Surface Pretreatment and Fabrication Technology of Braided Carbon Fiber Rope Aluminum Matrix Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pre-Treatment of the Braided Carbon Fibers Rope
2.3. Fabrication of the Composites
2.4. Characterization and Performance Testing
3. Results and Discussion
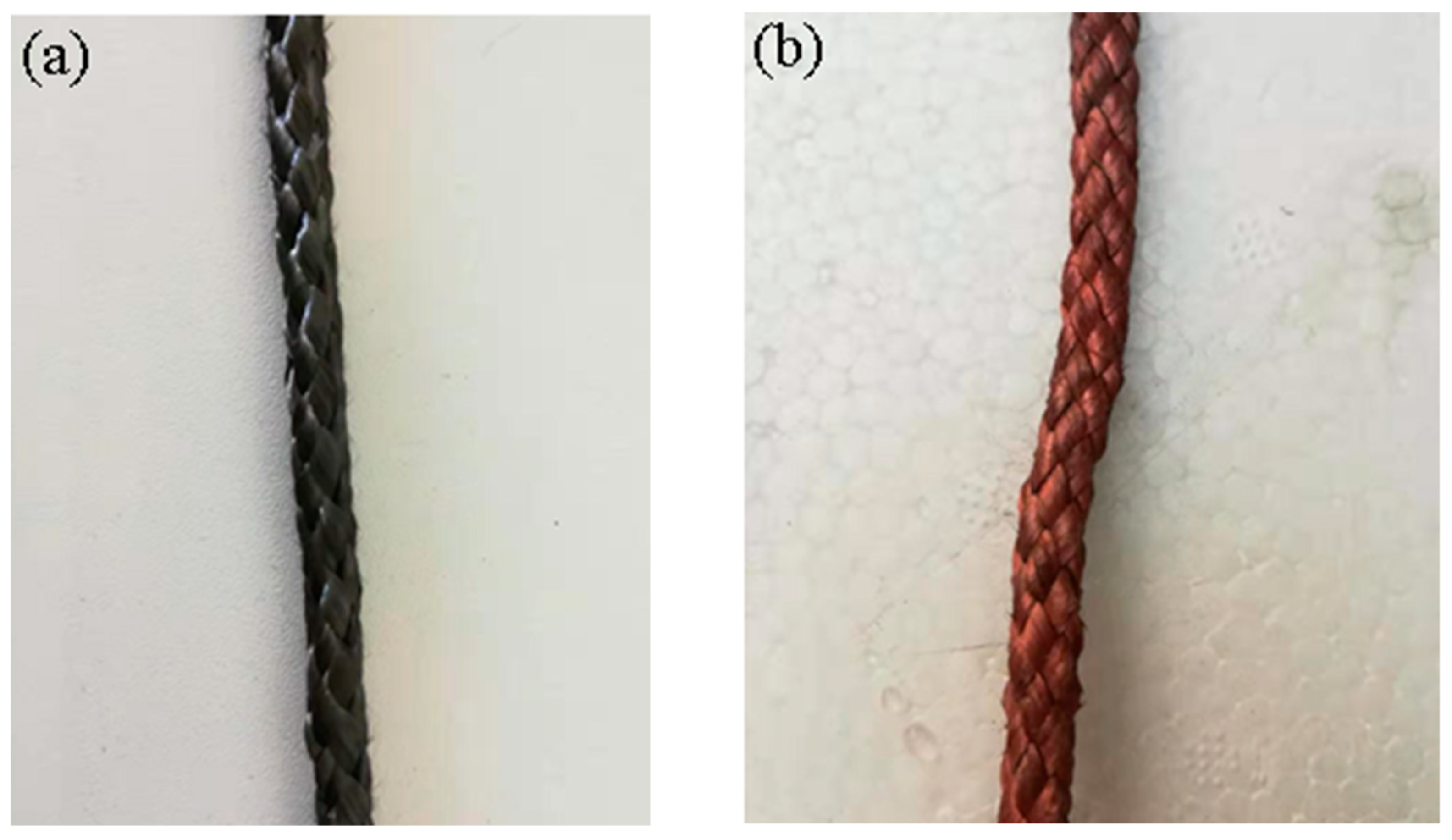
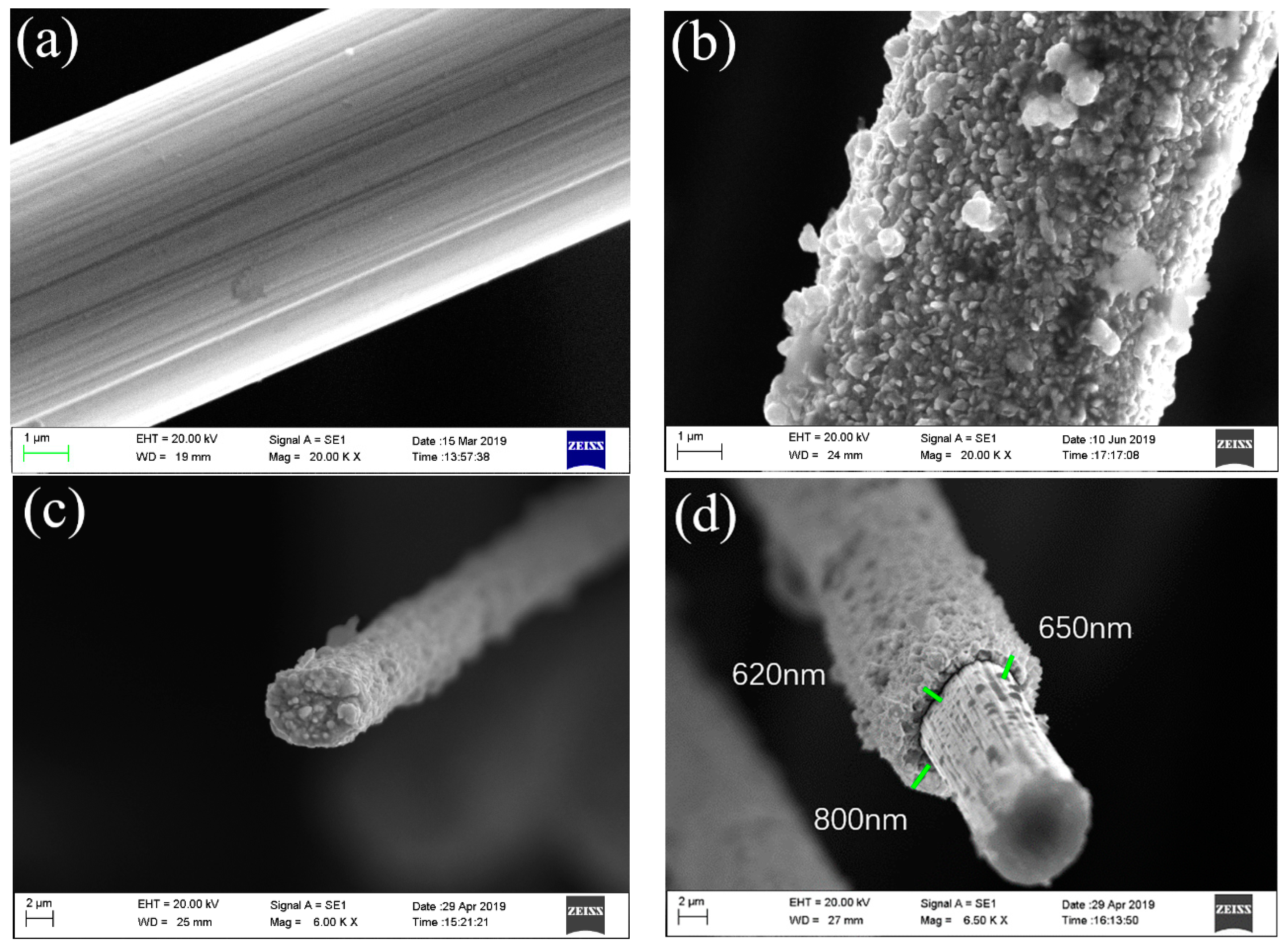
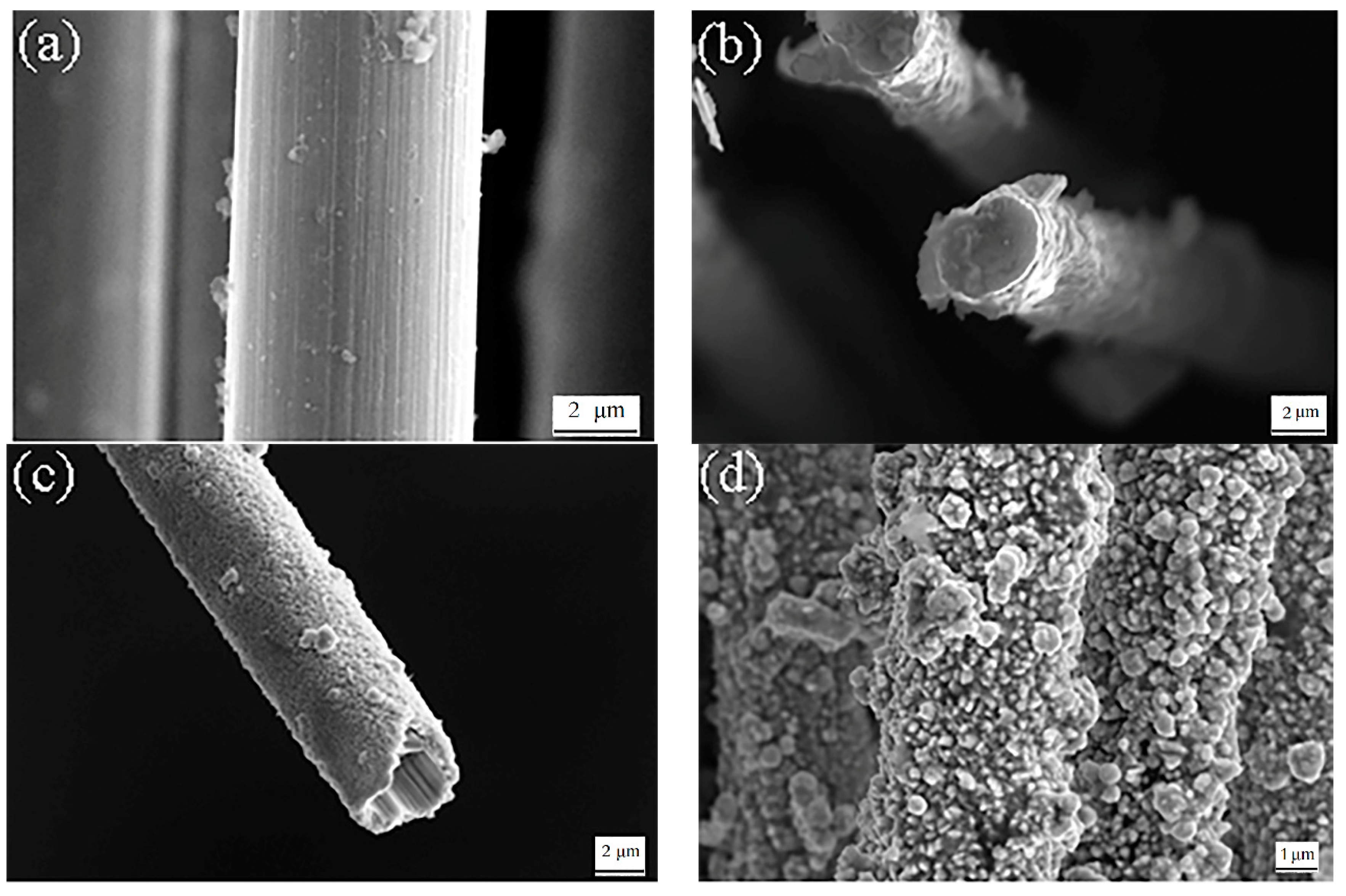
3.1. Morphology Observation
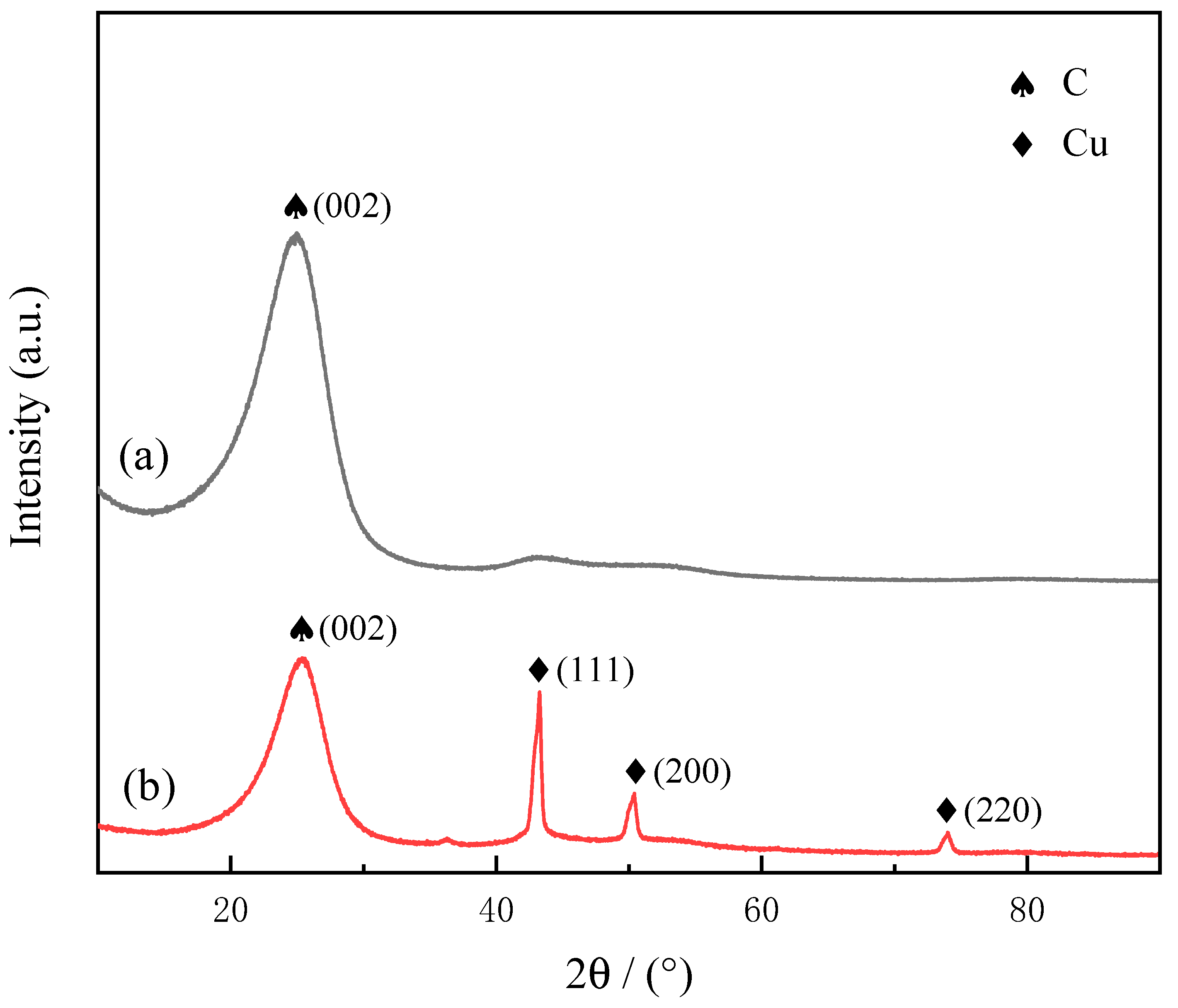
3.2. Structural Analysis
3.3. Effect of pH Value on Electroless Copper Plating Reaction
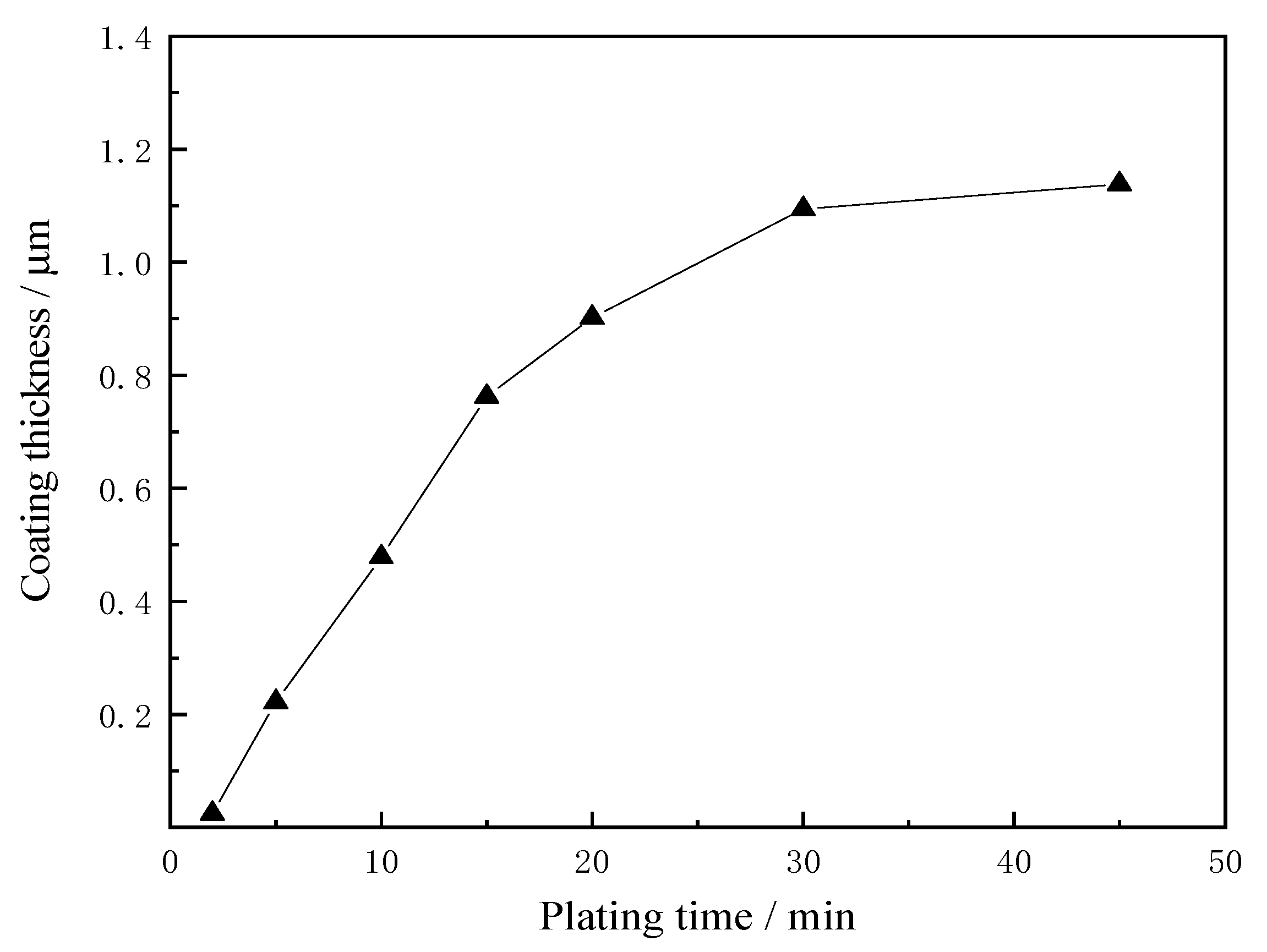
3.4. Effect of Plating Time on Coating
3.5. Mechanism of Electroless Copper Plating on Carbon Fiber Surface
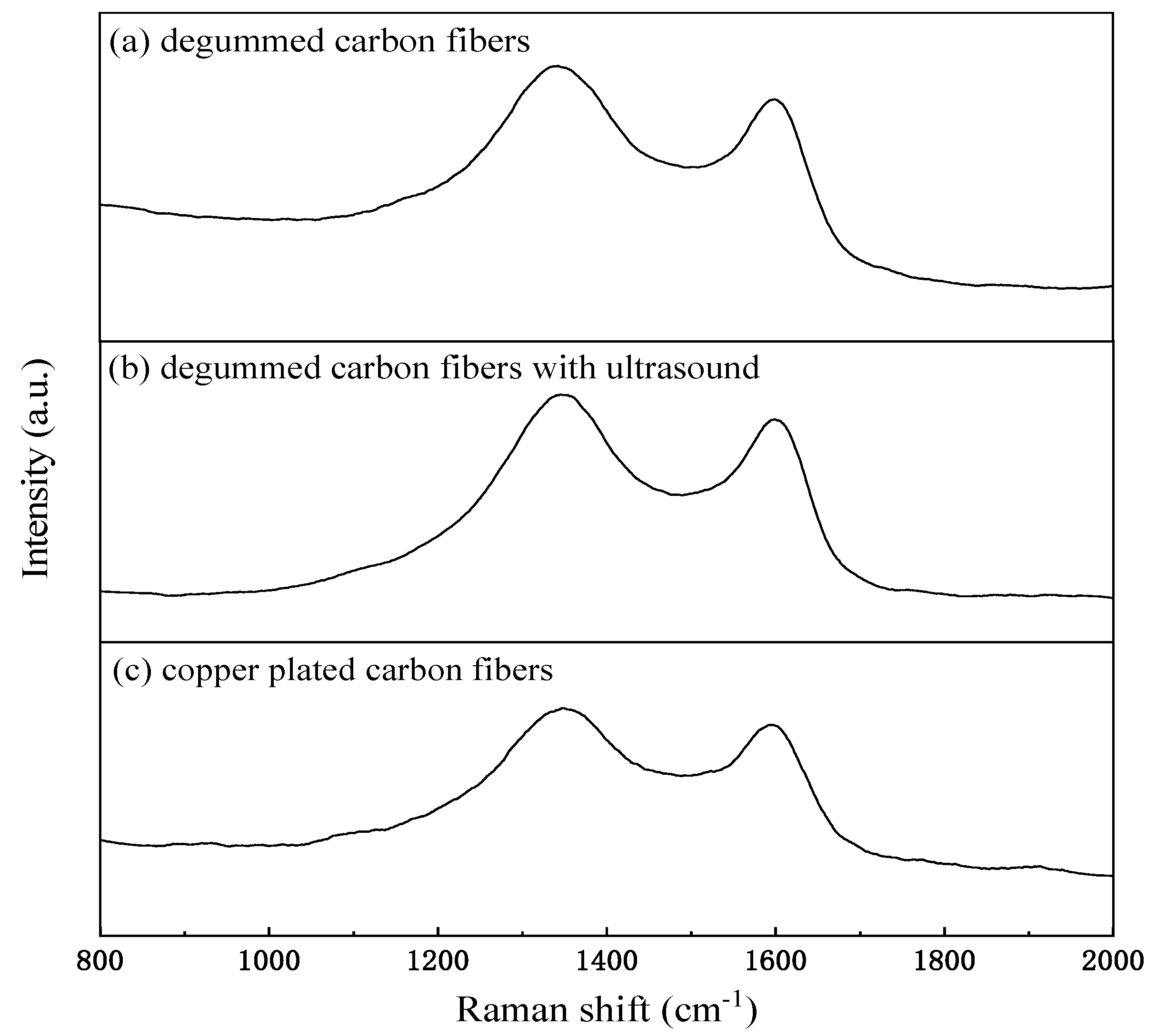
3.5.1. Raman Analysis
3.5.2. XPS Analysis
3.6. Interface and Mechanical Properties of Carbon Fiber and Aluminum Matrix Composites
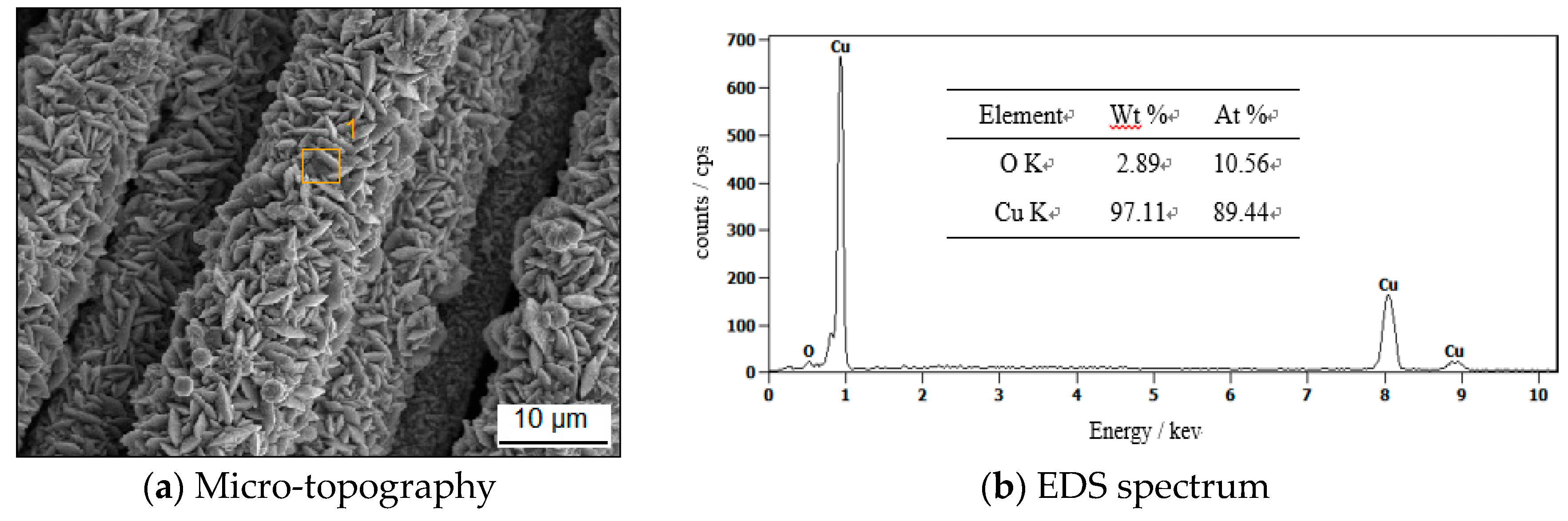
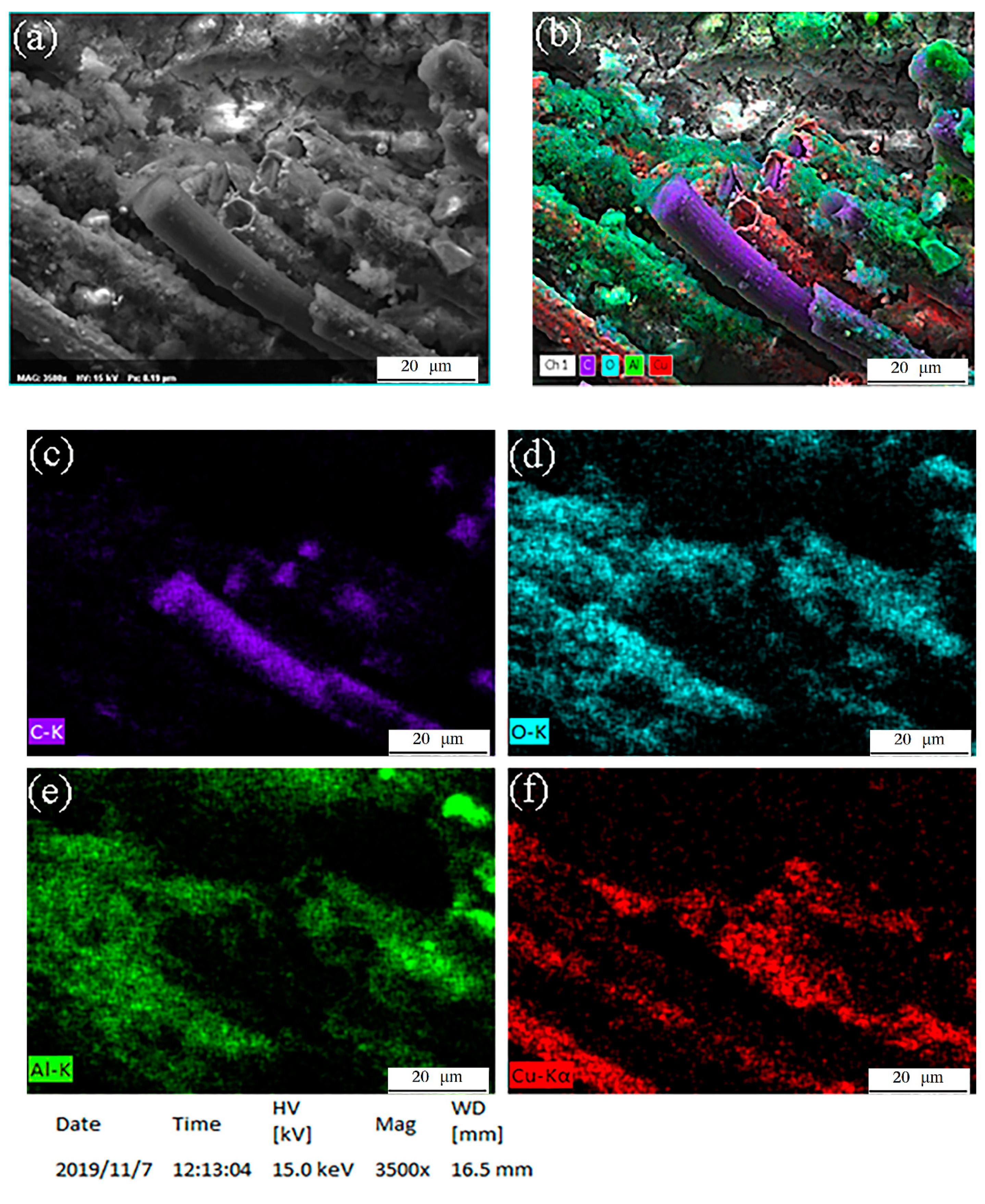
3.6.1. Element Distributions
3.6.2. Strengthening Effect of Braided Carbon Fiber Rope on Aluminum Matrix Composite
4. Conclusions
- (1)
- The surface of the braided carbon fiber rope treated with ultrasonic degumming and concentrated nitric acid becomes rough, and the axial groove-like streaks were obviously increased. By XPS analysis, the carbon fiber surface contained many hydrophilic oxygen-containing functional groups, such as C–OH, C=O, COOH, etc., which can effectively improve the wettability of carbon fiber and plating solution, as well as ensure the smooth progress of copper plating.
- (2)
- The tensile strength of the composite material was higher than that of the matrix material, and the reinforcing effect of the carbon fiber rope was better than that of the carbon fiber bundle. When the volume fraction of the reinforcing phase carbon fiber was 4%, the tensile strength of the carbon fiber bundle reinforced composite material was 14% higher than that of the matrix, and the tensile strength of the carbon fiber rope reinforced composite was 31% higher than that of the matrix.
- (3)
- The comparative analysis of the copper coating on the carbon fiber surface obtained from different pH of the plating solution shows that, when pH = 12, a continuous and uniform copper plating layer was formed on the carbon fiber surface, and the coating was dense without obvious peeling.
- (4)
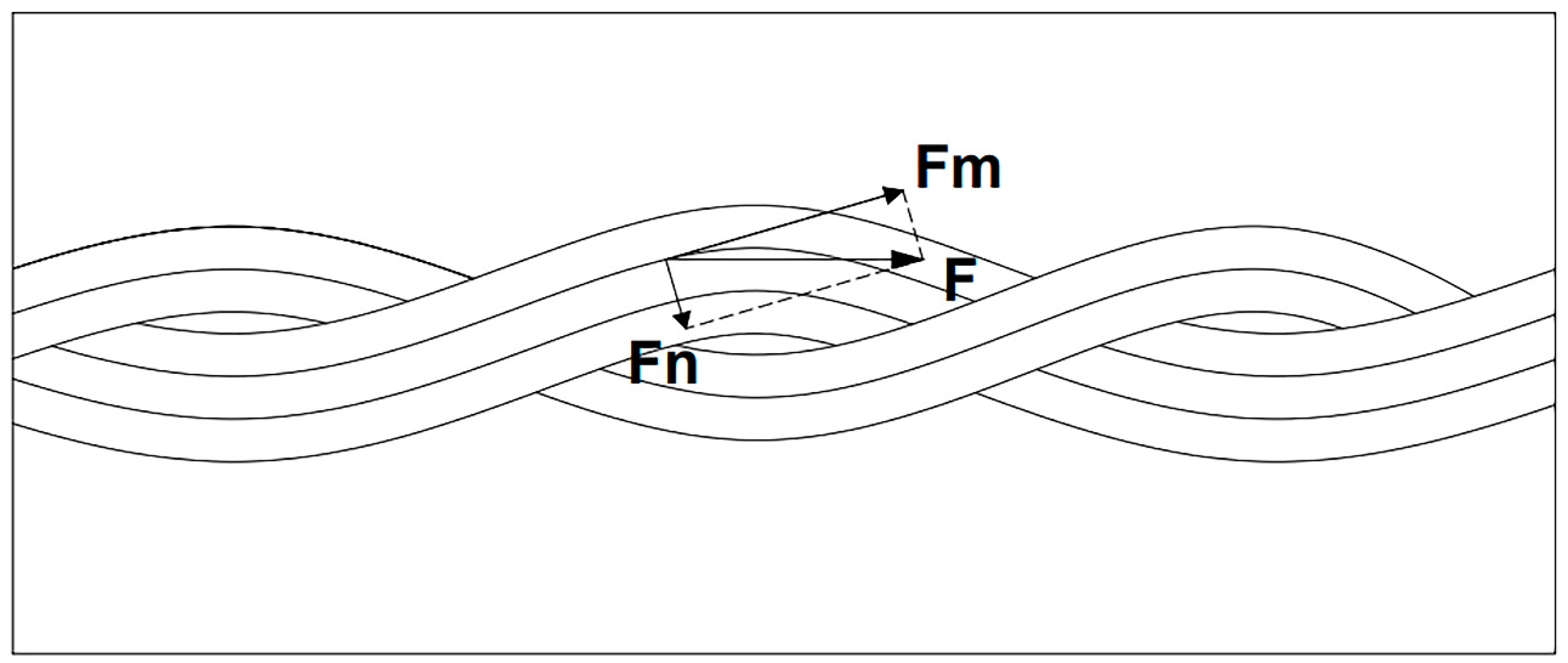
- The main way to strengthen the carbon fiber braided rope is that the surface of the carbon fiber braided rope is wavy and uneven, which increases the contact area with the aluminum matrix and reaches a state of physical engagement with the aluminum matrix, thereby forming a whole.
Author Contributions
Funding
Conflicts of Interest
References
- Shirvanimoghaddam, K.; Hamim, S.U.; Karbalaei Akbari, M.; Fakhrhoseini, S.M.; Khayyam, H.; Pakseresht, A.H.; Ghasali, E.; Zabet, M.; Munir, K.S.; Jia, S.; et al. Carbon fiber reinforced metal matrix composites: Fabrication processes and properties. Compos. Part A Appl. Sci. Manuf. 2017, 92, 70–96. [Google Scholar] [CrossRef]
- Xavior, M.A.; Kumar, J.P.A. Machinability of Hybrid Metal Matrix Composite—A Review. Procedia Eng. 2017, 174, 1110–1118. [Google Scholar] [CrossRef]
- Zhang, J.J.; Liu, S.C.; Chen, H.; Lu, Y.P.; Chen, Q.S.; Dong, Y.; Wang, T.M.; Li, T.J. Surface Pretreatment and Fabrication Technology of Woven Carbon Fiber Cloth Aluminium Alloy Matrix Composite. Mater. Sci. Forum 2015, 816, 3–8. [Google Scholar] [CrossRef]
- Barton, B.E.; Behr, M.J.; Patton, J.T.; Hukkanen, E.J.; Landes, B.G.; Wang, W.; Horstman, N.; Rix, J.E.; Keane, D.; Weigand, S.; et al. High-Modulus Low-Cost Carbon Fibers from Polyethylene Enabled by Boron Catalyzed Graphitization. Small 2017, 13, 1701926. [Google Scholar] [CrossRef] [PubMed]
- Newcomb, B.A. Processing, structure, and properties of carbon fibers. Compos. Part A Appl. Sci. Manuf. 2016, 91, 262–282. [Google Scholar] [CrossRef]
- Xiao, H.; Lu, Y.; Zhao, W.; Qin, X. The effect of heat treatment temperature and time on the microstructure and mechanical properties of PAN-based carbon fibers. J. Mater. Sci. 2014, 49, 794–804. [Google Scholar] [CrossRef]
- Hajjari, E.; Divandari, M.; Arabi, H. Effect of Applied Pressure and Nickel Coating on Microstructural Development in Continuous Carbon Fiber-Reinforced Aluminum Composites Fabricated by Squeeze Casting. Mater. Manuf. Process. 2011, 26, 599–603. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, L.; Li, W.; Shen, B.; Hu, W. Interface characteristics and mechanical properties of short carbon fibers/Al composites with different coatings. Appl. Surf. Sci. 2009, 255, 4393–4400. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Tang, Y.; Shen, B.; Hu, W. Friction and wear properties of short carbon fiber reinforced aluminum matrix composites. Wear 2009, 266, 733–738. [Google Scholar] [CrossRef]
- Liu, L.; Tang, Y.; Zhao, H.; Zhu, J.; Hu, W. Fabrication and properties of short carbon fibers reinforced copper matrix composites. J. Mater. Sci. 2008, 43, 974–979. [Google Scholar] [CrossRef]
- Panwar, N.; Chauhan, A. Fabrication methods of particulate reinforced Aluminium metal matrix composite-A review. Mater. Today Proc. 2018, 5, 5933–5939. [Google Scholar] [CrossRef]
- Akbarzadeh, E.; Picas, J.A.; Teresa Baile, M. Microstructure and properties of aluminum silicon/short fibre carbon composites fabricated by semi-solid thixomixing. Mater. Des. 2015, 88, 683–692. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Wang, W.; Hu, J.; Zhang, J.; Wu, S. Influence of fiber length on the tensile behavior of fiber metal laminates with discontinuous reinforcement. J. Reinf. Plast. Compos. 2015, 34, 1651–1661. [Google Scholar] [CrossRef]
- Liu, T.; He, X.; Liu, Q.; Ren, S.; Kang, Q.; Zhang, L.; Qu, X. Effect of chromium carbide coating on thermal properties of short graphite fiber/Al composites. J. Mater. Sci. 2014, 49, 6705–6715. [Google Scholar] [CrossRef]
- Shi, J.; Liu, Y.; Yao, G.; Yan, P.; Liu, H. Effect of Mg on Microstructure and Mechanical Properties of Copper-Coated Short Carbon Fiber Reinforced Al Alloy Matrix Composite. In Advanced Materials Research; Gao, S., Ed.; Trans Tech Publications Ltd.: Cambridge, UK, 2012; Volume 457, pp. 348–353. [Google Scholar]
- Zhang, Y.; Yan, L.; Miao, M.; Wang, Q.; Wu, G. Microstructure and mechanical properties of z-pinned carbon fiber reinforced aluminum alloy composites. Mater. Des. 2015, 86, 872–877. [Google Scholar] [CrossRef]
- Beronska, N.; Izdinsky, K.; Stefanik, P.; Kudela, S., Jr.; Simancik, F.; Vavra, I.; Krizanova, Z. Structure and thermal expansion behaviour of Al/C composites reinforced with unidirectionally aligned continuous high modulus C fibres. Met. Mater. 2011, 49, 435–446. [Google Scholar] [CrossRef]
- Rohatgi, P.K.; Tiwari, V.; Gupta, N. Squeeze infiltration processing of nickel coated carbon fiber reinforced Al-2014 composite. J. Mater. Sci. 2006, 41, 7232–7239. [Google Scholar] [CrossRef]
- Ramesh, C.S.; Adarsha, H.; Pramod, S.; Khan, Z. Tribological characteristics of innovative Al6061–carbon fiber rod metal matrix composites. Mater. Des. 2013, 50, 597–605. [Google Scholar] [CrossRef]
- Calderon, N.R.; Voytovych, R.; Narciso, J.; Eustathopoulos, N. Wetting dynamics versus interfacial reactivity of AlSi alloys on carbon. J. Mater. Sci. 2010, 45, 2150–2156. [Google Scholar] [CrossRef]
- Xue, Y.; Chen, W.; Zhao, Q.; Fu, Y. Electroless carbon fibers: A new route for improving mechanical property and wettability of composites. Surf. Coat. Technol. 2019, 358, 409–415. [Google Scholar] [CrossRef]
- Che, D.; Yao, G.; Cao, Z. A Precious Metal-Free Electroless Technique for the Deposition of Copper on Carbon Fibers. Metall. Mater. Trans. A 2012, 43, 4194–4199. [Google Scholar] [CrossRef]
- Kang, S.S.; Ji, H.; Gul, H.Z.; Sakong, W.K.; Kim, J.Y.; Kim, W.S.; Lee, J.; Han, S.; Park, M.; Choi, Y.C.; et al. Metal-coated carbon fiber for lighter electrical metal wires. Synth. Met. 2016, 222, 180–185. [Google Scholar] [CrossRef]
- Halouzka, V.; Halouzkova, B.; Jirovsky, D.; Hemzal, D.; Ondra, P.; Siranidi, E.; Kontos, A.G.; Falaras, P.; Hrbac, J. Copper nanowire coated carbon fibers as efficient substrates for detecting designer drugs using SERS. Talanta 2017, 165, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Asano, K. Mechanical Properties of Aluminum Composites Reinforced with PAN- and Pitch-Based Short Carbon Fibers. Mater. Trans. 2017, 58, 906–913. [Google Scholar] [CrossRef] [Green Version]
- Knowles, A.J.; Jiang, X.; Galano, M.; Audebert, F. Microstructure and mechanical properties of 6061 Al alloy based composites with SiC nanoparticles. J. Alloys Compd. 2014, 615, S401–S405. [Google Scholar] [CrossRef]
- Matsunaga, T.; Matsuda, K.; Hatayama, T.; Shinozaki, K.; Yoshida, M. Fabrication of continuous carbon fiber-reinforced aluminum–magnesium alloy composite wires using ultrasonic infiltration method. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1902–1911. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Zhang, Y.; Dong, Y.; Lu, Y.; Li, T. Fabrication of woven carbon fibers reinforced Al–Mg (95–5 wt%) matrix composites by an electromagnetic casting process. J. Mater. Process. Technol. 2015, 226, 78–84. [Google Scholar] [CrossRef]
- Parandoush, P.; Zhou, C.; Lin, D. 3D Printing of Ultrahigh Strength Continuous Carbon Fiber Composites. Adv. Eng. Mater. 2019, 21, 1800622. [Google Scholar] [CrossRef]
- Wang, L.; Nygren, G.; Karkkainen, R.L.; Yang, Q. A multiscale approach for virtual testing of highly aligned short carbon fiber composites. Compos. Struct. 2019, 230, 111462. [Google Scholar] [CrossRef]
- Liu, B.; Hu, X.; Li, Y.; Xiao, T.; Xu, F. Internal three-dimensional strain evolution of the failure process for short carbon fiber composite through in situ synchrotron radiation X-ray computed tomography. Carbon 2020, 157, 506–514. [Google Scholar] [CrossRef]
- Aytaç, A.; Gürbüz, M.; Sanli, A.E. Electrooxidation of hydrogen peroxide and sodium borohydride on Ni deposited carbon fiber electrode for alkaline fuel cells. Int. J. Hydrogen Energy 2011, 36, 10013–10021. [Google Scholar] [CrossRef]
- Abdel Gawad, O.; Abou Tabl, M.H.; Abdel Hamid, Z.; Mostafa, S.F. Electroplating of chromium and Cr-carbide coating for carbon fiber. Surf. Coat. Technol. 2006, 201, 1357–1362. [Google Scholar] [CrossRef]
- Skrabalak, S.E. Ultrasound-assisted synthesis of carbon materials. Phys. Chem. Chem. Phys. 2009, 11, 4930. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, S. Catalytic graphitization of electroless Ni–P coated PAN-based carbon fibers. Carbon 2006, 44, 1986–1993. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Qiao, X.; Liu, W.; Shi, S.; Qu, Y. Mechanism of ultrasonic treatment under nickel salt solution and its effect on electroless nickel plating of carbon fibers. Ultrason. Sonochem. 2019, 52, 493–504. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]




| Fiber Diameter/μm | Carbon Content/% | Linear Density/mg·m−1 | Bulk Density/g·cm−3 | Tensile Strength/GPa | Elastic Modulus/GPa | Elongation at Break/% |
|---|---|---|---|---|---|---|
| 7 | 93.0–95.0 | 800 | 1.80 | 4.90 | 230 | 2.10 |
| Component | Molecular Formula | Concentration | Function |
|---|---|---|---|
| Copper sulfate | CuSO4·5H2O | 10g·L−1 | Main salt |
| Seignette salt | KNaC4H4O6·4H2O | 10g·L−1 | Complexing agent |
| Formaldehyde | HCHO | 10mL·L−1 | Reducing agent |
| Sodium hydroxide | NaOH | variable | Adjusting pH |
| C=C | C–OH | C=O | COOH | ||||
|---|---|---|---|---|---|---|---|
| Eb | At.% | Ec | At.% | Ed | At.% | Ee | At.% |
| 284.75 | 70.86 | 286.30 | 24.83 | 287.48 | 1.71 | 288.90 | 2.59 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Wu, C.; Ping, H.; Wang, M.; Tang, W. Surface Pretreatment and Fabrication Technology of Braided Carbon Fiber Rope Aluminum Matrix Composite. Metals 2020, 10, 1212. https://doi.org/10.3390/met10091212
Liang J, Wu C, Ping H, Wang M, Tang W. Surface Pretreatment and Fabrication Technology of Braided Carbon Fiber Rope Aluminum Matrix Composite. Metals. 2020; 10(9):1212. https://doi.org/10.3390/met10091212
Chicago/Turabian StyleLiang, Jun, Chunjing Wu, Hang Ping, Ming Wang, and Weizhong Tang. 2020. "Surface Pretreatment and Fabrication Technology of Braided Carbon Fiber Rope Aluminum Matrix Composite" Metals 10, no. 9: 1212. https://doi.org/10.3390/met10091212
APA StyleLiang, J., Wu, C., Ping, H., Wang, M., & Tang, W. (2020). Surface Pretreatment and Fabrication Technology of Braided Carbon Fiber Rope Aluminum Matrix Composite. Metals, 10(9), 1212. https://doi.org/10.3390/met10091212





