Assessing the Potential of Tortistilus (Hemiptera: Membracidae) from Northern California Vineyards as Vector Candidates of Grapevine Red Blotch Virus
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
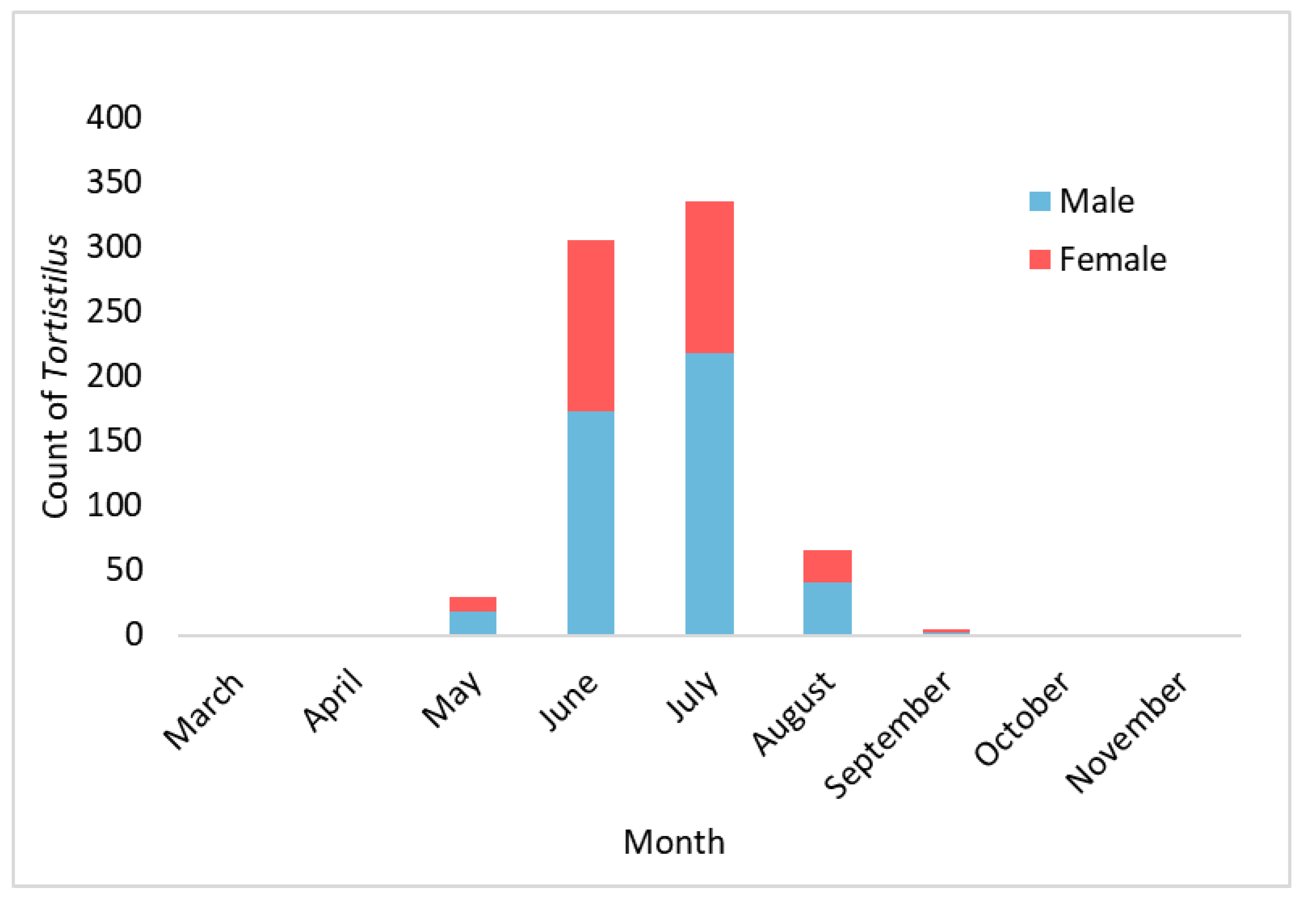
2.1. Tortistilus and Spissistilus Collections
2.2. Nucleic Acid Extraction and GRBV Testing Using PCR
2.3. Sequencing of Tortistilus ITS and mt-COI DNA Fragments
2.4. Sequence Analyses and Phylogenetic Relationships
2.5. Diagnostic PCR for Distinction between Tortistilus and Spissistilus Species
3. Results
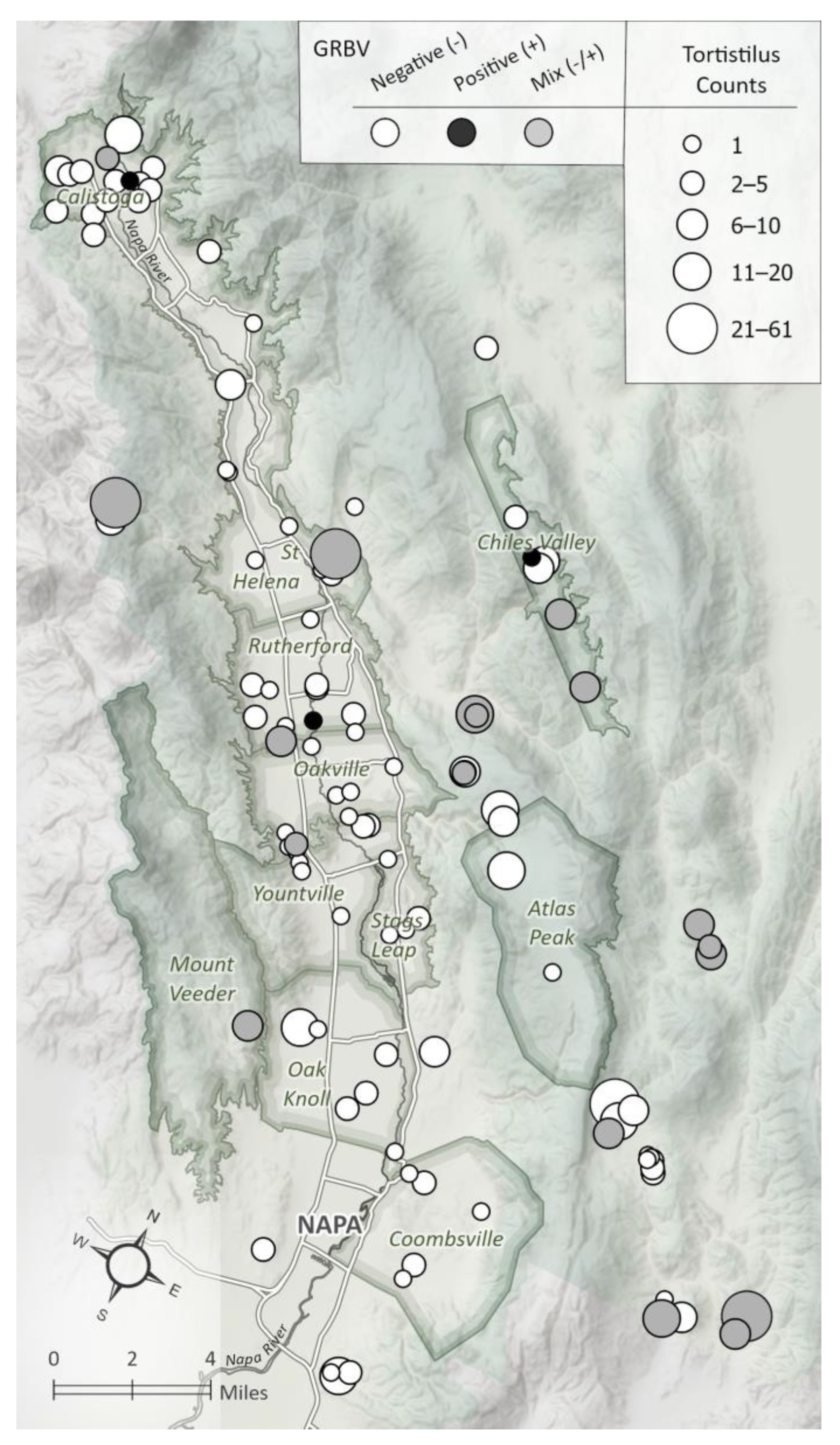
3.1. Vineyard Sites and Tortistilus Specimen Collections
3.2. Morphological Variability of Tortistilus Populations from Northern California Vineyards
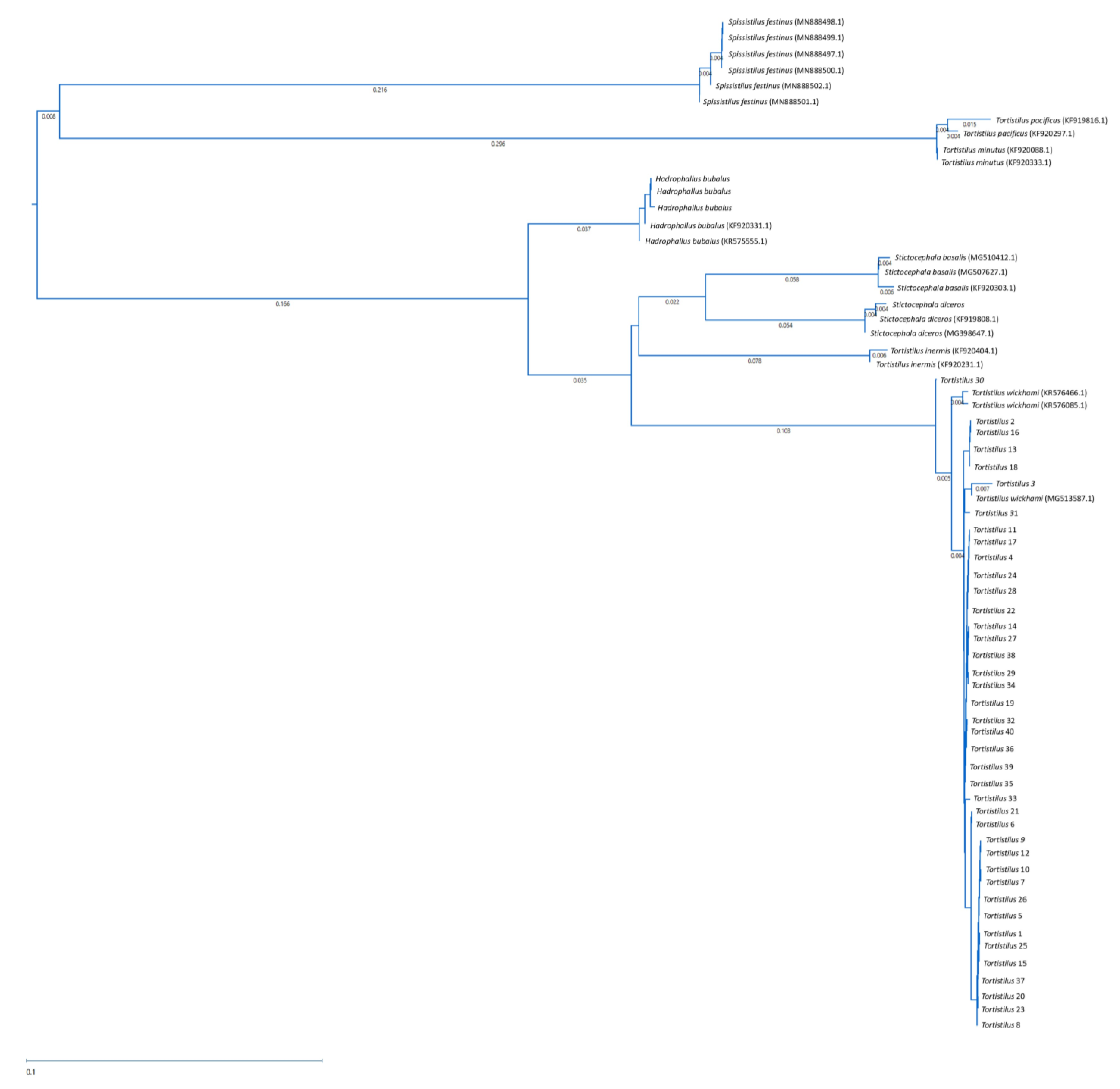
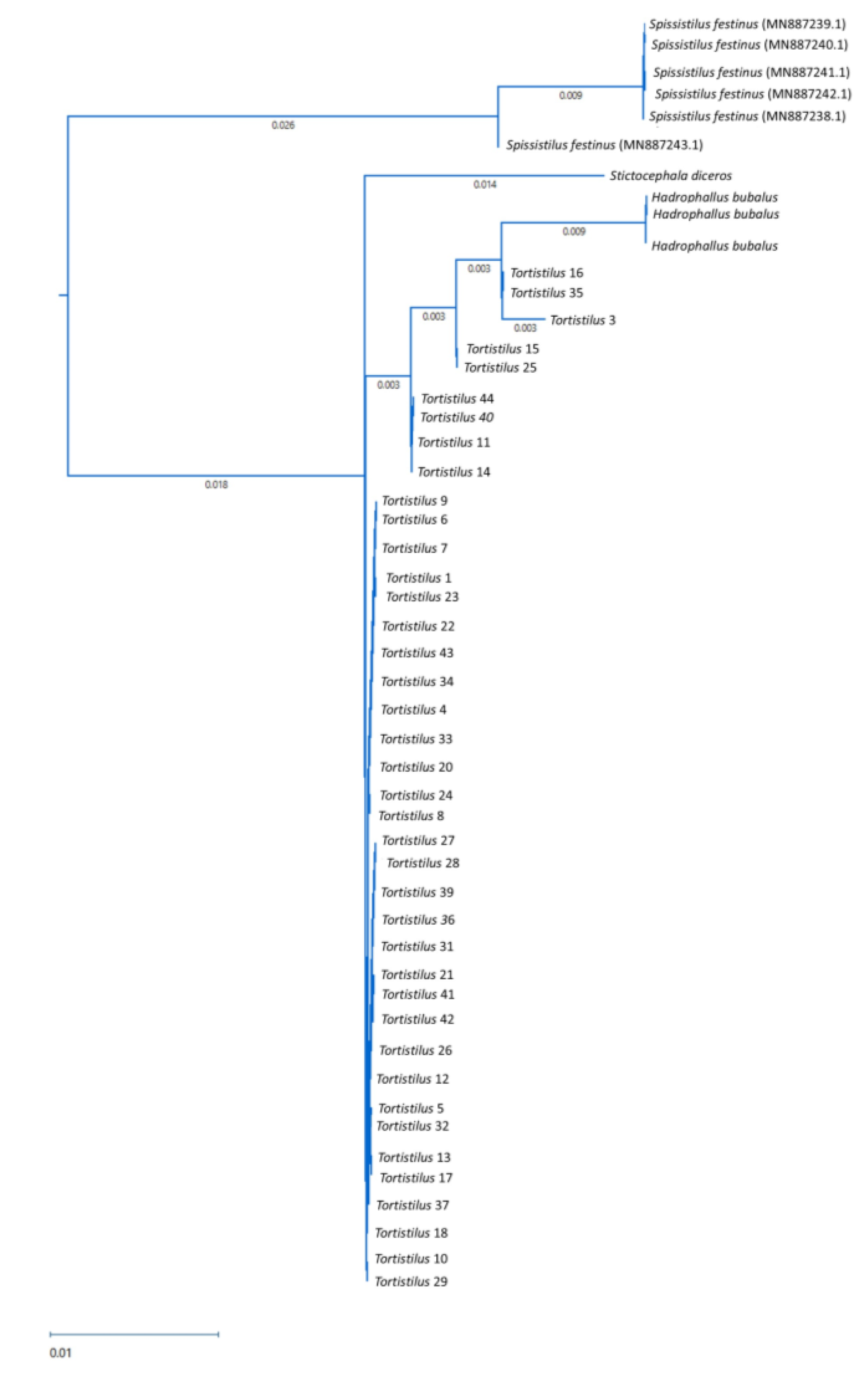
3.3. Taxonomic Identification of Tortistilus Populations from Northern California Vineyards
3.4. GRBV Acquisition by Tortistilus wickhami from Northern California Vineyards
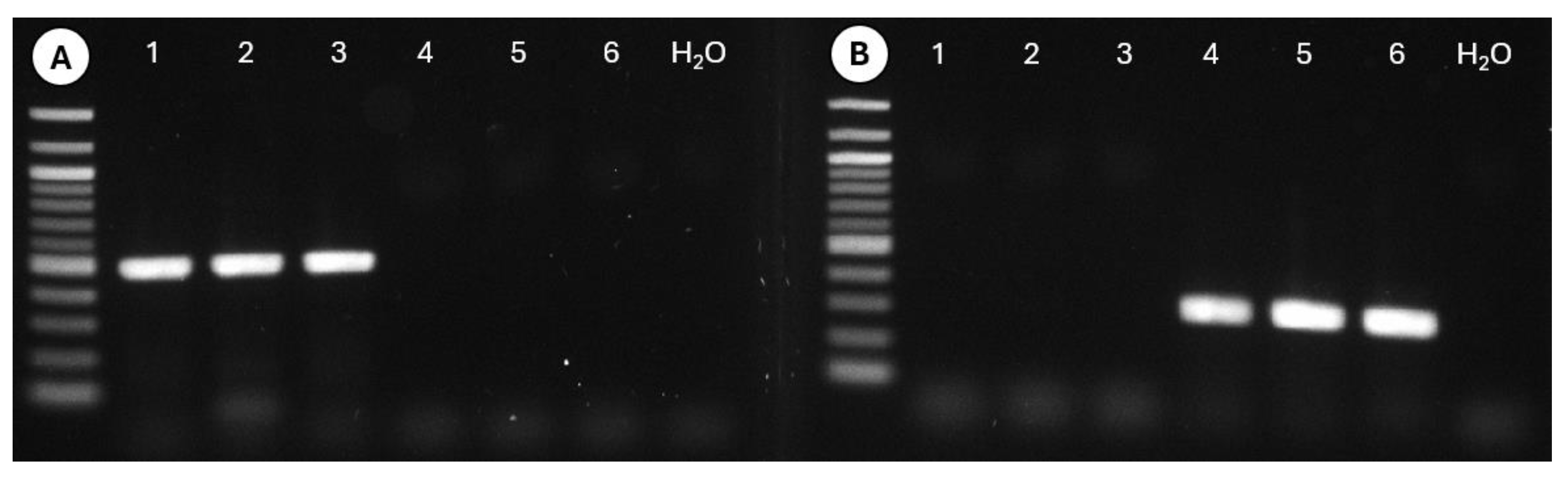
3.5. Diagnostic Polymerase Chain Reaction for Tortistilus wickhami
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bahder, B.W.; Zalom, F.G.; Sudarshana, M.R. An evaluation of the flora adjacent to wine grape vineyards for the presence of alternative host plants of grapevine red blotch-associated virus. Plant Dis. 2016, 100, 1571–1574. [Google Scholar] [CrossRef]
- Flasco, M.; Hoyle, V.; Cieniewicz, E.J.; Roy, B.G.; McLane, H.L.; Perry, K.L.; Loeb, G.; Nault, B.; Heck, M.; Fuchs, M. Grapevine red blotch virus is transmitted by the three-cornered alfalfa hopper in a circulative, nonpropagative transmission mode with unique attributes. Phytopathology 2021, 111, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Hoyle, V.; Flasco, M.; Choi, J.J.; Cieniewicz, E.J.; McLane, H.L.; Perry, K.L.; Dangl, G.; Al Rwahnih, M.; Heck, M.; Loeb, G.; et al. Transmission of grapevine red blotch virus by Spissistilus festinus [Say, 1830] (Hemiptera: Membracidae) between free-living vines and Vitis vinifera ‘Cabernet franc’. Viruses 2022, 14, 1156. [Google Scholar] [CrossRef]
- Yepes, L.M.; Cieniewicz, E.; Krenz, B.; McLane, H.; Thompson, J.R.; Perry, K.L.; Fuchs, M. Causative role of grapevine red blotch virus in red blotch disease. Phytopathology 2018, 108, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Cieniewicz, E.; Perry, K.; Fuchs, M. Grapevine red blotch: Molecular biology of the virus and management of the disease. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Meng, B., Martelli, G., Golino, D., Fuchs, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 303–314. [Google Scholar] [CrossRef]
- Rumbaugh, A.C.; Sudarshana, M.R.; Oberholster, A. Grapevine red blotch disease etiology and its impact on grapevine physiology and berry and wine composition. Horticulturae 2021, 7, 552. [Google Scholar] [CrossRef]
- Ricketts, K.D.; Gómez, M.I.; Fuchs, M.F.; Martinson, T.E.; Smith, R.J.; Cooper, M.L.; Moyer, M.M.; Wise, A. Mitigating the economic impact of grapevine red blotch: Optimizing disease management strategies in U.S. vineyards. Am. J. Enol. Vitic. 2017, 68, 127–135. [Google Scholar] [CrossRef]
- Flasco, M.T.; Hoyle, V.; Cieniewicz, E.J.; Loeb, G.; McLane, H.; Perry, K.; Fuchs, M.F. The three-cornered alfalfa hopper, Spissistilus festinus, is a vector of grapevine red blotch virus in vineyards. Viruses 2023, 15, 927. [Google Scholar] [CrossRef] [PubMed]
- Fiallo-Olivé, E.; Pan, L.-L.; Liu, S.-S.; Navas-Castillo, J. Transmission of begomoviruses and other whitefly-borne viruses: Dependence on the vector species. Phytopathology 2020, 110, 10–17. [Google Scholar] [CrossRef]
- Flasco, M.; Hoyle, V.; Cieniewicz, E.J.; Fuchs, M. Transmission of grapevine red blotch virus: A virologist’s perspective of the literature and a few recommendations. Am. J. Enol. Vitic. 2023, 74, 0740023. [Google Scholar] [CrossRef]
- Krenz, B.; Fuchs, M.; Thompson, J.R. Grapevine red blotch disease: A comprehensive Q&A guide. PLoS Pathog. 2023, 19, e1011671. [Google Scholar] [CrossRef]
- Cieniewicz, E.; Flasco, M.; Brunelli, M.; Onwumelu, A.; Wise, A.; Fuchs, M.F. Differential spread of grapevine red blotch virus in California and New York vineyards. Phytobiomes J. 2019, 3, 203–211. [Google Scholar] [CrossRef]
- Dalton, D.T.; Hilton, R.J.; Kopp, D.; Walton, V.M. Vouchers for treehoppers (Hemiptera: Membracidae) collected in Benton, Josephine, and Yamhill Counties, Oregon. Cat. Or. State Arthropod Collect. 2020, 4, 1–7. [Google Scholar]
- Kahl, D.; Úrbez-Torres, J.R.; Kits, J.; Hart, M.; Nyirfa, A.; Lowery, D.T. Identification of candidate insect vectors of Grapevine red blotch virus by means of an artificial feeding diet. Can. J. Plant Pathol. 2021, 43, 905–913. [Google Scholar] [CrossRef]
- LaFond, H.F.; Volenberg, D.S.; Schoelz, J.E.; Finke, D.L. Identification of potential grapevine red blotch virus vector in Missouri vineyards. Am. J. Enol. Vitic. 2022, 73, 247–255. [Google Scholar] [CrossRef]
- Wilson, H.; Hogg, B.N.; Blaisdell, G.K.; Andersen, J.C.; Yazdani, A.S.; Billings, A.C.; Ooi, K.M.; Soltani, N.; Almeida, R.P.P.; Cooper, M.L.; et al. Survey of vineyard insects and plants to identify potential insect vectors and non-crop reservoirs of grapevine red blotch virus. PhytoFrontiers 2022, 2, 66–73. [Google Scholar] [CrossRef]
- Cryan, J.R.; Wiegmann, B.M.; Deitz, L.L.; Dietrich, C.H. Phylogeny of the treehoppers (Insecta: Hemiptera: Membracidae): Evidence from two nuclear genes. Mol. Phylogenet. Evol. 2000, 17, 317–334. [Google Scholar] [CrossRef]
- Setiono, F.J.; Chatterjee, D.; Fuchs, M.; Perry, K.L.; Thompson, J.R. The distribution and detection of grapevine red blotch virus in its host depend on time of sampling and tissue type. Plant Dis. 2018, 102, 2187–2193. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Ji, Y.J.; Zhang, D.X.; He, L.J. Evolutionary conservation and versatility of a new set of primers for amplifying the ribosomal internal transcribed spacer regions in insects and other invertebrates. Mol. Ecol. Notes 2003, 3, 581–585. [Google Scholar] [CrossRef]
- Cieniewicz, E.; Poplaski, V.; Brunelli, M.; Dombroskie, J.; Fuchs, M. Two distinct genotypes of Spissistilus festinus (Say, 1830) (Hemiptera, Membracidae) in the United States revealed by phylogenetic and morphological analyses. Insects 2020, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Preto, C.R.; Bahder, B.W.; Bick, E.N.; Sudarshana, M.R.; Zalom, F.G. Seasonal dynamics of Spissistilus festinus (Hemiptera: Membracidae) in a Californian vineyard. J. Econ. Entomol. 2019, 112, 1138–1144. [Google Scholar] [CrossRef]
- Wilson, H.; Yazdani, A.S.; Daane, K.M. Influence of riparian habitat and ground covers on three-cornered alfalfa hopper (Hemiptera: Membracidae) populations in vineyards. J. Econ. Entomol. 2020, 113, 2354–2361. [Google Scholar] [CrossRef]
- Hoyle, V.; Headrick, H.; Cooper, W.R.; Fendell-Hummel, H.G.; Cooper, M.L.; Flasco, M.; Cieniewicz, E.; Heck, M.; Fuchs, M. Ecological connectivity between natural and cultivated plant communities in vineyard ecosystems for disease dynamics revealed by the dietary profiles and landscape-level movement of a treehopper. School of Integrative Plant Science, Plant Pathology and Plant-Microbe Biology, Cornell University, Geneva, NY 14456, USA, 2024, submitted.
- Simons, J.N. The pseudo-curly top disease in South Florida. J. Econ. Entomol. 1962, 55, 358–363. [Google Scholar] [CrossRef]
- Simons, J.N.; Coe, D.M. Transmission of pseudo-curly top virus in Florida by a treehopper. Virology 1958, 6, 43–48. [Google Scholar] [CrossRef]
- Caldwell, J.S. A generic revision of the treehoppers of the tribe Ceresini in America north of Mexico, based on a study of the male genitalia. Proc. U.S. Natl. Mus. 1949, 98, 491–521. [Google Scholar] [CrossRef]
- Wistrom, C.M.; Sisterson, S.; Pryor, M.P.; Hashim-Buckey, J.M.; Daane, K.M. Distribution of glassy-winged sharpshooter and three-cornered alfalfa hopper on plant hosts in the San Joaquin Valley, California. J. Econ. Entomol. 2010, 103, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Cieniewicz, E.; Pethybridge, S.J.; Gorny, A.; Madden, L.V.; McLane, H.; Perry, K.L.; Fuchs, M. Spatiotemporal spread of grapevine red blotch-associated virus in a California vineyard. Virus Res. 2017, 241, 156–162. [Google Scholar] [CrossRef]
- Van Duzee, E.P. Studies in North American Membracidae. Buffalo Soc. Nat. Hist. 1908, 9, 29–129. [Google Scholar]
- Dennis, C.J. Genitalia of the Membracidae of Wisconsin. Can. Entomol. 1952, 84, 157–173. [Google Scholar] [CrossRef]
- Kopp, D.D.; Yonke, T.R. A taxonomic review of the tribe Ceresini (Homoptera: Membracidae). Misc. Pub. Entomol. Soc. Am. 1979, 11, 1–97. [Google Scholar] [CrossRef]
- Sakakibara, A.M. Revalidation of Ilithucia Stål and descriptions of new species (Homoptera, Membracidae, Smiliinae). Rev. Bras. Zool. 2002, 19, 189–200. [Google Scholar] [CrossRef]
- Andrade, G.S. Vestistiloides, a novel genus of Ceresini (Hemiptera, Auchenorrhyncha, Membracidae). Rev. Bras. Zool. 2003, 20, 1–2. [Google Scholar] [CrossRef]
- Andrade, G.S. Species of the genus Ceresa Amyot & Serville (Hemiptera, Auchenorrhyncha, Membracidae). Rev. Bras. Zool. 2004, 21, 671–738. [Google Scholar] [CrossRef]
- Sakakibara, A.M.; Evangelista, O. New species and nomenclatural notes in the Ceresini (Hemiptera, Membracidae, Smiliinae). Zootaxa 2008, 1702, 52–60. [Google Scholar] [CrossRef]
- Florez-V, C.; Wolff, W.I.; Cardona-Duque, J. Contribution to the taxonomy of the family Membracidae Rafinesque (Hemiptera: Auchenorrhyncha) in Colombia. ZooTaxa 2015, 3910, 1–261. [Google Scholar] [CrossRef][Green Version]
- Cryan, J.R.; Wiegmann, B.M.; Deitz, L.L.; Dietrich, C.H.; Whiting, M.F. Treehopper trees: Phylogeny of Membracidae (Hemiptera: Cicadomorpha: Membracoidea) based on molecules and morphology. Syst. Entomol. 2004, 29, 441–454. [Google Scholar] [CrossRef]
- Dietrich, C.H.; McKamey, S.H.; Deitz, L.L. Morphology-based phylogeny of the treehopper family Membracidae (Hemiptera; Cicadomorpha: Membracoidea). Syst. Entomol. 2001, 26, 213–239. [Google Scholar] [CrossRef]




| Name | Location | Year | Morphology | Sex | GenBank Accession Number | |
|---|---|---|---|---|---|---|
| mt-COI | ITS2 | |||||
| Tortistilus 1 | Rutherford, CA, USA | 2023 | Unhorned | Female | PQ096090 | PQ108530 |
| Tortistilus 2 | Rutherford, CA, USA | 2023 | Horned | Female | PQ096091 | - |
| Tortistilus 3 | Oak Knoll District, CA, USA | 2023 | Horned | Male | PQ096092 | PQ108531 |
| Tortistilus 4 | Oak Knoll District, CA, USA | 2023 | Unhorned | Female | PQ096093 | PQ108532 |
| Tortistilus 5 | Oak Knoll District, CA, USA | 2023 | Unhorned | Female | PQ096094 | PQ108533 |
| Tortistilus 6 | Oak Knoll District, CA, USA | 2023 | Horned | Female | PQ096095 | PQ108534 |
| Tortistilus 7 | Chiles Valley, CA, USA | 2023 | Unhorned | Male | PQ096096 | PQ108535 |
| Tortistilus 8 | Chiles Valley, CA, USA | 2023 | Unhorned | Male | PQ096097 | PQ108536 |
| Tortistilus 9 | Chiles Valley, CA, USA | 2023 | Horned | Male | PQ096098 | PQ108537 |
| Tortistilus 10 | Chiles Valley, CA, USA | 2023 | Unhorned | Female | PQ096099 | PQ108538 |
| Tortistilus 11 | Chiles Valley, CA, USA | 2023 | Horned | Male | PQ096100 | PQ108539 |
| Tortistilus 12 | Chiles Valley, CA, USA | 2023 | Unhorned | Male | PQ096101 | PQ108540 |
| Tortistilus 13 | Chiles Valley, CA, USA | 2023 | Horned | Male | PQ096102 | PQ108541 |
| Tortistilus 14 | Chiles Valley, CA, USA | 2023 | Unhorned | Male | PQ096103 | PQ108542 |
| Tortistilus 15 | Chiles Valley, CA, USA | 2023 | Unhorned | Male | PQ096104 | PQ108543 |
| Tortistilus 16 | Chiles Valley, CA, USA | 2023 | Unhorned | Male | PQ096105 | PQ108544 |
| Tortistilus 17 | Calistoga, CA, USA | 2023 | Unhorned | Female | PQ096106 | PQ108545 |
| Tortistilus 18 | Calistoga, CA, USA | 2023 | Horned | Female | PQ096107 | PQ108546 |
| Tortistilus 19 | Spring Mtn. District, CA, USA | 2023 | Unhorned | Female | PQ096108 | - |
| Tortistilus 20 | Spring Mtn. District, CA, USA | 2023 | Horned | Male | PQ096070 | PQ108547 |
| Tortistilus 21 | Napa, CA, USA | 2022 | Horned | Male | PQ096071 | PQ108548 |
| Tortistilus 22 | Napa, CA, USA | 2022 | Unhorned | Male | PQ096072 | PQ108549 |
| Tortistilus 23 | Calistoga, CA, USA | 2022 | Horned | Male | PQ096073 | PQ108551 |
| Tortistilus 24 | St. Helena, CA, USA | 2022 | Unhorned | Female | PQ096074 | PQ108552 |
| Tortistilus 25 | Yountville, CA, USA | 2022 | Horned | Male | PQ096075 | PQ108553 |
| Tortistilus 26 | Gordon Valley, CA, USA | 2022 | Unhorned | Male | PQ096076 | PQ108554 |
| Tortistilus 27 | St. Helena, CA, USA | 2022 | Horned | Female | PQ096077 | PQ108556 |
| Tortistilus 28 | Coombsville, CA, USA | 2021 | Unhorned | Male | PQ096078 | PQ108557 |
| Tortistilus 29 | Gordon Valley, CA, USA | 2021 | Unhorned | Male | PQ096079 | PQ108558 |
| Tortistilus 30 | Oak Knoll District, CA, USA | 2021 | Unhorned | Female | PQ096080 | - |
| Tortistilus 31 | Pope Valley, CA, USA | 2021 | Unhorned | Female | PQ096109 | PQ108559 |
| Tortistilus 32 | Oak Knoll District, CA, USA | 2021 | Unhorned | Male | PQ096081 | PQ108560 |
| Tortistilus 33 | Pope Valley, CA, USA | 2021 | Unhorned | Male | PQ096082 | PQ108561 |
| Tortistilus 34 | Wooden Valley, CA, USA | 2021 | Unhorned | Female | PQ096083 | PQ108562 |
| Tortistilus 35 | Pope Valley, CA, USA | 2022 | Unhorned | Female | PQ096084 | PQ108563 |
| Tortistilus 36 | Pope Valley, CA, USA | 2022 | Unhorned | Male | PQ096085 | PQ108564 |
| Tortistilus 37 | Pope Valley, CA, USA | 2022 | Unhorned | Male | PQ096086 | PQ108565 |
| Tortistilus 38 | Pope Valley, CA, USA | 2022 | Unhorned | Male | PQ096087 | - |
| Tortistilus 39 | Pope Valley, CA, USA | 2022 | Unhorned | Female | PQ096088 | PQ108566 |
| Tortistilus 40 | Pope Valley, CA, USA | 2022 | Unhorned | Female | PQ096089 | PQ108567 |
| Tortistilus 41 | Chiles Valley, CA, USA | 2023 | Horned | Female | - | PQ108568 |
| Tortistilus 42 | Yountville, CA, USA | 2022 | Horned | Male | - | PQ108550 |
| Tortistilus 43 | St. Helena, CA, USA | 2022 | Unhorned | Female | - | PQ108555 |
| Tortistilus 44 | Pope Valley, CA, USA | 2022 | Unhorned | Female | - | PQ108569 |
| Hadrophallus bubalus | Geneva, NY, USA | 2022 | Horned | Female | PQ096110 | PQ108570 |
| Hadrophallus bubalus | Geneva, NY, USA | 2022 | Horned | Male | PQ096111 | PQ108571 |
| Hadrophallus bubalus | Geneva, NY, USA | 2022 | Horned | Female | PQ096112 | PQ108572 |
| Stictocephala diceros | Lyons, NY, USA | 2023 | Horned | Female | PQ096113 | - |
| Stictocephala diceros | Lyons, NY, USA | 2023 | Horned | Male | - | PQ108573 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoyle, V.J.; McGinnity Schneider, E.J.; McLane, H.L.; Wunsch, A.O.; Fendell-Hummel, H.G.; Cooper, M.L.; Fuchs, M.F. Assessing the Potential of Tortistilus (Hemiptera: Membracidae) from Northern California Vineyards as Vector Candidates of Grapevine Red Blotch Virus. Insects 2024, 15, 664. https://doi.org/10.3390/insects15090664
Hoyle VJ, McGinnity Schneider EJ, McLane HL, Wunsch AO, Fendell-Hummel HG, Cooper ML, Fuchs MF. Assessing the Potential of Tortistilus (Hemiptera: Membracidae) from Northern California Vineyards as Vector Candidates of Grapevine Red Blotch Virus. Insects. 2024; 15(9):664. https://doi.org/10.3390/insects15090664
Chicago/Turabian StyleHoyle, Victoria J., Elliot J. McGinnity Schneider, Heather L. McLane, Anna O. Wunsch, Hannah G. Fendell-Hummel, Monica L. Cooper, and Marc F. Fuchs. 2024. "Assessing the Potential of Tortistilus (Hemiptera: Membracidae) from Northern California Vineyards as Vector Candidates of Grapevine Red Blotch Virus" Insects 15, no. 9: 664. https://doi.org/10.3390/insects15090664
APA StyleHoyle, V. J., McGinnity Schneider, E. J., McLane, H. L., Wunsch, A. O., Fendell-Hummel, H. G., Cooper, M. L., & Fuchs, M. F. (2024). Assessing the Potential of Tortistilus (Hemiptera: Membracidae) from Northern California Vineyards as Vector Candidates of Grapevine Red Blotch Virus. Insects, 15(9), 664. https://doi.org/10.3390/insects15090664







