Prey Switching and Natural Pest Control Potential of Carabid Communities over the Winter Wheat Cropping Season
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Sampling
2.2. Carabids Selection for MGCA
2.3. DNA Extraction
2.4. Primer Design, Multiplex PCR Assay and PCR Amplification
2.5. Statistical Analysis
3. Results
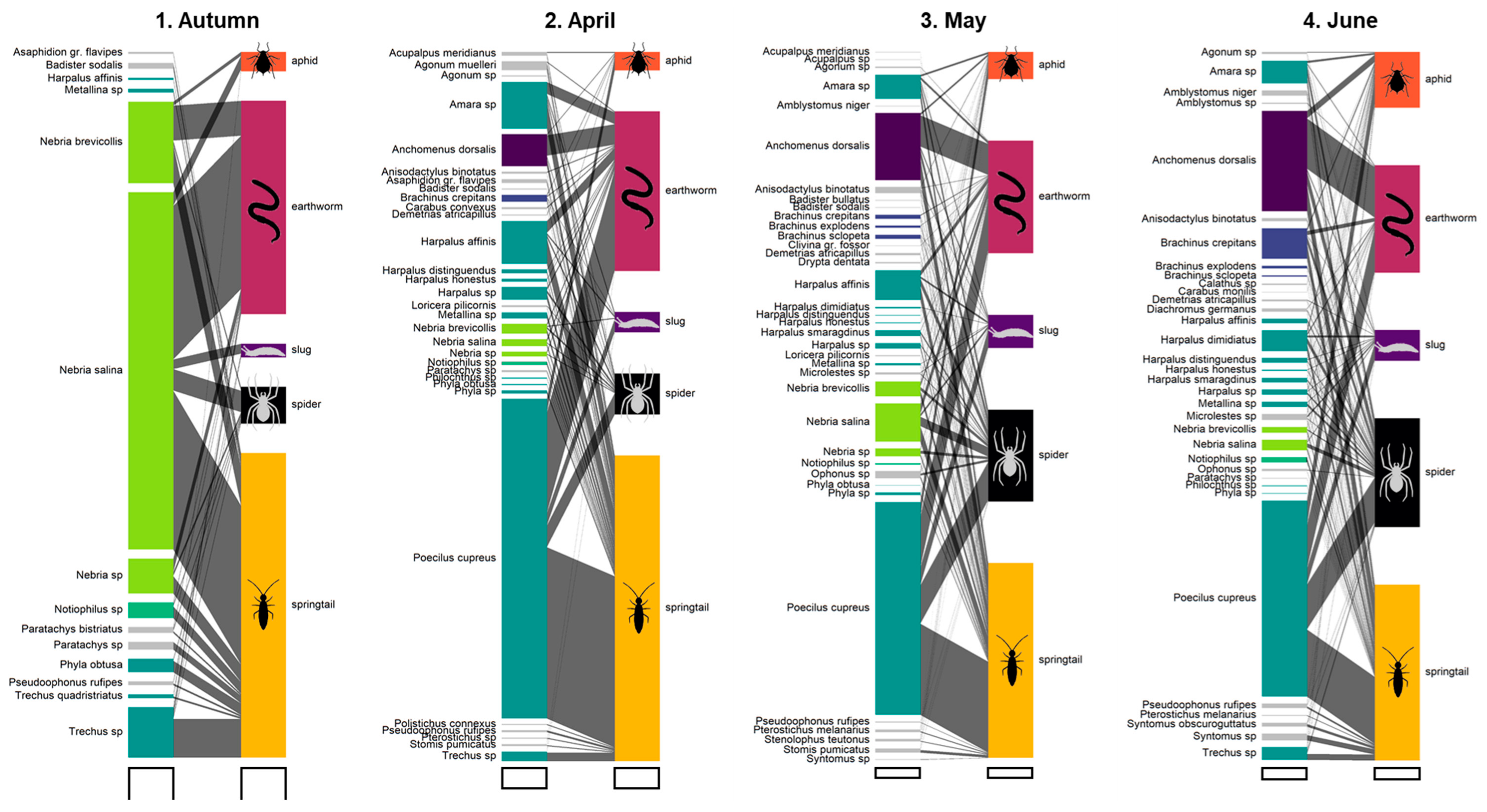
3.1. Carabid Communities
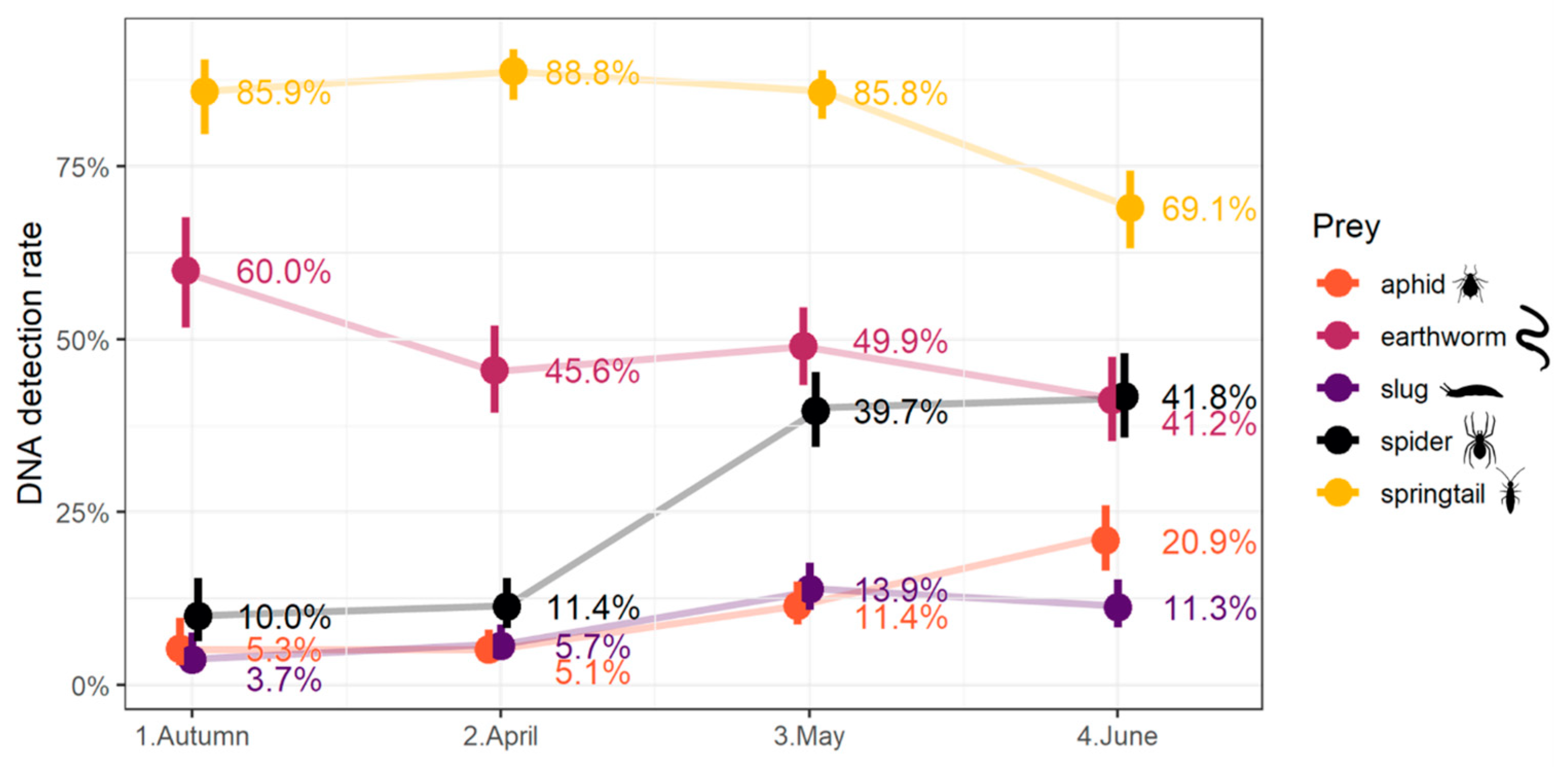
3.2. Detection Rates at Community Level
3.3. Factors Influencing Prey DNA Detection Rates at Carabid Community Scale
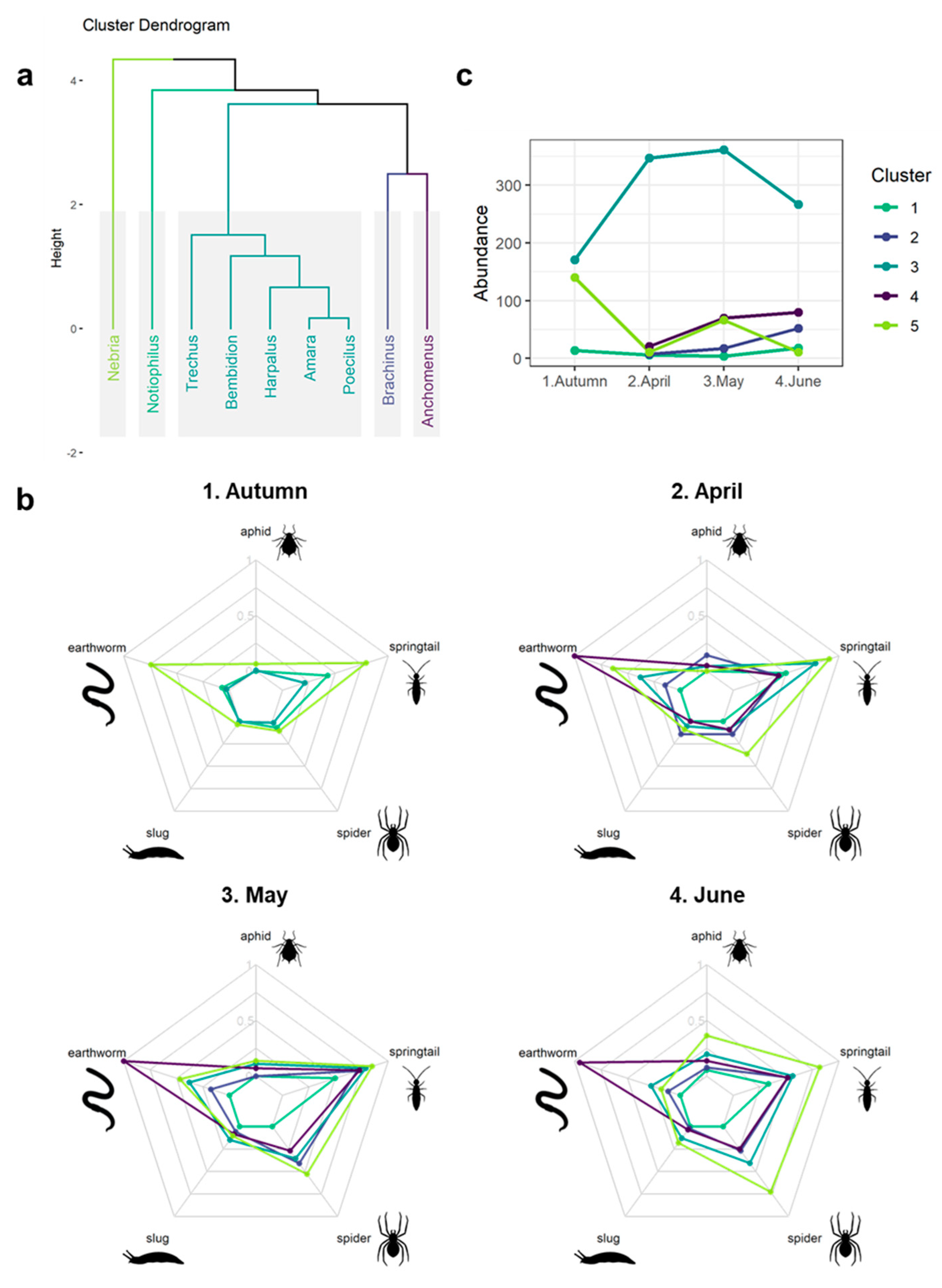
3.4. Seasonal Predation Profile of Predominant Carabid Genera
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Letourneau, D.; Jedlicka, J.; Bothwell, S.; Moreno, C. Effects of Natural Enemy Biodiversity on the Suppression of Arthropod Herbivores in Terrestrial Ecosystems. Annu. Rev. Ecol. Syst. 2009, 40, 573–592. [Google Scholar] [CrossRef]
- Snyder, W.E. Give predators a complement: Conserving natural enemy biodiversity to improve biocontrol. Biol. Control 2019, 135, 73–82. [Google Scholar] [CrossRef]
- Daouti, E.; Jonsson, M.; Vico, G.; Menegat, A. Seed predation is key to preventing population growth of the weed Alopecurus myosuroides. J. Appl. Ecol. 2022, 59, 471–482. [Google Scholar] [CrossRef]
- Yoo, H.J.S.; O’Neil, R.J. Temporal relationships between the generalist predator, Orius insidiosus, and its two major prey in soybean. Biol. Control 2009, 48, 168–180. [Google Scholar] [CrossRef]
- Kromp, B. Carabid beetles in sustainable agriculture: A review on pest control efficacy, cultivation impacts and enhancement. Agric. Ecosyst. Environ. 1999, 74, 187–228. [Google Scholar] [CrossRef]
- Lövei, G.L.; Sunderland, K.D. Ecology and Behavior of Ground Beetles (Coleoptera: Carabidae). Annu. Rev. Entomol. 1996, 41, 231–256. [Google Scholar] [CrossRef] [PubMed]
- Frei, B.; Guenay, Y.; Bohan, D.A.; Traugott, M.; Wallinger, C. Molecular analysis indicates high levels of carabid weed seed consumption in cereal fields across Central Europe. J. Pest. Sci. 2019, 92, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Greenstone, M.H.; Szendrei, Z.; Payton, M.E.; Rowley, D.L.; Coudron, T.C.; Weber, D.C. Choosing natural enemies for conservation biological control: Use of the prey detectability half-life to rank key predators of Colorado potato beetle. Entomol. Exp. Appl. 2010, 136, 97–107. [Google Scholar] [CrossRef]
- Reich, I.; Jessie, C.; Ahn, S.-J.; Choi, M.-Y.; Williams, C.; Gormally, M.; Mc Donnell, R. Assessment of the Biological Control Potential of Common Carabid Beetle Species for Autumn- and Winter-Active Pests (Gastropoda, Lepidoptera, Diptera: Tipulidae) in Annual Ryegrass in Western Oregon. Insects 2020, 11, 722. [Google Scholar] [CrossRef]
- King, R.A.; Vaughan, I.P.; Bell, J.R.; Bohan, D.A.; Symondson, W.O.C. Prey choice by carabid beetles feeding on an earthworm community analysed using species- and lineage-specific PCR primers. Mol. Ecol. 2010, 19, 1721–1732. [Google Scholar] [CrossRef]
- Davey, J.S.; Vaughan, I.P.; King, R.A.; Bell, J.R.; Bohan, D.A.; Bruford, M.W.; Holland, J.M.; Symondson, W.O.C. Intraguild predation in winter wheat: Prey choice by a common epigeal carabid consuming spiders. J. Appl. Ecol. 2013, 50, 271–279. [Google Scholar] [CrossRef]
- Staudacher, K.; Jonsson, M.; Traugott, M. Diagnostic PCR assays to unravel food web interactions in cereal crops with focus on biological control of aphids. J. Pest. Sci. 2016, 89, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Carbonne, B.; Petit, S.; Neidel, V.; Foffova, H.; Daouti, E.; Frei, B.; Skuhrovec, J.; Řezáč, M.; Saska, P.; Wallinger, C.; et al. The resilience of weed seedbank regulation by carabid beetles, at continental scales, to alternative prey. Sci. Rep. 2020, 10, 19315. [Google Scholar] [CrossRef] [PubMed]
- Honek, A.; Martinkova, Z.; Saska, P.; Pekar, S. Size and taxonomic constraints determine the seed preferences of Carabidae (Coleoptera). Basic. Appl. Ecol. 2007, 8, 343–353. [Google Scholar] [CrossRef]
- Charalabidis, A.; Dechaume-Moncharmont, F.-X.; Carbonne, B.; Bohan, D.A.; Petit, S. Diversity of foraging strategies and responses to predator interference in seed-eating carabid beetles. Basic. Appl. Ecol. 2019, 36, 13–24. [Google Scholar] [CrossRef]
- Loughridge, A.H.; Luff, M.L. Aphid Predation by Harpalus rufipes (Degeer) (Coleoptera: Carabidae) in the Laboratory and Field. J. Appl. Ecol. 1983, 20, 451–462. [Google Scholar] [CrossRef]
- Hajek, A.E.; Hannam, J.J.; Nielsen, C.; Bell, A.J.; Liebherr, J.K. Distribution and Abundance of Carabidae (Coleoptera) Associated with Soybean Aphid (Hemiptera: Aphididae) Populations in Central New York. Ann. Entomol. Soc. Am. 2007, 100, 876–886. [Google Scholar] [CrossRef][Green Version]
- Symondson, W.O.C.; Sunderland, K.D.; Greenstone, M.H. Can generalist predators be effective biocontrol agents? Annu. Rev. Entomol. 2002, 47, 561–594. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.; Filser, J.; Henschel, J.R. Predation by ground beetles and wolf spiders on herbivorous insects in a maize crop. Agric. Ecosyst. Environ. 1999, 72, 189–199. [Google Scholar] [CrossRef]
- Birkhofer, K.; Bylund, H.; Dalin, P.; Ferlian, O.; Gagic, V.; Hambäck, P.A.; Klapwijk, M.; Mestre, L.; Roubinet, E.; Schroeder, M.; et al. Methods to identify the prey of invertebrate predators in terrestrial field studies. Ecol. Evol. 2017, 7, 1942–1953. [Google Scholar] [CrossRef]
- Roubinet, E.; Birkhofer, K.; Malsher, G.; Staudacher, K.; Ekbom, B.; Traugott, M.; Jonsson, M. Diet of generalist predators reflects effects of cropping period and farming system on extra- and intraguild prey. Ecol. Appl. 2017, 27, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, K.; Rubbmark, O.R.; Birkhofer, K.; Malsher, G.; Sint, D.; Jonsson, M.; Traugott, M. Habitat heterogeneity induces rapid changes in the feeding behaviour of generalist arthropod predators. Funct. Ecol. 2018, 32, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, B.; Gratton, C. Temporal Resource (Dis)continuity for Conservation Biological Control: From Field to Landscape Scales. Front. Sustain. Food Syst. 2020, 4, 127. [Google Scholar] [CrossRef]
- Gray, C.; Ma, A.; McLaughlin, O.; Petit, S.; Woodward, G.; Bohan, D.A. Ecological plasticity governs ecosystem services in multilayer networks. Commun. Biol. 2021, 4, 75. [Google Scholar] [CrossRef] [PubMed]
- Roger, J.-L.; Jambon, O.; Bouger, G. Clé de Détermination Des Carabides—Paysages Agricoles du Nord Ouest de la France; SAD-Paysage, Institut National de Recherche Agronomique: Rennes, France, 2013. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 9 August 2024).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Hartig, F.; Lohse, L. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models, R Package Version 0.4.6. 2022. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 9 August 2024).
- Fox, J.; Weisberg, S.; Price, B.; Adler, D.; Bates, D.; Baud-Bovy, G.; Bolker, B.; Ellison, S.; Firth, D.; Friendly, M.; et al. Car: Companion to Applied Regression 2019, R Package Version 3.1.2. Available online: https://CRAN.R-project.org/package=car (accessed on 9 August 2024).
- Lenth, R.V.; Buerkner, P.; Herve, M.; Love, J.; Riebl, H.; Singmann, H. Emmeans: Estimated Marginal Means, Aka Least-Squares Means 2020, R Package Version 1.10.0. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 9 August 2024).
- Dormann, C.; Gruber, B.; Fründ, J. Introducing the bipartite Package: Analysing Ecological Networks. R News 2008, 8/2, 8–11. [Google Scholar]
- Husson, F.; Josse, J.; Le, S.; Mazet, J. FactoMineR: Multivariate Exploratory Data Analysis and Data Mining 2018, R Package Version 2.10. Available online: https://CRAN.R-project.org/package=FactoMineR (accessed on 9 August 2024).
- Barnier, J. Explor: Interactive Interfaces for Results Exploration 2019, R Package Version 0.3.10. Available online: https://CRAN.R-project.org/package=explor (accessed on 9 August 2024).
- Scaccini, D.; Panini, M.; Chiesa, O.; Nicoli Aldini, R.; Tabaglio, V.; Mazzoni, E. Slug Monitoring and Impacts on the Ground Beetle Community in the Frame of Sustainable Pest Control in Conventional and Conservation Agroecosystems. Insects 2020, 11, 380. [Google Scholar] [CrossRef] [PubMed]
- Janssen, A.; Montserrat, M.; HilleRisLambers, R.; Roos AM de Pallini, A.; Sabelis, M.W. Intraguild Predation Usually does not Disrupt Biological Control. In Trophic and Guild in Biological Interactions Control; Brodeur, J., Boivin, G., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2006; pp. 21–44. [Google Scholar] [CrossRef]
- Rosenheim, J.A.; Harmon, J.P. The Influence of Intraguild Predation on the Suppression of a Shared Prey Population: An Empirical Reassessment. In Trophic and Guild in Biological Interactions Control; Brodeur, J., Boivin, G., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2006; pp. 1–20. [Google Scholar] [CrossRef]
- Sunderland, K.D. The Diet of some Predatory Arthropods in Cereal Crops. J. Appl. Ecol. 1975, 12, 507–515. [Google Scholar] [CrossRef]
- Settle, W.H.; Ariawan, H.; Astuti, E.T.; Cahyana, W.; Hakim, A.L.; Hindayana, D.; Lestari, A.S. Managing Tropical Rice Pests Through Conservation of Generalist Natural Enemies and Alternative Prey. Ecology 1996, 77, 1975–1988. [Google Scholar] [CrossRef]
- Kamenova, S.; Leroux, C.; Polin, S.E.; Plantegenest, M. Community-wide stable isotope analysis reveals two distinct trophic groups in a service-providing carabid community. Bull. Entomol. Res. 2018, 108, 130–139. [Google Scholar] [CrossRef]
- McKemey, A.R.; Symondson, W.O.C.; Glen, D.M.; Brain, P. Effects of Slug Size on Predation by Pterostichus melanarius (Coleoptera: Carabidae). Biocontrol Sci. Technol. 2001, 11, 81–91. [Google Scholar] [CrossRef]
- Oberholzer, F.; Frank, T. Predation by the Carabid Beetles Pterostichus melanarius and Poecilus cupreus on Slugs and Slug Eggs. Biocontrol Sci. Technol. 2003, 13, 99–110. [Google Scholar] [CrossRef]
- McKemey, A.R.; Symondson, W.O.C.; Glen, D.M. Predation and prey size choice by the carabid beetle Pterostichus melanarius (Coleoptera: Carabidae): The dangers of extrapolating from laboratory to field. Bull. Entomol. Res. 2003, 93, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Honek, A.; Martinkova, Z.; Jarosik, V. Ground beetles (Carabidae) as seed predators. EJE 2003, 100, 531–544. [Google Scholar] [CrossRef]
- Saska, P.; Honek, A. Development of the beetle parasitoids, Brachinus explodens and B. crepitans (Coleoptera: Carabidae). J. Zool. 2004, 262, 29–36. [Google Scholar] [CrossRef]
- Berg K von Traugott, M.; Symondson, W.O.C.; Scheu, S. The effects of temperature on detection of prey DNA in two species of carabid beetle. Bull. Entomol. Res. 2008, 98, 263–269. [Google Scholar] [CrossRef]
- Sheppard, S.K.; Bell, J.; Sunderland, K.D.; Fenlon, J.; Skervin, D.; Symondson, W.O.C. Detection of secondary predation by PCR analyses of the gut contents of invertebrate generalist predators. Mol. Ecol. 2005, 14, 4461–4468. [Google Scholar] [CrossRef]
- Harper, G.L.; King, R.A.; Dodd, C.S.; Harwood, J.D.; Glen, D.M.; Bruford, M.W.; Symondson, W.O.C. Rapid screening of invertebrate predators for multiple prey DNA targets. Mol. Ecol. 2005, 14, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Guenay, Y.; Trager, H.; Glarcher, I.; Traugott, M.; Wallinger, C. Limited detection of secondarily consumed plant food by DNA-based diet analysis of omnivorous carabid beetles. Environ. DNA 2021, 3, 426–434. [Google Scholar] [CrossRef]
- Harwood, J.D.; Phillips, S.W.; Sunderland, K.D.; Symondson, W.O.C. Secondary predation: Quantification of food chain errors in an aphid–spider–carabid system using monoclonal antibodies. Mol. Ecol. 2001, 10, 2049–2057. [Google Scholar] [CrossRef]
- Ferrante, M.; Barone, G.; Lövei, G.L. The carabid Pterostichus melanarius uses chemical cues for opportunistic predation and saprophagy but not for finding healthy prey. BioControl 2017, 62, 741–747. [Google Scholar] [CrossRef]
- von Berg, K.; Traugott, M.; Scheu, S. Scavenging and active predation in generalist predators: A mesocosm study employing DNA-based gut content analysis. Pedobiologia 2012, 55, 1–5. [Google Scholar] [CrossRef]
- Mair, J.; Port, G.R. Predation by the carabid beetles Pterostichus madidus and Nebria brevicollis is affected by size and condition of the prey slug Deroceras reticulatum. Agric. For. Entomol. 2001, 3, 99–106. [Google Scholar] [CrossRef]
- Baulechner, D.; Jauker, F.; Neubauer, T.A.; Wolters, V. Convergent evolution of specialized generalists: Implications for phylogenetic and functional diversity of carabid feeding groups. Ecol. Evol. 2020, 10, 11100–11110. [Google Scholar] [CrossRef] [PubMed]
- Baulechner, D.; Jauker, F.; Wolters, V. Carabid adaptation to a collembolan diet: Hunting efficiency and nutritional value. Ecol. Entomol. 2022, 47, 242–248. [Google Scholar] [CrossRef]
- Hintzpeter, U.; Bauer, T. The antennal setal trap of the Ground beetle Loricera pilicornis: A specialization for feeding on Collembola. J. Zool. 1986, 208, 615–630. [Google Scholar] [CrossRef]
| Fixed Effect | Chisq | Df | Pr (>Chisq) |
|---|---|---|---|
| (Intercept) | 75.224 | 1 | <0.001 |
| Prey group | 302.143 | 4 | <0.001 |
| Session | 49.939 | 3 | <0.001 |
| Prey group: Session | 236.196 | 12 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacco--Martret de Préville, A.; Staudacher, K.; Traugott, M.; Bohan, D.A.; Plantegenest, M.; Canard, E. Prey Switching and Natural Pest Control Potential of Carabid Communities over the Winter Wheat Cropping Season. Insects 2024, 15, 610. https://doi.org/10.3390/insects15080610
Sacco--Martret de Préville A, Staudacher K, Traugott M, Bohan DA, Plantegenest M, Canard E. Prey Switching and Natural Pest Control Potential of Carabid Communities over the Winter Wheat Cropping Season. Insects. 2024; 15(8):610. https://doi.org/10.3390/insects15080610
Chicago/Turabian StyleSacco--Martret de Préville, Ambre, Karin Staudacher, Michael Traugott, David A. Bohan, Manuel Plantegenest, and Elsa Canard. 2024. "Prey Switching and Natural Pest Control Potential of Carabid Communities over the Winter Wheat Cropping Season" Insects 15, no. 8: 610. https://doi.org/10.3390/insects15080610
APA StyleSacco--Martret de Préville, A., Staudacher, K., Traugott, M., Bohan, D. A., Plantegenest, M., & Canard, E. (2024). Prey Switching and Natural Pest Control Potential of Carabid Communities over the Winter Wheat Cropping Season. Insects, 15(8), 610. https://doi.org/10.3390/insects15080610








