujo, R.O.; Di Giovanni, F. Description of the first species of Nemeritis Holmgren (Hymenoptera: Ichneumonidae: Campopleginae) from the Southern Hemisphere, with a key to the New World species. Zootaxa 2021, 5023, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Lamborot, L.; Arretz, P.; Guerrero, M.A.; Araya, J.E. Parasitism of eggs and larvae of Copitarsia turbata (Herrich & Schaffer) (Lepidoptera: Noctuidae) on horticultural crops in the Metropolitan Region of Chile. Acta Ent. Ch. 1995, 19, 129–133. [Google Scholar]
- Porter, C.C. First record of Phrudinae (Hymenoptera: Ichneumonidae) from South America with notice of a new genus and species from Chile. J. N. Y. Entomol. Soc. 1993, 101, 130–134. [Google Scholar]
- Porter, C.C. Picrocryptoides: A new genus of the tribe Mesostenini from southern South America (Hymenoptera, Ichneumonidae). Psyche 1965, 72, 167–174. [Google Scholar] [CrossRef]
- Porter, C.C. A revision of the South American species of Coccygomimus (Hymenoptera, Ichneumonidae). Studia Ent. 1970, 13, 1–192. [Google Scholar]
- Neira, C.M.; Ruff, F.J.; Mundaca, B.N. Natural enemies of Pieris brassicae L. (Lepidoptera: Pieridae) from cultivated crucifers in Valdivia. Agro-Ciencia 1989, 5, 5–10. [Google Scholar]
- Lanfranco, L.D.; Cerda, L. Coccygomimus fuscipes (Hym.: Ichneumonidae): Un parasitoide nativo de la Polilla del Brote, Rhyacionia buoliana (Lep.: Tortricidae). Bosque 1986, 7, 36–37. [Google Scholar] [CrossRef]
- Araujo, R.O.; Vivallo, F.; Santos, B.F. Discovery of two new Andean species of Scolomus (Townes & Townes), with a key to all known species (Hymenoptera: Ichneumonidae: Metopiinae). Zootaxa 2018, 4429, 189–194. [Google Scholar] [PubMed]
- Walkley, L.M. A second species of Ichneumonidae belonging to Scolomus Townes (Hymenoptera). Proc. Entomol. Soc. Wash. 1962, 64, 231–233. [Google Scholar]
- Cameron, P. On some Hymenoptera (chiefly undescribed) from Japan and the Pacific. Proc. Trans. Nat. Hist. Soc. Glasg. 1886, 1, 263–276. [Google Scholar]
- Cameron, P. Descriptions of one new genus and some new species of parasitic Hymenoptera. Mem. Proc. Manch. Lit. Phil. Soc. 1887, 26, 117–136. [Google Scholar]
- Porter, C.C. Certonotus Kriechbaumer (Hymenoptera: Ichneumonidae), an Australian genus newly recorded in South America. Fla Entomol. 1981, 64, 235–244. [Google Scholar] [CrossRef]
- Porter, C.C. Trachysphyrus and the new genus Aeliopotes in the coastal desert of Peru and north Chile (Hymenoptera: Ichneumonidae). Psyche 1985, 92, 513–545. [Google Scholar] [CrossRef]
- Diller, E.; Riedel, M.; Melzer, R.R.; Schoenitzer, K. Neotropische Phaeogenini mit Beschreibung neuer Arten der Gattung Tycherus Föerster, 1869 (Hymenoptera, Ichneumonidae, Ichneumoninae, Phaeogenini). Mitt. Müench. Entomol. Ges. 2009, 99, 3–95. [Google Scholar]
- Diller, E. Neue Taxa der Gattung Tycherus Foerster, (1869), aus der Neotropis, ein Nachtrag (Insecta; Hymenoptera, Ichneumonidae, Ichneumoninae, Phaeogenini). Entomofauna 2010, 31, 413–428. [Google Scholar]
- Porter, C.C. A new genus of the tribe Mesostenini from Chile (Hymenoptera, Ichneumonidae). Psyche 1963, 70, 117–119. [Google Scholar] [CrossRef]
- Durán, M.L. Las cuncunas de los pinos, un problema de entomología forestal. Agric. Tec. 1944, 4, 17–25. [Google Scholar]

| Elements | Genera (No.) | % | |
|---|---|---|---|
| 1 | Cosmopolitan | 50 | 36 |
| 2 | Endemic | 29 | 21 |
| 3 | Neotropical | 22 | 16 |
| 4 | Holarctic–Oriental | 19 | 14 |
| 5 | South-temperate | 16 | 11 |
| 6 | Australasian | 3 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pádua, D.G.; Moreira-Muñoz, A.; Morales-Fierro, V.; Araujo, R.O. Chilean Darwin Wasps (Ichneumonidae): Biogeographic Relationships and Distribution Patterns. Insects 2024, 15, 415. https://doi.org/10.3390/insects15060415
Pádua DG, Moreira-Muñoz A, Morales-Fierro V, Araujo RO. Chilean Darwin Wasps (Ichneumonidae): Biogeographic Relationships and Distribution Patterns. Insects. 2024; 15(6):415. https://doi.org/10.3390/insects15060415
Chicago/Turabian StylePádua, Diego G., Andrés Moreira-Muñoz, Vanezza Morales-Fierro, and Rodrigo O. Araujo. 2024. "Chilean Darwin Wasps (Ichneumonidae): Biogeographic Relationships and Distribution Patterns" Insects 15, no. 6: 415. https://doi.org/10.3390/insects15060415
APA StylePádua, D. G., Moreira-Muñoz, A., Morales-Fierro, V., & Araujo, R. O. (2024). Chilean Darwin Wasps (Ichneumonidae): Biogeographic Relationships and Distribution Patterns. Insects, 15(6), 415. https://doi.org/10.3390/insects15060415






