The Influence of Alloy Composition and Liquid Phase on Foaming of Al–Si–Mg Alloys
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Laboratory X-Ray Radioscopy and Tomography
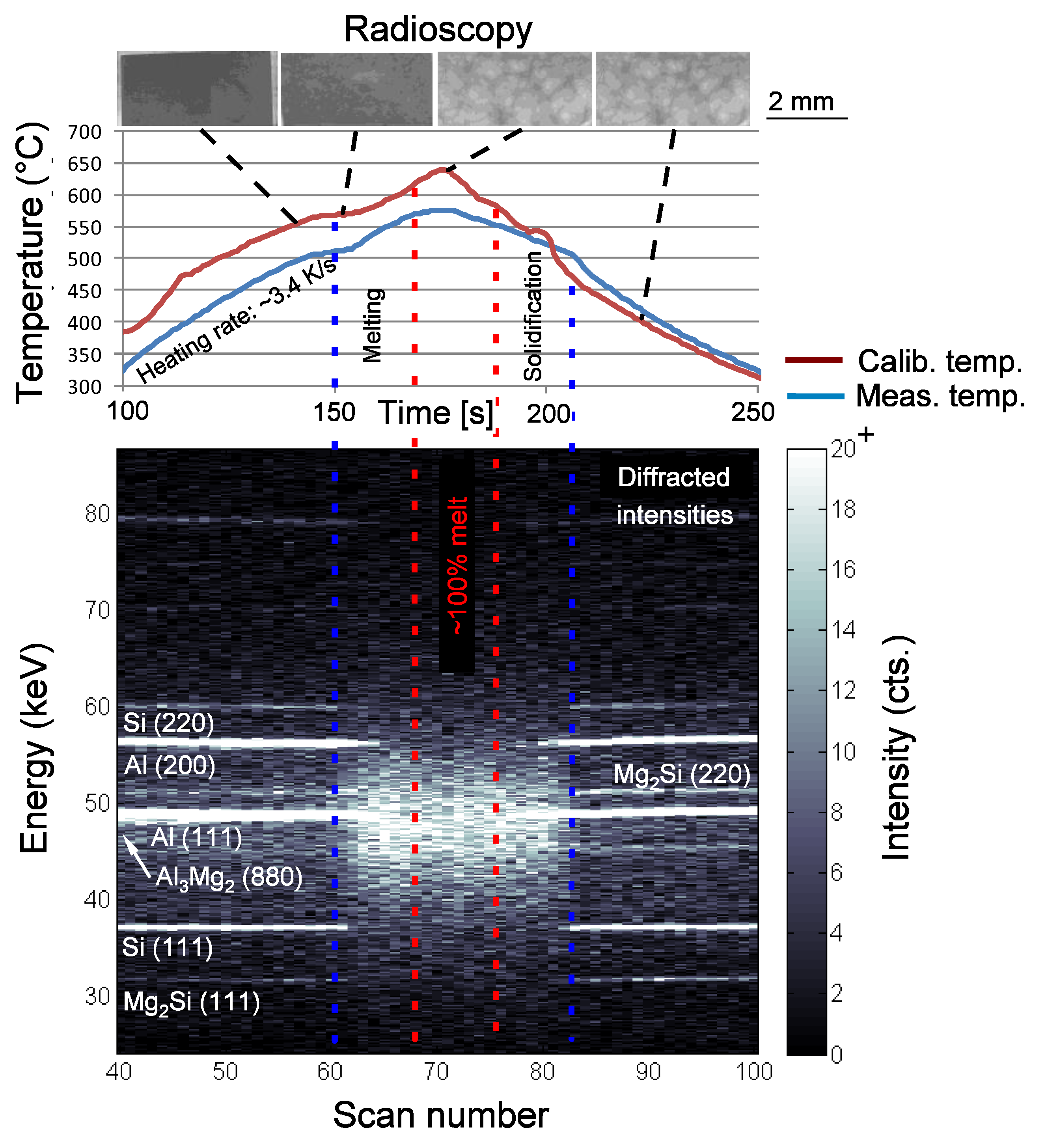
2.3. Synchrotron X-Ray Radioscopy and Diffraction
2.4. Synchrotron X-Ray Tomoscopy
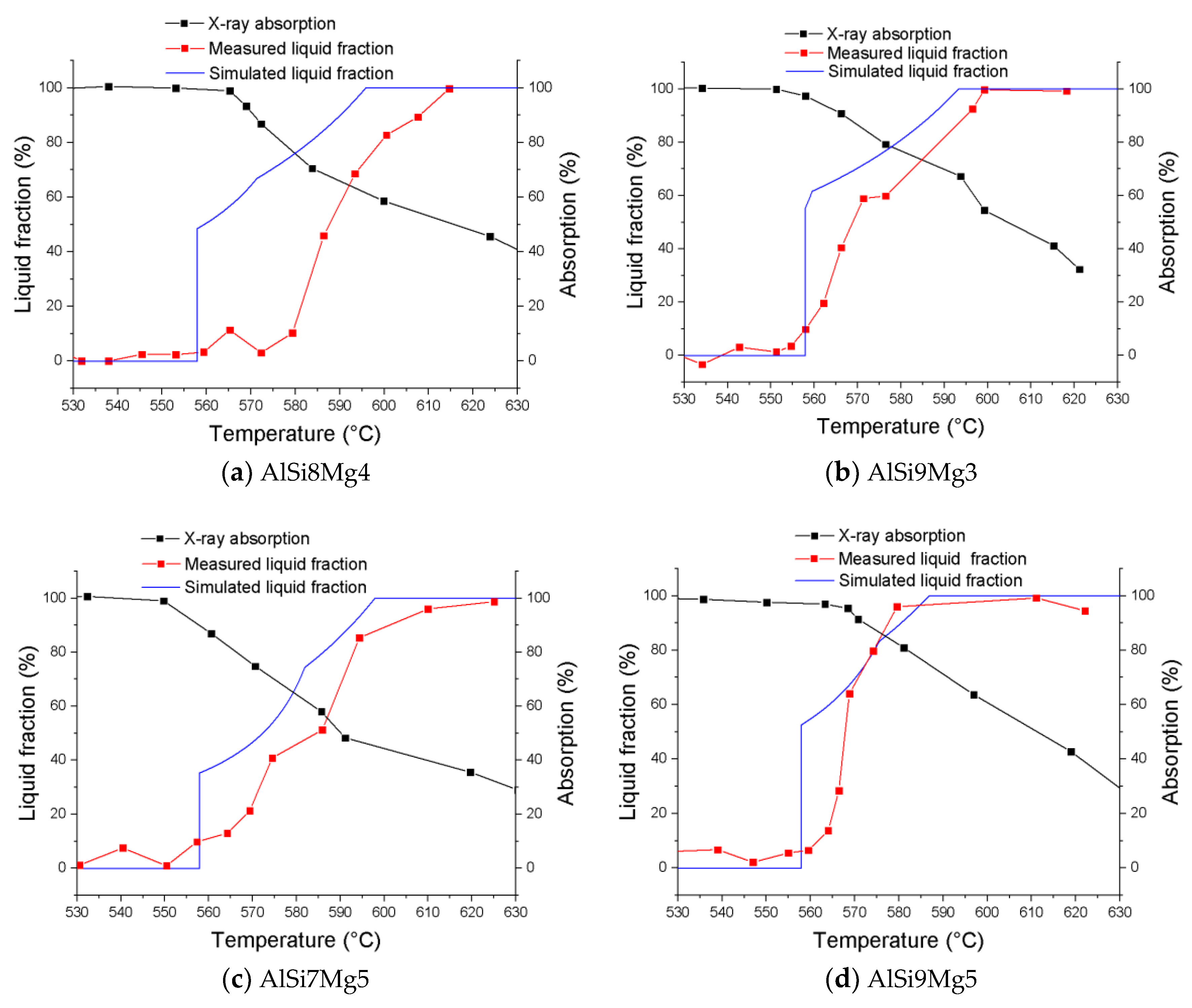
2.5. Calculation of the Liquid Fraction
3. Results
3.1. Gas Nucleation and Bubble Evolution
3.2. Expansion Kinetics
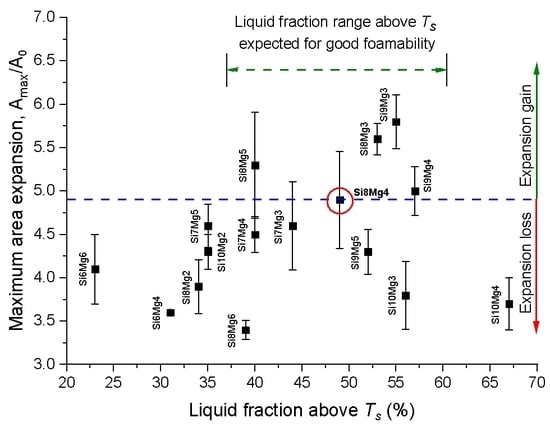
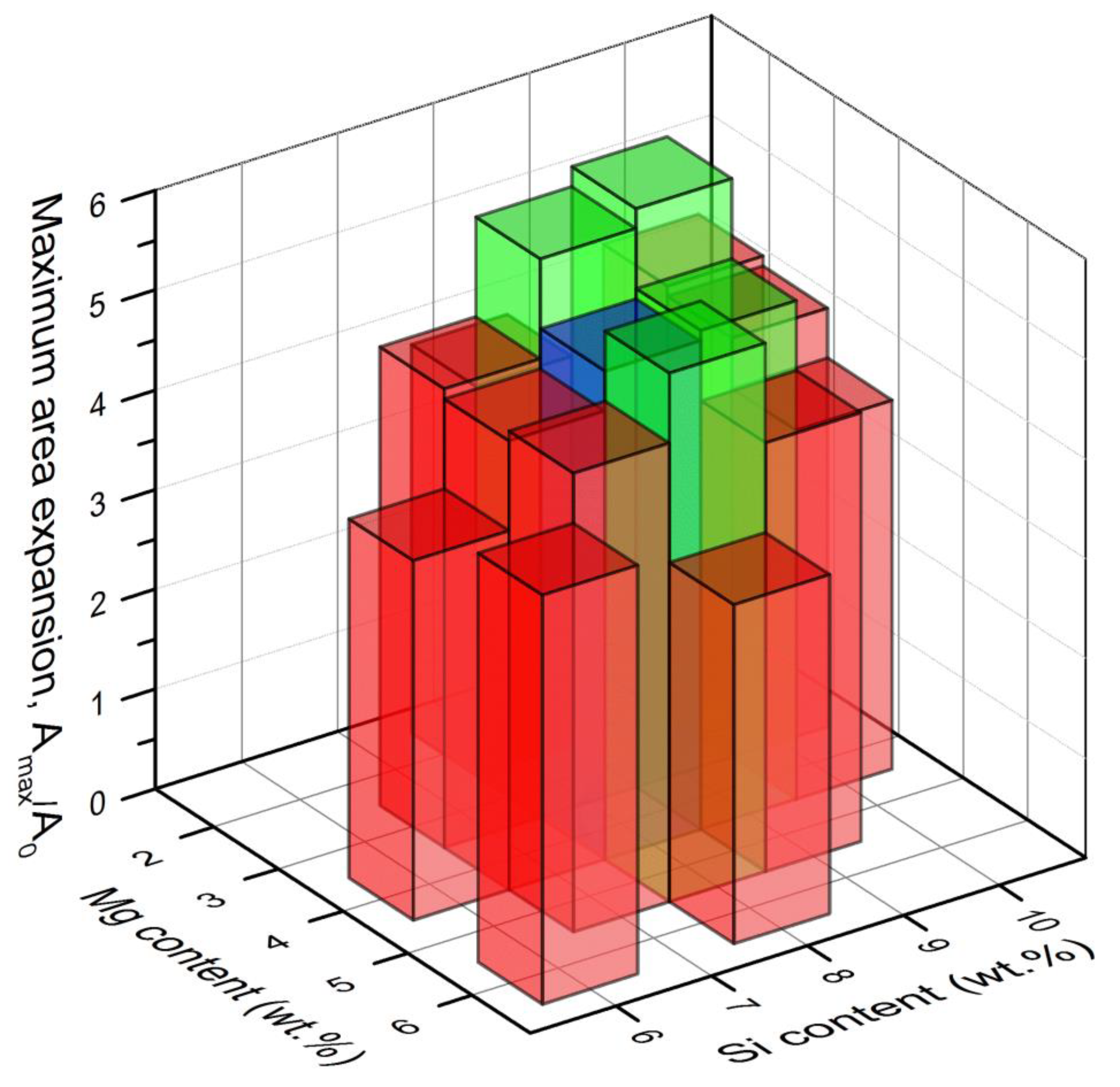
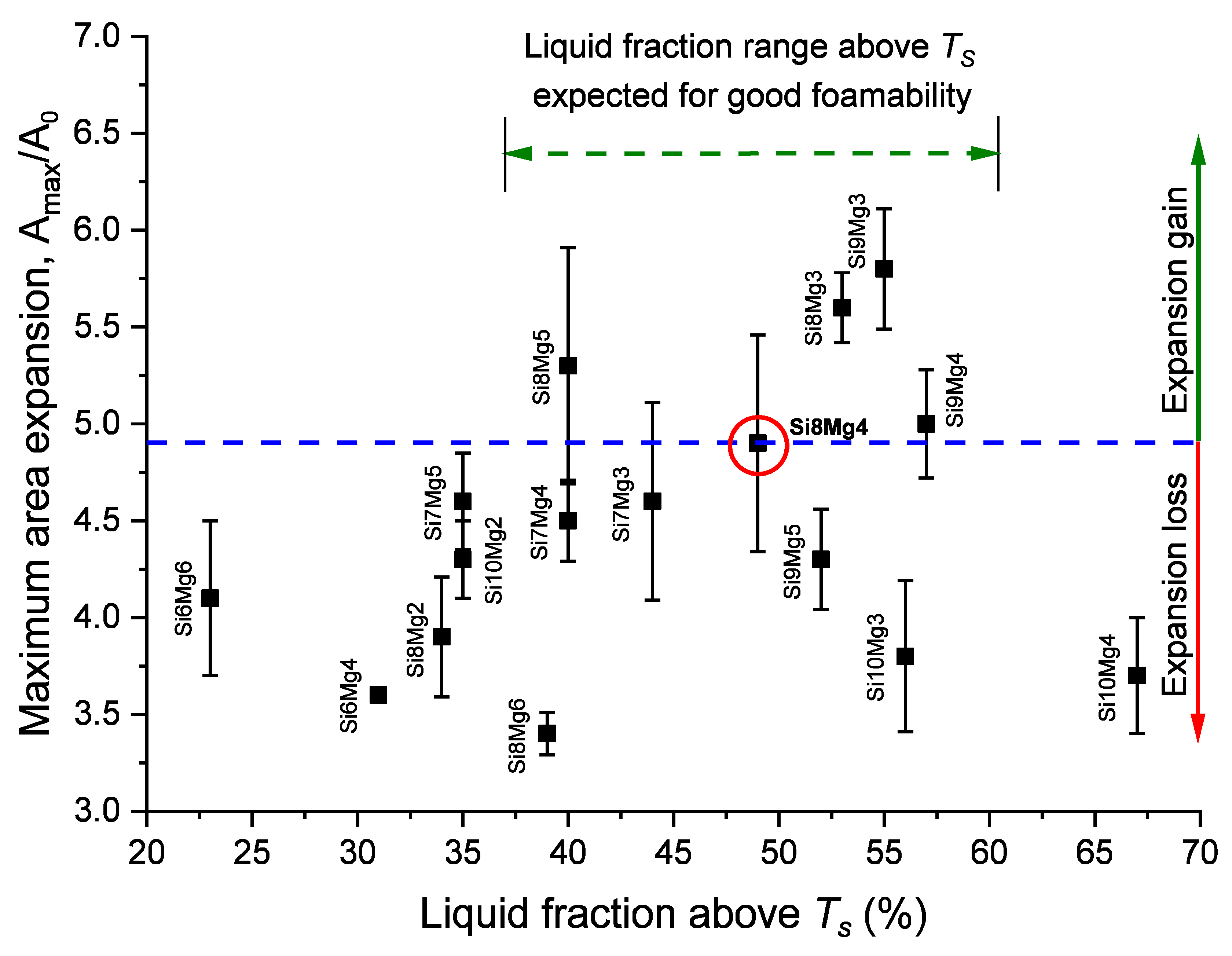
3.3. Maximum Area Expansion
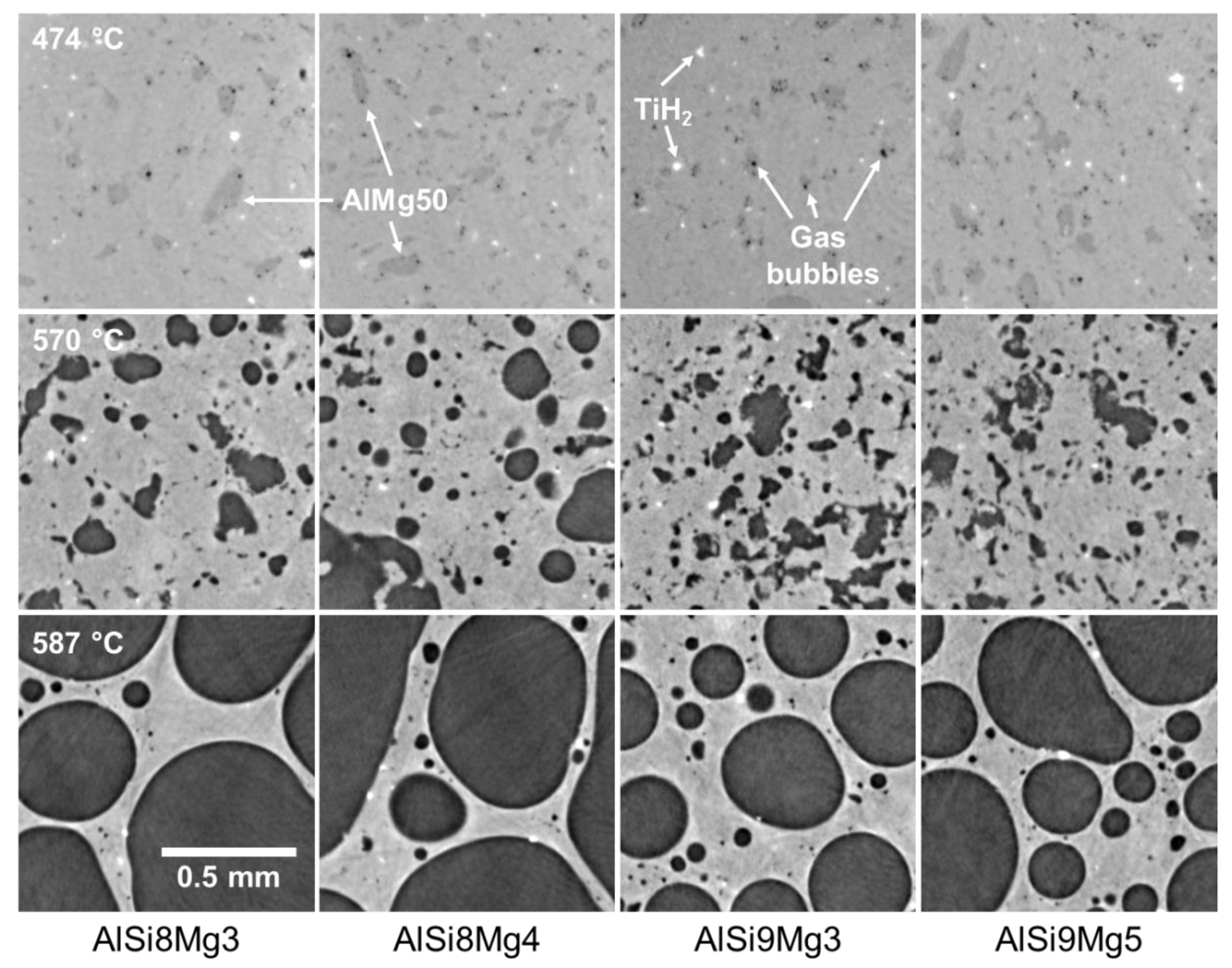
3.4. Morphology
3.5. Liquid Phase Evolution
4. Discussion
5. Conclusions
- An optimisation of alloy composition in the AlSiXMgY system for foaming was performed for compositions around the reference AlSi8Mg4.
- A tendency to higher expansions was observed for higher Si and lower Mg contents than in the reference system.
- A correlation between increased liquid fraction and high foam expansion was found up to a preferred range for the liquid fraction of 40–60% just above TS. Even higher liquid fractions had an adverse effect on foamability.
- AlSi9Mg3 shows the highest expansion of all alloys with an acceptable foam structure, while AlSi9Mg5 has the best pore structure with an acceptable expansion. Therefore 4% Mg appears as a good compromise. Within the “island of good foaming” around AlSi8Mg4 cited in the introduction, the best point might lie slightly on the Si-rich side.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B

Appendix C


Appendix D

References
- García-Moreno, F. Commercial applications of metal foams: Their properties and production. Materials 2016, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Banhart, J. Manufacturing routes for metallic foams. JOM 2000, 52, 22–27. [Google Scholar] [CrossRef]
- Körner, C.; Arnold, M.; Singer, R.F. Metal foam stabilization by oxide network particles. Mater. Sci. Eng. A 2005, 396, 28–40. [Google Scholar] [CrossRef]
- Rack, A.; Helwig, H.M.; Bütow, A.; Rueda, A.; Matijašević-Lux, B.; Helfen, L.; Goebbels, J.; Banhart, J. Early pore formation in aluminium foams studied by synchrotron-based microtomography and 3-D image analysis. Acta Mater. 2009, 57, 4809–4821. [Google Scholar] [CrossRef]
- Kamm, P.H. Der Schäumprozess von Aluminiumlegierungen: Tomoskopische Untersuchung der Gasnukleation. Ph.D. Thesis, Technische University Berlin, Berlin, Germany, August 2017. [Google Scholar]
- Neu, T.R. Einfluss der Kompaktierung auf das Schäumverhalten von pulvermetallurgisch hergestellten Metallhalbzeugen. Ph.D. Thesis, Technische Universität Berlin, Berlin, Germany, July 2018. [Google Scholar]
- Duarte, I.; Banhart, J. A study of aluminium foam formation-kinetics and microstructure. Acta Mater. 2000, 48, 2349–2362. [Google Scholar] [CrossRef]
- Weigand, P. Untersuchung der Einflussfaktoren auf die pulvermetallurgische Herstellung von Aluminiumschäumen. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, May 1999. [Google Scholar]
- Lázaro, J.; Solórzano, E.; Rodríguez-Pérez, M.A.; Kennedy, A.R. Effect of solidification rate on pore connectivity of aluminium foams and its consequences on mechanical properties. Mater. Sci. Eng. A 2016, 672, 236–246. [Google Scholar] [CrossRef]
- Simancik, F.; Behulova, K.; Bors, L. Effect of Ambient Atmosphere on the Foam Expansion, Cellular Metals and Metal Foaming Technology: Metfoam, Bremen, 2001; Banhart, J., Ashby, M.F., Eds.; MIT Publishing: Bremen, Germany, 2001; pp. 89–92. [Google Scholar]
- Banhart, J.; Baumeister, J. Deformation characteristics of metal foams. J. Mater. Sci. 1998, 33, 1431–1440. [Google Scholar] [CrossRef]
- Sahm, H. Untersuchung des Einflusses von Magnesium auf die Schäumbarkeit von Aluminiumlegierungen; Engineer Report; TU Bergakademie Freiberg: Freiberg, Germany, March 1994. [Google Scholar]
- Helwig, H.M.; Garcia-Moreno, F.; Banhart, J. A study of Mg and Cu additions on the foaming behaviour of Al-Si alloys. J. Mater. Sci. 2011, 46, 5227–5236. [Google Scholar] [CrossRef]
- Zhiqiang, G.; Donghui, M.; Xiaoguang, Y.; Xue, D. Effect of Mg addition on the foaming behaviour of AlSi7 based alloy prepared by powder metallurgy method. Rare Metal Mater. Eng. 2016, 45, 3068–3073. [Google Scholar] [CrossRef]
- Lehmhus, D.; Busse, M. Potential new matrix alloys for production of PM aluminium foams. Adv. Eng. Mater. 2004, 6, 391–396. [Google Scholar] [CrossRef]
- Helwig, H.M.; Banhart, J.; Seeliger, H.-W. Metal foams made of an aluminium alloy, use and manufacturing route. Patent EP 2 143 809, 14 September 2011. [Google Scholar]
- Pohltec Metalfoam. Available online: http://metalfoam.de/ (accessed on 18 December 2019).
- Nagelberg, A.S.; Antolin, S.; Urquhart, A.W. Formation of Al2O3/metal composites by the directed oxidation of molten aluminum-magnesium-silicon alloys: Part ii, growth kinetics. J. Am. Ceram. Soc. 1992, 75, 455–462. [Google Scholar] [CrossRef]
- Jiménez, C.; Garcia-Moreno, F.; Pfretzschner, B.; Klaus, M.; Wollgarten, M.; Zizak, I.; Schumacher, G.; Tovar, M.; Banhart, J. Decomposition of TiH2 studied in situ by synchrotron X-ray and neutron diffraction. Acta Mater. 2011, 59, 6318–6330. [Google Scholar] [CrossRef]
- Helwig, H.M.; Hiller, S.; Garcia-Moreno, F.; Banhart, J. Influence of compaction conditions on the foamability of AlSi8Mg4 alloy. Metall. Mater. Trans. B 2009, 40, 755–767. [Google Scholar] [CrossRef]
- Garcia-Moreno, F.; Fromme, M.; Banhart, J. Real-time x-ray radioscopy on metallic foams using a compact micro-focus source. Adv. Eng. Mater. 2004, 6, 416–420. [Google Scholar] [CrossRef]
- Genzel, C.; Denks, I.A.; Gibmeier, J.; Klaus, M.; Wagener, G. The materials science synchrotron beamline EDDI for energy-dispersive diffraction analysis. Nucl. Instrum. Methods Phys. Res. Sect. A 2007, 578, 23–33. [Google Scholar] [CrossRef]
- Jiménez, C.; Plaepow, M.; Kamm, P.H.; Neu, T.; Klaus, M.; Wagener, G.; Banhart, J.; Genzel, C.; García-Moreno, F. Simultaneous X-ray radioscopy/tomography and energy-dispersive diffraction applied to liquid aluminium alloy foams. J. Synchrotron Rad. 2018, 25, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Warren, B.E. X-Ray Diffraction; Dover: New York, NY, USA, 1990. [Google Scholar]
- Garcia-Moreno, F.; Solorzano, E.; Banhart, J. Kinetics of coalescence in liquid aluminium foams. Soft Matter 2011, 7, 9216–9223. [Google Scholar] [CrossRef]
- García-Moreno, F.; Kamm, P.H.; Neu, T.R.; Bülk, F.; Mokso, R.; Schlepütz, C.M.; Stampanoni, M.; Banhart, J. Using X-ray tomoscopy to explore the dynamics of foaming metal. Nat. Commun. 2019, 10, 3762. [Google Scholar] [CrossRef]
- Fife, J.L.; Rappaz, M.; Pistone, M.; Celcer, T.; Mikuljan, G.; Stampanoni, M. Development of a laser-based heating system for in situ synchrotron-based X-ray tomographic microscopy. J. Synchrotron Rad. 2012, 19, 352–358. [Google Scholar] [CrossRef]
- Mokso, R.; Schlepütz, C.M.; Theidel, G.; Billich, H.; Schmid, E.; Celcer, T.; Mikuljan, G.; Sala, L.; Marone, F.; Schlumpf, N.; et al. GigaFRoST: The gigabit fast readout system for tomography. J. Synchrotron Rad. 2017, 24, 1250–1259. [Google Scholar] [CrossRef]
- Buhrer, M.; Stampanoni, M.; Rochet, X.; Buchi, F.; Eller, J.; Marone, F. High-numerical-aperture macroscope optics for time-resolved experiments. J. Synchrotron Rad. 2019, 26, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Paganin, D.; Mayo, S.C.; Gureyev, T.E.; Miller, P.R.; Wilkins, S.W. Simultaneous phase and amplitude extraction from a single defocused image of a homogeneous object. J. Microsc. 2002, 206, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Marone, F.; Stampanoni, M. Regridding reconstruction algorithm for real-time tomographic imaging. J. Synchrotron Rad. 2012, 19, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.O.; Helander, T.; Höglund, L.; Shi, P.; Sundman, B. Thermo-Calc & DICTRA, computational tools for materials science. Calphad 2002, 26, 273–312. [Google Scholar]
- Schaffnit, P.; Stallybrass, C.; Konrad, J.; Stein, F.; Weinberg, M. A Scheil–Gulliver model dedicated to the solidification of steel. Calphad 2015, 48, 184–188. [Google Scholar] [CrossRef]
- Mukherjee, M.; Garcia-Moreno, F.; Banhart, J. Solidification of metal foams. Acta Mater. 2010, 58, 6358–6370. [Google Scholar] [CrossRef]
- Helfen, L.; Baumbach, T.; Stanzick, H.; Banhart, J.; Elmoutaouakkil, A.; Cloetens, P. Viewing the early stage of metal foam formation by computed tomography using synchrotron radiation. Adv. Eng. Mater. 2002, 4, 808–813. [Google Scholar] [CrossRef]
- Radtke, L. Optimierung einer AlSiMg-Legierung für die Metallschaumherstellung. Master’s Thesis, Technische Universität Berlin, Berlin, Germany, January 2017. [Google Scholar]
- Lumley, R.; Sercombe, T.; Schaffer, G. Surface oxide and the role of magnesium during the sintering of aluminum. Metall. Mater. Trans. A 1999, 30, 457–463. [Google Scholar] [CrossRef]
- Paes, M.; Zoqui, E.J. Semi-solid behavior of new Al–Si–Mg alloys for thixoforming. Mater. Sci. Eng. A 2005, 406, 63–73. [Google Scholar] [CrossRef]
- Di Sabatino, M. Fluidity of aluminium foundry alloys. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, September 2005. [Google Scholar]





| Powder | Provider | Purity | D50 (µm) |
|---|---|---|---|
| Al | Aluminium Powder Company Ltd. | 99.7 | 63.9 |
| Si | Elkem AS | 97.5 | 25.7 |
| AlMg50 | Possehl Erzkontor | - | 63.8 |
| TiH2 | Chemetall GmbH | 98.8 | 14.4 |
| Alloy | Mean Pore Size (mm) | n | V (mm3) | n/V (1/mm3) |
|---|---|---|---|---|
| AlSi8Mg4 | 2.00 ± 0.47 | 1189 | 1704 | 0.69 |
| AlSi7Mg5 | 2.44 ± 0.59 | 1086 | 1240 | 0.88 |
| AlSi9Mg3 | 3.19 ± 0.81 | 448 | 2181 | 0.21 |
| AlSi9Mg5 | 1.61 ± 0.42 | 1276 | 1355 | 0.94 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Moreno, F.; Radtke, L.A.; Neu, T.R.; Kamm, P.H.; Klaus, M.; Schlepütz, C.M.; Banhart, J. The Influence of Alloy Composition and Liquid Phase on Foaming of Al–Si–Mg Alloys. Metals 2020, 10, 189. https://doi.org/10.3390/met10020189
García-Moreno F, Radtke LA, Neu TR, Kamm PH, Klaus M, Schlepütz CM, Banhart J. The Influence of Alloy Composition and Liquid Phase on Foaming of Al–Si–Mg Alloys. Metals. 2020; 10(2):189. https://doi.org/10.3390/met10020189
Chicago/Turabian StyleGarcía-Moreno, Francisco, Laurenz Alexander Radtke, Tillmann Robert Neu, Paul Hans Kamm, Manuela Klaus, Christian Matthias Schlepütz, and John Banhart. 2020. "The Influence of Alloy Composition and Liquid Phase on Foaming of Al–Si–Mg Alloys" Metals 10, no. 2: 189. https://doi.org/10.3390/met10020189
APA StyleGarcía-Moreno, F., Radtke, L. A., Neu, T. R., Kamm, P. H., Klaus, M., Schlepütz, C. M., & Banhart, J. (2020). The Influence of Alloy Composition and Liquid Phase on Foaming of Al–Si–Mg Alloys. Metals, 10(2), 189. https://doi.org/10.3390/met10020189