Elevated Plasmodium Sporozoite Infection Rates in Primary and Secondary Malaria Vectors in Anopheles stephensi-Infested Areas of Ethiopia
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Period
2.2. Mosquito Survey
2.3. Mosquito Specimen Processing
2.4. Mosquito DNA Extraction
2.5. Molecular Genotyping of Species, Bloodmeal, and Parasite Infections
2.6. Data Analysis
2.7. Ethical Considerations
3. Results
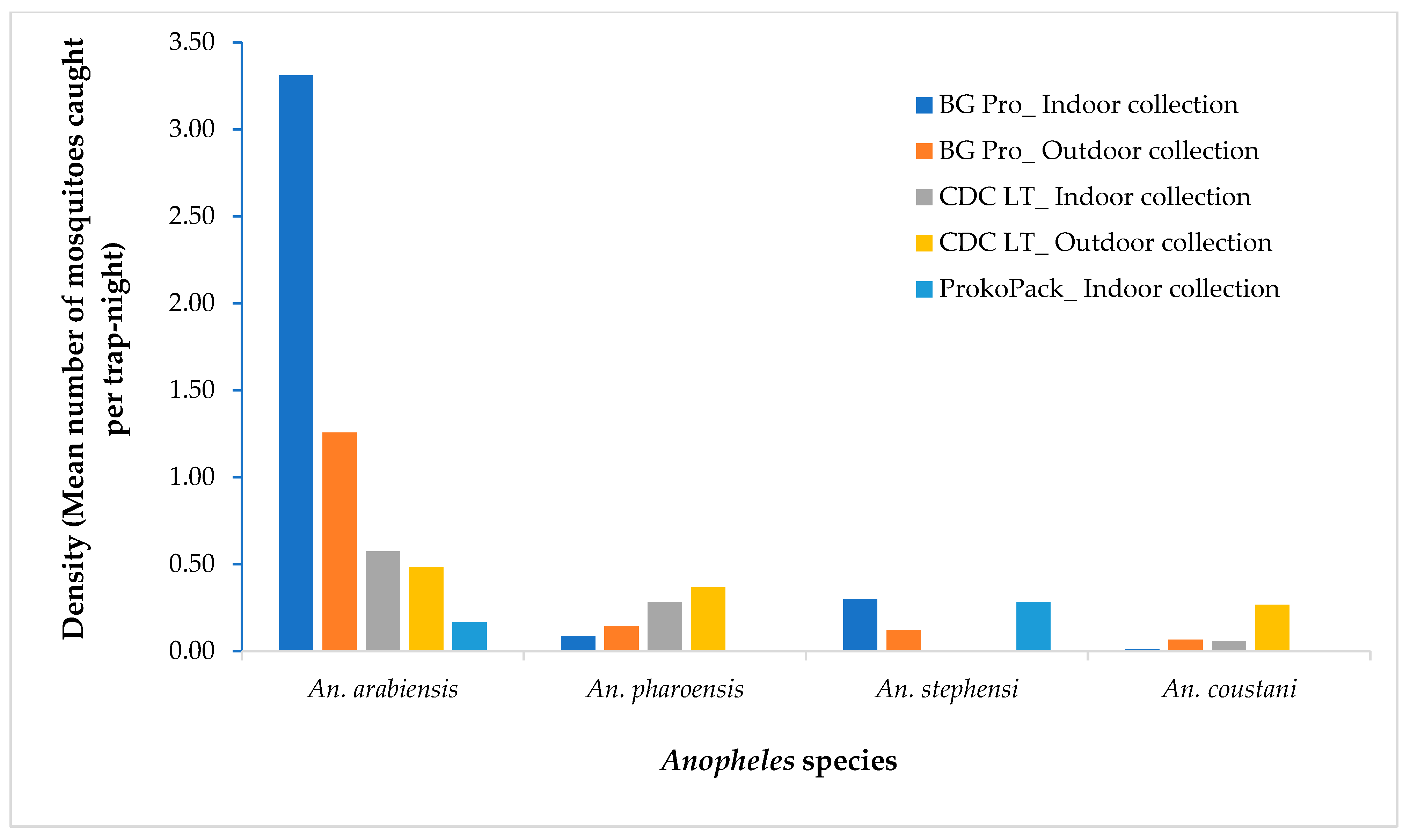
3.1. Anopheles Species and Density
3.2. Bloodmeal Source
3.3. Sporozoite Infection and Entomological Inoculation Rates
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sinka, M.E. Global Distribution of the Dominant Vector Species of Malaria, Anopheles mosquitoes. New Insights into Malar Vectors; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar]
- Adugna, F.; Wale, M.; Nibret, E. Review of Anopheles Mosquito Species, Abundance, and Distribution in Ethiopia. J. Trop. Med. 2021, 2021, 6726622. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, F.G.; Ashine, T.; Teka, H.; Esayas, E.; Messenger, L.A.; Chali, W.; Meerstein-Kessel, L.; Walker, T.; Behaksra, S.W.; Lanke, K.; et al. Anopheles stephensi Mosquitoes as Vectors of Plasmodium vivax and falciparum, Horn of Africa, 2019. Emerg. Infect. Dis. 2021, 27, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Balkew, M.; Mumba, P.; Yohannes, G.; Abiy, E.; Getachew, D.; Yared, S.; Worku, A.; Gebresilassie, A.; Tadesse, F.G.; Gadisa, E.; et al. An update on the distribution, bionomics, and insecticide susceptibility of Anopheles stephensi in Ethiopia, 2018–2020. Malar. J. 2021, 20, 263. [Google Scholar] [CrossRef]
- Carter, T.E.; Yared, S.; Gebresilassie, A.; Bonnell, V.; Damodaran, L.; Lopez, K.; Ibrahim, M.; Mohammed, S.; Janies, D. First detection of Anopheles stephensi Liston, 1901 (Diptera: Culicidae) in Ethiopia using molecular and morphological approaches. Acta Trop. 2018, 188, 180–186. [Google Scholar] [CrossRef]
- Afrane, Y.A.; Bonizzoni, M.; Yan, G. Secondary Malaria Vectors of Sub-Saharan Africa: Threat to Malaria Elimination on the Continent? Current Topics in Malaria; InTech: London, UK, 2016. [Google Scholar] [CrossRef]
- Gueye, A.; Ngom, E.H.M.; Diagne, A.; Ndoye, B.B.; Dione, M.L.; Sambe, B.S.; Sokhna, C.; Diallo, M.; Niang, M.; Dia, I. Host feeding preferences of malaria vectors in an area of low malaria transmission. Sci. Rep. 2023, 13, 16410. [Google Scholar] [CrossRef]
- Animut, A.; Balkew, M.; Gebre-michael, T.; Lindtjørn, B. Blood meal sources and entomological inoculation rates of anophelines along a highland altitudinal transect in south-central Ethiopia. Malar. J. 2013, 12, 76. [Google Scholar] [CrossRef]
- Katusi, G.C.; Hermy, M.R.G.; Makayula, S.M.; Ignell, R.; Govella, N.J.; Hill, S.R.; Mnyone, L.L. Seasonal variation in abundance and blood meal sources of primary and secondary malaria vectors within Kilombero Valley, Southern Tanzania. Parasites Vectors 2022, 15, 479. [Google Scholar] [CrossRef] [PubMed]
- Mlacha, Y.P.; Chaki, P.P.; Muhili, A.; Massue, D.J.; Tanner, M.; Majambere, S.; Killen, G.F.; Govella, N.J. Reduced human—Biting preferences of the African malaria vectors Anopheles arabiensis and Anopheles gambiae in an urban context: Controlled, competitive host—Preference experiments in Tanzania. Malar. J. 2020, 19, 418. [Google Scholar] [CrossRef]
- Yewhalaw, D.; Kelel, M.; Getu, E.; Temam, S.; Wessel, G. Blood Meal Sources and Sporozoite Rates of Anophelines in Gilgel-Gibe Dam Area, Southwestern . Afr. J. Vector Biol. 2014. Available online: https://www.researchgate.net/publication/292615632_Blood_meal_sources_and_sporozoite_rates_of_Anophelines_in_Gilgel-Gibe_dam_area_Southwestern_Ethiopia (accessed on 25 April 2025).
- Asale, A.; Duchateau, L.; Devleesschauwer, B.; Huisman, G.; Yewhalaw, D. Zooprophylaxis as a control strategy for malaria caused by the vector Anopheles arabiensis (Diptera: Culicidae): A systematic review. Infect. Dis. Poverty 2017, 7, 131. [Google Scholar] [CrossRef]
- Fornadel, C.; Norris, L.; Glass, G.; Norris, D. Analysis of Anopheles arabiensis blood feeding behavior in southern zambia during the two years after introduction of insecticide-treated bed nets. Am. J. Trop. Med. Hyg. 2010, 83, 848–853. [Google Scholar] [CrossRef]
- Mahande, A.; Mosha, F.; Mahande, J.; Kweka, E. Feeding and resting behaviour of malaria vector, Anopheles arabiensis with reference to zooprophylaxis. Malar. J. 2007, 6, 100. [Google Scholar] [CrossRef] [PubMed]
- Garrett-Jones, C.; Boreham, P.; Pant, C. Feeding habits of anophelines (Diptera: Culicidae) in 1971–1978, with reference to the human blood index: A review. Bull. Entomol. Res. 1980, 70, 165–185. [Google Scholar] [CrossRef]
- Sinka, M.E.; Bangs, M.J.; Manguin, S.; Rubio-Palis, Y.; Chareonviriyaphap, T.; Coetzee, M.; Mbogo, C.M.; Hemingway, J.; Patil, A.P.; Temperley, W.H.; et al. A global map of dominant malaria vectors. Parasites Vectors 2012, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Ravishankaran, S.; Justin, N.A.J.A.; Asokan, A.; Mathai, M.T.; Valecha, N.; Montgomery, J.; Thomas, M.B.; Eapen, A. Resting and feeding preferences of Anopheles stephensi in an urban setting, perennial for malaria. Malar. J. 2017, 16, 111. [Google Scholar] [CrossRef]
- Mustapha, A.; Musembi, S.; Nyamache, A. Secondary malaria vectors in western Kenya include novel species with unexpectedly high densities and parasite infection rates. Parasites Vectors 2021, 14, 252. [Google Scholar] [CrossRef]
- Hawaria, D.; Kibret, S.; Zhong, D.; Lee, M.-C.; Lelisa, K.; Bekele, B.; Birhanu, M.; Mengesha, M.; Solomon, H.; Yewhalaw, D.; et al. First report of Anopheles stephensi from southern Ethiopia. Malar. J. 2023, 22, 373. [Google Scholar] [CrossRef] [PubMed]
- Hawassa City Administration Health Department. Annual Malaria Morbidity Report; Hawassa City Administration Health Department: Hawassa, Ethiopia, 2022. [Google Scholar]
- Coetzee, M. Key to the females of Afrotropical Anopheles mosquitoes (Diptera: Culicidae). Malar. J. 2020, 19, 70. [Google Scholar] [CrossRef]
- Zhong, D.; Hemming-Schroeder, E.; Wang, X.; Kibret, S.; Zhou, G.; Atieli, H.; Lee, M.-C.; Afrane, Y.A.; Githeko, A.K.; Yan, G. Extensive new Anopheles cryptic species involved in human malaria transmission in western Kenya. Sci. Rep. 2020, 10, 16139. [Google Scholar] [CrossRef]
- Scott, J.; Brogdon, W.; Collins, F. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am. J. Trop. Med. Hyg. 1993, 49, 520–529. [Google Scholar] [CrossRef]
- Mbewe, R.B.; Keven, J.B.; Mzilahowa, T.; Mathanga, D.; Wilson, M.; Cohee, L.; Laufer, M.K.; Walker, E.D. Blood-feeding patterns of Anopheles vectors of human malaria in Malawi: Implications for malaria transmission and effectiveness of LLIN interventions. Malar. J. 2022, 21, 67. [Google Scholar] [CrossRef]
- Keven, J.; Artzberger, G.; Gillies, M.; Mbewe, R.; Walker, E. Probe-based multiplex qPCR identifies blood-meal hosts in Anopheles mosquitoes from Papua New Guinea. Parasites Vectors 2020, 13, 111. [Google Scholar] [CrossRef] [PubMed]
- Shokoples, S.E.; Ndao, M.; Kowalewska-Grochowska, K.; Yanow, S.K. Multiplexed real-time PCR assay for discrimination of Plasmodium species with improved sensitivity for mixed infections. J. Clin. Microbiol. 2009, 47, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, N.; Mwingira, F.; Shekalaghe, S.; Robinson, L.J.; Mueller, I.; Felger, I. Ultra-Sensitive Detection of Plasmodium falciparum by Amplification of Multi-Copy Subtelomeric Targets. PLoS Med. 2015, 12, e1001788. [Google Scholar] [CrossRef]
- Veron, V.; Simon, S.; Carme, B. Multiplex real-time PCR detection of P. falciparum, P. vivax and P. malariae in human blood samples. Exp. Parasitol. 2009, 121, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Drakeley, C.; Schellenberg, D.; Kihonda, J.; Sousa, C.A.; Arez, A.P.; Lopes, D.; Lines, J.; Mshinda, H.; Lengeler, C.; Schellenberg, J.A.; et al. An estimation of the entomological inoculation rate for Ifakara: A semi-urban area in a region of intense malaria transmission in Tanzania. Trop. Med. Int. Health 2003, 8, 767–774. [Google Scholar] [CrossRef]
- Ogola, E.; Villinger, J.; Mabuka, D.; Omondi, D.; Orindi, B.; Mutunga, J.; Owino, V.; Masiga, D.K. Composition of Anopheles mosquitoes, their blood—Meal hosts, and Plasmodium falciparum infection rates in three islands with disparate bed net coverage in Lake Victoria, Kenya. Malar. J. 2017, 16, 360. [Google Scholar] [CrossRef]
- Adugna, T.; Yewhelew, D.; Getu, E. Bloodmeal sources and feeding behavior of anopheline mosquitoes in Bure district, northwestern Ethiopia. Parasites Vectors 2021, 14, 166. [Google Scholar] [CrossRef]
- Aschale, Y.; Getachew, A.; Yewhalaw, D.; Cristofaro ADe Sciarretta, A. Systematic review of sporozoite infection rate of Anopheles mosquitoes in Ethiopia, 2001–2021. Parasites Vectors 2023, 16, 437. [Google Scholar] [CrossRef]
- Kibret, S.; Lautze, J.; Boelee, E.; Mccartney, M. How does an Ethiopian dam increase malaria ? Entomological determinants around the Koka reservoir. Trop. Med. Int. Health 2012, 17, 1320–1328. [Google Scholar] [CrossRef]
- Yewhalaw, D.; Legesse, W.; Van Bortel, W.; Gebre-Selassie, S.; Kloos, H.; Duchateau, L.; Speybroeck, N. Malaria and water resource development: The case of Gilgel-Gibe hydroelectric dam in Ethiopia. Malar. J. 2009, 8, 21. [Google Scholar] [CrossRef]
- Bedasso, A.H.; Gutto, A.A.; Waldetensai, A.; Eukubay, A.; Bokore, G.E.; Kinde, S.; Gemechu, F.; Debebe, Y.; Aklilu, M.; Tasew, G.; et al. Malaria vector feeding, peak biting time and resting place preference behaviors in line with Indoor based intervention tools and its implication: Scenario from selected sentinel sites of Ethiopia. Hellyon 2022, 8, e12178. [Google Scholar] [CrossRef] [PubMed]
- Kibret, S.; Alemu, Y.; Boelee, E.; Tekie, H.; Alemu, D.; Petros, B. The impact of a small-scale irrigation scheme on malaria transmission in Ziway area, Central Ethiopia. Trop. Med. Int. Health 2010, 15, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Msugupakulya, B.; Urio, N.; Jumanne, M.; Ngowo, H.; Selvaraj, P. Changes in contributions of different Anopheles vector species to malaria transmission in east and southern Africa from 2000 to 2022. Parasites Vectors 2023, 16, 408. [Google Scholar] [CrossRef] [PubMed]
- Abduselam, N.; Zeynudin, A.; Berens-Riha, N.; Seyoum, D.; Pritsch, M.; Tibebu, H.; Eba, K.; Hoelscher, M.; Wieser, A.; Yewhalaw, D. Similar trends of susceptibility in Anopheles arabiensis and Anopheles pharoensis to Plasmodium vivax infection in Ethiopia. Parasites Vectors 2016, 9, 552. [Google Scholar] [CrossRef]
- Ashine, T.; Eyasu, A.; Asmamaw, Y.; Simma, E.; Zemene, E.; Epstein, A.; Brown, R.; Negash, N.; Kochora, A.; Reynolds, A.M.; et al. Spatiotemporal distribution and bionomics of Anopheles stephensi in different eco—Epidemiological settings in Ethiopia. Parasites Vectors 2024, 17, 166. [Google Scholar] [CrossRef]
- Emiru, T.; Getachew, D.; Murphy, M.; Sedda, L.; Ejigu, L.A.; Bulto, M.G.; Byrne, I.; Demisse, M.; Abdo, M.; Chali, W.; et al. Evidence for a role of Anopheles stephensi in the spread of drug- and diagnosis-resistant malaria in Africa. Nat. Med. 2023, 29, 3203–3211. [Google Scholar] [CrossRef]
- de Santi, V.P.; Khaireh, B.A.; Chiniard, T.; Pradines, B.; Taudon, N.; Larréché, S.; Mohamed, A.B.; de Laval, F.; Berger, F.; Gala, F.; et al. Role of Anopheles stephensi Mosquitoes in Malaria Outbreak, Djibouti, 2019. Emerg. Infect. Dis. 2021, 27, 1697–1700. [Google Scholar] [CrossRef]


| Host | An. arabiensisn (%) | An. pharoensis n (%) | An. stephensi n (%) | An. coustani n (%) |
|---|---|---|---|---|
| Human | 35 (10.6) | 14 (16.5) | 1 (3.3) | 1 (10.0) |
| Human + cow | 18 (5.4) | 3 (3.5) | 1 (3.3) | 1 (10.0) |
| Human + cow + goat | 1 (0.3) | 2 (2.3) | - | - |
| Human + goat | 5 (1.5) | 2 (2.4) | - | - |
| Cow | 116 (35.0) | 12 (14.1) | 16 (53.3) | 3 (30.0) |
| Cow + goat | 19 (5.7) | 3 (3.5) | 1 (3.3) | - |
| Cow + goat + dog | 1 (0.3) | - | - | - |
| Dog | 1 (0.3) | - | - | - |
| Goat | 11 (3.3) | 13 (15.3) | 3 (10.0) | 2 (20.0) |
| Not detected | 124 (37.5) | 36 (42.4) | 8 (26.7) | 3 (30.0) |
| Total | 331 | 85 | 30 | 10 |
| Anopheles Species | Number Tested | Sporozoite Positive n (%) | Parasite Species | ||||
|---|---|---|---|---|---|---|---|
| Indoor Collection | Outdoor Collection | Plasmodium falciparum | Plasmodium vivax | ||||
| Indoors | Outdoors | Indoors | Outdoors | ||||
| An. arabiensis | 331 | 13 (4.0) | 13 (4.0) | 7 | 6 | 6 | 7 |
| An. pharoensis | 85 | 3 (3.5) | 1(1.2) | 1 | 0 | 2 | 1 |
| An. stephensi | 30 | 0 | 0 | 0 | 0 | 0 | 0 |
| An. coustani | 11 | 0 | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawaria, D.; Amanuel, T.; Anbesie, A.; Zhong, D.; Kibret, S.; Lee, M.-C.; Zhou, G.; Wang, C.; Chen, J.; Matewos, T.; et al. Elevated Plasmodium Sporozoite Infection Rates in Primary and Secondary Malaria Vectors in Anopheles stephensi-Infested Areas of Ethiopia. Insects 2025, 16, 462. https://doi.org/10.3390/insects16050462
Hawaria D, Amanuel T, Anbesie A, Zhong D, Kibret S, Lee M-C, Zhou G, Wang C, Chen J, Matewos T, et al. Elevated Plasmodium Sporozoite Infection Rates in Primary and Secondary Malaria Vectors in Anopheles stephensi-Infested Areas of Ethiopia. Insects. 2025; 16(5):462. https://doi.org/10.3390/insects16050462
Chicago/Turabian StyleHawaria, Dawit, Timotwos Amanuel, Abraham Anbesie, Daibin Zhong, Solomon Kibret, Ming-Chieh Lee, Guofa Zhou, Chloe Wang, Jiale Chen, Tafesse Matewos, and et al. 2025. "Elevated Plasmodium Sporozoite Infection Rates in Primary and Secondary Malaria Vectors in Anopheles stephensi-Infested Areas of Ethiopia" Insects 16, no. 5: 462. https://doi.org/10.3390/insects16050462
APA StyleHawaria, D., Amanuel, T., Anbesie, A., Zhong, D., Kibret, S., Lee, M.-C., Zhou, G., Wang, C., Chen, J., Matewos, T., Ejeso, A., Ayele, C., Yosef, T., Yewhalaw, D., & Yan, G. (2025). Elevated Plasmodium Sporozoite Infection Rates in Primary and Secondary Malaria Vectors in Anopheles stephensi-Infested Areas of Ethiopia. Insects, 16(5), 462. https://doi.org/10.3390/insects16050462







