Laser Polishing of Ti6Al4V Fabricated by Selective Laser Melting
Abstract
1. Introduction
2. Materials and Methods
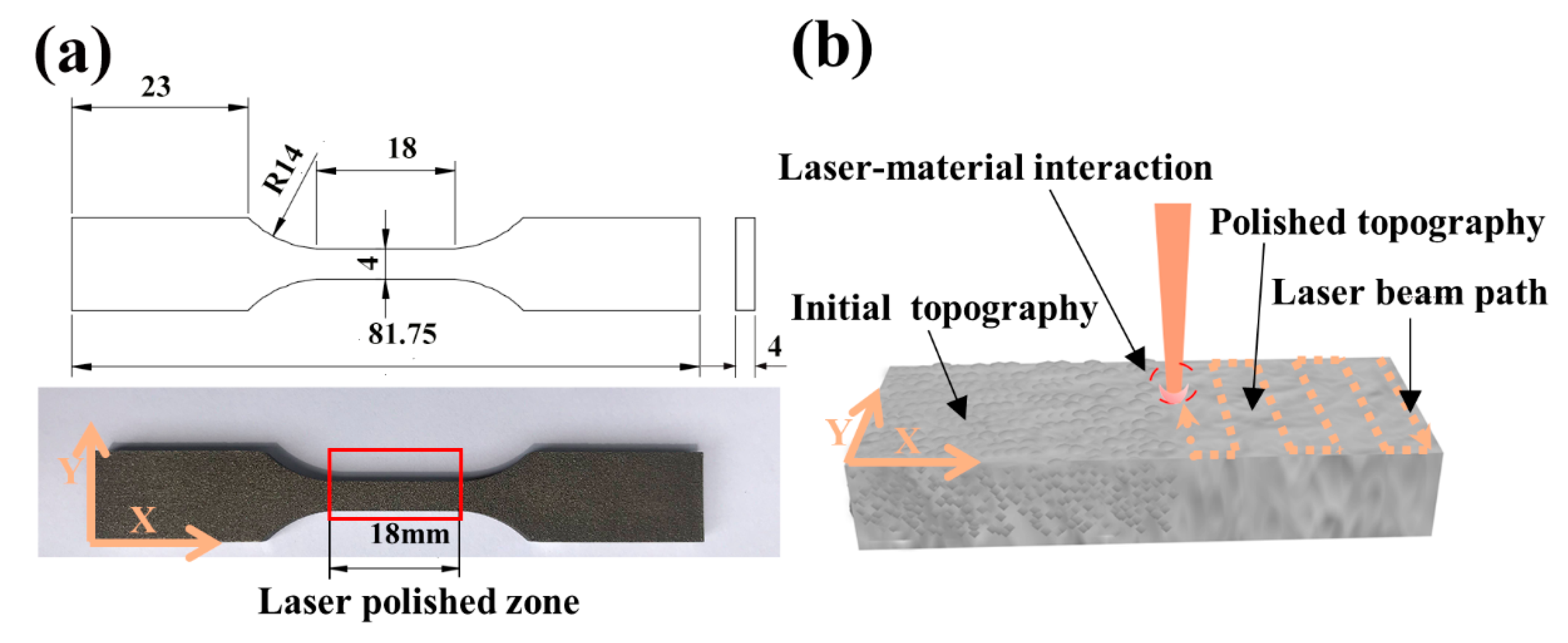
2.1. Material Preparation
2.2. Characterization
2.3. Cell Culture and Morphology
2.4. Cell proliferation
3. Results and Discussion
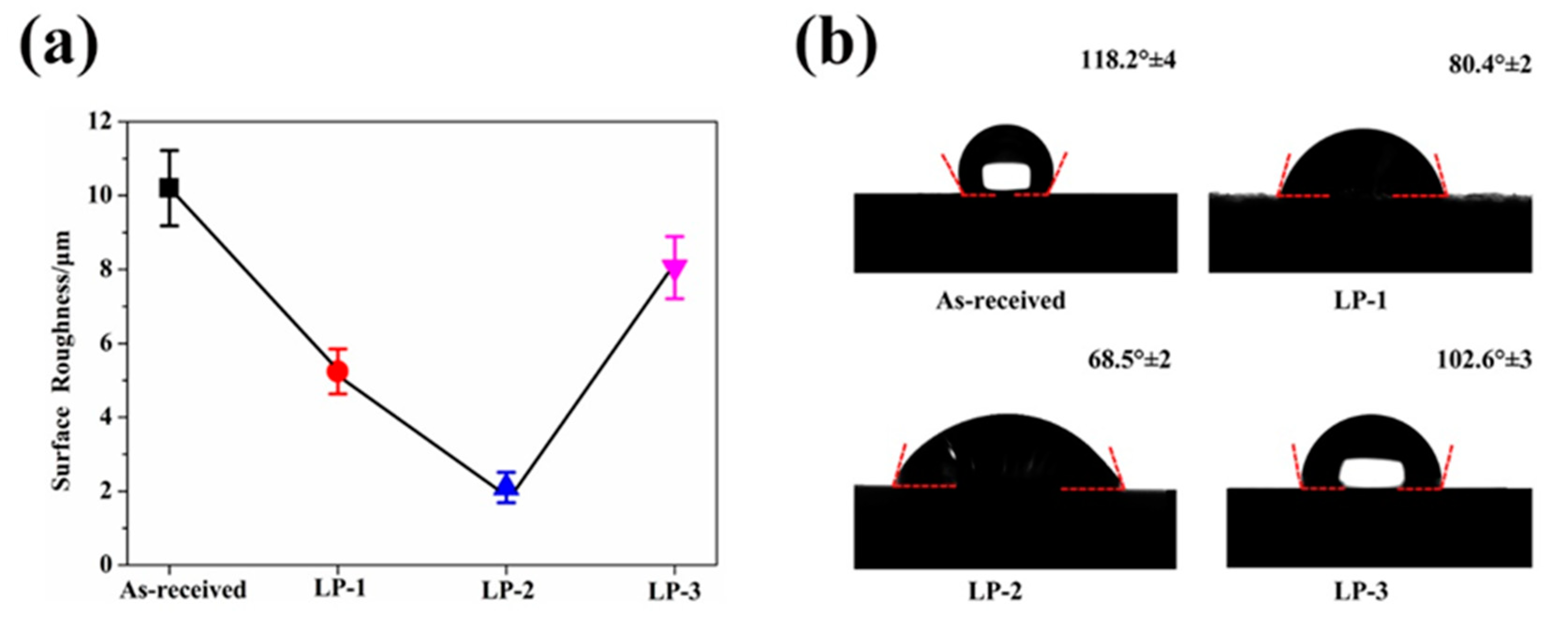
3.1. Surface Morphology
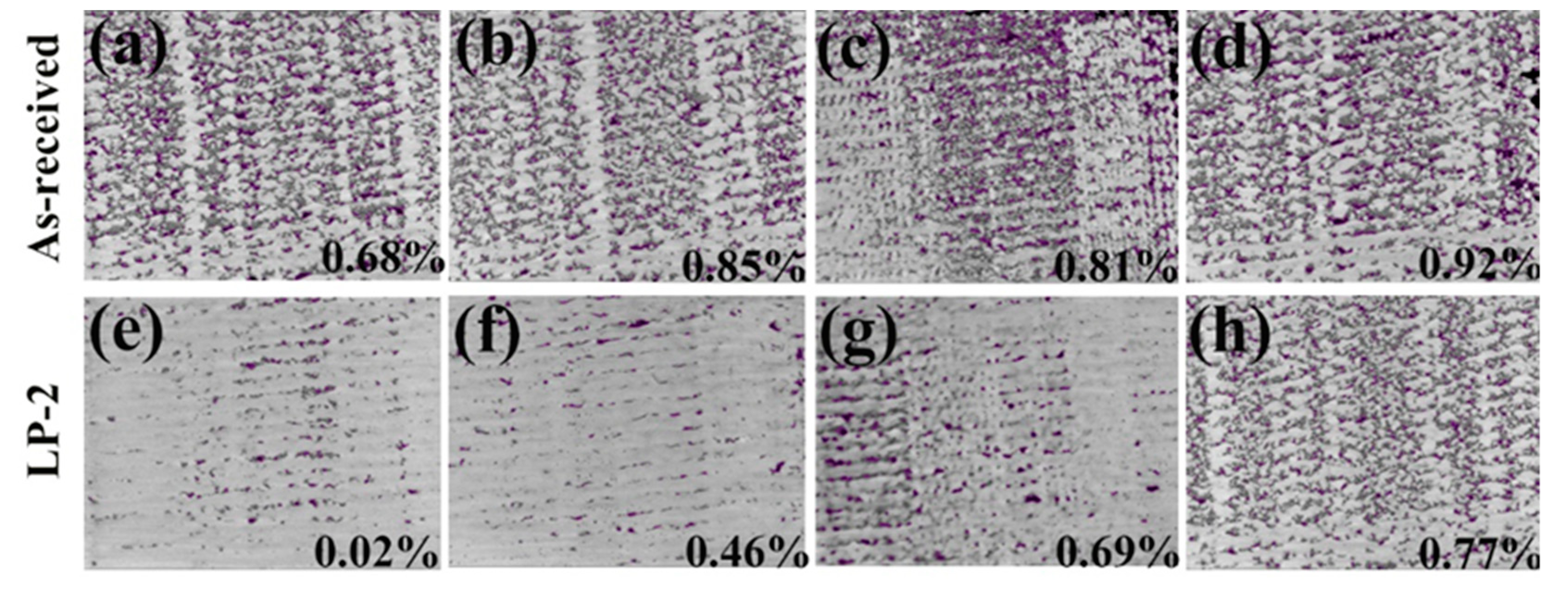
3.2. Surface Porosity
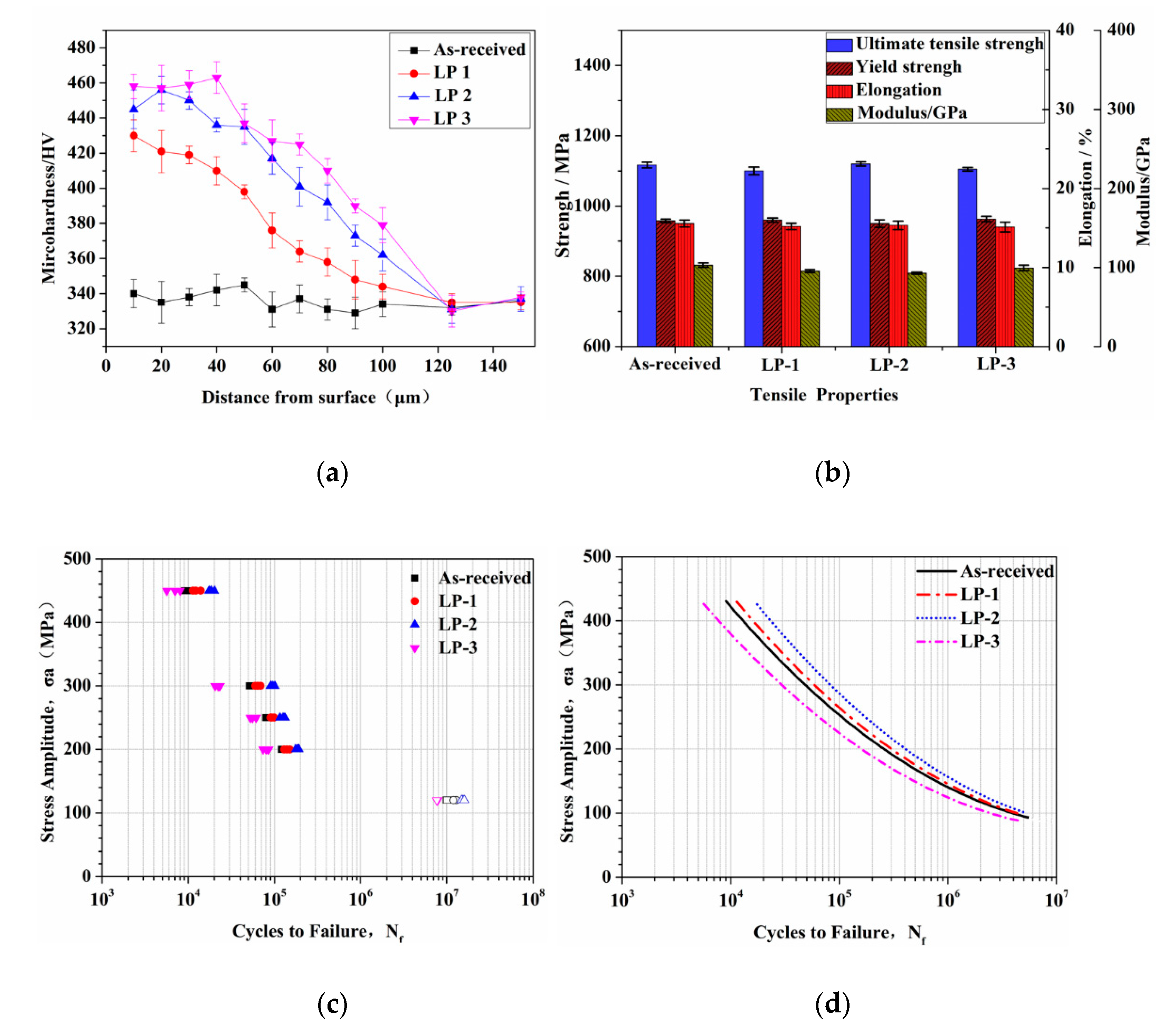
3.3. Mechanical Properties
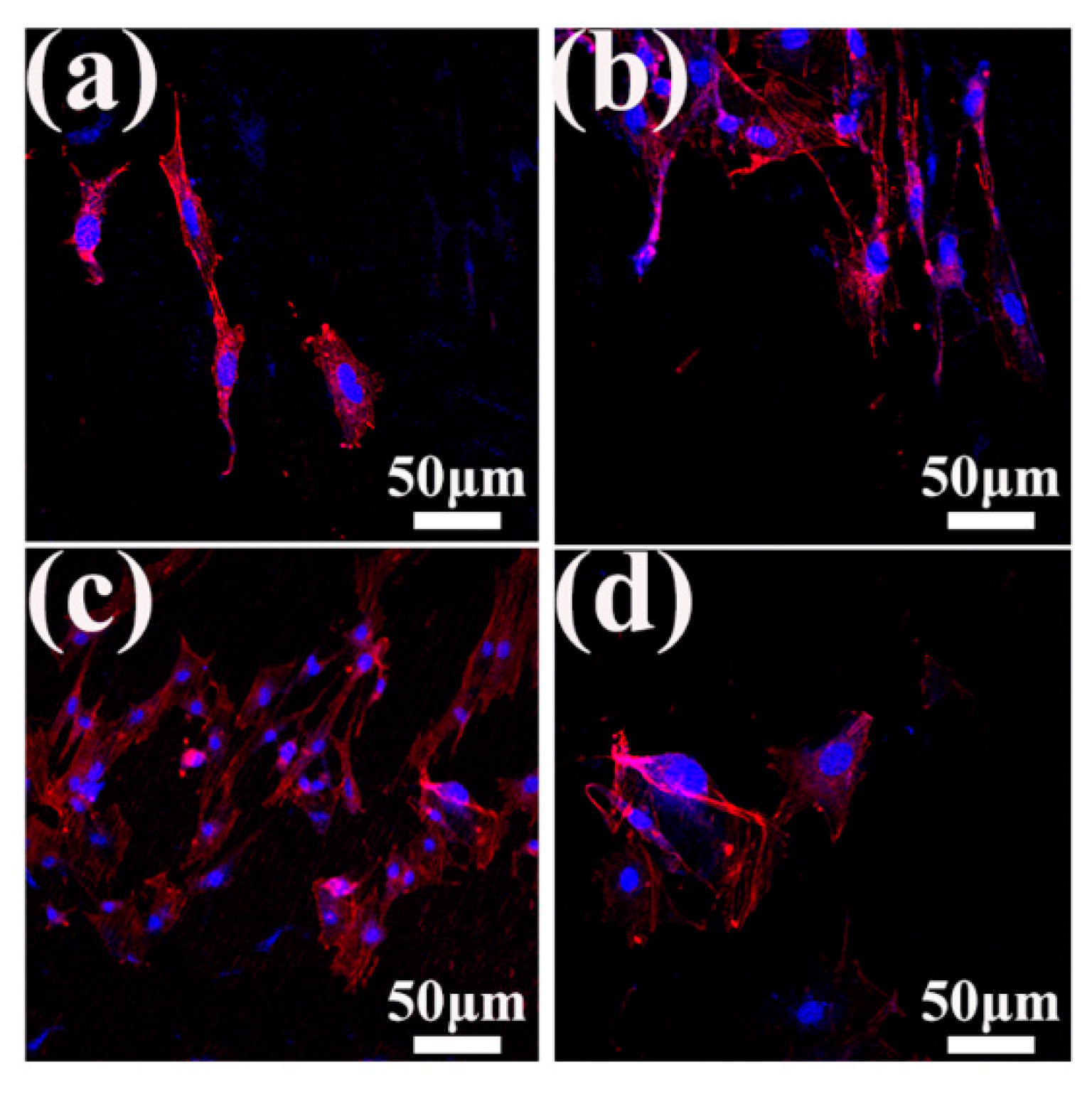
3.4. Cell Morphology and Proliferation Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hamid, K.S.; Parekh, S.G.; Adams, S.B. Salvage of Severe Foot and Ankle Trauma with a 3D Printed Scaffold. Foot Ankle Int. 2016, 37, 433–439. [Google Scholar] [CrossRef]
- Wong, K.C.; Kumta, S.M.; Geel, N.V.; Demol, J. One-step reconstruction with a 3D-printed, biomechanically evaluated custom implant after complex pelvic tumor resection. Comput. Aided Surg. 2015, 20, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Strano, G.; Hao, L.; Everson, R.M.; Evans, K.E. Surface roughness analysis, modelling and prediction in selective laser melting. J. Mater. Process. Technol. 2013, 213, 589–597. [Google Scholar] [CrossRef]
- Raines, A.L.; Olivares-Navarrete, R.; Wieland, M.; Cochran, D.L.; Schwartz, Z.; Boyan, B.D. Regulation of angiogenesis during osseointegration by titanium surface microstructure and energy. Biomaterials 2010, 31, 4909–4917. [Google Scholar] [CrossRef] [PubMed]
- Wall, I.; Donos, N.; Carlqvist, K.; Jones, F.; Brett, P. Modified titanium surfaces promote accelerated osteogenic differentiation of mesenchymal stromal cells in vitro. Bone 2009, 45, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Habibzadeh, S.; Li, L.; Shum-Tim, D.; Davis, E.C.; Omanovic, S. Electrochemical polishing as a 316L stainless steel surface treatment method: Towards the improvement of biocompatibility. Corros. Sci. 2014, 87, 89–100. [Google Scholar] [CrossRef]
- Lyczkowska, E.; Szymczyk, P.; Dybala, B.; Chlebus, E. Chemical polishing of scaffolds made of Ti-6Al-7Nb alloy by additive manufacturing. Arch. Civ. Mech. Eng. 2014, 14, 586–594. [Google Scholar] [CrossRef]
- Balla, V.K.; Soderlind, J.; Bose, S.; Bandyopadhyay, A. Microstructure, mechanical and wear properties of laser surface melted Ti6Al4V alloy. J. Mech. Behav. Biomed. Mater. 2014, 32, 335–344. [Google Scholar] [CrossRef]
- Aviles, R.; Albizuri, J.; Lamikiz, A.; Ukar, E.; Aviles, A. Influence of laser polishing on the high cycle fatigue strength of medium carbon AISI 1045 steel. Int. J. Fatigue 2011, 33, 1477–1489. [Google Scholar] [CrossRef]
- Marimuthu, S.; Triantaphyllou, A.; Antar, M.; Wimpenny, D.; Morton, H.; Beard, M. Laser polishing of selective laser melted components. Int. J. Mach. Tool Manu. 2015, 95, 97–104. [Google Scholar] [CrossRef]
- Ma, C.P.; Guan, Y.C.; Zhou, W. Laser polishing of additive manufactured Ti alloys. Opt. Lasers Eng. 2017, 93, 171–177. [Google Scholar] [CrossRef]
- Yung, K.C.; Xiao, T.Y.; Choy, H.S.; Wang, W.J.; Cai, Z.X. Laser polishing of additive manufactured CoCr alloy components with complex surface geometry. J. Mater. Process. Technol. 2018, 262, 53–64. [Google Scholar] [CrossRef]
- Bhaduri, D.; Penchev, P.; Batal, A.; Dimov, S.; Soo, S.L.; Sten, S.; Harrysson, U.; Zhang, Z.; Dong, H. Laser polishing of 3D printed mesoscale components. Appl. Surf. Sci. 2017, 405, 29–46. [Google Scholar] [CrossRef]
- Aviles, R.; Albizuri, J.; Ukar, E.; Lamikiz, A.; Aviles, A. Influence of laser polishing in an inert atmosphere on the high cycle fatigue strength of AISI 1045 steel. Int. J. Fatigue 2014, 68, 67–79. [Google Scholar] [CrossRef]
- Qin, L.; Wu, H.; Guo, J.; Feng, X.; Dong, G.; Shao, J.; Zeng, Q.; Zhang, Y.; Qin, Y. Fabricating hierarchical micro and nano structures on implantable Co-Cr-Mo alloy for tissue engineering by one-step laser ablation. Colloids Surf. B 2018, 161, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Moridi, A.; Demir, A.G.; Caprio, L.; Hart, A.J.; Previtali, B.; Colosimo, B.M. Deformation and failure mechanisms of Ti-6Al-4V as built by selective laser melting. Mater. Sci. Eng. A 2019, 768, 138456. [Google Scholar] [CrossRef]
- Qian, G.; Jian, Z.; Pan, X.; Berto, F. In-situ investigation on fatigue behaviors of Ti-6Al-4V manufactured by selective laser melting. Int. J. Fatigue 2020, 133, 105424. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, G.; Zhang, W.; Hu, J. Investigating the effect of picosecond laser texturing on microstructure and biofunctionalization of titanium alloy. J. Mater. Process. Technol. 2018, 255, 129–136. [Google Scholar] [CrossRef]
- Klotzbach, A.; Hauser, M.; Beyer, E. Laser Cutting of Carbon Fiber Reinforced Polymers using Highly Brilliant Laser Beam Sources. Physics Procedia 2011, 12, 572–577. [Google Scholar] [CrossRef]
- Kancharla, V. Applications review: Materials processing with fiber lasers under 1kW. International Congress on Applications of Lasers & Electro-Optics 2006, 2006, 1301. [Google Scholar]
- Ahmmed, K.M.T.; Grambow, C.; Kietzig, A.-M. Fabrication of Micro/Nano Structures on Metals by Femtosecond Laser Micromachining. Micromachines 2014, 5, 1219–1253. [Google Scholar] [CrossRef]
- Chichkov, B.N.; Momma, C.; Nolte, S.; von Alvensleben, F.; Tünnermann, A. Femtosecond, picosecond and nanosecond laser ablation of solids. Appl. Phys. A 1996, 63, 109–115. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Y.; Lu, S.; Chen, T.; Zhao, Y.; Chen, H.; Tang, Z. Fabrication and characterization of selective laser melting printed Ti-6Al-4V alloys subjected to heat treatment for customized implants design. Prog. Nat. Sci. Mater. Int. 2016, 26, 671–677. [Google Scholar] [CrossRef]
- Bouet, G.; Cabanettes, F.; Bidron, G.; Guignandon, A.; Peyroche, S.; Bertrand, P.; Vico, L.; Dumas, V. Laser-Based Hybrid Manufacturing of Endosseous Implants: Optimized Titanium Surfaces for Enhancing Osteogenic Differentiation of Human Mesenchymal Stem Cells. ACS Biomater. Sci. Eng. 2019, 5, 4376–4385. [Google Scholar] [CrossRef]
- Liang, C.; Wang, H.; Yang, J.; Cai, Y.; Hu, X.; Yang, Y.; Li, B.; Li, H.; Li, H.; Li, C.; et al. Femtosecond Laser-Induced Micropattern and Ca/P Deposition on Ti Implant Surface and Its Acceleration on Early Osseointegration. ACS Appl. Mater. Interfaces 2013, 5, 8179–8186. [Google Scholar] [CrossRef]
- Lamikiz, A.; Sanchez, J.A.; Lopez de Lacalle, L.N.; Arana, J.L. Laser polishing of parts built up by selective laser sintering. Int. J. Mach. Tool Manu. 2007, 47, 2040–2050. [Google Scholar] [CrossRef]
- Pfefferkorn, F.E.; Duffie, N.A.; Li, X.; Vadali, M.; Ma, C. Improving surface finish in pulsed laser micro polishing using thermocapillary flow. CIRP Ann-Manuf. Technol. 2013, 62, 203–206. [Google Scholar] [CrossRef]
- Kou, S.; Limmaneevichitr, C.; Wei, P.S. Oscillatory Marangoni Flow: A Fundamental Study by Conduction-Mode Laser Spot Welding. Weld. J. 2011, 90, 229S–240S. [Google Scholar]
- Truong, V.K.; Lapovok, R.; Estrin, Y.S.; Rundell, S.; Wang, J.Y.; Fluke, C.J.; Crawford, R.J.; Ivanova, E.R. The influence of nano-scale surface roughness on bacterial adhesion to ultrafine-grained titanium. Biomaterials 2010, 31, 3674–3683. [Google Scholar] [CrossRef]
- Park, J.-W.; Kim, Y.-J.; Park, C.H.; Lee, D.-H.; Ko, Y.G.; Jang, J.-H.; Lee, C.S. Enhanced osteoblast response to an equal channel angular pressing-processed pure titanium substrate with microrough surface topography. Acta Biomater. 2009, 5, 3272–3280. [Google Scholar] [CrossRef]
- Koohkan, M.R.; Attarnejad, R.; Nasseri, M. Time domain analysis of dam-reservoir interaction Using coupled differential quadrature and finite difference methods. Eng. Comput. 2010, 27, 280–294. [Google Scholar] [CrossRef]
- Mullen, L.; Stamp, R.C.; Fox, P.; Jones, E.; Ngo, C.; Sutcliffe, C.J. Selective laser melting: A unit cell approach for the manufacture of porous, titanium, bone in-growth constructs, suitable for orthopedic applications. II. Randomized structures. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 92B, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Pobloth, A.-M.; Checa, S.; Razi, H.; Petersen, A.; Weaver, J.C.; Schmidt-Bleek, K.; Windolf, M.; Tatai, A.Á.; Roth, C.P.; Schaser, K.-D.; et al. Mechanobiologically optimized 3D titanium-mesh scaffolds enhance bone regeneration in critical segmental defects in sheep. Sci. Transl. Med. 2018, 10, eaam8828. [Google Scholar] [CrossRef]
- Biggs, M.J.P.; Richards, R.G.; Dalby, M.J. Nanotopographical modification: A regulator of cellular function through focal adhesions. Nanomed.-Nanotechnol. Biol. Med. 2010, 6, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Chehroudi, B.; Ghrebi, S.; Murakami, H.; Waterfield, J.D.; Owen, G.; Brunette, D.M. Bone formation on rough, but not polished, subcutaneously implanted Ti surfaces is preceded by macrophage accumulation. J. Biomed. Mater. Res. Part A 2010, 93A, 724–737. [Google Scholar] [CrossRef]
- Tangpasuthadol, V.; Pongchaisirikul, N.; Hoven, V.P. Surface modification of chitosan films. Effects of hydrophobicity on protein adsorption. Carbohydr. Res. 2003, 338, 937–942. [Google Scholar] [CrossRef]
- Gitlin, I.; Carbeck, J.D.; Whitesides, G.M. Why are proteins charged? Networks of charge-charge interactions in proteins measured by charge ladders and capillary electrophoresis. Chem.-Int. Edit 2006, 45, 3022–3060. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Lu, T.; Wen, J.; Qian, S.; Cao, H.; Ning, C.; Pan, X.; Jiang, X.; Liu, X.; Chu, P.K. Enhanced osteointegration on tantalum-implanted polyetheretherketone surface with bone-like elastic modulus. Biomaterials 2015, 51, 173–183. [Google Scholar] [CrossRef]
- Jimbo, R.; Sawase, T.; Shibata, Y.; Hirata, K.; Hishikawa, Y.; Tanaka, Y.; Bessho, K.; Ikeda, T.; Atsuta, M. Enhanced osseointegration by the chemotactic activity of plasma fibronectin for cellular fibronectin positive cells. Biomaterials 2007, 28, 3469–3477. [Google Scholar] [CrossRef]





| Ti | AL | V | O | N | C |
|---|---|---|---|---|---|
| Balance | 5.5–6.75 | 3.5–4.5 | <2000 ppm | <3000 ppm | <3000 ppm |
| Process Variables | Samples | ||
|---|---|---|---|
| LP-1 | LP-2 | LP-3 | |
| Energy density (W/cm2) | 0.853 × 105 | 1.703 × 105 | 2.503 × 105 |
| Pulse overlap factor along X (%) | 50 | 50 | 50 |
| Pulse overlap factor along Y (%) | 50 | 50 | 50 |
| Sample Comparation | p Value | ||
|---|---|---|---|
| 1 Day | 4 Day | 7 Day | |
| As-received VS LP-1 | 0.483 | 0.581 | 0.394 |
| As-received VS LP-2 | 0.154 | 0.036 | 0.041 |
| As-received VS LP-3 | 0.267 | 0.147 | 0.328 |
| As-received VS LP-4 | 0.065 | 0.391 | 0.247 |
| LP-2 VS LP-3 | 0.091 | 0.029 | 0.030 |
| LP-2 VS LP-4 | 0.338 | 0.022 | 0.024 |
| LP-3 VS LP-4 | 0.431 | 0.067 | 0.473 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, C.; Hu, Y.; Liu, N.; Zou, X.; Wang, H.; Zhang, X.; Fu, Y.; Hu, J. Laser Polishing of Ti6Al4V Fabricated by Selective Laser Melting. Metals 2020, 10, 191. https://doi.org/10.3390/met10020191
Liang C, Hu Y, Liu N, Zou X, Wang H, Zhang X, Fu Y, Hu J. Laser Polishing of Ti6Al4V Fabricated by Selective Laser Melting. Metals. 2020; 10(2):191. https://doi.org/10.3390/met10020191
Chicago/Turabian StyleLiang, Chunyong, Yazhou Hu, Ning Liu, Xianrui Zou, Hongshui Wang, Xinping Zhang, Yulan Fu, and Jingyun Hu. 2020. "Laser Polishing of Ti6Al4V Fabricated by Selective Laser Melting" Metals 10, no. 2: 191. https://doi.org/10.3390/met10020191
APA StyleLiang, C., Hu, Y., Liu, N., Zou, X., Wang, H., Zhang, X., Fu, Y., & Hu, J. (2020). Laser Polishing of Ti6Al4V Fabricated by Selective Laser Melting. Metals, 10(2), 191. https://doi.org/10.3390/met10020191






