Consumption and Digestion of Plastics by Greater Hive Moth Larvae
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining Biological Material and Plastic
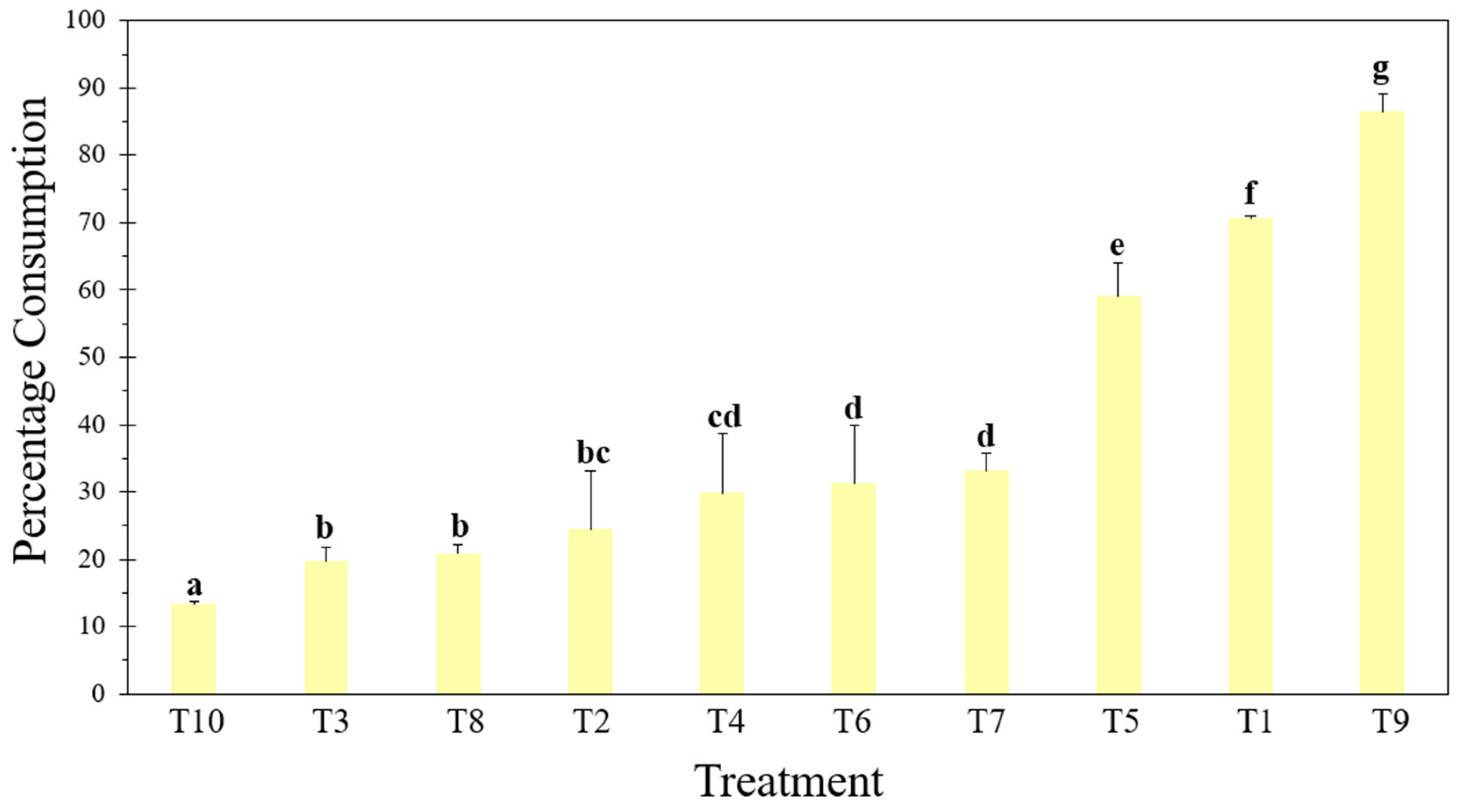
2.2. Percentage Consumed
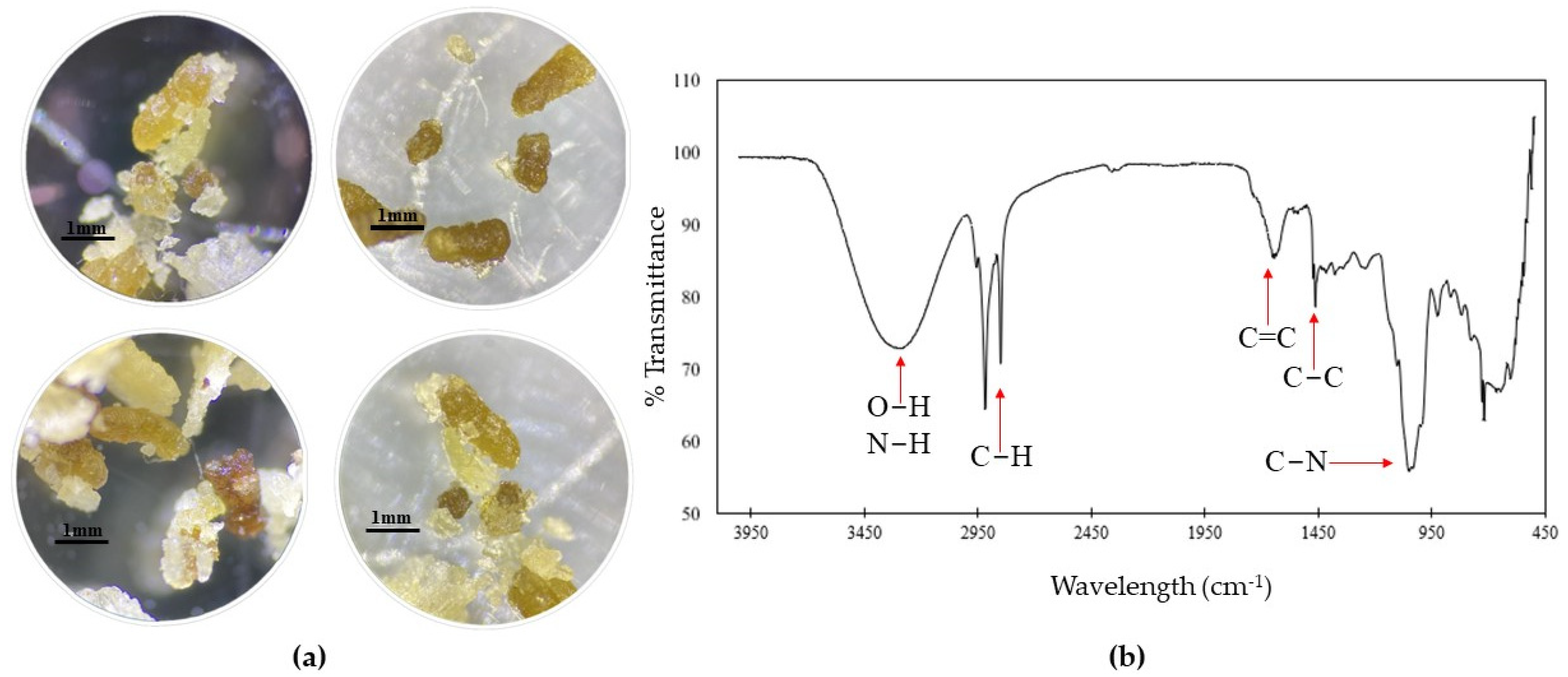
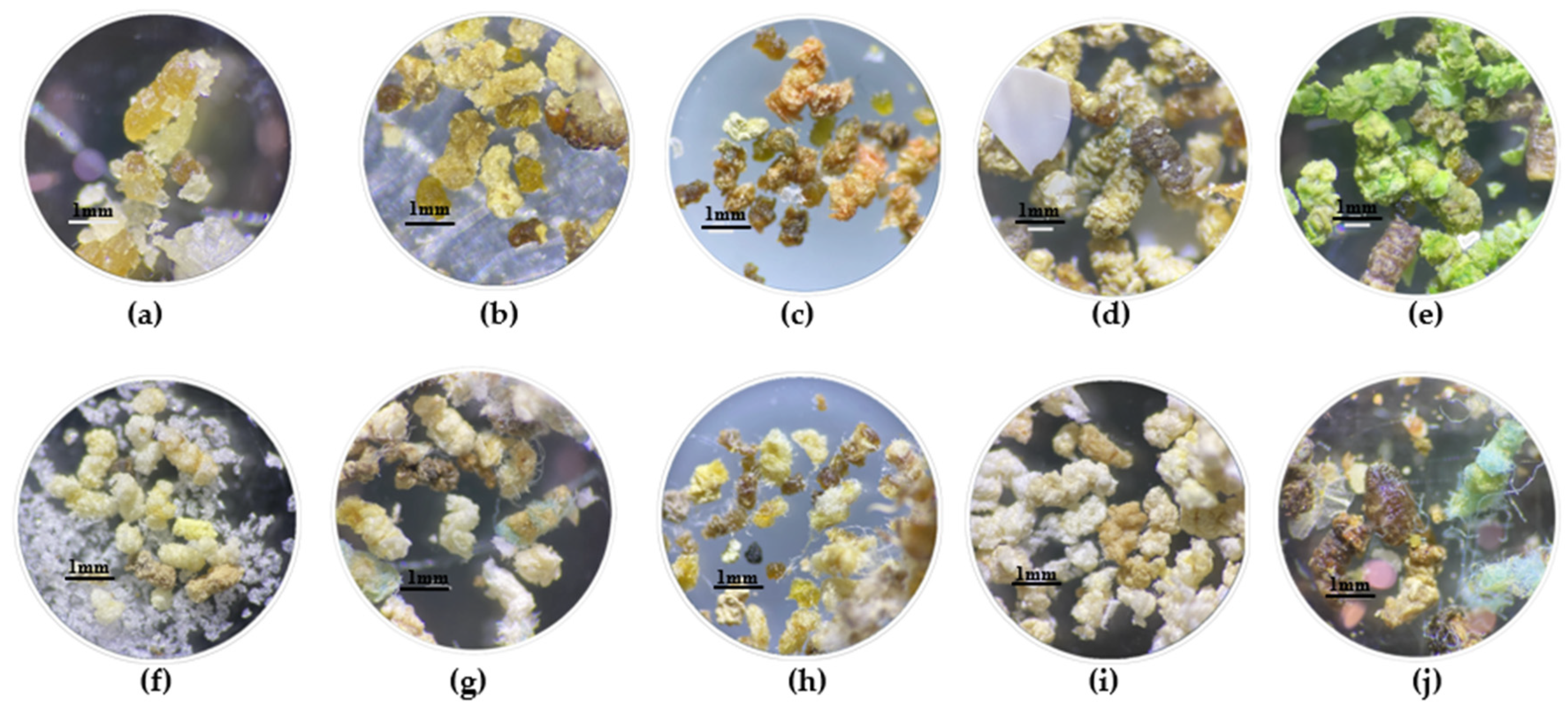
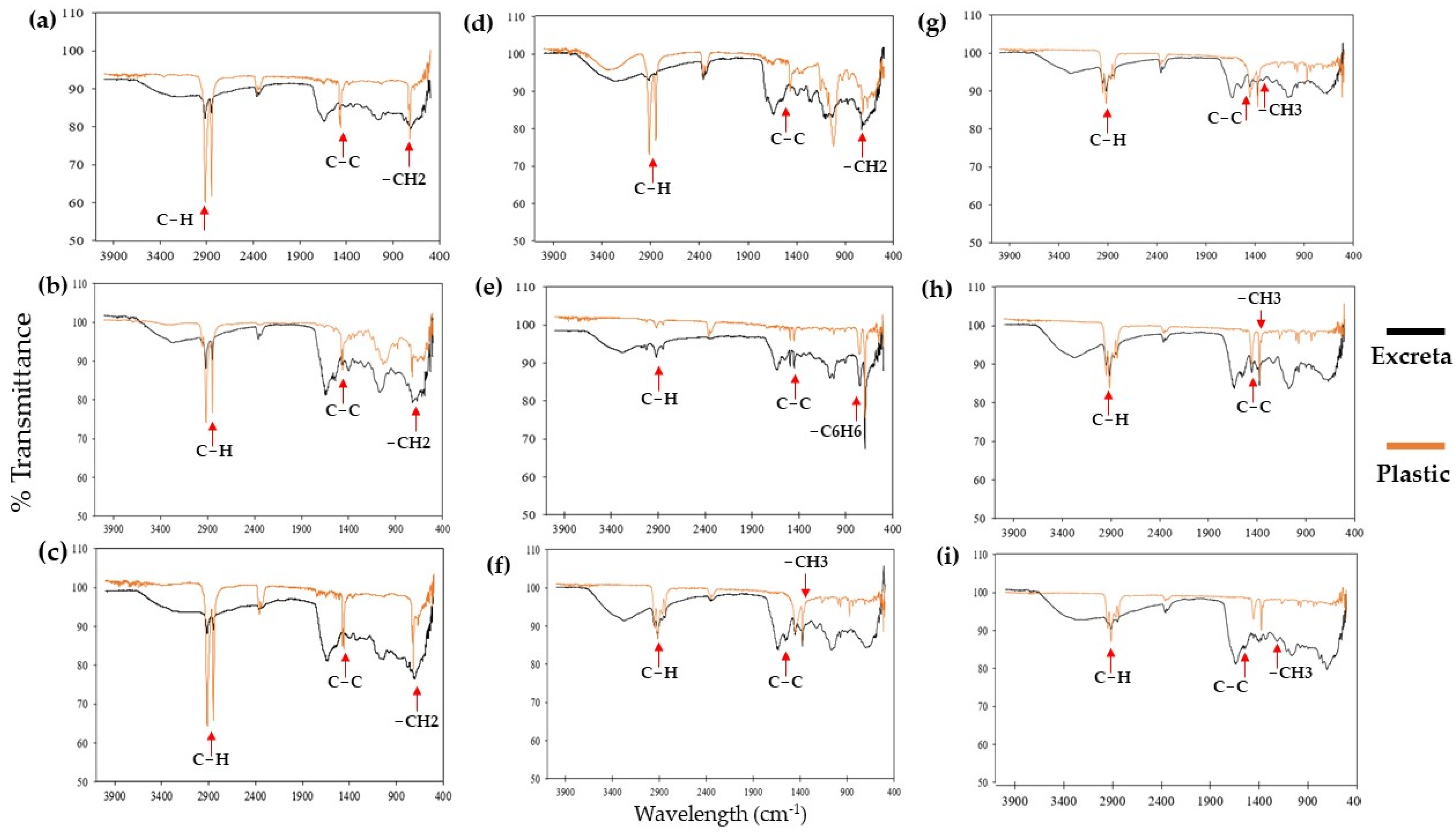
2.3. Digestion of Plastics by G. mellonella
2.4. Life Cycle Continuity
2.5. Statistical Analysis
3. Results
3.1. Percentage Consumed
3.2. Digestion of Plastics by G. mellonella
3.3. Life Cycle Continuity
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, R.C.; Moore, C.J.; Vom Saal, F.S.; Swan, S.H. Plastics, the environment, and human health: Current consensus and future trends. Philos. Trans. R. Soc. B 2009, 364, 2153–2166. [Google Scholar] [CrossRef]
- Alabi, O.A.; Ologbonjaye, K.I.; Awosolu, O.; Alalade, O.E. Public and Environmental Health Effects of Plastic Wastes Disposal: A Review. J. Toxicol. Risk Assess. 2019, 5, 021. [Google Scholar] [CrossRef]
- Shams, M.; Alam, I.; Mahbub, M.S. Plastic Pollution during COVID-19: Plastic Waste Directives and Its Long-Term Impact on the Environment. Environ. Adv. 2021, 5, 100119. [Google Scholar] [CrossRef]
- Lin, Z.; Jin, T.; Zou, T.; Xu, L.; Xi, B.; Xu, D.; He, J.; Xiong, L.; Tang, C.; Peng, J.; et al. Current Progress on Plastic/Microplastic Degradation: Fact Influences and Mechanism. Environ. Pollut. 2022, 304, 119159. [Google Scholar] [CrossRef]
- Manzoor, S.; Naqash, N.; Rashid, G.; Singh, R. Plastic Material Degradation and Formation of Microplastic in the Environment: A Review. Mater. Today Proc. 2022, 56, 3254–3260. [Google Scholar] [CrossRef]
- Walker, T.R.; Fequet, L. Current Trends of Unsustainable Plastic Production and Micro(Nano)Plastic Pollution. Trends Anal. Chem. 2023, 160, 116984. [Google Scholar] [CrossRef]
- Webb, H.K.; Arnott, J.; Crawford, R.J.; Ivanova, E.P. Plastic Degradation and Its Environmental Implications with Special Reference to Poly(Ethylene Terephthalate). Polymers 2013, 5, 1–18. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, N. Mechanistic Implications of Plastic Degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- Cai, Z.; Li, M.; Zhu, Z.; Wang, X.; Huang, Y.; Li, T.; Gong, H.; Yan, M. Biological Degradation of Plastics and Microplastics: A Recent Perspective on Associated Mechanisms and Influencing Factors. Microorganisms 2023, 11, 1661. [Google Scholar] [CrossRef]
- Temporiti, M.E.E.; Nicola, L.; Nielsen, E.; Tosi, S. Fungal Enzymes Involved in Plastics Biodegradation. Microorganisms 2022, 10, 1180. [Google Scholar] [CrossRef]
- NCBI Taxonomy. Available online: https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?id=7137 (accessed on 18 March 2024).
- Bombelli, P.; Howe, C.J.; Bertocchini, F. Polyethylene Bio-Degradation by Caterpillars of the Wax Moth Galleria mellonella. Curr. Biol. 2017, 27, 292–293. [Google Scholar] [CrossRef]
- Peydaei, A.; Bagheri, H.; Gurevich, L.; de Jonge, N.; Nielsen, J.L. Mastication of Polyolefins Alters the Microbial Composition in Galleria mellonella. Environ. Pollut. 2021, 280, 116877. [Google Scholar] [CrossRef]
- Sanluis-Verdes, A.; Colomer-Vidal, P.; Rodriguez-Ventura, F.; Bello-Villarino, M.; Spinola-Amilibia, M.; Ruiz-Lopez, E.; Illanes-Vicioso, R.; Castroviejo, P.; Cigliano, R.A.; Montoya, M.; et al. Wax worm saliva and the enzymes therein are the key to polyethylene degradation by Galleria mellonella. Nat. Commun. 2022, 13, 5568. [Google Scholar] [CrossRef]
- Cassone, B.J.; Grove, H.C.; Elebute, O.; Villanueva, S.M.P.; LeMoine, C.M.R. Role of the Intestinal Microbiome in Low-Density Polyethylene Degradation by Caterpillar Larvae of the Greater Wax Moth, Galleria mellonella. Proc. R. Soc. B. 2020, 287, 20200112. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Men, L.; Zhang, Z.; Guan, F.; Tian, J.; Wang, B.; Wang, J.; Zhang, Y.; Zhang, W. Biodegradation of Polyethylene by Enterobacter sp. D1 from the Guts of Wax Moth Galleria mellonella. Int. J. Environ. Res. Public Health 2019, 16, 1941. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, D.; Li, Q.; Zhao, Y.; Li, L.; Lin, H.; Bi, Q.; Zhao, Y. Biodegradation of Polyethylene Microplastic Particles by the Fungus Aspergillus flavus from the Guts of Wax Moth Galleria mellonella. Sci. Total Environ. 2020, 704, 135931. [Google Scholar] [CrossRef]
- Billen, P.; Khalifa, L.; Van Gerven, F.; Tavernier, S.; Spatari, S. Technological Application Potential of Polyethylene and Polystyrene Biodegradation by Macro-Organisms Such as Mealworms and Wax Moth Larvae. Sci. Total Environ. 2020, 735, 139521. [Google Scholar] [CrossRef]
- Ruiz Barrionuevo, J.M.; Martín, E.; Galindo Cardona, A.; Malizia, A.; Chalup, A.; de Cristóbal, R.E.; Monmany Garzia, A.C. Consumption of low-density polyethylene, polypropylene, and polystyrene materials by larvae of the greater wax moth, Galleria mellonella L. (Lepidoptera, Pyralidae), impacts on their ontogeny. Environ. Sci. Pollut. Res. Int. 2022, 29, 68132–68142. [Google Scholar] [CrossRef]
- Edo, C.; Fernández-Alba, A.R.; Vejsnæs, F.; van der Steen, J.J.M.; Fernández-Piñas, F.; Rosal, R. Honeybees as active samplers for microplastics. Sci. Total Environ. 2021, 767, 144481. [Google Scholar] [CrossRef]
- Jorjão, A.L.; Oliveira, L.D.; Scorzoni, L.; Figueiredo-Godoi, L.M.; Prata, M.C.A.; Olavo, J.A.; Junqueira, J.C. From moths to caterpillars: Ideal conditions for Galleria mellonella rearing for in vivo microbiological studies. Virulence 2018, 9, 383–389. [Google Scholar] [CrossRef]
- Garzón, M.G.; León Enciso, J.; Parga, J.; Padilla Saavedra, L.D.; Peñuela Franco, D.; Pinzón Ruiz, C.E.; Lugo Cohetato, D.J. Descripción histológica de Galleria mellonella Contribución a su implementación como modelo animal: Contribution to its implementation as an animal model. Hechos Microbiológicos 2022, 13, 1–7. [Google Scholar] [CrossRef]
- Kwadha, C.A.; Ong’Amo, G.O.; Ndegwa, P.N.; Raina, S.K.; Fombong, A.T. The Biology and Control of the Greater Wax Moth, Galleria mellonella. Insects 2017, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Nation, J.L. Insect Physiology and Biochemistry, 4th ed.; CRC Press: Boca Raton, FL, USA, 2022; ISBN 978-1-00-327982-2. [Google Scholar] [CrossRef]
- Velandia Cabra, J.R. Identificación de polímeros por espectroscopía infrarroja. Rev. Ontare 2018, 5, 115–140. [Google Scholar] [CrossRef][Green Version]
- Réjasse, A.; Waeytens, J.; Deniset-Besseau, A.; Crapart, N.; Nielsen-Leroux, C.; Sandt, C. Plastic Biodegradation: Do Galleria mellonella Larvae Bioassimilate Polyethylene? A Spectral Histology Approach Using Isotopic Labeling and Infrared Microspectroscopy. Environ. Sci. Techol. 2022, 56, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, C.M.; Grove, H.C.; Smith, C.M.; Cassone, B.J. A Very Hungry Caterpillar: Polyethylene Metabolism and Lipid Homeostasis in Larvae of the Greater Wax Moth (Galleria mellonella). Environ. Sci. Technol. 2020, 54, 14706–14715. [Google Scholar] [CrossRef] [PubMed]
- Ajibola, A.; Chamunorwa, J.P.; Erlwanger, K.H. Nutraceutical values of natural honey and its contribution to human health and wealth. Nutr. Metab. 2012, 9, 61. [Google Scholar] [CrossRef]
- Lan, Y. Waxes. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 312–316. ISBN 978-0-12-814045-1. [Google Scholar] [CrossRef]
- Pandit, P.; Maity, S.; Singha, K.; Annu; Uzun, M.; Shekh, M.; Ahmed, S. Potential Biodegradable Face Mask to Counter Environmental Impact of COVID-19. Clean. Eng. Technol. 2021, 4, 100218. [Google Scholar] [CrossRef]
- Taranto, F.; Pasqualone, A.; Mangini, G.; Tripodi, P.; Miazzi, M.M.; Pavan, S.; Montemurro, C. Polyphenol Oxidases in Crops: Biochemical, Physiological and Genetic Aspects. Int. J. Mol. Sci. 2017, 18, 377. [Google Scholar] [CrossRef] [PubMed]
- Wojda, I.; Staniec, B.; Sułek, M.; Kordaczuk, J. The Greater Wax Moth Galleria mellonella: Biology and Use in Immune Studies. Pathog. Dis. 2020, 78, ftaa057. [Google Scholar] [CrossRef]
- Sehnal, F.; Delbecque, J.P.; Maróy, P.; Malá, J. Ecdysteroid titres during larval life and metamorphosis of Galleria mellonella. In Ecdysone; Bownes, M., Ed.; Pergamon: Oxford, UK, 1986; pp. 157–162. ISBN 978-0-08-032016-8. [Google Scholar] [CrossRef]
- Pignataro, E.; Pini, F.; Barbanente, A.; Arnesano, F.; Palazzo, A.; Marsano, R.M. Flying toward a plastic-free world: Can Drosophila serve as a model organism to develop new strategies of plastic waste management? Sci. Total Environ. 2024, 914, 169942. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias-González, A.F.; Gómez-Méndez, L.D.; Sáenz-Aponte, A. Consumption and Digestion of Plastics by Greater Hive Moth Larvae. Insects 2024, 15, 645. https://doi.org/10.3390/insects15090645
Arias-González AF, Gómez-Méndez LD, Sáenz-Aponte A. Consumption and Digestion of Plastics by Greater Hive Moth Larvae. Insects. 2024; 15(9):645. https://doi.org/10.3390/insects15090645
Chicago/Turabian StyleArias-González, Andrés Felipe, Luis David Gómez-Méndez, and Adriana Sáenz-Aponte. 2024. "Consumption and Digestion of Plastics by Greater Hive Moth Larvae" Insects 15, no. 9: 645. https://doi.org/10.3390/insects15090645
APA StyleArias-González, A. F., Gómez-Méndez, L. D., & Sáenz-Aponte, A. (2024). Consumption and Digestion of Plastics by Greater Hive Moth Larvae. Insects, 15(9), 645. https://doi.org/10.3390/insects15090645





