Functional Characterization of an Odorant Receptor Expressed in Newly Hatched Larvae of Fall Armyworm Spodoptera frugiperda
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Tissue Collection
2.2. RNA Isolation and cDNA Synthesis
2.3. Phylogenetic Analysis
2.4. qPCR
2.5. OR Cloning and Sequence Analysis
2.6. Expression Vector Construction and cRNA Synthesis
2.7. Chemicals
2.8. Gene Expression in Xenopus Oocytes and Electrophysiological Recordings
2.9. Behavior Assays
3. Results
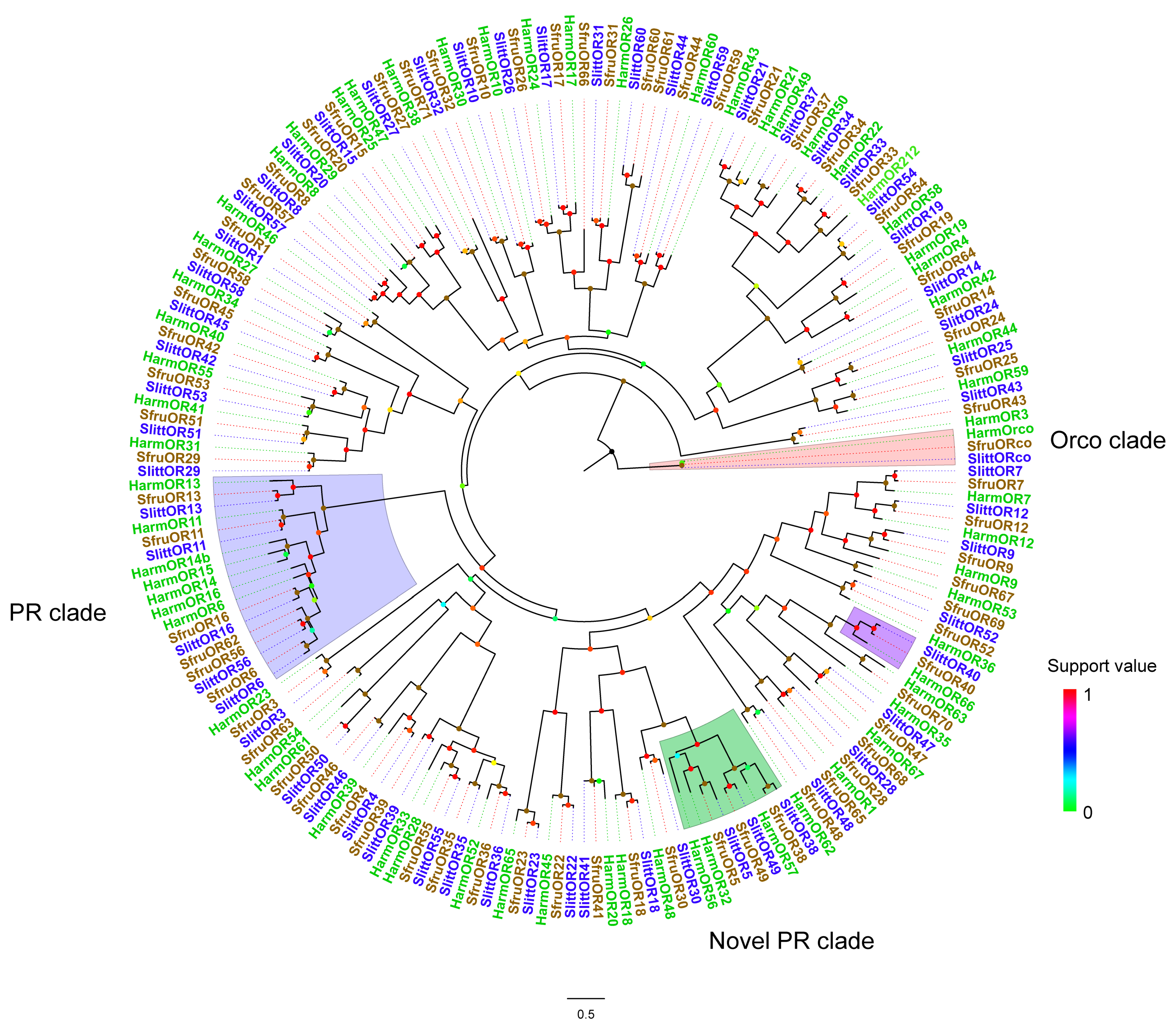
3.1. Identification of a Candidate OR Expressed in Newly Hatched Larvae of Noctuidae Moths Using Phylogenetic Analysis
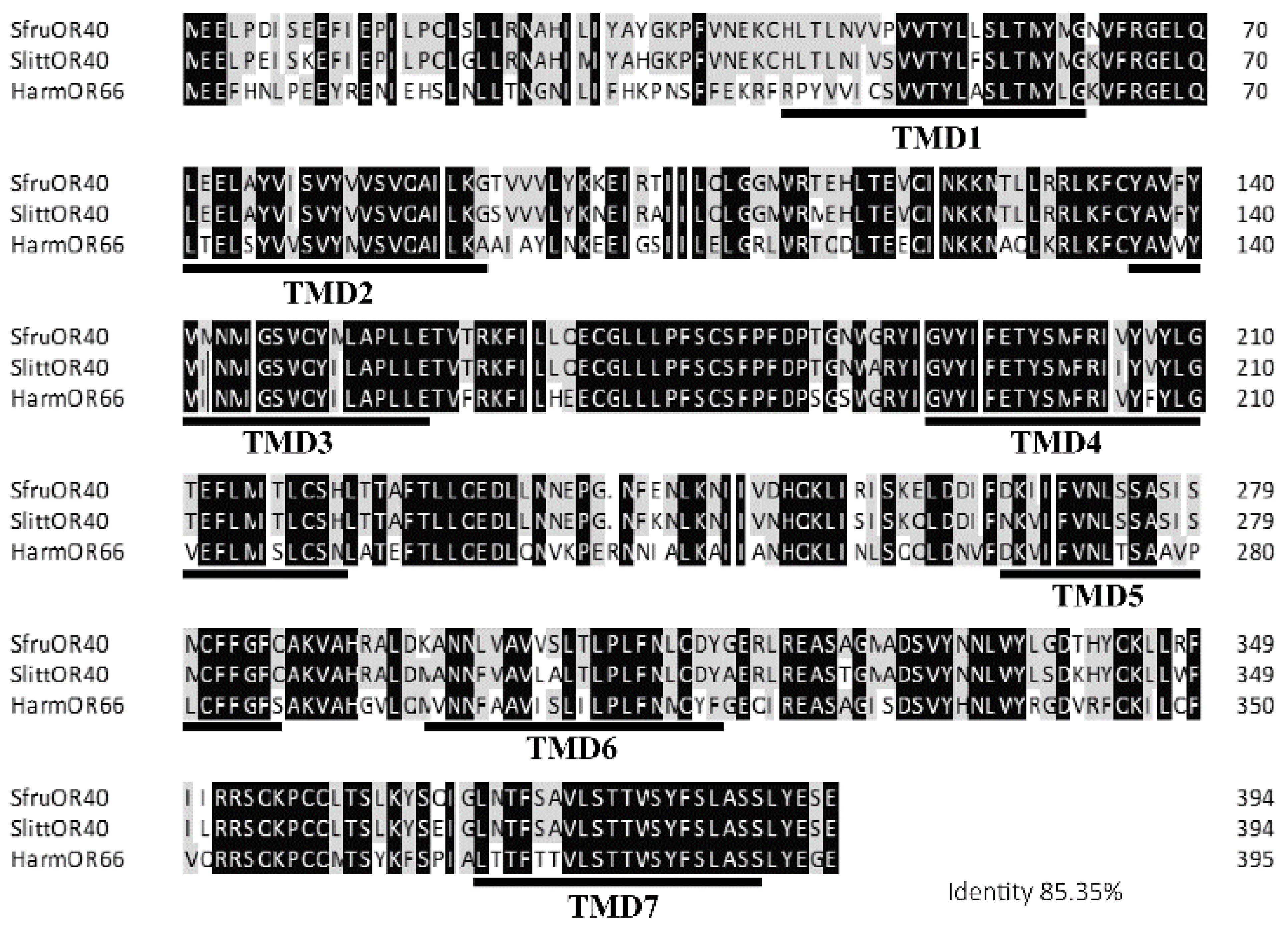
3.2. Gene Cloning and Sequence Analysis of Sfruor40
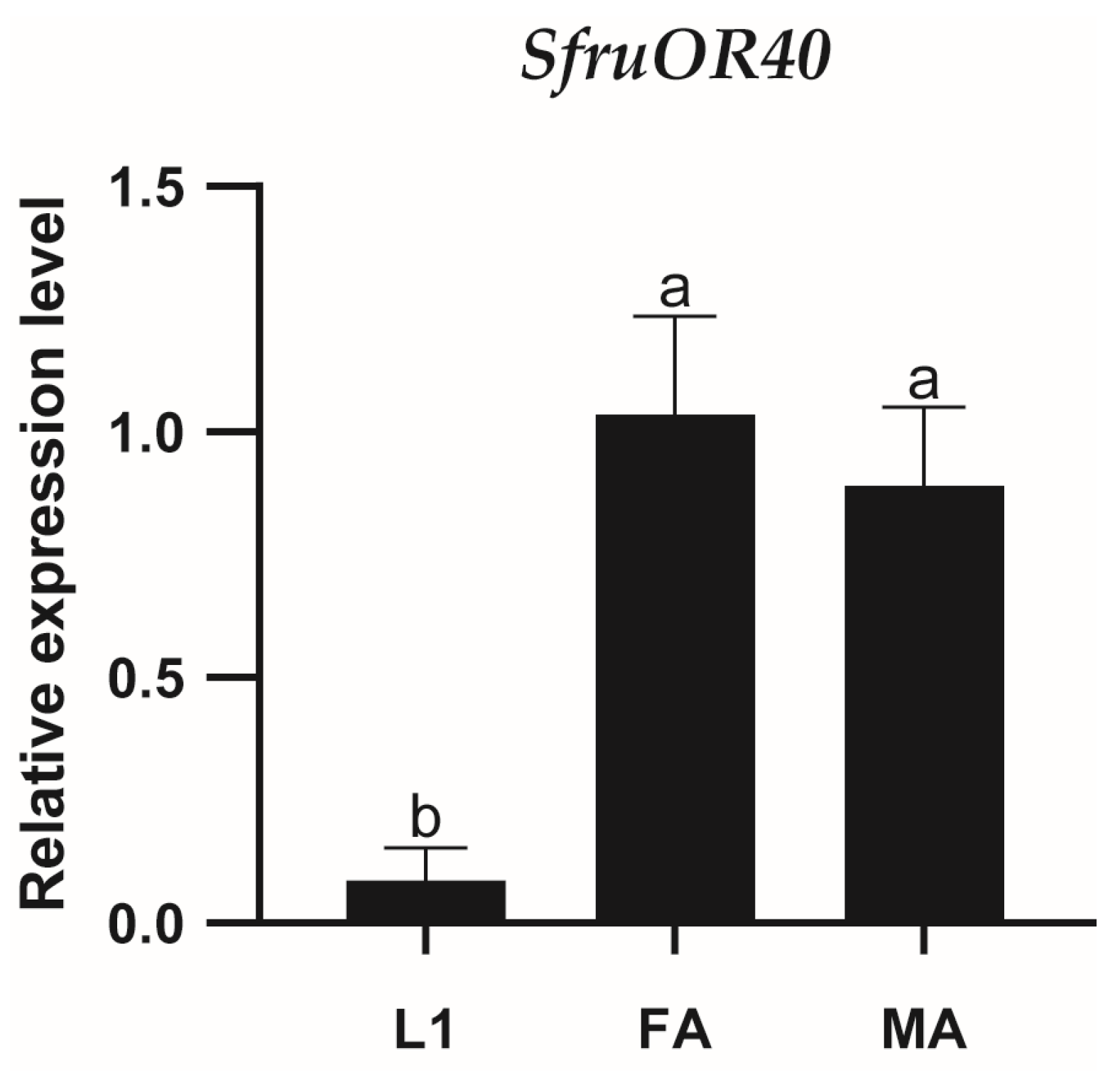
3.3. Expression Profile of SfruOR40 in S. frugiperda
3.4. Functional Characterization of SfruOR40
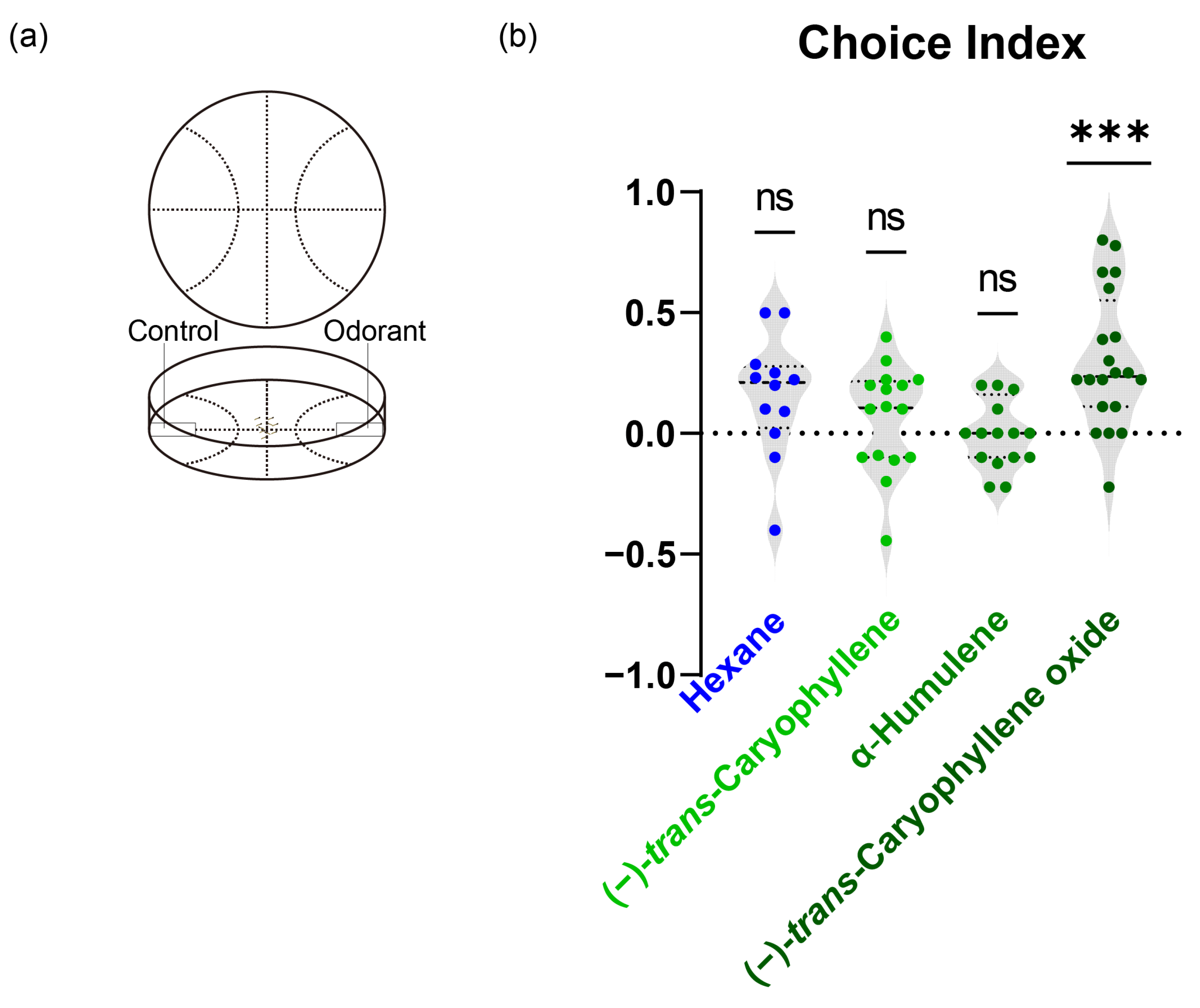
3.5. Attractiveness of Host Plant Volatiles to S. frugiperda Larvae
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hildebrand, J.G. Analysis of Chemical Signals by Nervous Systems. Proc. Natl. Acad. Sci. USA 1995, 92, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Masson, C.; Mustaparta, H. Chemical Information Processing in the Olfactory System of Insects. Physiol. Rev. 1990, 70, 199–245. [Google Scholar] [CrossRef] [PubMed]
- Leal, W.S. Odorant Reception in Insects: Roles of Receptors, Binding Proteins, and Degrading Enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef] [PubMed]
- Kurtovic, A.; Widmer, A.; Dickson, B.J. A Single Class of Olfactory Neurons Mediates Behavioural Responses to a Drosophila Sex Pheromone. Nature 2007, 446, 542–546. [Google Scholar] [CrossRef]
- Krieger, J.; Grosse-Wilde, E.; Gohl, T.; Dewer, Y.M.E.; Raming, K.; Breer, H. Genes Encoding Candidate Pheromone Receptors in a Moth (Heliothis virescens). Proc. Natl. Acad. Sci. USA 2004, 101, 11845–11850. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.; Heidel, A.J.; Heckel, D.G.; Groot, A.T. Transcriptome Analysis of the Sex Pheromone Gland of the Noctuid Moth Heliothis virescens. BMC Genom. 2010, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Poivet, E.; Gallot, A.; Montagné, N.; Glaser, N.; Legeai, F.; Jacquin-Joly, E. A Comparison of the Olfactory Gene Repertoires of Adults and Larvae in the Noctuid Moth Spodoptera littoralis. PLoS ONE 2013, 8, e60263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, B.; Dong, S.L.; Cao, D.P.; Dong, J.F.; Walker, W.B.; Liu, Y.; Wang, G.R. Antennal Transcriptome Analysis and Comparison of Chemosensory Gene Families in Two Closely Related Noctuidae Moths, Helicoverpa armigera and H. assulta. PLoS ONE 2015, 10, e0117054. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gu, S.; Zhang, Y.; Guo, Y.; Wang, G. Candidate Olfaction Genes Identified within the Helicoverpa armigera Antennal Transcriptome. PLoS ONE 2012, 7, e48260. [Google Scholar] [CrossRef]
- Liu, Y.; Heath, J.J.; Zhang, S.; Wijk, M.V.; Wang, G.R.; Buellesbach, J.; Wada-Katsumata, A.; Groot, A.T.; Schal, C. A Mosaic of Endogenous and Plant-Derived Courtship Signals in Moths. Curr. Biol. 2023, 33, 3529–3535. [Google Scholar] [CrossRef]
- Cao, S.; Huang, T.Y.; Shen, J.; Liu, Y.; Wang, G.R. An Orphan Pheromone Receptor Affects the Mating Behavior of Helicoverpa armigera. Front. Physiol. 2020, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Sun, D.D.; Liu, Y.; Yang, Q.; Wang, G.R. Mutagenesis of Odorant Coreceptor Orco Reveals the Distinct Role of Olfaction between Sexes in Spodoptera frugiperda. J. Integr. Agric. 2023, 22, 2162–2172. [Google Scholar] [CrossRef]
- Corcoran, J.A.; Jordan, M.D.; Thrimawithana, A.H.; Crowhurst, R.N.; Newcomb, R.D. The Peripheral Olfactory Repertoire of the Lightbrown Apple Moth, Epiphyas postvittana. PLoS ONE 2015, 10, e0128596. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.Z.; Zhou, Q.Z.; Liu, T.T.; Fang, S.M.; Wang, Y.-W.; Fang, X.; Huang, C.L.; Yu, Q.Y.; Chen, C.H.; Zhang, Z. Evidence of Peripheral Olfactory Impairment in the Domestic Silkworms: Insight from the Comparative Transcriptome and Population Genetics. BMC Genom. 2018, 19, 788. [Google Scholar] [CrossRef] [PubMed]
- Adden, A.; Stewart, T.C.; Webb, B.; Heinze, S. A Neural Model for Insect Steering Applied to Olfaction and Path Integration. Neural Comput. 2022, 34, 2205–2231. [Google Scholar] [CrossRef] [PubMed]
- Rouyar, A.; Deisig, N.; Dupuy, F.; Limousin, D.; Wycke, M.-A.; Renou, M.; Anton, S. Unexpected Plant Odor Responses in a Moth Pheromone System. Front. Physiol. 2015, 6, 148. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.B.; Du, L.X.; Chen, Q.Y.; Feng, Y.L.; Zhang, J.; Zhang, X.X.; Tian, K.; Cao, S.; Huang, T.Y.; Jacquin-Joly, E.; et al. Odorant Receptors for Detecting Flowering Plant Cues Are Functionally Conserved across Moths and Butterflies. Mol. Biol. Evol. 2021, 38, 1413–1427. [Google Scholar] [CrossRef] [PubMed]
- de Fouchier, A.; Walker, W.B.; Montagné, N.; Steiner, C.; Binyameen, M.; Schlyter, F.; Chertemps, T.; Maria, A.; François, M.-C.; Monsempes, C.; et al. Functional Evolution of Lepidoptera Olfactory Receptors Revealed by Deorphanization of a Moth Repertoire. Nat. Commun. 2017, 8, 15709. [Google Scholar] [CrossRef]
- Hansson, B.S.; Stensmyr, M.C. Evolution of Insect Olfaction. Neuron 2011, 72, 698–711. [Google Scholar] [CrossRef]
- Di, C.; Ning, C.; Huang, L.Q.; Wang, C.Z. Design of Larval Chemical Attractants Based on Odorant Response Spectra of Odorant Receptors in the Cotton Bollworm. Insect Biochem. Mol. Biol. 2017, 84, 48–62. [Google Scholar] [CrossRef]
- Tanaka, K.; Uda, Y.; Ono, Y.; Nakagawa, T.; Suwa, M.; Yamaoka, R.; Touhara, K. Highly Selective Tuning of a Silkworm Olfactory Receptor to a Key Mulberry Leaf Volatile. Curr. Biol. 2009, 19, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Revadi, S.V.; Giannuzzi, V.A.; Rossi, V.; Hunger, G.M.; Conchou, L.; Rondoni, G.; Conti, E.; Anderson, P.; Walker, W.B.; Jacquin-Joly, E.; et al. Stage-Specific Expression of an Odorant Receptor Underlies Olfactory Behavioral Plasticity in Spodoptera littoralis Larvae. BMC Biol. 2021, 19, 231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Liu, Y.; Guo, M.B.; Sun, D.D.; Zhang, M.J.; Chu, X.; Berg, B.G.; Wang, G.R. A Female-Specific Odorant Receptor Mediates Oviposition Deterrence in the Moth Helicoverpa armigera. Curr. Biol. 2024, 34, 1–11.e4. [Google Scholar] [CrossRef] [PubMed]
- Itagaki, H.; Hildebrand, J.G. Olfactory Interneurons in the Brain of the Larval Sphinx Moth Manduca sexta. J. Comp. Physiol. A 1990, 167, 309–320. [Google Scholar] [CrossRef]
- Zalucki, M.P.; Clarke, A.R.; Malcolm, S.B. Ecology and Behavior of First Instar Larval Lepidoptera. Annu. Rev. Entomol. 2002, 47, 361–393. [Google Scholar] [CrossRef] [PubMed]
- Tay, W.T.; Meagher, R.L.; Czepak, C.; Groot, A.T. Spodoptera frugiperda: Ecology, Evolution, and Management Options of an Invasive Species. Annu. Rev. Entomol. 2023, 68, 299–317. [Google Scholar] [CrossRef] [PubMed]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First Report of Outbreaks of the Fall Armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a New Alien Invasive Pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.X.; Hu, C.X.; Jia, H.R.; Wu, Q.L.; Shen, X.J.; Zhao, S.Y.; Jiang, Y.Y.; Wu, K.M. Case Study on the First Immigration of Fall Armyworm, Spodoptera frugiperda Invading into China. J. Integr. Agric. 2021, 20, 664–672. [Google Scholar] [CrossRef]
- WU, Q.L.; Jiang, Y.Y.; Hu, G.; Wu, K.M. Analysis on Spring and Summer Migration Routes of Fall Armyworm (Spodoptera frugiperda) from Tropical and Southern Subtropical Zones of China. Plant Prot. 2019, 45, 1–9. [Google Scholar] [CrossRef]
- Wu, Q.L.; Jiang, Y.Y.; Wu, K.M. Analysis of Migration Routes of the Fall Armyworm Spodoptera frugiperda (J. E. Smith) from Myanmar to China. J. Plant Prot. 2019, 45, 1–6. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, Q.L.; Zhang, H.W.; Wu, K.M. Spread of Invasive Migratory Pest Spodoptera frugiperda and Management Practices throughout China. J. Integr. Agric. 2021, 20, 637–645. [Google Scholar] [CrossRef]
- Cheruiyot, D.; Chiriboga Morales, X.; Chidawanyika, F.; Bruce, T.J.A.; Khan, Z.R. Potential Roles of Selected Forage Grasses in Management of Fall Armyworm (Spodoptera frugiperda) through Companion Cropping. Entomol. Exp. Appl. 2021, 169, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Midega, C.A.O.; Pittchar, J.O.; Pickett, J.A.; Hailu, G.W.; Khan, Z.R. A Climate-Adapted Push-Pull System Effectively Controls Fall Armyworm, Spodoptera frugiperda (J E Smith), in Maize in East Africa. Crop Prot. 2018, 105, 10–15. [Google Scholar] [CrossRef]
- Scheidegger, L.; Niassy, S.; Midega, C.; Chiriboga, X.; Delabays, N.; Lefort, F.; Zürcher, R.; Hailu, G.; Khan, Z.; Subramanian, S. The Role of Desmodium intortum, Brachiaria sp. and Phaseolus vulgaris in the Management of Fall Armyworm Spodoptera frugiperda (J. E. Smith) in Maize Cropping Systems in Africa. Pest Manag. Sci. 2021, 77, 2350–2357. [Google Scholar] [CrossRef] [PubMed]
- Firake, D.M.; Behere, G.T. Natural Mortality of Invasive Fall Armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) in Maize Agroecosystems of Northeast India. Biol. Control 2020, 148, 104303. [Google Scholar] [CrossRef]
- Hussain, A.G.; Wennmann, J.T.; Goergen, G.; Bryon, A.; Ros, V.I.D. Viruses of the Fall Armyworm Spodoptera frugiperda: A Review with Prospects for Biological Control. Viruses 2021, 13, 2220. [Google Scholar] [CrossRef] [PubMed]
- Groot, A.T.; Dekker, T.; Heckel, D.G. The Genetic Basis of Pheromone Evolution in Moths. Annu. Rev. Entomol. 2016, 61, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, F.; Yang, B.; Liu, Y.; Wang, G.R. Functional Characterization of Sex Pheromone Receptors in Spodoptera frugiperda, S. exigua, and S. litura Moths. Insect Sci. 2023, 30, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Jacquin-Joly, E.; Montagné, N.; Liu, F.; Liu, Y.; Wang, G.R. Identification of an Odorant Receptor Responding to Sex Pheromones in Spodoptera frugiperda Extends the Novel Type-I PR Lineage in Moths. Insect Sci. 2023, 31, 489–502. [Google Scholar] [CrossRef]
- Dong, J.F.; Yang, H.B.; Li, D.X.; Yu, H.Q.; Tian, C.H. Identification and Expression Analysis of Chemosensory Receptors in the Tarsi of Fall Armyworm, Spodoptera frugiperda (J. E. Smith). Front. Physiol. 2023, 14, 1177297. [Google Scholar] [CrossRef]
- Wang, J.X.; Wei, Z.Q.; Chen, M.D.; Yan, Q.; Zhang, J.; Dong, S.L. Conserved Odorant Receptors Involved in Nonanal-Induced Female Attractive Behavior in Two Spodoptera Species. J. Agric. Food Chem. 2023, 71, 13795–13804. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.L.; Jiang, P.S.; Dong, B.X.; Tian, C.H.; Dong, J.F. Candidate Chemosensory Receptors in the Antennae and Maxillae of Spodoptera frugiperda (J. E. Smith) Larvae. Front. Physiol. 2022, 13, 970915. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, B.; Zheng, W.G.; Liu, C.H.; Zhang, D.D.; Zhao, S.Y.; Li, Z.Y.; Xu, P.J.; Wilson, K.; Withers, A.; et al. Genetic Structure and Insecticide Resistance Characteristics of Fall Armyworm Populations Invading China. Mol. Ecol. Resour. 2020, 20, 1682–1696. [Google Scholar] [CrossRef]
- Gouin, A.; Bretaudeau, A.; Nam, K.; Gimenez, S.; Aury, J.-M.; Duvic, B.; Hilliou, F.; Durand, N.; Montagné, N.; Darboux, I.; et al. Two Genomes of Highly Polyphagous Lepidopteran Pests (Spodoptera frugiperda, Noctuidae) with Different Host-Plant Ranges. Sci. Rep. 2017, 7, 11816. [Google Scholar] [CrossRef] [PubMed]
- Koutroumpa, F.A.; Monsempes, C.; François, M.-C.; Severac, D.; Montagné, N.; Meslin, C.; Jacquin-Joly, E. Description of Chemosensory Genes in Unexplored Tissues of the Moth Spodoptera littoralis. Front. Ecol. Evol. 2021, 9, 678277. [Google Scholar] [CrossRef]
- Meslin, C.; Mainet, P.; Montagné, N.; Robin, S.; Legeai, F.; Bretaudeau, A.; Johnston, J.S.; Koutroumpa, F.; Persyn, E.; Monsempès, C.; et al. Spodoptera littoralis Genome Mining Brings Insights on the Dynamic of Expansion of Gustatory Receptors in Polyphagous Noctuidae. G3 Genes Genomes Genet. 2022, 12, jkac131. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML Version 8: A Tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinform. Oxf. Engl. 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Shimodaira, H.; Hasegawa, M. Multiple Comparisons of Log-Likelihoods with Applications to Phylogenetic Inference. Mol. Biol. Evol. 1999, 16, 1114. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Liu, Y.P.; Zhang, S.; Cao, S.; Jacquin-Joly, E.; Zhou, Q.; Liu, Y.; Wang, G.R. An Odorant Receptor Mediates the Avoidance of Plutella xylostella against Parasitoid. BMC Biol. 2024, 22, 61. [Google Scholar] [CrossRef]
- Poivet, E.; Rharrabe, K.; Monsempes, C.; Glaser, N.; Rochat, D.; Renou, M.; Marion-Poll, F.; Jacquin-Joly, E. The Use of the Sex Pheromone as an Evolutionary Solution to Food Source Selection in Caterpillars. Nat. Commun. 2012, 3, 1047. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Liu, Y.; Guo, M.B.; Wang, G.R. A Conserved Odorant Receptor Tuned to Floral Volatiles in Three Heliothinae Species. PLoS ONE 2016, 11, e0155029. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Shi, L.F.; Khashaveh, A.; Shan, S.; Lv, B.B.; Gu, S.H.; Zhang, Y.J. Loss of Binding Capabilities in an Ecologically Important Odorant Receptor of the Fall Armyworm, Spodoptera frugiperda, by a Single Point Mutation. J. Agric. Food Chem. 2023, 71, 13003–13013. [Google Scholar] [CrossRef]
- Benton, R.; Sachse, S.; Michnick, S.W.; Vosshall, L.B. Atypical Membrane Topology and Heteromeric Function of Drosophila Odorant Receptors in vivo. PLoS Biol. 2006, 4, e20. [Google Scholar] [CrossRef] [PubMed]
- Legeai, F.; Malpel, S.; Montagné, N.; Monsempes, C.; Cousserans, F.; Merlin, C.; François, M.-C.; Maïbèche-Coisné, M.; Gavory, F.; Poulain, J.; et al. An Expressed Sequence Tag Collection from the Male Antennae of the Noctuid Moth Spodoptera littoralis: A Resource for Olfactory and Pheromone Detection Research. BMC Genom. 2011, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Krieger, J.; Raming, K.; Dewer, Y.M.E.; Bette, S.; Conzelmann, S.; Breer, H. A Divergent Gene Family Encoding Candidate Olfactory Receptors of the Moth Heliothis virescens. Eur. J. Neurosci. 2002, 16, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Hoballah, M.E.; Turlings, T.C.J. The Role of Fresh versus Old Leaf Damage in the Attraction of Parasitic Wasps to Herbivore-Induced Maize Volatiles. J. Chem. Ecol. 2005, 31, 2003–2018. [Google Scholar] [CrossRef] [PubMed]
- Zakir, A.; Sadek, M.; Bengtsson, M.; Hansson, B.; Witzgall, P. Herbivore-Induced Plant Volatiles Provide Associational Resistance against an Ovipositing Herbivore. J. Ecol. 2013, 101, 410–417. [Google Scholar] [CrossRef]
- Binder, B.F.; Robbins, J.C. Effect of Terpenoids and Related Compounds on the Oviposition Behavior of the European Corn Borer, Ostrinia nubilalis (Lepidoptera: Pyralidae). J. Agric. Food Chem. 1997, 45, 980–984. [Google Scholar] [CrossRef]
- De Moraes, C.M.; Mescher, M.C.; Tumlinson, J.H. Caterpillar-Induced Nocturnal Plant Volatiles Repel Conspecific Females. Nature 2001, 410, 577–580. [Google Scholar] [CrossRef]
- Konstantopoulou, M.A.; Krokos, F.D.; Mazomenos, B.E. Chemical Composition of Corn Leaf Essential Oils and Their Role in the Oviposition Behavior of Sesamia nonagrioide Females. J. Chem. Ecol. 2004, 30, 2243–2256. [Google Scholar] [CrossRef] [PubMed]
- Witzgall, P.; Ansebo, L.; Yang, Z.; Angeli, G.; Sauphanor, B.; Bengtsson, M. Plant Volatiles Affect Oviposition by Codling Moths. Chemoecology 2005, 15, 77–83. [Google Scholar] [CrossRef]
- Carroll, M.J.; Schmelz, E.A.; Meagher, R.L.; Teal, P.E.A. Attraction of Spodoptera frugiperda Larvae to Volatiles from Herbivore-Damaged Maize Seedlings. J. Chem. Ecol. 2006, 32, 1911–1924. [Google Scholar] [CrossRef] [PubMed]

| Usage | Primer Name | Primer Sequences |
|---|---|---|
| Gene cloning | SfruOrco-F | ATGATGACCAAAGTGAAAGCCC |
| SfruOrco-R | TTACTTGAGCTGCACCAACACC | |
| SfruOR40-F | ATGGAAGAGTTGCCTGATATTTCA | |
| SfruOR40-R | TCAAATTTCACTTTCGTATAGACTTGAA | |
| Vector construction | SfruOrco-F | TCAGGGCCCgccaccATGATGACCAAAGTGAAAGCCC (Apa I) |
| SfruOrco-R | TCAGCGGCCGCTTACTTGAGCTGCACCAACACC (Not I) | |
| SfruOR40-F | TCAGGGCCCgccaccATGGAAGAGTTGCCTGATATTTCA (Apa I) | |
| SfruOR40-R | TCAGCGGCCGCTCAAATTTCACTTTCGTATAGACTTGAA (Not I) | |
| qPCR | SfruOR40-F | TCGGATTTTGTGCTAAGGTCG |
| SfruOR40-R | TCTCAACCGCTCCCCGTAAT | |
| SfruActin-F | GGTTGGTATGGGTCAGAAGG | |
| SfruActin-R | AGCTCGTTGTAGAAGGTGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, X.; Liu, W.; Chen, R.; Liu, Y. Functional Characterization of an Odorant Receptor Expressed in Newly Hatched Larvae of Fall Armyworm Spodoptera frugiperda. Insects 2024, 15, 564. https://doi.org/10.3390/insects15080564
Wang Z, Wang X, Liu W, Chen R, Liu Y. Functional Characterization of an Odorant Receptor Expressed in Newly Hatched Larvae of Fall Armyworm Spodoptera frugiperda. Insects. 2024; 15(8):564. https://doi.org/10.3390/insects15080564
Chicago/Turabian StyleWang, Zhiqiang, Xiaoqing Wang, Weihao Liu, Run Chen, and Yang Liu. 2024. "Functional Characterization of an Odorant Receptor Expressed in Newly Hatched Larvae of Fall Armyworm Spodoptera frugiperda" Insects 15, no. 8: 564. https://doi.org/10.3390/insects15080564






