Dynamics of Bactrocera dorsalis Resistance to Seven Insecticides in South China
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Maintenance
2.2. Insecticides
2.3. Determination of Toxicity
2.4. Statistical Analysis
3. Results
3.1. Toxicity of Insecticides against the SS
3.2. Dynamics of Resistance to Beta-Cypermethrin among Different Populations
3.3. Dynamics of Resistance to Cyhalothrin among Different Populations
3.4. Dynamics of Resistance to Trichlorfon among Different Populations
3.5. Dynamics of Resistance to Spinosad among Different Populations
3.6. Dynamics of Resistance to Emamectin Benzoate among Different Populations
3.7. Dynamics of Resistance to Chlorpyrifos among Different Populations
3.8. Dynamics of Resistance to Avermectin among Different Populations
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paini, D.R.; Sheppard, A.W.; Cook, D.C.; De Barro, P.J.; Worner, S.P.; Thomas, M.B. Global threat to agriculture from invasive species. Proc. Natl. Acad. Sci. USA 2016, 113, 7575–7579. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, D.; Xu, Y.; Wang, L.; Cheng, D.; Qi, Y.; Zeng, L.; Lu, Y. Invasion, expansion, and control of Bactrocera dorsalis (Hendel) in China. J. Integr. Agric. 2019, 18, 771–787. [Google Scholar] [CrossRef]
- Hassan, B.; Siddiqui, J.A.; Xu, Y.J. Vertically Transmitted Gut Bacteria and Nutrition Influence the Immunity and Fitness of Bactrocera dorsalis Larvae. Front. Microbiol. 2020, 11, 596352. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.P.; Zeng, L.; Lu, Y.Y. Monitoring of resistance of oriental fruit fly adults to insecticides in South China. J. South China Agric. Univ. 2005, 4, 23–26. [Google Scholar]
- Ye, H.; Liu, J.H. Population dynamics of the oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae) in the Kunming area, southwestern China. Insect Sci. 2005, 12, 387–392. [Google Scholar] [CrossRef]
- Dang, X.L.; Tian, J.H.; Yi, H.Y.; Wang, W.X.; Zheng, M.; Li, Y.F.; Cao, Y. Inducing and isolation of antibacterial peptides from oriental fruit fly, Bactrocera dorsalis Hendel. Insect Sci. 2006, 13, 257–262. [Google Scholar] [CrossRef]
- Zeng, Y.; Reddy, G.V.; Li, Z.; Qin, Y.; Wang, Y.; Pan, X.; Jiang, F.; Gao, F. Global distribution and invasion pattern of oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). J. Appl. Entomol. 2019, 143, 165–176. [Google Scholar] [CrossRef]
- Dohino, T.; Hallman, G.J.; Grout, T.G.; Clarke, A.R.; Follett, P.A.; Cugala, D.R.; Minh Tu, D.; Murdita, W.; Hernandez, E.; Pereira, R.; et al. Phytosanitary Treatments Against Bactrocera dorsalis (Diptera: Tephritidae): Current Situation and Future Prospects. J. Econ Entomol. 2017, 110, 67–79. [Google Scholar]
- Wei, D.D.; He, W.; Lang, N.; Miao, Z.Q.; Xiao, L.F.; Dou, W.; Wang, J.J. Recent research status of Bactrocera dorsalis: Insights from resistance mechanisms and population structure. Arch. Insect Biochem. Physiol. 2019, 102, e21601. [Google Scholar] [CrossRef]
- Guo, T.D.; Gong, Q.T.; YE, B.H.; Wu, H.B.; Sun, R.H. Advances in the research of Bactrocera dorsalis in China. Deciduous Fruits 2019, 51, 43–46. [Google Scholar]
- Zhou, W.C.; Li, W.F.; Zhan, K.R.; Zhao, S.X.; Chen, S.L.; Ning, Z.Z.; Zhang, H. Field Population dynamics of oriental fruit fly in Fujian province. Entomol. J. East China 2008, 1, 26–30. [Google Scholar]
- Ye, H.; Liu, J.H. Population dynamics of Bactrocera dorsalis (Diptera:Tephritidae) in Xishuangbanna of Southern Yunnan. Chin. J. Appl. Ecol. 2005, 7, 1330–1334. [Google Scholar]
- Clarke, A.R.; Armstrong, K.F.; Carmichael, A.E.; Milne, J.R.; Raghu, S.; Roderick, G.K.; Yeates, D.K. Invasive phytophagous pests arising through a recent tropical evolutionary radiation: The Bactrocera dorsalis complex of fruit flies. Annu. Rev. Entomol. 2005, 50, 293–319. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.C.; Feng, H.T.; Wu, W.J.; Geib, S.M.; Mao, C.H.; Vontas, J. Truncated transcripts of nicotinic acetylcholine subunit gene Bdα6 are associated with spinosad resistance in Bactrocera dorsalis. Insect Biochem. Mol. Biol. 2012, 42, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Wu, J.; Cai, X.Y.; Li, D.D.; Cheng, D.F.; Lu, Y.Y. Lethal and sublethal effects of broflanilide on four tephritid pests (Diptera: Tephritidae). Pest Manag. Sci. 2023, 79, 2862–2868. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cai, X.; Qi, Y.; Lu, Y.; Li, X. Lethal, Sublethal, and Offspring Effects of Fluralaner and Dinotefuran on Three Species of Bactrocera Fruit Flies. Insects 2024, 15, 440. [Google Scholar] [CrossRef]
- Li, X.; Qi, Y.; Lu, Y. Advances for the metabolic detoxification genes in major Tephritidae species. J. Plant Prot. 2004, 49, 351–365. [Google Scholar]
- Li, J.Y.; Liu, J.; Chi, B.J.; Kong, F.L.; Liu, Y.J. Population dynamics in different fruit orchards and toxicity of sic insecticides to Bactrocera dorsalis in laboratory. Shandong Agric. Sci. 2020, 52, 120–123. [Google Scholar]
- Hu, W.Y.; Li, Z.; Li, K.; Li, Z.Y.; Xi, Y.Q.; Yin, X.M. Screening of insecticides and resistance monitoring of Bactrocera dorsalis adults. J Henan Agric. Univ. 2023, 57, 269–276+287. [Google Scholar]
- Hsu, J.C.; Feng, H.T.; Wu, W.J. Resistance and synergistic effects of insecticides in Bactrocera dorsalis (Diptera: Tephritidae) in Taiwan. J. Econ. Entomol. 2004, 97, 1682–1688. [Google Scholar] [CrossRef]
- Hsu, J.C.; Feng, H.T. Development of resistance to spinosad in oriental fruit fly (Diptera: Tephritidae) in laboratory selection and cross-resistance. J. Econ. Entomol. 2006, 99, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Lin, Y.Y.; Jin, Q.A.; Wen, H.B.; Peng, Z.Q. Population Susceptibility to Insecticides and the Development of Resistance in Bactrocera cucurbitae (Diptera: Tephritidae). J. Econ. Entomol. 2016, 109, 837–846. [Google Scholar] [CrossRef]
- Guo, Z.J.; Lu, Y.Y.; Yang, F.; Zeng, L.; Liang, G.W.; Xu, Y.J. Transmission modes of a pesticide-degrading symbiont of the oriental fruit fly Bactrocera dorsalis (Hendel). Appl. Microbiol. Biotechnol. 2017, 101, 8543–8556. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lu, X.P.; Wang, L.L.; Wei, D.; Feng, Z.J.; Zhang, Q.; Xiao, L.F.; Dou, W.; Wang, J.J. Functional characterization of NADPH-cytochrome P450 reductase from Bactrocera dorsalis: Possible involvement in susceptibility to malathion. Sci. Rep. 2015, 5, 18394. [Google Scholar] [CrossRef]
- Baxter, S.W.; Chen, M.; Dawson, A.; Zhao, J.Z.; Vogel, H.; Shelton, A.M.; Heckel, D.G.; Jiggins, C.D. Mis-spliced transcripts of nicotinic acetylcholine receptor alpha6 are associated with field evolved spinosad resistance in Plutella xylostella (L.). PLoS Genet 2010, 6, e1000802. [Google Scholar] [CrossRef] [PubMed]
- Ureña, E.; Guillem-Amat, A.; Couso-Ferrer, F.; Beroiz, B.; Perera, N.; López-Errasquín, E.; Castañera, P.; Ortego, F.; Hernández-Crespo, P. Multiple mutations in the nicotinic acetylcholine receptor Ccalpha6 gene associated with resistance to spinosad in medfly. Sci. Rep. 2019, 9, 2961. [Google Scholar] [CrossRef]
- Wang, J.; Ma, H.H.; Zuo, Y.Y.; Yang, Y.H.; Wu, Y.D. CRISPR-mediated gene knockout reveals nicotinic acetylcholine receptor (nAChR) subunit α6 as a target of spinosyns in Helicoverpa armigera. Pest Manag. Sci. 2020, 76, 2925–2931. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Zeng, L.; Lu, Y.Y.; Liang, G.W. Monitoring of Insecticide Resistance of Bactrocera dorsalis Adults in South China. J. South China Agric. Univ. 2007, 28, 20–23. [Google Scholar]
- Zhang, Y.P.; Zeng, L.; Lu, Y.Y.; Liang, G.W. Monitoring of Insecticides Resistance of Oriental Fruit Fly Field Populations in South China. J. Huazhong Agric. Univ. 2008, 27, 456–459. [Google Scholar]
- Zhang, Y.P.; Zeng, L.; Lu, Y.Y.; Liang, G.W. Genetic Analysis of Bactrocera dorsalis Resistance to Trichlorphon. J. South China Agric. Univ. 2008, 16, 29–37. [Google Scholar]
- Zhang, Y.P.; Zeng, L.; Lu, Y.Y.; Liang, G.W. Resistance stability and re-growth in adults of the oriental fruit fly, Bactrocera dorsalis(Diptera:Tephritidae) to trichlorphon. Acta Entomol. Sin. 2008, 51, 1044–1049. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Mutamiswa, R.; Nyamukondiwa, C.; Chikowore, G.; Chidawanyika, F. Overview of oriental fruit fly, Bactrocera dorsalis (Hendel)(Diptera: Tephritidae) in Africa: From invasion, bio-ecology to sustainable management. Crop. Prot. 2021, 141, 105492. [Google Scholar] [CrossRef]
- Hansen, J.; Sato, M.; Ruedy, R.; Lo, K.; Lea, D.W.; Medina-Elizade, M. Global temperature change. Proc. Natl. Acad. Sci. USA 2006, 103, 14288–14293. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ren, L.; Xie, M.; Gao, Y.; He, M.; Hassan, B.; Lu, Y.; Cheng, D. Egg-Surface Bacteria Are Indirectly Associated with Oviposition Aversion in Bactrocera dorsalis. Curr. Biol. 2020, 30, 4432–4440.e4434. [Google Scholar] [CrossRef]
- Pineda, S.; Smagghe, G.; Schneider, M.I.; Del Estal, P.; Vinuela, E.; Martinez, A.M.; Budia, F. Toxicity and pharmacokinetics of spinosad and methoxyfenozide to Spodoptera littoralis (Lepidoptera : Noctuidae). Environ. Entomol. 2006, 35, 856–864. [Google Scholar] [CrossRef]
- Shen, G.M.; Dou, W.; Niu, J.Z.; Jiang, H.B.; Yang, W.J.; Jia, F.X.; Hu, F.; Cong, L.; Wang, J.J. Transcriptome analysis of the oriental fruit fly (Bactrocera dorsalis). PLoS ONE 2011, 6, e29127. [Google Scholar] [CrossRef]
- Vontas, J.; Hernández-Crespo, P.; Margaritopoulos, J.T.; Ortego, F.; Feng, H.T.; Mathiopoulos, K.D.; Hsu, J.C. Insecticide resistance in Tephritid flies. Pest Biochem. Physiol. 2011, 100, 199–205. [Google Scholar] [CrossRef]
- Shen, G.M.; Wang, X.N.; Dou, W.; Wang, J.J. Biochemical and molecular characterisation of acetylcholinesterase in four field populations of Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Pest Manag. Sci. 2012, 68, 1553–1563. [Google Scholar] [CrossRef]
- Wang, L.L.; Lu, X.P.; Meng, L.W.; Huang, Y.; Wei, D.; Jiang, H.B.; Smagghe, G.; Wang, J.J. Functional characterization of an alpha-esterase gene involving malathion detoxification in Bactrocera dorsalis (Hendel). Pestic. Biochem. Physiol. 2016, 130, 44–51. [Google Scholar] [CrossRef]
- Meng, L.W.; Yuan, G.R.; Lu, X.P.; Jing, T.X.; Zheng, L.S.; Yong, H.X.; Wang, J.J. Two delta class glutathione S-transferases involved in the detoxification of malathion in Bactrocera dorsalis (Hendel). Pest Manag. Sci. 2019, 75, 1527–1538. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Meng, Q.Q.; Li, Z.Q.; Zhang, S.Q.; Zeng, L.; Lu, Y.Y. Monitoring of resistance in field populations of Bactrocera dorsalis in Shenzhen. China Plant Prot. 2015, 35, 63–66. [Google Scholar]
- He, F.M.; An, C.C.; Zhang, Y.K.; Li, Y.F.; Tan, H.H. Toxicity of insecticides to adults of Bactrocera dorsalis (Hendel) and insecticides resistance in field populations in Guangxi. Plant Prot. 2020, 5, 270–275. [Google Scholar]
- Sun, Z.Y. Study on Pesticide Sensitivity Monitoring and Occurrence Regularity of Bactrocera dorsalis in Shandong Province. Master Thesis, Yantai University, Yantai, China, 2023. [Google Scholar]
- Stockton, D.G.; Kraft, L.; Dombrowski, P.; Doucette, L.; Bosch, M.; Gutierrez-Coarite, R.; Manandhar, R.; Uyeda, J.; Silva, J.; Hawkins, J.; et al. Persistence of widespread moderate spinosad resistance among wild melon fly (Zeugodacus cucurbitae) and oriental fruit fly (Bactrocera dorsalis) populations on the major Hawaiian islands. Pest Manag. Sci. 2024, 12. [Google Scholar] [CrossRef]
- Maino, J.L.; Umina, P.A.; Hoffmann, A.A. Climate contributes to the evolution of pesticide resistance. Global Ecol. Biogeogr. 2018, 27, 223–232. [Google Scholar] [CrossRef]
- Wang, P.; Yang, F.; Wang, Y.; Zhou, L.L.; Luo, H.B.; Zhang, S.; Si, S.Y. Monitoring the resistance of the beet armyworm (Lepidoptera: Noctuidae) to four insecticides in Southern China from 2014 to 2018. J. Econ. Entomol. 2021, 114, 332–338. [Google Scholar] [CrossRef]
- Wang, X.; Xiang, X.; Yu, H.; Liu, S.; Yin, Y.; Cui, P.; Wu, Y.; Yang, J.; Jiang, C.; Yang, Q. Monitoring and biochemical characterization of beta-cypermethrin resistance in Spodoptera exigua (Lepidoptera: Noctuidae) in Sichuan Province, China. Pestic. Biochem. Physiol. 2018, 146, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.Y.; Ren, X.M.; Ma, X.Y.; Ma, Y.J.; Song, J.P.; Wang, D.; Li, H.H.; Ma, Y. Resistance monitoring of cotton bollworm Helicoverpa armigera to three insecticides in northern China. J. Plant Prot. 2021, 4, 900–906. [Google Scholar]
- Cilek, J.E.; Steelman, C.D.; Knapp, F.W. Horn fly (Diptera: Muscidae) insecticide resistance in Kentucky and Arkansas. J. Econ. Entomol. 1991, 84, 756–762. [Google Scholar] [CrossRef]
- Wu, P.Y.; Zhang, Y.M.; Zhang, J.; Qin, N.; Wang, W.; Li, P.Y.; Li, J.Y.; Hao, L.Y. Surveillance of the resistance of Culex pipiens pallens and Musca domestica to insecticides in Tianjin in 2010. Chin. J. Vector Biol. Cont. 2012, 23, 122–124. [Google Scholar]
- Jin, T.; Zeng, L.; Lin, Y.; Lu, Y.; Liang, G. Insecticide resistance of the oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae), in mainland China. Pest Manag. Sci. 2011, 67, 370–376. [Google Scholar] [CrossRef]
- Comins, H.N. The management of pesticide resistance. J. Theor. Biol. 1977, 65, 399–420. [Google Scholar] [CrossRef]
- De Souza, K.; Holt, J.; Colvin, J. Diapause, migration and pyrethroid-resistance dynamics in the cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). Ecol. Entomol. 1995, 20, 333–342. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Lu, Y.Y.; Zeng, L.; Liang, G.W. Population life parameters and relative fitness of alphamethrin-resistant Bactrocera dorsalis strain. Ying Yong Sheng Tai Xue Bao 2009, 20, 381–386. [Google Scholar] [PubMed]
- Nisha, A. Antibiotic residues-a global health hazard. Vet. World 2008, 1, 375. [Google Scholar] [CrossRef]
- Wang, Y.H.; Cang, T.; Zhao, X.P.; Yu, R.X.; Chen, L.P.; Wu, C.X.; Wang, Q. Comparative acute toxicity of twenty-four insecticides to earthworm, Eisenia fetida. Ecotoxicol. Environ. Saf. 2012, 79, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.M.; Dahlgren, L.; Siegfried, B.D.; Ellis, M.D. Acaricide, fungicide and drug interactions in honey bees (Apis mellifera). PLoS ONE 2013, 8, e54092. [Google Scholar] [CrossRef] [PubMed]
- Gomiero, T. Food quality assessment in organic vs. conventional agricultural produce: Findings and issues. Appl. Soil. Ecol. 2018, 123, 714–728. [Google Scholar] [CrossRef]
- Meng, X.; Rui, C.; Zhao, J.; Fan, X.; Cen, W. Changes of pyrethroids resistance frequencies in cotton bollworm (Helicoverpa armigera) with gene flow. J. Plant Prot. 2000, 27, 273–276. [Google Scholar]
- Sun, X.; Barrett, B.; Biddinger, D. Fecundity and fertility reductions in adult leafrollers exposed to surfaces treated with the ecdysteroid agonists tebufenozide and methoxyfenozide. Entomol. Exp. Appl. 2000, 94, 75–83. [Google Scholar] [CrossRef]
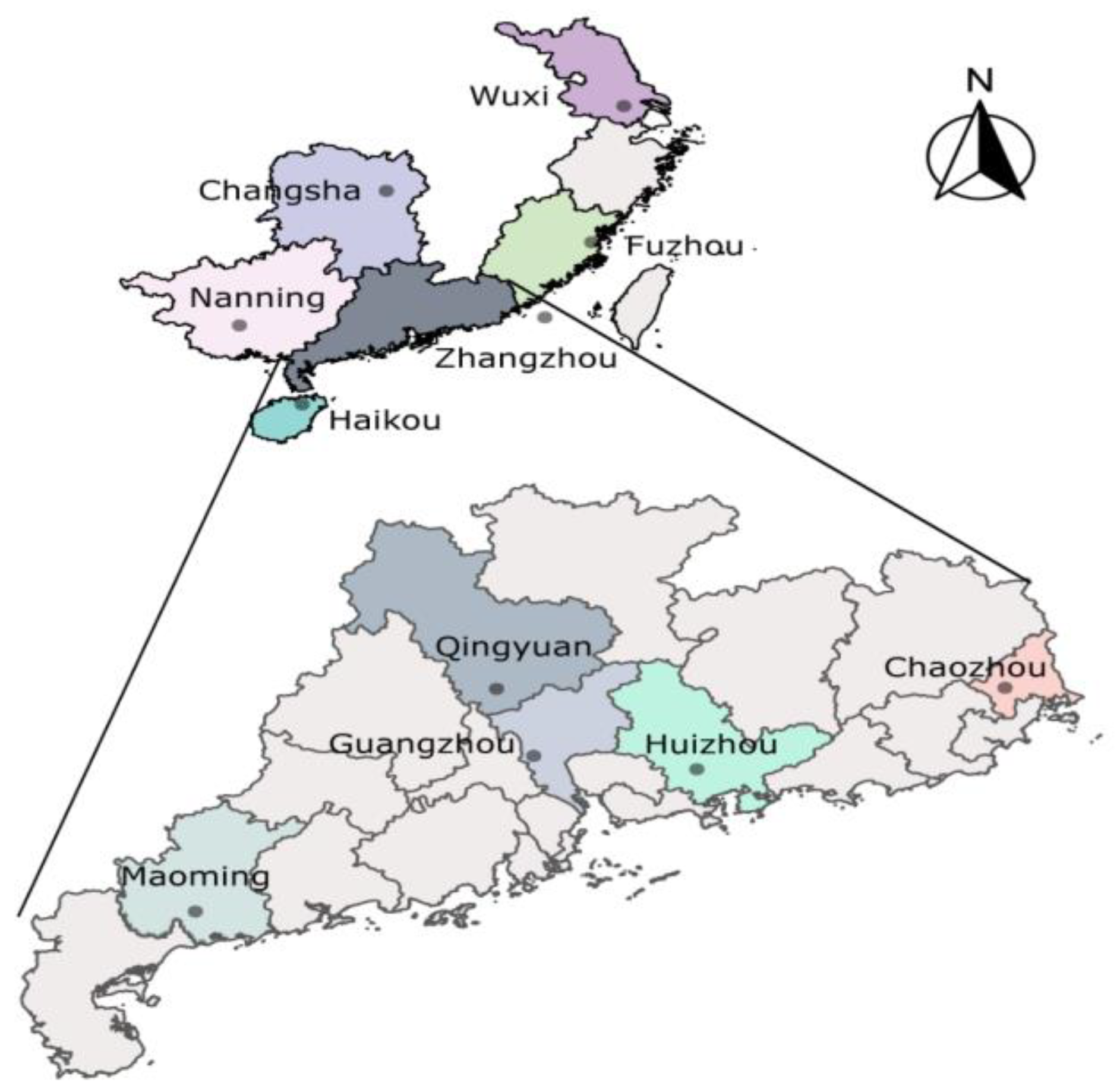
| Populations | Province | Longitude (E)/Latitude (N) |
|---|---|---|
| Guangzhou (GZ) | Guangdong | 113°17′/23°8′ |
| Qingyuan (QY) | Guangdong | 113°16′/23°49′ |
| Huizhou (HZ) | Guangdong | 114°27′/23°10′ |
| Maoming (MM) | Guangdong | 110°50′/21°54′ |
| Chaozhou (CZ) | Guangdong | 116°38′/23°40′ |
| Nanning (NN) | Guangxi | 108°19′/22°49′ |
| Zhangzhou (ZZ) | Fujian | 117°59′/23°8′ |
| Fuzhou (FZ) | Fujian | 119°28′/26°08′ |
| Wuxi (WX) | Jiangsu | 120°29′/31°34′ |
| Changsha (CS) | Hunan | 112°58′/28°11′ |
| Haikou (HK) | Hainan | 110°19′/20°1′ |
| Area | 2010 | 2011 | 2012 | 2013 | 2023 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| LC50/mg·L−1 (95% CI) | RR | LC50/mg·L−1 (95% CI) | RR | LC50/mg·L−1 (95% CI) | RR | LC50/mg·L−1 (95% CI) | RR | LC50/mg·L−1 (95% CI) | RR | |
| GZ | 38.27 (34.88–41.98) | 15.21 | 40.03 (36.61–43.77) | 15.91 | 35.63 (33.96–37.39) | 14.14 | 48.50 (40.15–62.19) | 19.25 | 32.02 (22.21–43.01) | 12.71 |
| HZ | 70.15 (65.15–75.545) | 27.88 | 83.32 (77.83–89.21) | 33.11 | 65.79 (62.33–69.45) | 26.11 | 75.37 (57.79–120.59) | 29.91 | 57.69 (45.10–79.81) | 22.89 |
| QY | 53.45 (49.96–57.18) | 21.24 | 48.21 (45.34–51.28) | 19.16 | 58.47 (53.98–33.33) | 23.20 | 47.97 (39.46–62.03) | 19.04 | 33.45 (24.73–44.86) | 13.27 |
| CZ | 45.85 (42.95–48.94) | 18.22 | 58.03 (54.84–61.41) | 23.06 | 68.53 (64.19–73.16) | 27.19 | 47.69 (38.38–64.38) | 18.92 | 50.63 (43.72–59.47) | 20.09 |
| MM | 69.05 (61.456–77.58) | 27.44 | 70.78 (64.348–77.86) | 28.13 | 55.61 (51.02–60.61) | 22.07 | 66.81 (57.07–82.54) | 26.51 | 57.44 (49.64–66.95) | 42.55 |
| NN | 55.86 (53.29–58.55) | 22.20 | 71.26 (66.20–76.70) | 28.32 | 60.79 (55.63–66.43) | 24.12 | 88.94 (79.09–100.01) | 35.29 | 59.37 (50.06–73.14) | 23.56 |
| HK | 68.31 (63.25–73.77) | 27.15 | 70.19 (65.24–75.52) | 27.90 | 78.04 (73.67–82.68) | 30.97 | 53.59 (42.00–77.31) | 21.27 | 47.56 (37.80–61.39) | 18.87 |
| FZ | 41.11 (37.84–44.67) | 16.34 | 37.11 (34.31–40.13) | 14.75 | 43.16 (40.22–46.31) | 17.13 | 59.08 (49.12–73.54) | 23.44 | 25.76 (13.31–44.17) | 10.22 |
| ZZ | 49.49 (45.23–54.15) | 19.67 | 57.65 (52.61–63.18) | 22.91 | 49.44 (46.03–53.10) | 19.62 | 56.55 (43.45–85.83) | 22.44 | 32.73 (21.16–45.91) | 12.99 |
| CS | 39.02 (36.12–42.16) | 15.51 | 41.62 (38.17–45.39) | 16.54 | 46.15 (42.66–49.91) | 18.31 | 49.09 (39.64–66.00) | 19.48 | 41.45 (35.27–49.32) | 16.45 |
| WX | 35.44 (32.63–38.49) | 14.09 | 38.34 (35.34–41.59) | 15.24 | 39.78 (37.58–42.12) | 15.79 | 63.35 (52.79–80.26) | 25.14 | 56.68 (47.62–69.84) | 22.49 |
| SS | 2.52 (2.21–2.88) | |||||||||
| Area | 2010 | 2011 | 2012 | 2013 | 2023 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| LC50/mg·L−1 (95% CI) | RR | LC50/mg·L−1 (95% CI) | RR | LC50/mg·L−1 (95% CI) | RR | LC50/mg·L−1 (95% CI) | RR | LC50/mg·L−1 (95% CI) | RR | |
| GZ | 35.84 (33.01–38.91) | 26.55 | 38.57 (35.57–41.83) | 28.57 | 34.39 (32.67–36.19) | 25.47 | 41.74 (34.20–53.22) | 30.92 | 26.60 (21.78–33.78) | 19.71 |
| HZ | 44.75 (42.19–47.47) | 33.15 | 52.75 (48.42–57.46) | 39.07 | 38.83 (35.95–41.93) | 28.76 | 57.09 (46.36–78.02) | 42.29 | 7.41 (6.32–8.89) | 5.49 |
| QY | 45.50 (42.87–48.29) | 33.70 | 32.01 (29.45–34.79) | 23.71 | 31.55 (29.64–33.59) | 23.37 | 45.55 (36.79–60.32) | 33.74 | 3.34 (1.92–5.09) | 2.47 |
| CZ | 46.14 (43.24–49.23) | 34.18 | 38.96 (35.38–42.89) | 28.86 | 43.98 (40.64–47.60) | 32.58 | 43.05 (33.92–58.52) | 31.89 | 38.99 (32.31–46.85) | 28.88 |
| MM | 36.39 (31.72–41.76) | 26.96 | 37.81 (32.7–43.71) | 28.00 | 39.03 (36.63–41.59) | 28.91 | 35.39 (29.03–43.69) | 26.21 | 52.26 (38.88–70.53) | 38.71 |
| NN | 21.95 (18.50–26.04) | 16.26 | 29.18 (26.20–32.50) | 21.61 | 31.69 (28.69–35.00) | 23.47 | 30.10 (23.74–37.50) | 22.30 | 58.07 (44.47–78.12) | 43.01 |
| HK | 24.73 (22.30–27.43) | 18.32 | 24.48 (21.84–37.44) | 18.13 | 25.69 (23.36–28.26) | 19.03 | 34.26 (29.27–40.09) | 25.38 | 38.14 (29.29–49.04) | 28.25 |
| FZ | 12.81 (10.88–15.10) | 9.49 | 14.44 (12.63–16.51) | 10.70 | 27.77 (25.71–30.01) | 20.57 | 20.01 (15.95–28.74) | 14.82 | 5.70 (4.17–7.72) | 4.22 |
| ZZ | 22.36 (19.90–25.14) | 16.56 | 27.54 (23.89–31.74) | 20.40 | 30.63 (27.95–3.76) | 22.69 | 34.66 (28.27–42.88) | 25.67 | 39.34 (24.67–60.38) | 29.14 |
| CS | 39.02 (36.12–42.16) | 28.90 | 26.61 (23.65–29.95) | 19.71 | 26.51 (24.61–28.55) | 19.64 | 23.09 (19.55–26.60) | 17.10 | 19.98 (16.92–23.59) | 14.80 |
| WX | 15.14 (12.91–17.76) | 11.21 | 24.6 (21.99–27.63) | 18.22 | 32.42 (28.99–36.27) | 24.02 | 30.69 (26.60–38.26) | 22.73 | 75.09 (63.14–94.97) | 55.62 |
| SS | 1.35 (0.97–1.89) | |||||||||
| Area | 2010 | 2011 | 2012 | 2013 | 2023 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| LC50/mg·L−1 (95% CI) | RR | LC50/mg·L−1 (95% CI) | RR | LC50/mg·L−1 (95% CI) | RR | LC50/mg·L−1 (95% CI) | RR | LC50/mg·L−1 (95% CI) | RR | |
| GZ | 35.81 (29.68–34.50) | 22.38 | 34.61 (31.91–37.53) | 21.63 | 26.08 (24.21–28.09) | 16.30 | 24.68 (22.14–28.89) | 15.42 | 15.414 (13.00–17.97) | 9.63 |
| HZ | 34.02 (31.80–36.40) | 21.24 | 30.82 (28.64–33.17) | 19.24 | 26.51 (24.43–28.78) | 16.57 | 27.91 (25.23–32.56) | 17.44 | 8.81 (7.14–11.92) | 5.51 |
| QY | 33.44 (31.14–35.90) | 20.88 | 38.84 (35.25–42.79) | 24.25 | 33.43 (30.25–36.94) | 20.89 | 24.34 (21.75–28.67) | 15.21 | 4.82 (4.12–5.74) | 3.01 |
| CZ | 38.00 (34.30–42.10) | 23.72 | 37.06 (34.07–0.31) | 23.14 | 41.05 (38.24–44.07) | 25.66 | 21.33 (18.94–24.81) | 13.33 | 46.59 (40.78–53.34) | 29.12 |
| MM | 44.02 (41.07–47.18) | 27.48 | 47.09 (43.63–50.81) | 29.40 | 42.12 (39.28–45.17) | 26.33 | 35.45 (28.90–48.02) | 22.16 | 16.65 (15.91–17.43) | 10.41 |
| NN | 35.97 (29.56–36.22) | 22.48 | 34.71 (32.01–37.63) | 21.67 | 37.04 (35.08–39.10) | 23.15 | 25.38 (21.96–32.19) | 15.86 | 41.52 (36.95–46.90) | 25.95 |
| HK | 20.57 (18.22–23.22) | 12.84 | 20.98 (18.68–23.56) | 13.10 | 25.03 (23.25–26.94) | 15.64 | 20.28 (18.69–22.22) | 12.68 | 43.80 (37.27–51.76) | 27.38 |
| FZ | 23.05 (20.44–25.99) | 14.39 | 32.58 (29.86–35.56) | 20.34 | 30.20 (27.58–33.07) | 18.88 | 28.02 (24.73–33.39) | 17.51 | 3.33 (3.01–3.70) | 2.08 |
| ZZ | 33.91 (31.35–36.67) | 21.17 | 32.38 (29.86–35.12) | 20.24 | 33.35 (30.19–36.84) | 20.84 | 27.25 (23.92–32.55) | 17.03 | 19.62 (17.18–22.44) | 12.26 |
| CS | 31.81 (29.55–34.25) | 19.86 | 35.30 (32.97–37.80) | 22.04 | 25.43 (23.67–27.32) | 15.89 | 26.90 (24.64–30.61) | 16.81 | 3.42 (2.90–4.01) | 2.14 |
| WX | 27.73 (24.63–31.22) | 17.33 | 32.09 (27.79–37.06) | 20.06 | 28.74 (26.37–31.31) | 17.96 | 23.80 (20.97–27.00) | 14.88 | 19.55 (17.12–22.30) | 12.22 |
| SS | 1.60 (1.37–1.87) | |||||||||
| Area | 2010 | 2011 | 2012 | 2013 | 2023 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| LC50/mg·L−1 (95% CI) | RR | LC50/mg·L−1 (95% CI) | RR | LC50/mg·L−1 (95% CI) | RR | LC50/mg·L−1 (95% CI) | RR | LC50/mg·L−1 (95% CI) | RR | |
| GZ | 4.18 (3.44–5.07) | 6.97 | 4.87 (3.92–6.06) | 8.12 | 5.55 (4.73–6.52) | 9.25 | 5.21 (3.93–8.14) | 8.68 | 23.00 (18.18–29.00) | 38.33 |
| HZ | 5.16 (4.12–6.46) | 8.60 | 4.82 (4.00–5.80) | 8.03 | 5.78 (5.29–6.33) | 9.63 | 5.86 (4.88–7.79) | 9.77 | 23.99 (16.84–33.08) | 39.98 |
| QY | 4.69 (3.80–5.78) | 7.82 | 5.85 (5.05–6.77) | 9.75 | 6.62 (5.99–7.32) | 11.03 | 7.12 (5.59–10.80) | 11.87 | 12.63 (5.44–18.74) | 21.05 |
| CZ | 4.49 (3.98–5.05) | 7.48 | 4.53 (4.03–5.08) | 7.55 | 5.49 (5.07–5.95) | 9.15 | 7.37 (6.24–9.45) | 12.28 | 11.07 (6.03–16.34) | 18.45 |
| MM | 4.41 (3.71–5.24) | 7.35 | 4.96 (4.10–6.00) | 8.27 | 7.10 (6.30–8.01) | 11.83 | 6.73 (5.68–8.48) | 11.22 | 14.85 (8.67–21.35) | 23.92 |
| NN | 3.95 (3.35–4.66) | 6.58 | 5.25 (4.52–6.10) | 8.75 | 6.83 (6.08–7.67) | 11.38 | 4.70 (3.80–6.26) | 7.83 | 17.73 (15.83–20.01) | 29.55 |
| HK | 5.36 (4.33–6.64) | 8.93 | 4.98 (4.12–6.03) | 8.30 | 6.47 (5.82–7.18) | 10.78 | 6.20 (5.42–7.09) | 10.33 | 16.36 (11.53–22.05) | 27.27 |
| FZ | 4.12 (3.40–5.00) | 6.87 | 4.96 (4.22–6.87) | 8.97 | 4.86 (4.51–5.25) | 8.10 | 6.18 (4.70–9.74) | 10.30 | 51.88 (44.54–61.74) | 86.47 |
| ZZ | 5.53 (4.74–6.47) | 9.22 | 4.25 (3.77–4.79) | 7.08 | 5.35 (4.92–5.82) | 8.92 | 4.65 (3.63–6.55) | 7.75 | 40.94 (27.78–60.43) | 68.23 |
| CS | 3.67 (3.02–4.45) | 6.12 | 4.75 (3.90–5.78) | 7.92 | 4.34 (3.84–4.91) | 7.23 | 6.91 (5.19–11.37) | 11.52 | 6.44 (5.14–8.41) | 10.73 |
| WX | 4.68 (3.77–5.80) | 7.80 | 5.23 (4.31–6.36) | 8.72 | 5.13 (4.73–5.56) | 8.55 | 5.63 (4.46–8.00) | 9.38 | 55.99 (48.91–65.24) | 93.32 |
| SS | 0.60 (0.51–0.71) | |||||||||
| Area | 2010 | 2011 | 2012 | 2013 | 2023 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| LC50/mg·L−1 (95% CI) | RR | LC50/mg·L−1 (95% CI) | RR | LC50/mg·L−1 (95% CI) | RR | LC50/mg·L−1 (95% CI) | RR | LC50/mg·L−1 (95% CI) | RR | |
| GZ | 7.47 (6.47–8.62) | 8.30 | 9.94 (8.80–11.24) | 10.99 | 11.33 (10.51–12.22) | 12.59 | 12.41 (9.89–17.73) | 13.79 | 11.78 (5.23–17.051) | 13.09 |
| HZ | 6.04 (5.25–6.95) | 6.71 | 7.18 (6.25–8.24) | 7.98 | 10.06 (9.04–11.20) | 11.18 | 6.22 (5.04–8.69) | 6.91 | 13.06 (7.65–18.13) | 14.51 |
| QY | 4.95 (4.41–5.55) | 5.50 | 7.70 (7.09–8.35) | 8.56 | 7.78 (7.34–8.26) | 8.64 | 6.21 (4.71–9.85) | 6.90 | 3.78 (1.66–5.62) | 4.20 |
| CZ | 5.86 (5.04–6.81) | 6.51 | 6.26 (5.39–7.27) | 6.96 | 7.59 (6.72–8.57) | 8.43 | 8.50 (6.90–12.20) | 9.44 | 22.00 (15.11–33.15) | 24.44 |
| MM | 5.95 (5.31–6.66) | 6.61 | 6.29 (5.57–7.11) | 6.99 | 10.85 (10.23–11.50) | 12.06 | 5.74 (4.90–6.87) | 6.38 | 24.20 (16.59–39.39) | 26.89 |
| NN | 5.04 (4.43–5.72) | 5.60 | 5.63 (4.97–6.38) | 6.26 | 6.98 (6.48–7.52) | 7.76 | 6.09 (4.55–9.97) | 6.77 | 28.16 (18.31–38.00) | 31.29 |
| HK | 5.53 (4.85–6.31) | 6.14 | 5.35 (4.57–6.27) | 5.94 | 7.67 (6.85–8.59) | 8.52 | 6.38 (5.00–8.13) | 7.09 | 14.67 (11.25–20.67) | 16.30 |
| FZ | 2.23 (1.96–2.54) | 2.48 | 2.33 (1.98–2.75) | 2.59 | 6.31 (5.74–6.92) | 7.01 | 6.35 (4.47–12.54) | 7.06 | 17.82 (9.33–28.17) | 19.80 |
| ZZ | 2.06 (1.67–2.55) | 2.29 | 2.59 (2.17–3.08) | 2.88 | 7.58 (6.65–8.65) | 8.43 | 2.93 (2.40–3.52) | 3.26 | 67.68 (57.47–83.42) | 75.20 |
| CS | 3.95 (3.59–4.35) | 4.39 | 3.55 (3.24–3.90) | 3.94 | 6.07 (5.50–6.69) | 6.74 | 4.95 (3.93–6.86) | 5.50 | 12.56 (10.71–14.99) | 13.96 |
| WX | 4.00 (3.44–4.66) | 4.44 | 7.31 (6.38–8.38) | 10.99 | 8.73 (8.05–9.47) | 9.70 | 11.30 (9.45–13.17) | 12.56 | 93.41 (74.26–131.76) | 103.79 |
| SS | 0.90 (0.69–1.17) | |||||||||
| Area | 2010 | 2011 | 2012 | 2023 | ||||
|---|---|---|---|---|---|---|---|---|
| LC50/mg·L−1 (95% CI) | RR | LC50/mg·L−1 (95% CI) | RR | LC50/mg·L−1 (95% CI) | RR | LC50/mg·L−1 (95% CI) | RR | |
| GZ | 2.60 (2.32~2.90) | 2.86 | 3.20 (2.76~3.72) | 3.52 | 3.21 (2.90~3.55) | 3.53 | 2.41 (1.86–3.03) | 2.65 |
| HZ | 3.00 (2.61~3.44) | 3.30 | 4.06 (3.68~4.49) | 4.46 | 6.40 (5.79~7.07) | 7.03 | 1.98 (1.15–2.84) | 2.18 |
| QY | 4.38 (3.93~4.89) | 4.81 | 6.01 (5.43~6.65) | 6.60 | 4.41 (4.15~4.69) | 4.85 | 2.45 (2.10–2.84) | 2.69 |
| CZ | 3.71 (3.31~4.16) | 4.08 | 4.86 (4.32~5.48) | 5.34 | 6.35 (5.76~6.99) | 6.98 | 4.69 (3.40–6.50) | 5.15 |
| MM | 2.94 (2.56~3.36) | 3.49 | 4.07 (3.69~4.48) | 4.47 | 6.65 (5.94~7.45) | 7.31 | 2.52 (2.09–3.05) | 2.77 |
| NN | 3.18 (2.73~3.71) | 3.49 | 4.28 (3.84~4.76) | 4.70 | 5.43 (5.03~5.86) | 5.97 | 1.71 (1.14–2.40) | 1.88 |
| HK | 3.10 (2.640~3.54) | 3.41 | 4.74 (4.31~5.22) | 5.21 | 4.19 (3.92~4.48) | 4.60 | 2.62 (1.80–3.57) | 2.88 |
| FZ | 2.52 (2.24~2.84) | 2.77 | 2.92 (2.56~3.34) | 3.21 | 3.99 (3.75~4.25) | 4.38 | 2.76 (1.79–3.91) | 3.03 |
| ZZ | 2.58 (2.27~2.92) | 2.84 | 4.52 (4.12~4.97) | 4.97 | 5.13 (4.77~5.53) | 5.64 | 1.77 (1.02–2.46) | 1.95 |
| CS | 2.17 (1.92~2.45) | 2.38 | 3.04 (2.6~3.52 | 3.34 | 2.71 (2.49~2.96) | 2.98 | 1.97 (1.47–2.61) | 2.16 |
| WX | 2.16 (1.91~2.44) | 2.37 | 4.81 (4.33~5.35) | 5.29 | 3.93 (3.60~4.29) | 4.32 | 1.34 (1.12–1.59) | 1.47 |
| SS | 0.91 (0.75~1.10) | |||||||
| Area | 2013 | 2023 | ||
|---|---|---|---|---|
| LC50/mg·L−1 (95% CI) | RR | LC50/mg·L−1 (95% CI) | RR | |
| GZ | 6.10 (4.49~10.35) | 10.04 | 13.66 (2.20–24.54) | 22.39 |
| HZ | 5.31 (4.05~8.00) | 8.74 | 59.31 (47.95–78.15) | 97.23 |
| QY | 6.28 (4.83~9.55) | 10.34 | 47.21 (34.66–67.70) | 77.39 |
| CZ | 8.87 (7.30~11.80) | 14.60 | 74.71 (65.63–86.95) | 122.48 |
| MM | 11.81 (9.13~18.91) | 19.44 | 47.48 (39.33–57.18) | 77.84 |
| NN | 3.28 (2.61~4.12) | 5.40 | 76.51 (63.97–91.11) | 125.43 |
| HK | 8.50 (6.85~11.65) | 13.99 | 50.05 (43.51–56.79) | 82.05 |
| FZ | 9.16 (7.35~13.67) | 15.08 | 32.56 (21.96–44.70) | 53.38 |
| ZZ | 15.07 (9.85~41.71) | 24.81 | 56.32 (45.26–74.29) | 92.33 |
| CS | 13.62 (10.16~22.78) | 22.42 | 52.19 (40.82–67.45) | 85.56 |
| WX | 10.25 (7.82~16.60) | 16.88 | 55.45 (43.93–75.62) | 90.90 |
| SS | 0.61 (0.47–0.92) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Li, P.; Li, D.; Cai, X.; Gu, S.; Zeng, L.; Cheng, D.; Lu, Y. Dynamics of Bactrocera dorsalis Resistance to Seven Insecticides in South China. Insects 2024, 15, 679. https://doi.org/10.3390/insects15090679
Li X, Li P, Li D, Cai X, Gu S, Zeng L, Cheng D, Lu Y. Dynamics of Bactrocera dorsalis Resistance to Seven Insecticides in South China. Insects. 2024; 15(9):679. https://doi.org/10.3390/insects15090679
Chicago/Turabian StyleLi, Xinlian, Peizheng Li, Doudou Li, Xinyan Cai, Shiwei Gu, Ling Zeng, Daifeng Cheng, and Yongyue Lu. 2024. "Dynamics of Bactrocera dorsalis Resistance to Seven Insecticides in South China" Insects 15, no. 9: 679. https://doi.org/10.3390/insects15090679
APA StyleLi, X., Li, P., Li, D., Cai, X., Gu, S., Zeng, L., Cheng, D., & Lu, Y. (2024). Dynamics of Bactrocera dorsalis Resistance to Seven Insecticides in South China. Insects, 15(9), 679. https://doi.org/10.3390/insects15090679






