Acute Hemodynamic, Metabolic, and Hormonal Responses to a Boxing Exergame with and without Blood Flow Restriction in Non-Athlete Young Individuals
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Study Design and Exercise Protocol
2.3. Measurement of HRV
2.4. Sampling of Glucose, Lactate, Growth Hormone, and Serum Normetanephrine
2.5. Statistical Analyses
3. Results
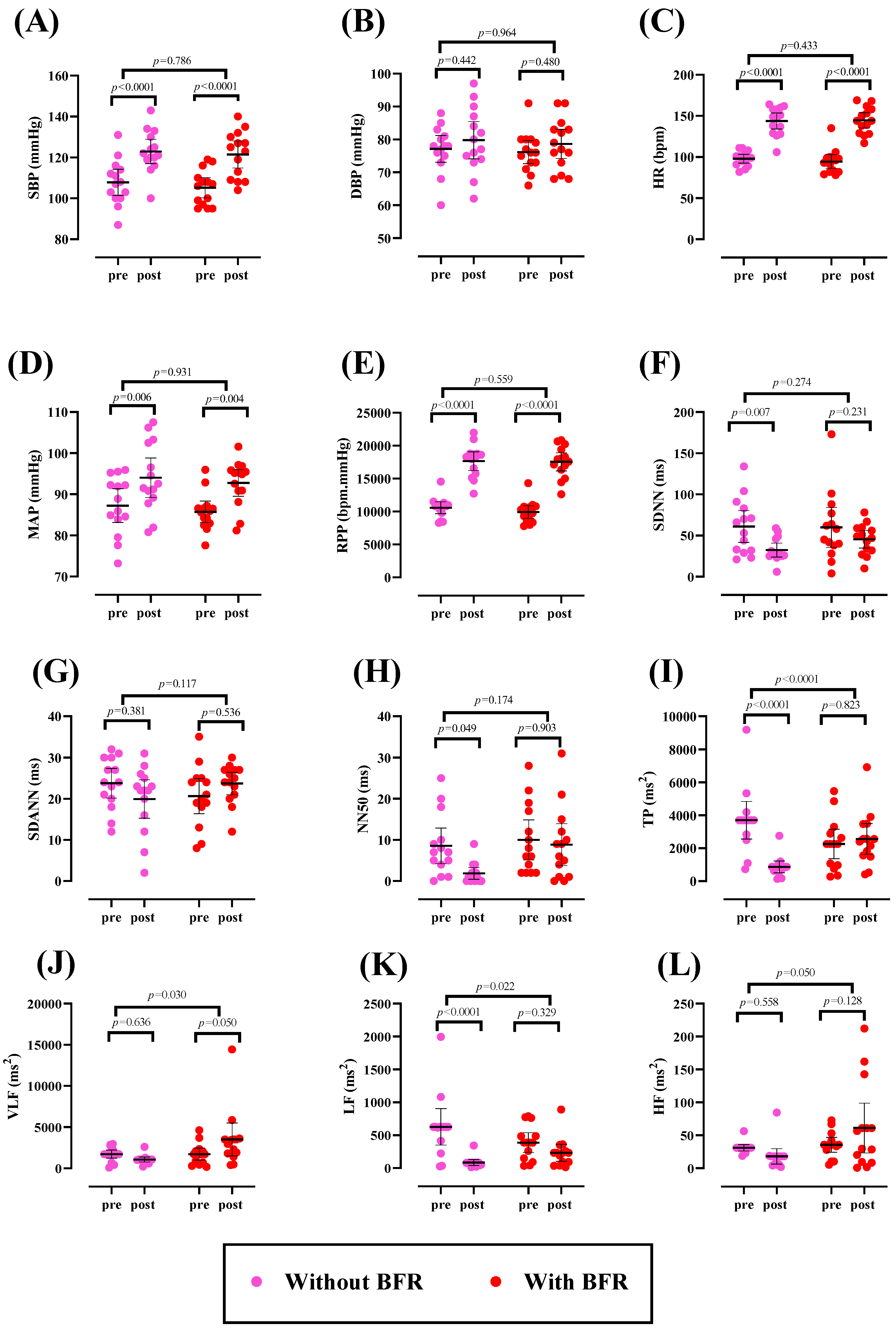
3.1. Hemodynamic and HRV Parameters
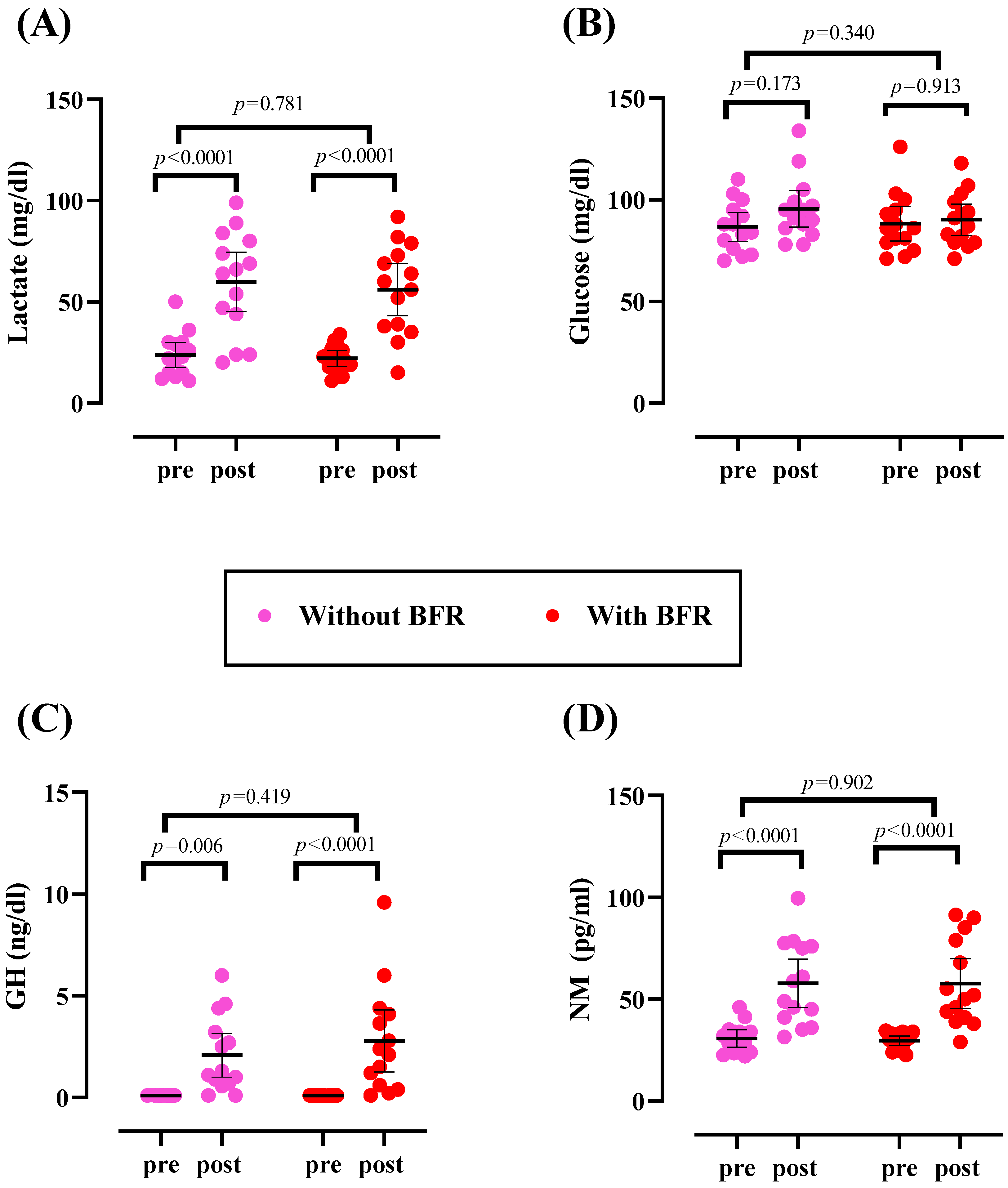
3.2. Metabolic Parameters
3.3. Hormones
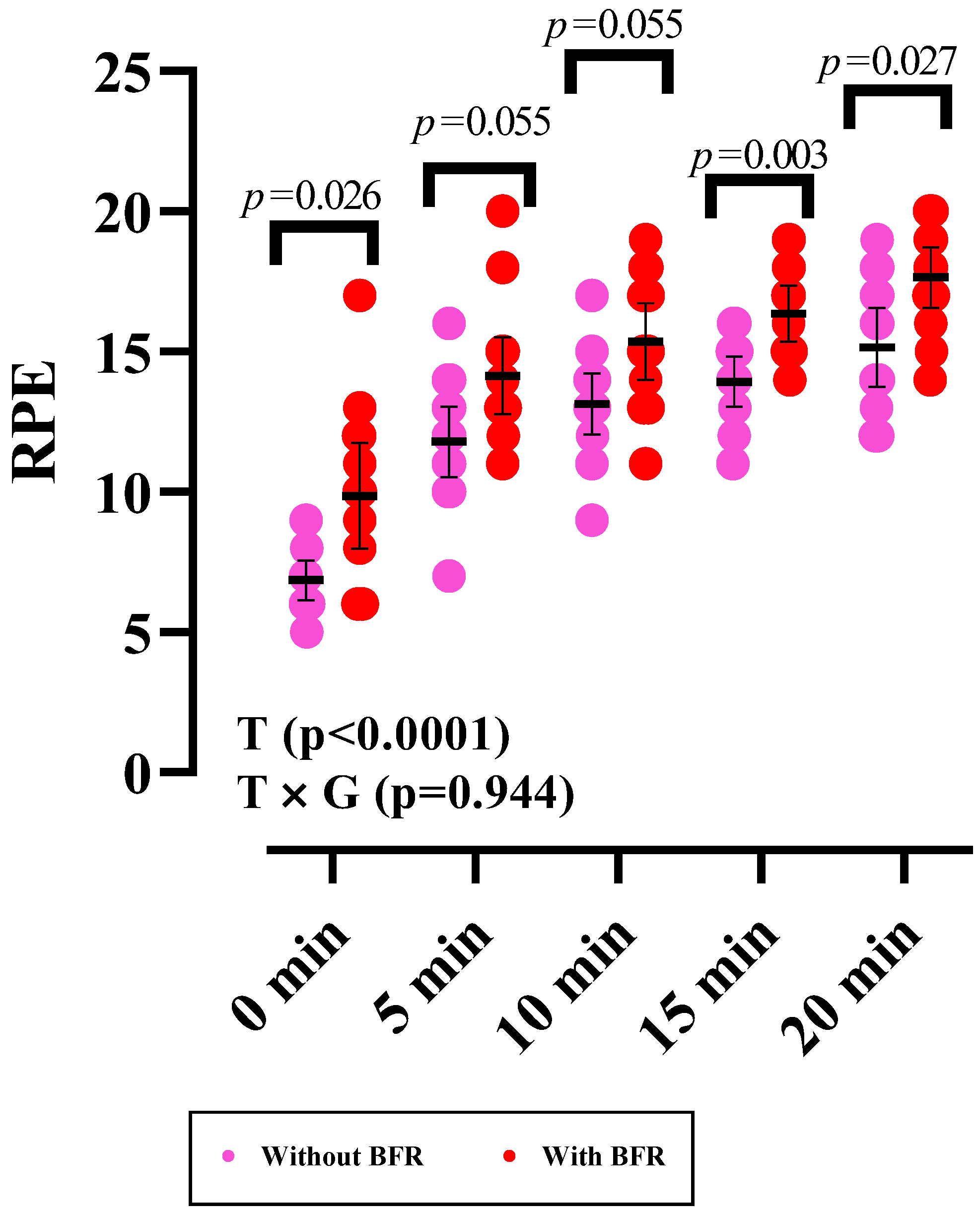
3.4. RPE
4. Discussion
5. Conclusions
6. Future Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | 5′AMP-activated protein kinase | BFR | Blood flow restriction |
| BP | Blood pressure | CLIA | Chemiluminescence technology |
| DBP | Diastolic blood pressure | GLUT4 | Glucose transporter |
| GH | Growth hormone | HF | High frequency |
| HR | Heart rate | HRV | Heart rate variability |
| LF | Low frequency | LC-MS/MS | Liquid chromatography–mass spectrometry |
| MAP | Mean arterial pressure | MPAK | Mitogen-activated protein kinase |
| mTOR | Mechanical target of rapamycin | NM | Normetanephrine |
| NN50 | Number of pairs of adjacent NN intervals differing by more than 50 ms | PAR-Q PKC | Physical Activity Readiness Questionnaire Protein kinase C |
| RPE | Rating of perceived exertion | RPP | Rate pressure product |
| SBP | Systolic blood pressure | SDANN | Standard deviation of the average NN intervals |
| SDNN | Standard deviation of NN intervals | SNA | Sympathetic nerve activity |
| TP | Total power | VLF | Very low frequency |
References
- Haskell, W.L.; Lee, I.M.; Pate, R.R.; Powell, K.E.; Blair, S.N.; Franklin, B.A.; Macera, C.A.; Heath, G.W.; Thompson, P.D.; Bauman, A. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc. 2007, 39, 1423–1434. [Google Scholar] [CrossRef]
- Cortis, C.; Giancotti, G.; Rodio, A.; Bianco, A.; Fusco, A. Home is the new gym: Exergame as a potential tool to maintain adequate fitness levels also during quarantine. Hum. Mov. 2020, 21, 79–87. [Google Scholar] [CrossRef]
- Ambrosino, P.; Fuschillo, S.; Papa, A.; Di Minno, M.N.D.; Maniscalco, M. Exergaming as a Supportive Tool for Home-Based Rehabilitation in the COVID-19 Pandemic Era; Mary Ann Liebert, Inc.: Larchmont, NY, USA, 2020; Volume 9, pp. 311–313. [Google Scholar]
- Sween, J.; Wallington, S.F.; Sheppard, V.; Taylor, T.; Llanos, A.A.; Adams-Campbell, L.L. The role of exergaming in improving physical activity: A review. J. Phys. Act. Health 2014, 11, 864–870. [Google Scholar] [CrossRef]
- Wu, P.-T.; Wu, W.-L.; Chu, I.-H. Energy expenditure and intensity in healthy young adults during exergaming. Am. J. Health Behav. 2015, 39, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Amani-Shalamzari, S.; Rajabi, S.; Rajabi, H.; Gahreman, D.E.; Paton, C.; Bayati, M.; Rosemann, T.; Nikolaidis, P.T.; Knechtle, B. Effects of blood flow restriction and exercise intensity on aerobic, anaerobic, and muscle strength adaptations in physically active collegiate women. Front. Physiol. 2019, 10, 810. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.G.; Pereira Neto, E.A.; Pfeiffer, P.A.S.; Neto, G.R.; Rodrigues, A.S.; Bemben, M.G.; Patterson, S.D.; Batista, G.R.; Cirilo-Sousa, M.S. Acute and chronic responses of aerobic exercise with blood flow restriction: A systematic review. Front. Physiol. 2019, 10, 1239. [Google Scholar] [CrossRef] [PubMed]
- Davids, C.J.; Roberts, L.A.; Bjørnsen, T.; Peake, J.M.; Coombes, J.S.; Raastad, T. Where Does Blood Flow Restriction Fit in the Toolbox of Athletic Development? A Narrative Review of the Proposed Mechanisms and Potential Applications. Sports Med. 2023, 53, 2077–2093. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.R.; Loenneke, J.P.; Slattery, K.M.; Dascombe, B.J. Exercise with blood flow restriction: An updated evidence-based approach for enhanced muscular development. Sports Med. 2015, 45, 313–325. [Google Scholar] [CrossRef]
- Chua, M.T.; Sim, A.; Burns, S.F. Acute and Chronic Effects of Blood Flow Restricted High-Intensity Interval Training: A Systematic Review. Sports Med.-Open 2022, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Gobel, F.L.; Norstrom, L.; Nelson, R.R.; Jorgensen, C.R.; Wang, Y. The rate-pressure product as an index of myocardial oxygen consumption during exercise in patients with angina pectoris. Circulation 1978, 57, 549–556. [Google Scholar] [CrossRef]
- Mayo, J.J.; Kravitz, L. A review of the acute cardiovascular responses to resistance exercise of healthy young and older adults. J. Strength. Cond. Res. 1999, 13, 90–96. [Google Scholar]
- Cardona, J.E.M.; Gouveia, E.; Cameirao, M.S.; i Badia, S.B. Heart Rate Variability in Exergaming-Feasibility and Benefits of Physiological Adaptation for Cardiorespiratory Training in Older Adults by Means of Smartwatches. icSPORTS 2017, 145–150. [Google Scholar]
- Pecanha, T.; Paula-Ribeiro, M.D.; Nasario-Junior, O.; Lima, J.R.P.D. Post-exercise heart rate variability recovery: A time-frequency analysis. Acta Cardiol. 2013, 68, 607–613. [Google Scholar] [CrossRef]
- Khajehlandi, M.; Janbozorgi, M. Comparison of the effect of one session of resistance training with and without blood-flow restriction of arm on changes in serum levels of growth hormone and lactate in athlete females. KAUMS J. 2018, 22, 318–324. [Google Scholar]
- Hatami, M.; Nikooie, R.; Enhesari, A. Presentation of Lacto-Resistance Training Method and Comparing Its Effect on Muscle Hypertrophy with Traditional Resistance Training In Professional Bodybuilders. J. Appl. Exerc. Physiol. 2019, 15, 169–181. [Google Scholar]
- Ravasi, A.; Kazemi, F.; Rajab, A.; Radkani, M. Comparing the Effect of a Single-Session Aerobic and Resistance Exercise on Blood Glucose in Women with Type 2 Diabetes. SSU_J. 2012, 19, 775–783. [Google Scholar]
- Huang, C.-J.; Webb, H.E.; Zourdos, M.C.; Acevedo, E.O. Cardiovascular reactivity, stress, and physical activity. Front. Physiol. 2013, 4, 314. [Google Scholar] [CrossRef] [PubMed]
- Tokinoya, K.; Shishikura, Y.; Sekine, N.; Aoyagi, A.; Yoshida, Y.; Aita, Y.; Sugasawa, T.; Nabekura, Y.; Takekoshi, K. Plasma free metanephrine and normethanephrine levels correlated to plasma catecholamine after acute running in amateur runner. J. Exerc. Sci. Fit. 2021, 19, 178–181. [Google Scholar] [CrossRef]
- Pussard, E.; Chaouch, A.; Said, T. Plasma metanephrines responses to adreno-sympathetic stress. Am. J. Physiol. Biochem. Pharmacol. 2014, 3, 154. [Google Scholar] [CrossRef]
- Akbarnejad, A.; Yari, M.; Mohamadi, M.; Rajabi, A. Comparison of the Low-Intensity Resistance Exercise with Blood Flow Restriction and High Intensity Resistance Exercise on Serum Levels of VEGF-A In Adolescent Athletes. J. Appl. Exerc. Physiol. 2018, 14, 99–110. [Google Scholar] [CrossRef]
- Warburton, D.; Jamnik, V.; Bredin, S.; Shephard, R.; Gledhill, N. The 2021 Physical Activity Readiness Questionnaire for Everyone (PAR-Q+) and electronic Physical Activity Readiness Medical Examination (ePARmed-X+): 2021 PAR-Q+. Health Fit. J. Can. 2021, 14, 83–87. [Google Scholar] [CrossRef]
- Dorneles, G.P.; Colato, A.S.; Galvão, S.L.; Ramis, T.R.; Ribeiro, J.L.; Romão, P.R.; Peres, A. Acute response of peripheral CC r5 chemoreceptor and NK cells in individuals submitted to a single session of low-intensity strength exercise with blood flow restriction. Clin. Physiol. Funct. Imaging 2016, 36, 311–317. [Google Scholar] [CrossRef]
- DeMers, D.; Wachs, D. Physiology, Mean Arterial Pressure; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Makivić, B.; Nikić Djordjević, M.; Willis, M.S. Heart Rate Variability (HRV) as a tool for diagnostic and monitoring performance in sport and physical activities. J. Exerc. Physiol. Online 2013, 16, 103–131. [Google Scholar]
- Electrophysiology, Task Force of the European Society of Cardiology the North American Society of Pacing. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Moghadam, B.H.; Bagheri, R.; Roozbeh, B.; Ashtary-Larky, D.; Gaeini, A.A.; Dutheil, F.; Wong, A. Impact of saffron (Crocus Sativus Linn) supplementation and resistance training on markers implicated in depression and happiness levels in untrained young males. Physiol. Behav. 2021, 233, 113352. [Google Scholar] [CrossRef]
- Ochi, G.; Kuwamizu, R.; Fujimoto, T.; Ikarashi, K.; Yamashiro, K.; Sato, D. The Effects of Acute Virtual Reality Exergaming on Mood and Executive Function: Exploratory Crossover Trial. JMIR Serious Games 2022, 10, e38200. [Google Scholar] [CrossRef]
- Viana, R.B.; de Lira, C.A.B. Exergames as Coping Strategies for Anxiety Disorders During the COVID-19 Quarantine Period. Games Health J. 2020, 9, 147–149. [Google Scholar] [CrossRef]
- Vila-Chã, C.; Moyano-Cuevas, J.; Esteban, F.; Serra, N.; Vaz, C.; Pagador, J.B. Hemodynamic responses in active older adults to an acute Exergame session based on a grape harvesting context. In Proceedings of the International Association of Gerontology and Geriatrics European Region Congress, Gothenburg, Sweden, 23–25 May 2019. [Google Scholar] [CrossRef]
- Danese, E.; Tarperi, C.; Salvagno, G.L.; Guzzo, A.; Sanchis-Gomar, F.; Festa, L.; Bertinato, L.; Montagnana, M.; Schena, F.; Lippi, G. Sympatho-adrenergic activation by endurance exercise: Effect on metanephrines spillover and its role in predicting athlete’s performance. Oncotarget 2018, 9, 15650–15657. [Google Scholar] [CrossRef]
- Schranner, D.; Kastenmüller, G.; Schönfelder, M.; Römisch-Margl, W.; Wackerhage, H. Metabolite concentration changes in humans after a bout of exercise: A systematic review of exercise metabolomics studies. Sports Med.-Open 2020, 6, 11. [Google Scholar] [CrossRef]
- Fisher, J.P.; Young, C.N.; Fadel, P.J. Autonomic Adjustments to Exercise in Humans. Compr. Physiol. 2015, 5, 475–512. [Google Scholar] [CrossRef]
- May, A.K.; Brandner, C.R.; Warmington, S.A. Hemodynamic responses are reduced with aerobic compared with resistance blood flow restriction exercise. Physiol. Rep. 2017, 5, e13142. [Google Scholar] [CrossRef]
- Schamne, J.C.; Ferreira Junior, A.; Araújo, A.C.D.; Lima-Silva, A.E.; Bertuzzi, R.C.d.M.; Okuno, N.M. Cardiac autonomic responses during and after a single session of aerobic exercise with and without blood flow restriction. Mot. Rev. Educ. Física 2019, 25. [Google Scholar] [CrossRef]
- Michael, S.; Graham, K.S.; Davis, G.M. Cardiac Autonomic Responses during Exercise and Post-exercise Recovery Using Heart Rate Variability and Systolic Time Intervals—A Review. Front. Physiol. 2017, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Hunt, K.J.; Saengsuwan, J. Changes in heart rate variability with respect to exercise intensity and time during treadmill running. Biomed. Eng. Online 2018, 17, 128. [Google Scholar] [CrossRef]
- Kloter, E.; Barrueto, K.; Klein, S.D.; Scholkmann, F.; Wolf, U. Heart Rate Variability as a Prognostic Factor for Cancer Survival—A Systematic Review. Front. Physiol. 2018, 9, 623. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.L.; Fernhall, B. Advanced Cardiovascular Exercise Physiology; Human Kinetics: Champaign, IL, USA, 2022. [Google Scholar]
- Berntson, G.; Bigger, J.; Eckberg, D.; Grossman, P.; Kaufmann, P.; Malik, M.; Nagaraja, H.; Porges, S.; Saul, P.; Stone, P.; et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology 1997, 34, 623–648. [Google Scholar] [CrossRef]
- Iranpour, A.; Bolboli, L. Evaluation of Heart Rate Fluctuations with Two Frequency and Time Domain Methods Following Aerobic Training in Academic Active Men. Sci. J. Nurs. Midwifery Paramed. Fac. 2019, 4, 30–45. [Google Scholar]
- Bernardi, L.; Valle, F.; Coco, M.; Calciati, A.; Sleight, P. Physical activity influences heart rate variability and very-low-frequency components in Holter electrocardiograms. Cardiovasc. Res. 1996, 32, 234–237. [Google Scholar] [CrossRef]
- Kleiger, R.E.; Miller, J.P.; Bigger, J.T., Jr.; Moss, A.J. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am. J. Cardiol. 1987, 59, 256–262. [Google Scholar] [CrossRef]
- O’Donohoe, P.B.; Pandit, J.J. Neurological and humoral control of blood pressure. Anaesth. Intensive Care Med. 2016, 17, 253–257. [Google Scholar] [CrossRef]
- Freitas, E.D.S.; Karabulut, M.; Bemben, M.G. The Evolution of Blood Flow Restricted Exercise. Front. Physiol. 2021, 12, 2179. [Google Scholar] [CrossRef]
- Ferguson, B.S.; Rogatzki, M.J.; Goodwin, M.L.; Kane, D.A.; Rightmire, Z.; Gladden, L.B. Lactate metabolism: Historical context, prior misinterpretations, and current understanding. Eur. J. Appl. Physiol. 2018, 118, 691–728. [Google Scholar] [CrossRef]
- Miller, B.C.; Tirko, A.W.; Shipe, J.M.; Sumeriski, O.R.; Moran, K. The Systemic Effects of Blood Flow Restriction Training: A Systematic Review. Int. J. Sports Phys. Ther. 2021, 16, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Okita, K.; Takada, S.; Morita, N.; Takahashi, M.; Hirabayashi, K.; Yokota, T.; Kinugawa, S. Resistance training with interval blood flow restriction effectively enhances intramuscular metabolic stress with less ischemic duration and discomfort. Appl. Physiol. Nutr. Metab. 2019, 44, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Corvino, R.B.; Rossiter, H.B.; Loch, T.; Martins, J.C.; Caputo, F. Physiological responses to interval endurance exercise at different levels of blood flow restriction. Eur. J. Appl. Physiol. 2017, 117, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.J.; Scott, B.R.; Peiffer, J.J. Acute physiological responses to low-intensity blood flow restriction cycling. J. Sci. Med. Sport 2018, 21, 969–974. [Google Scholar] [CrossRef]
- Lockhart, C.; Scott, B.R.; Thoseby, B.; Dascombe, B.J. Acute Effects of Interset Rest Duration on Physiological and Perceptual Responses to Resistance Exercise in Hypoxia. J. Strength. Cond. Res. 2020, 34, 2241–2249. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, B.; Barjaste, A.; Rahmani-nia, F. The effect of aerobic exercise with and without blood flow restriction on lactate, cortisol and PGC-1α response in human skeletal muscle. Metab. Exerc. 2019, 9, 27–37. [Google Scholar] [CrossRef]
- Kubera, B.; Hubold, C.; Otte, S.; Lindenberg, A.-S.; Zeiß, I.; Krause, R.; Steinkamp, M.; Klement, J.; Entringer, S.; Pellerin, L. Rise in plasma lactate concentrations with psychosocial stress: A possible sign of cerebral energy demand. Obes. Facts 2012, 5, 384–392. [Google Scholar] [CrossRef]
- Baiamonte, B.A.; Kraemer, R.R.; Chabreck, C.N.; Reynolds, M.L.; McCaleb, K.M.; Shaheen, G.L.; Hollander, D.B. Exercise-induced hypoalgesia: Pain tolerance, preference and tolerance for exercise intensity, and physiological correlates following dynamic circuit resistance exercise. J. Sports Sci. 2017, 35, 1831–1837. [Google Scholar] [CrossRef]
- Eliakim, M.; Bodner, E.; Eliakim, A.; Nemet, D.; Meckel, Y. Effect of motivational music on lactate levels during recovery from intense exercise. J. Strength. Cond. Res. 2012, 26, 80–86. [Google Scholar] [CrossRef]
- Trefts, E.; Williams, A.S.; Wasserman, D.H. Chapter Nine—Exercise and the Regulation of Hepatic Metabolism. In Progress in Molecular Biology and Translational Science; Bouchard, C., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 135, pp. 203–225. [Google Scholar]
- Saatmann, N.; Zaharia, O.-P.; Loenneke, J.P.; Roden, M.; Pesta, D.H. Effects of Blood Flow Restriction Exercise and Possible Applications in Type 2 Diabetes. Trends Endocrinol. Metab. 2021, 32, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Gregg, L.D.; Kim, L.; Sherk, V.D.; Bemben, M.G.; Bemben, D.A. Hormone responses to an acute bout of low intensity blood flow restricted resistance exercise in college-aged females. J. Sports Sci. Med. 2014, 13, 91–96. [Google Scholar] [PubMed]
- Ozaki, H.; Kakigi, R.; Kobayashi, H.; Loenneke, J.P.; Abe, T.; Naito, H. Effects of walking combined with restricted leg blood flow on mTOR and MAPK signalling in young men. Acta Physiol. 2014, 211, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Raymond, C. High Intensity Strength Training in Conjunction with Vascular Occlusion. Master’s Thesis, Texas State University-San Marcos, San Marcos, TX, USA, 2013. [Google Scholar]
- Wideman, L.; Weltman, J.Y.; Hartman, M.L.; Veldhuis, J.D.; Weltman, A. Growth hormone release during acute and chronic aerobic and resistance exercise: Recent findings. Sports Med. 2002, 32, 987–1004. [Google Scholar] [CrossRef]
| Variable | With BFR | Without BFR |
|---|---|---|
| Measure | ||
| Anthropometry | ||
| Mean ± SD | ||
| Age (y) | 29.93 ± 7.04 | |
| Body mass (kg) | 63.30 ± 14.04 | |
| Stature (cm) | 171.30 ± 8.53 | |
| BMI (kg.m−2) | 22.40 ± 3.469 | |
| Hemodynamic and HRV | ||
| SBP (mmHg) | 105.2 ± 8.58 | 107.7 ± 11.0 |
| DBP (mmHg) | 76.14 ± 6.04 | 77.14 ± 7.01 |
| HR (bpm) | 92.00 ± 9.29 | 98 ± 9.11 |
| MAP (mmHg) | 85.73 ± 4.52 | 87.25 ± 7.06 |
| RPP (bpm.mmHg) | 9666.1 ± 1120 | 10,576 ± 1588.2 |
| SDNN (ms) | 59.8 ± 41.9 | 61 ± 33.7 |
| SDANN (ms) | 20.6 ± 7.65 | 23.7 ± 6.50 |
| NN50 (ms) | 7.27 ± 6.16 | 7.17 ± 6.30 |
| TP (ms2) | 2253.3 ± 1559.7 | 3149.34 ± 835.12 |
| VLF (ms2) | 1721.6 ± 1264.2 | 1717.3 ± 855.73 |
| LF (ms2) | 388.03 ± 259.02 | 500.17 ± 230.94 |
| HF (ms2) | 35.80 ± 24.9 | 31.1 ± 13.2 |
| Metabolic parameters | ||
| Lactate (mg/dL) | 22.14 ± 6.75 | 23.79 ± 10.85 |
| Glucose (mg/dL) | 88.29 ± 14.64 | 86.71 ± 12.24 |
| Hormones | ||
| GH (ng/dL) | 0.104 ± 0.01 | 0.107 ± 0.01 |
| NM (pg/mL) | 29.59 ± 4.02 | 30.68 ± 7.29 |
| RPE | ||
| 0 min | 9.86 ± 3.27 | 6.86 ± 1.23 |
| 5 min | 14.14 ± 2.38 | 11.79 ± 2.19 |
| 10 min | 15.36 ± 2.37 | 13.14 ± 1.87 |
| 15 min | 16.36 ± 1.73 | 13.93 ± 1.54 |
| 20 min | 17.64 ± 1.86 | 15.14 ± 2.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karimi, Z.; Mousavi, Z.; Nordvall, M.; Wong, A.; Bagheri, R.; Dutheil, F. Acute Hemodynamic, Metabolic, and Hormonal Responses to a Boxing Exergame with and without Blood Flow Restriction in Non-Athlete Young Individuals. Sports 2024, 12, 68. https://doi.org/10.3390/sports12030068
Karimi Z, Mousavi Z, Nordvall M, Wong A, Bagheri R, Dutheil F. Acute Hemodynamic, Metabolic, and Hormonal Responses to a Boxing Exergame with and without Blood Flow Restriction in Non-Athlete Young Individuals. Sports. 2024; 12(3):68. https://doi.org/10.3390/sports12030068
Chicago/Turabian StyleKarimi, Zohreh, Zeynabalsadat Mousavi, Michael Nordvall, Alexei Wong, Reza Bagheri, and Frederic Dutheil. 2024. "Acute Hemodynamic, Metabolic, and Hormonal Responses to a Boxing Exergame with and without Blood Flow Restriction in Non-Athlete Young Individuals" Sports 12, no. 3: 68. https://doi.org/10.3390/sports12030068
APA StyleKarimi, Z., Mousavi, Z., Nordvall, M., Wong, A., Bagheri, R., & Dutheil, F. (2024). Acute Hemodynamic, Metabolic, and Hormonal Responses to a Boxing Exergame with and without Blood Flow Restriction in Non-Athlete Young Individuals. Sports, 12(3), 68. https://doi.org/10.3390/sports12030068








