Abstract
The significant increase in the demand for efficient electric energy storage during the past decade has promoted an increase in the production and use of Cd-containing batteries. On the one hand, the amount of toxic Cd-containing used batteries is growing, while on the other hand, Cd is on a list of critical raw materials (for Europe). Both of these factors call for the development of effective technology for Cd recovery from spent batteries. The present paper is aimed at providing a short review of the recent progress in Cd recovery from spent batteries. Statistical data from the past decade on the source of Cd, its global production, and Ni-Cd battery recycling are given in the introduction. A short overview of the pyro-and hydro-metallurgical methods of metal production is provided. Recent progress in Cd recovery by commercial methods during the past decade is reviewed.
1. Introduction
Lead acid batteries take the top place in today’s market due to their low cost and good performance with proven industrial processing methods. Alkaline nickel-cadmium (Ni-Cd) batteries are widely used as autonomous sources of industrial and household current (power banks) due to a successful combination of feasibility studies and achieved sustainable electrical characteristics [1]. In recent decades, the market of secondary current sources for portable equipment has undergone significant changes, which leads to an intensive replacement of Ni-Cd batteries with lithium-ion (LIB) and nickel-metal-hydride (Ni-MH). However, the Ni-Cd accumulators with a specific energy of 40–60 W·h·kg−1, high charge/discharge efficiency of up to 70–90% retain a significant market share among the industrial secondary sources of current.
LIB, Ni-MH, and Ni-Cd batteries have a more complex composition and structure, and their recycling requires further research when compared with lead acid batteries [2]. It is very important to recycle spent Ni-MH, LIB, and Ni-Cd batteries separately because the amount (and type) of metal in them is different [3].
1.1. Critical Raw Materials (CRMs)
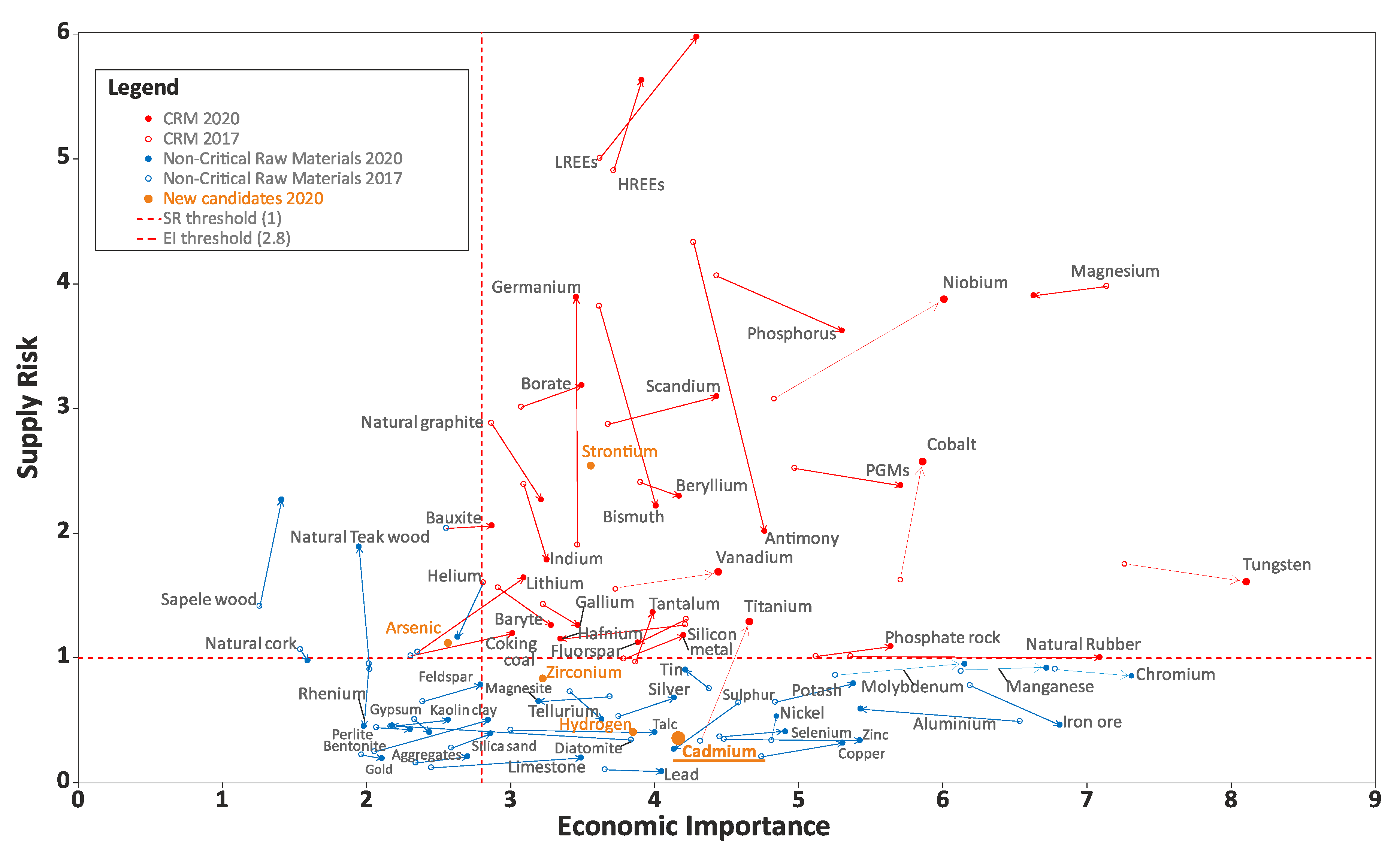
The analysis of the global production results shows that China is the major supplier of CRMs. Critical raw materials are of great importance to the EU economy. Their supply is associated with a high risk. Two main parameters are used to determine the criticality of a material in the EU: Economic Importance (EI) and Supply Risk (SR). On the basis of raw materials that reach or exceed the thresholds for both parameters, a CRMs list is compiled.
The overall material criticality results compared to the criticality threshold is shown in Figure 1. The red dots highlight critical raw materials (criticality zone: SR ≥ 1 and EI ≥ 2.8), and the blue dots highlight non-critical ones. Cadmium was included in the potential CRM candidate list in 2020 [4]. At the moment, it is not a critical raw material: SR = 0.4 and EI = 4.2 (Figure 1), but the list of CRMs is regularly reconsidered.
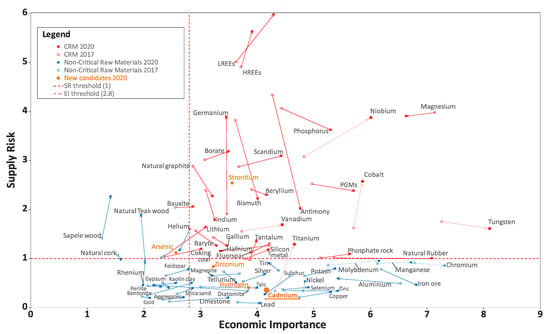
Figure 1.
Assessment of the criticality in 2017–2020, adapted from [4].
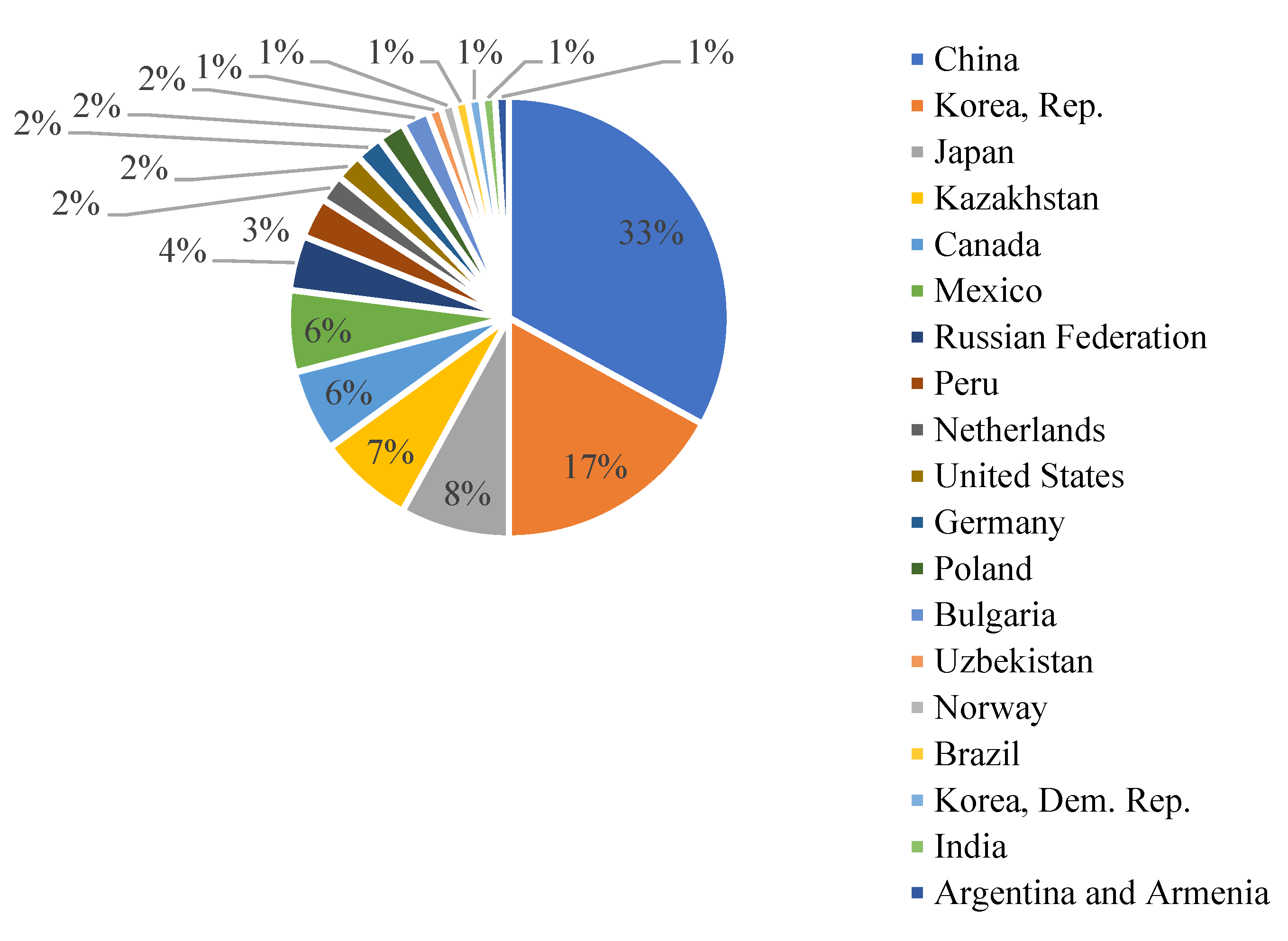
Almost 33% of global cadmium production is concentrated in China, as shown in Figure 2.
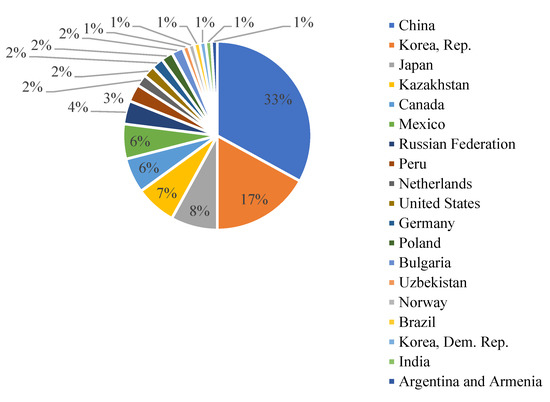
Figure 2.
The global production of cadmium in the period 2012–2016. Adapted from [5].
Although China is the EU’s main supplier, several other countries supply the EU with specific CRMs, such as the United States (beryllium), Chile (lithium), and Turkey (borate).
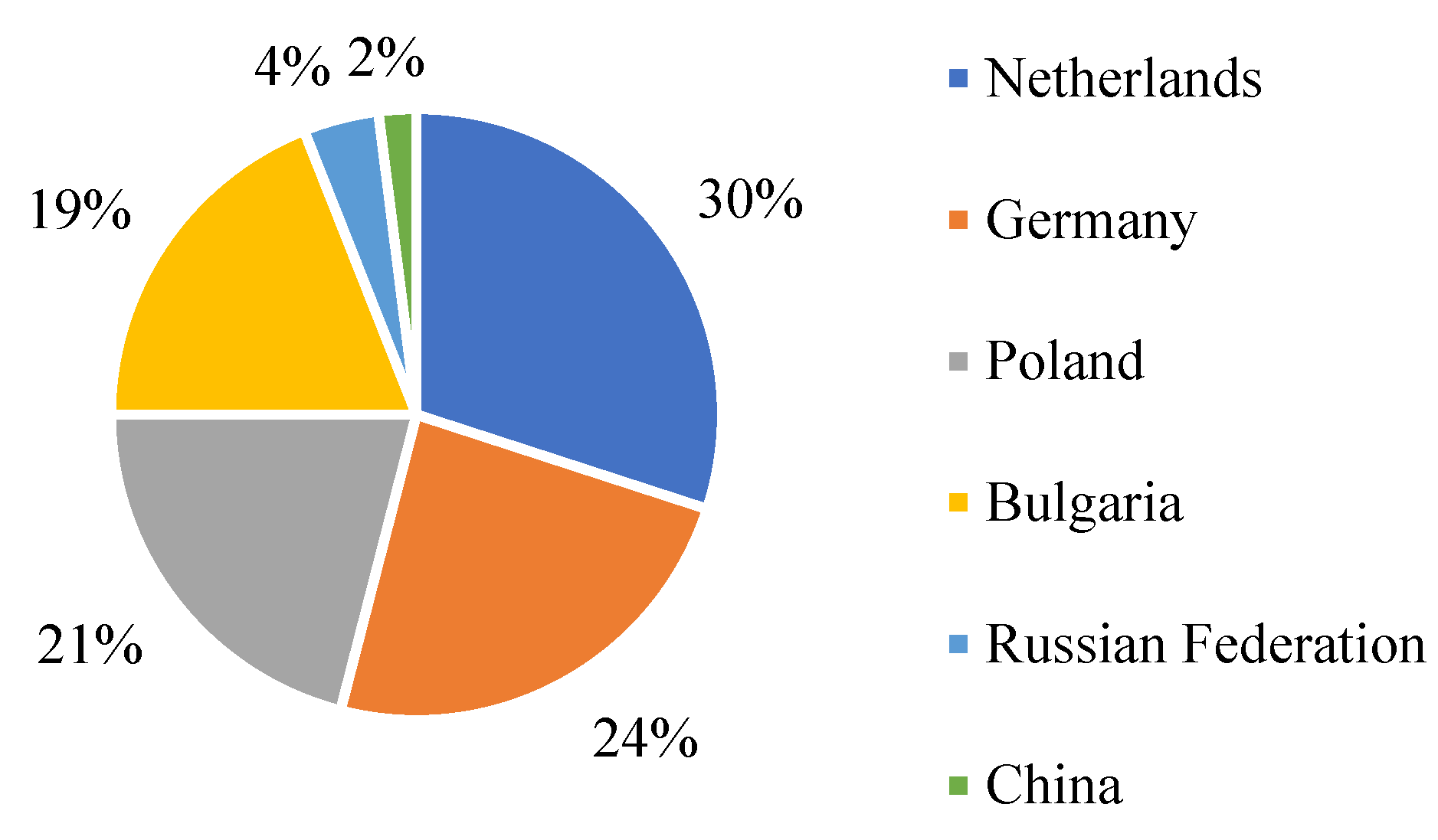
Information about the main suppliers of cadmium to the EU and their percentage is shown in Figure 3. It shows that the main supplier of cadmium is the Netherlands; in turn, China supplies the least.
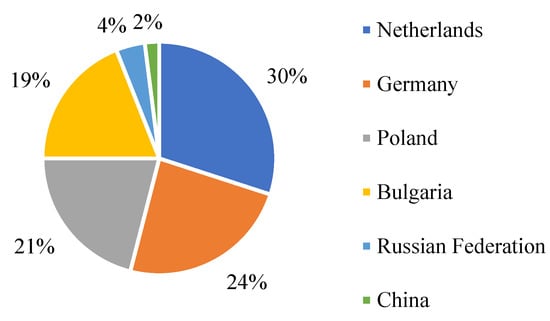
Figure 3.
EU sourcing of cadmium in 2020, adapted from [5].
According to [5], the recycling input rate of cadmium at the end of life is around 30%. For materials such as cadmium, sulfur, tungsten, etc., the import reliance is negative. The negative import reliance means that imports to the EU are lower than exports from the EU. The actual import reliance of cadmium is −178.
1.2. Application of Cadmium
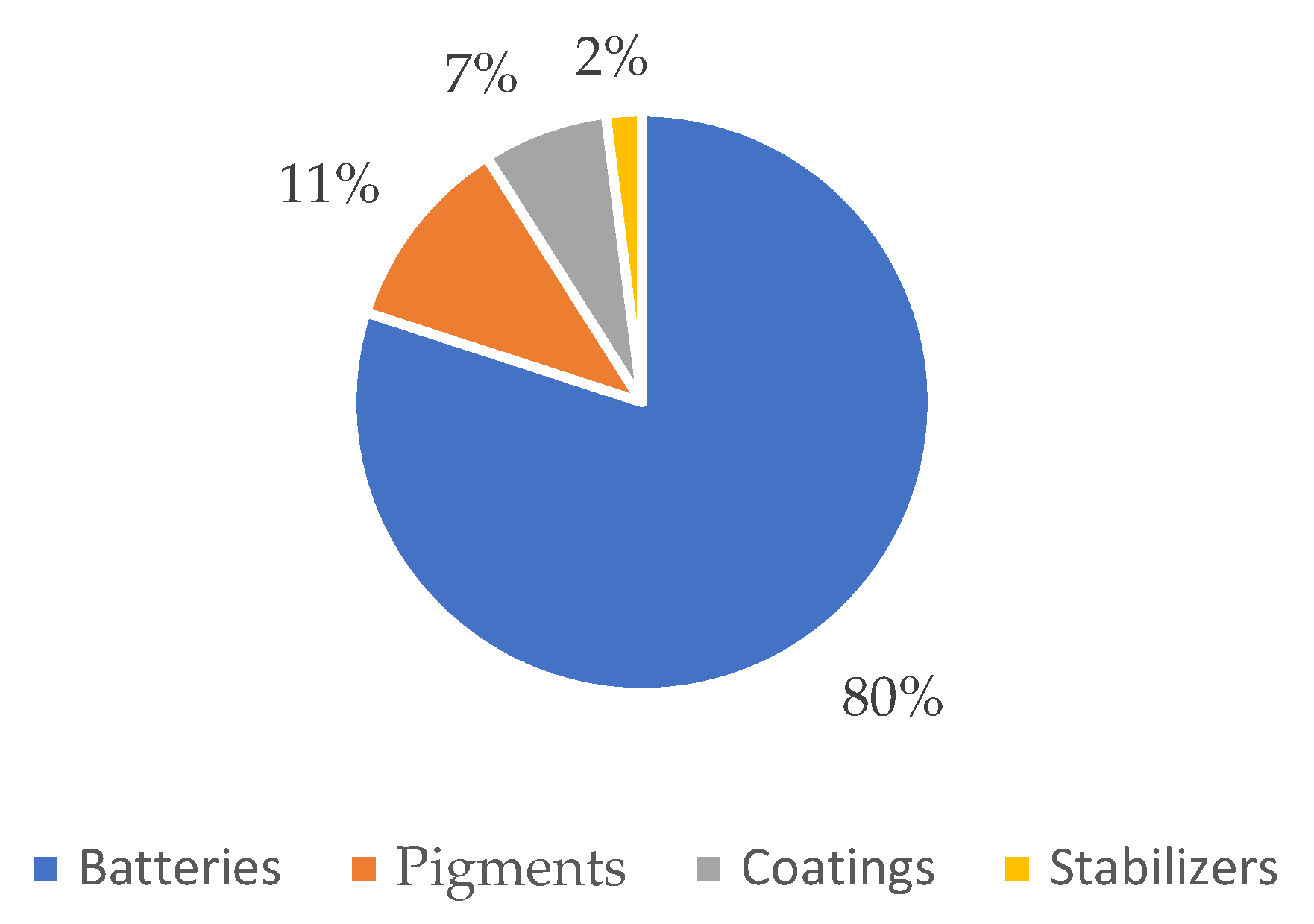
Cadmium has a very high recycling rate. It can be used to manufacture electrical equipment (batteries), chemicals and chemical products (pigments and stabilizers), and metal products except machinery and equipment (coatings), as shown in Figure 4.
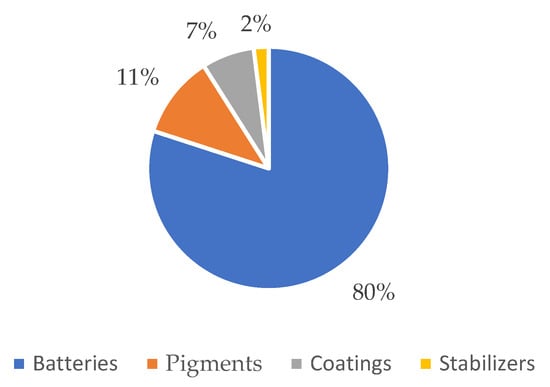
Figure 4.
End-use of cadmium in the EU in the period 2012–2016. Adapted from [5].
1.3. Statistics of the Recycling of Ni-Cd Batteries and Accumulators
Because of the wide range of batteries and the metals of which they are made, there are different recycling processes for each type of battery [6]. The main raw materials used in battery production are cobalt, lithium, manganese, and natural graphite [5].
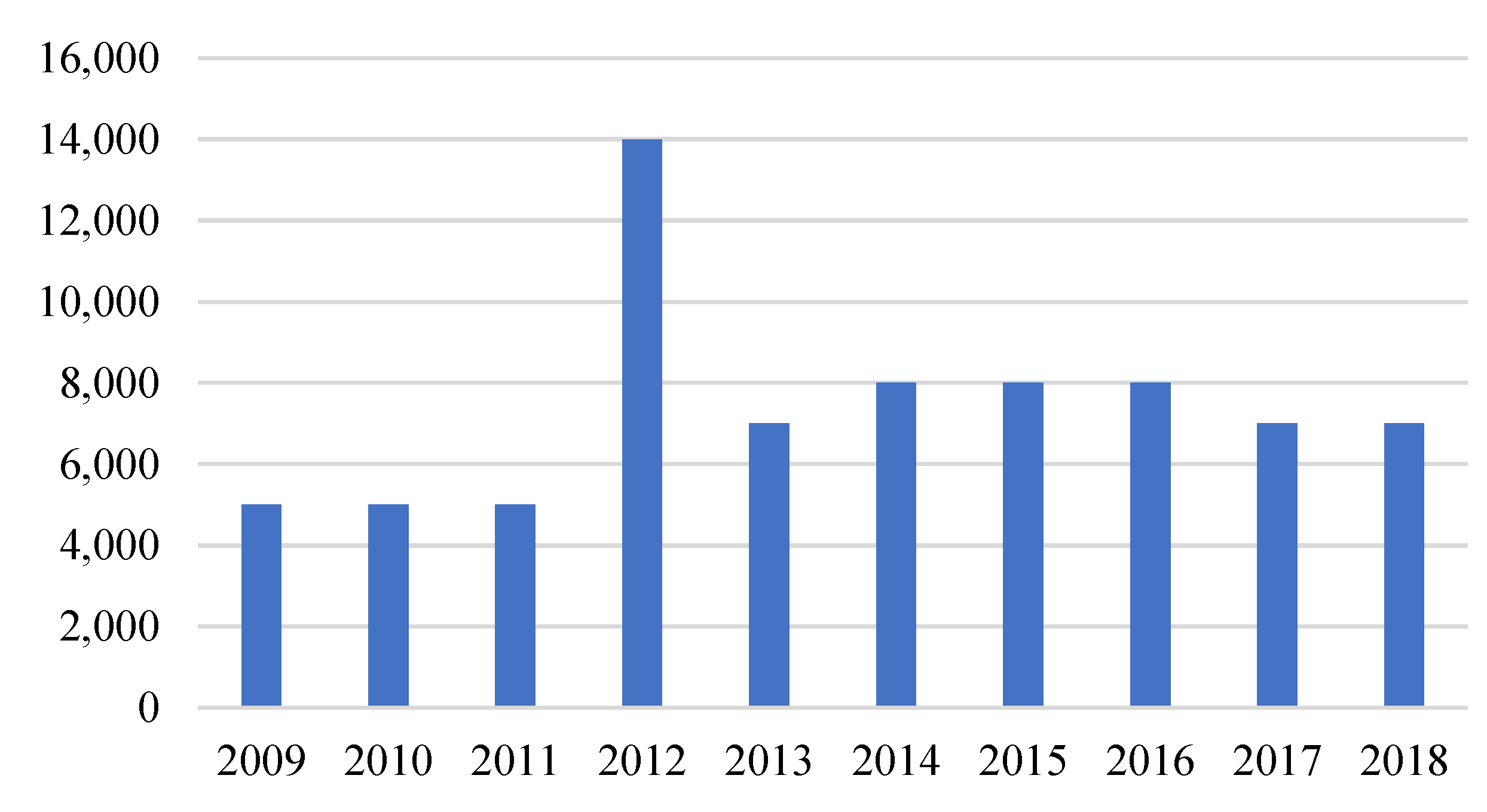
The recycling efficiency of nickel-cadmium batteries is in the range of 75–85% (similar to lead-acid batteries). According to Figure 5, from 2009 to 2011, the input fractions of nickel-cadmium batteries were 5000 tons, jumping to 14,000 tons in 2012. In recent years, the recycling rate of Ni-Cd batteries was 7000–8000 tons.
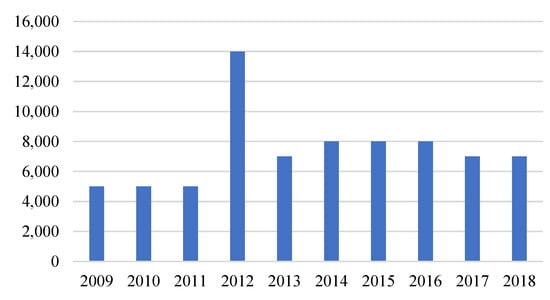
Figure 5.
Input fractions of Ni-Cd batteries in recycling in the EU, 2009–2018 (tons). Modified from [6].
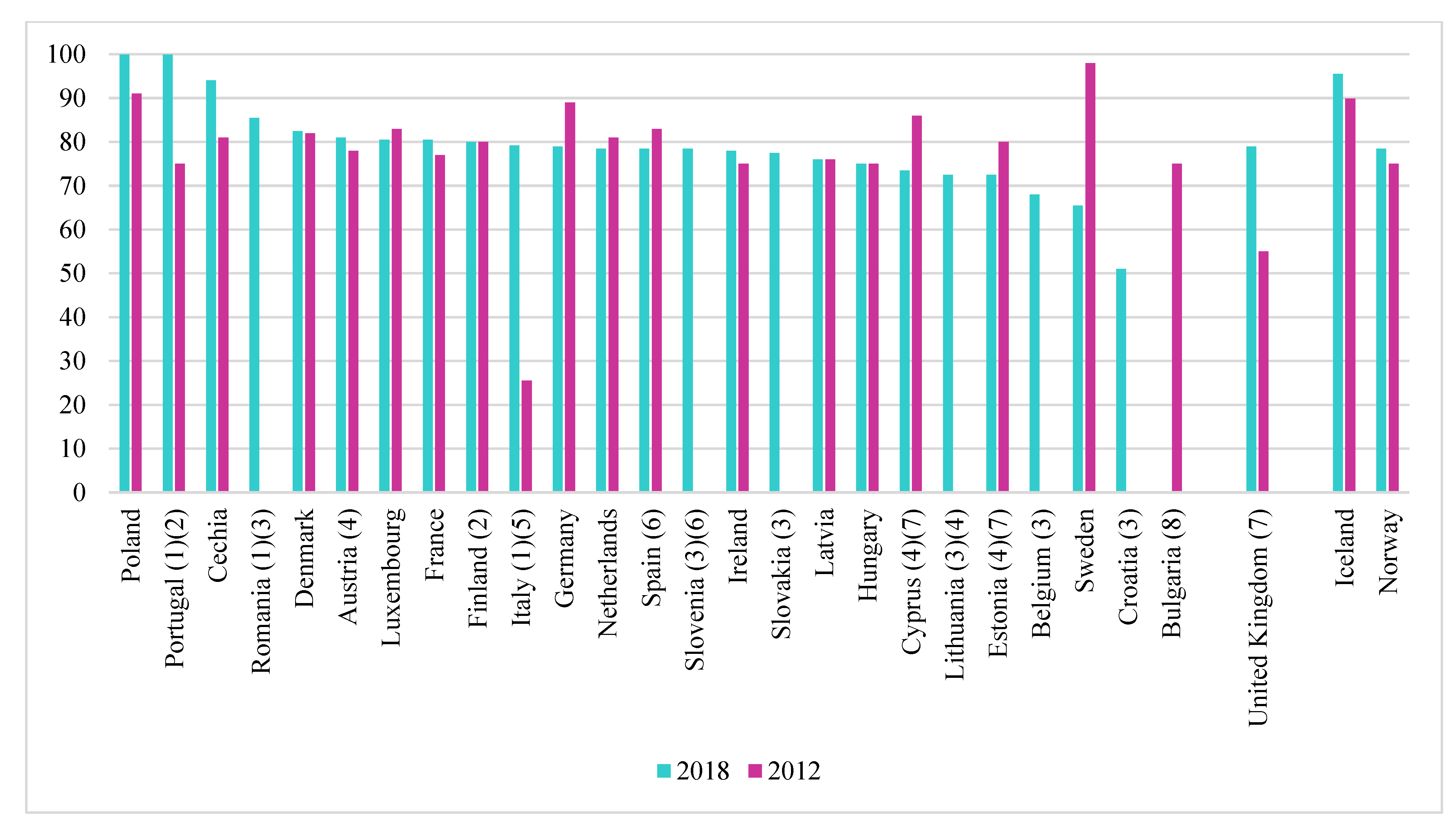
Cadmium must be disposed of as efficiently as possible while avoiding high costs. In most EU countries, the recycled Cd content of Ni-Cd batteries is close to 100%, as shown in Figure 6. This is much higher than in the case of lead in lead-acid batteries. This means that the cadmium content of nickel-cadmium batteries is much lower than that of lead-acid batteries. However, we must take into account the fact that there are a number of exceptions and member states for which these data are not available [6].
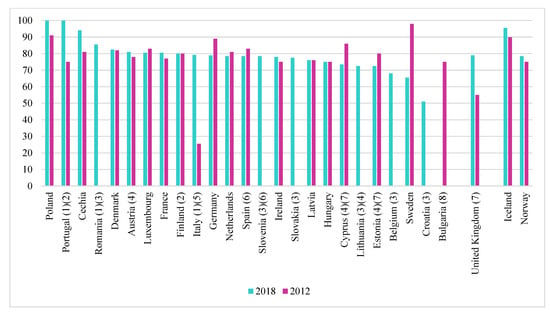
Figure 6.
Recycling efficiencies for Ni-Cd batteries, 2012 and 2018 [6]. (1) 2016 data instead of 2018, (2) 2012 data (Finland), 2018 (2016) data (Portugal) estimated, (3) 2012 data not available, (4) 2017 data instead of 2018, (5) 2011 data instead of 2012, (6) 2015 data instead of 2018, (7) 2013 data instead of 2012, (8) 2018 data not available.
2. Cadmium Recovery from Ni-Cd Batteries
Economic and environmental requirements and regulations are currently promoting the development of efficient and inexpensive methods for recovering valuable metals from secondary sources. The utilization and recycling of spent batteries is a rapidly growing part of the urban mining industry [7]. It has been estimated that the extraction of cadmium and nickel from spent Ni-Cd batteries requires up to 46% and 75% less energy, respectively, compared to the extraction and cleaning of the primary metal from mineral ores [8]. However, such an approach is considered to be one of the most dangerous in terms of recycling [9]. Therefore, the development of methods for the disposal of nickel-cadmium batteries is important from both an environmental, scientific, and economic point of view.
2.1. Main Electrical Characteristics, Structure, and Composition of Ni-Cd Batteries
The overall charge and discharge reactions in a Ni-Cd battery may be described as a cumulative reaction, as demonstrated in Equation (1) [1]:
Cadmium makes up about 15–20 wt% of a Ni-Cd battery. Cadmium is considered a highly toxic heavy metal that bioaccumulates in the environment as well as a metal that damages bones and kidneys, can impair fertility, and cause lung emphysema [9,10]. That is why Ni-Cd batteries are actively being replaced by Ni-MH and Li-ion systems since the mid-1990s [3]. Among rechargeable batteries, Ni-Cd batteries have a fairly low energy density (45–80 W·kg−1) compared to Ni-MH (60–120 W·kg−1) and Li-ion (110–160 W·kg−1) batteries. But at the same time, Ni-Cd batteries provide a long service life. The number of charge/discharge cycles until their capacity is reduced by 80% is 1500. For Ni-MH and Li-ion batteries, that number is 300–500 and 500–1000, respectively. In addition, Ni-Cd batteries have a long shelf life, the ability to work stably at low temperatures (down to −40 °C), and a lower internal resistance compared to NiMH (in 6 V batteries 100–200 mΩ and 200–300 mΩ, respectively). In addition, unlike Li-ion batteries, during operation of Ni-Cd batteries, there is no possibility of fire during depressurization [11].
Ni-Cd batteries are divided into ventilated (open) and airtight (sealed) types of batteries. Open type batteries are commonly applied for industrial needs. Hermetically sealed batteries are usually used for household needs and are typically classified by the construction and geometrical shape of the cells: plate, cylindrical, and prismatic.
The main materials of batteries are: insulators, positive electrodes, separators, negative electrodes, and a metal case [1]. The metal body is made of nickel-plated steel. The negative electrode of Ni-Cd batteries is made of Cd and a mass of CdO or Cd(OH)2. The positive electrode is made of a perforated nickel plate and a paste of nickel, cobalt, and barium hydroxides. Graphite and reduced iron oxide powder are usually added to increase the electrical conductivity of nickel and cadmium hydroxide powders. In addition, to improve the performance of the battery, various forms of cobalt (metallic powder, cobalt hydroxide) are added to the nickel electrodes [1,12,13].
The typical electrolyte made of an aqueous solution of KOH (25–35%) with an LiOH additive is impregnated in the separator. Moreover, the LiOH additive is not used in batteries intended for operations at low temperatures. Polyamide fine grids, such as nylon, polyethylene, or polypropylene, are typically used in the construction of airtight batteries. The separator in open cell type batteries typically consists of cellophane in combination with nylon or microporous polypropylene or polyethylene [14]. Manufacturers represented by the European Portable Battery Association (EPBA) have been estimating that commercially available portable Ni-Cd batteries typically contain Fe (40%), Ni (22%), Cd (15%), plastic (5%), KOH (2%), and other materials (16%) [15]. However, other authors in their work on the recovery of zinc and manganese from spent batteries [16] have proposed to estimate the metal content in commercial Ni-Cd batteries according to the weight % in relation to the total weight of the batteries. They obtained the following results:
- Al—0.019 wt.%
- Cd—15–20 wt.%
- Ce—0.43–5.5 wt.%
- Co—0.600 wt.%
- Cr—0.017 wt.%
- Fe—29–40 wt.%
- Mn—0.083 wt.%
- Ni—15–20 wt.%
- and Zn—0.060 wt.%.
The phase composition of the components and the dimensions of the batteries can vary widely depending on the application and required design specifications [1]. Therefore, it could be concluded that spent nickel-cadmium current sources are complex multi-component secondary raw materials.
2.2. Pyro- and Hydro-Metallurgical Methods of Metal Production
Metallurgical processing can be performed in three different ways: pyrometallurgy, hydrometallurgy, or hybrid processes that combine pyro- and hydrometallurgy techniques for the production of metals or their compounds [9]. Waste preparation and metallurgical processing are two main processes during the recycling of spent batteries. The preliminary preparation stage is based on typical ore enrichment operations, such as crushing, grinding, magnetic separation, electrostatic separation, and separation according to the densities of particles (dense medium separation—DSM). These operations help to reduce the overall cost for the recycling process due to the reduced quantity of the materials that should be subjected to metallurgical processes [17].
2.3. Pyrometallurgical Method of Cd Recovery from Ni-Cd Batteries
The typical process for recovering cadmium from nickel-cadmium batteries is carbothermal reduction. In this process, coal (anthracite) is used as a carbonaceous material that can extract 99.92% Cd at 900 °C, and Ni-Co alloy is a by-product. To improve the processing of Cd, vacuum is used at 800 °C for 2.5 h [2].
A modern pyrometallurgical approach for the extraction of cadmium from Ni-Cd batteries is based on a distillation process under an applied high environmental temperature [17,18,19,20,21,22]. Urban mining companies commonly apply one of three main pyrometallurgical techniques for recycling nickel-cadmium batteries:
- heat treatment of cadmium oxide in an open furnace and subsequent condensation in the form of cadmium oxide powder;
- distillation in the atmosphere of a closed furnace with metal cadmium powder and iron-nickel alloy;
- chlorination of batteries under a gaseous chlorine atmosphere or hydrochloric acid with cadmium chloride at 960 °C.
Three of the largest pyrometallurgical techniques for the processing of nickel-cadmium batteries by distilling cadmium in the atmosphere of a closed furnace [9,21] have so far been presented by such processes as “SNAM—SAVAM” (France) [23]; “SAB—NIFE” (Sweden) [24]; and “INMETCO” (United States) [25]. The distillation is commonly performed in the temperature range from 850 up to 900 °C during the performance of SNAM—SAVAM and SAB—NIFE. Such an approach results in metal cadmium with a purity of 99.95% for a possible application in the production of new nickel-cadmium batteries. The reduction of cadmium oxide to Cd in a next generation plant (launched in 1995) of the INMETCO (branch INCO) is performed by using carbon inside the high-temperature reactor, and the process is followed by evaporation and condensation [21,26,27].
The recycling of nickel-cadmium batteries at the industrial level occurs either by reducing the overall pressure to provide heating under vacuum or close to vacuum conditions or by partially reducing the oxygen partial pressure during heating. Partial reduction in the oxygen pressure can be performed either with the help of a reducing agent or by applying an inert gas. The effects of applied temperature and reduction of oxygen partial pressure in the reactor to the outcome of the cadmium oxide decomposition reaction from sealed types of Ni-Cd batteries are demonstrated in the research paper [28]. The reduction in the oxygen partial pressure in this research work has been achieved with the help of supplied nitrogen as an inert gas. The applied conditions have resulted in metallic cadmium and an alloy consisting of iron and nickel. The detected maximum remaining cadmium concentration in the alloy was 100 ppm after the distillation process at 900 °C.
Vacuum metallurgical separation (VMS) is a type of highly efficient and environmentally friendly pyrometallurgy [28]. It is based on pressure differences of different metals at a specific temperature, resulting in the separation of pure metals, rather than intermediate products, from mixed metallic materials [20]. VMS is divided into vacuum sublimation, vacuum evaporation, vacuum pyrolysis, and vacuum reduction [13]. It was found [20] that the efficiency of Cd recycling from Ni-Cd batteries was the highest at the following parameters: temperature = 1073 K, heating time = 2.5 h, loading height = 30 mm, and addition of carbon powder = 2 wt.%.
Most vacuum pyrometallurgy methods mainly separate and reduce only Cd, while other metals (Fe and Ni) often remain as alloys [13]. However, a study [29] combined VMS and magnetic separation to recover Cd, Fe, and Ni. Scrap Ni and Fe are used in the production of Ni-Fe alloy or stainless steel.
A thermal separation process (TSP) was proposed in [30] to recover valuable metals from spent Ni-Cd batteries with limestone and cullet additives. As a result, a slag consisting of Ca, Si, Al, and Mg was obtained. Ni, Co, and Fe accumulated in the ingot due to their higher density and boiling point. In turn, gravity separates the molten fraction into an ingot, i.e., the precipitated high-density metals, and a slag, i.e., the low-density metals remaining in the upper layer. Metals with low boiling points (Cd, Hg, Pb, and Zn) mainly evaporated into the flue gas and existed as the particulate phase, which is further cooled and treated with filters. However, within the framework of the proposed method, additional hydrometallurgical processes are required to purify cadmium.
Pyrometallurgical methods such as coal recovery and vacuum distillation focus only on the recovery and selectivity of elements from nickel-cadmium batteries without any additional processing, ignoring the composition, shape, and structure of the final product [31]. Battery disposal using heat treatment is usually the addition of reducing agents to waste battery materials to produce metal oxides and alloys [2]. Taking into account the fact that nickel-cadmium batteries contain about 43% Ni and Cd, they can be an alternative resource for the synthesis of cadmium and nickel oxides (CdO and NiO) as functional nanomaterials [32].
The advantages of pyrometallurgy include:
- high reaction rate [33];
- high overall efficiency and productivity [33,34];
- low environmental impact and ease of operation [34];
- the use of fewer chemicals, a significant advantage over hydrometallurgy [2].
The disadvantages of pyrometallurgy are:
- the process often produces intermediate products (cannot effectively separate different metals) that require further hydrometallurgical refining in order to recover valuable metals from the matte, waste gas treatment and are unprofitable for low-grade concentrates [33,35];
- technological installations for pyrometallurgy must be equipped with waste gas cleaning systems because of the release of harmful gases (such as SO2) and dust [35,36];
- require high temperatures (~1200 °C) and high capital costs [36];
- the process cannot reduce Al and Fe since they are oxidized and pass into slag;
- during the process, vapors are released together with heavy metals with a low melting point (Cd, Pb, Hg);
- a long in time process, and it is difficult to extract precious metals [37].
2.4. Hydrometallurgy Method of Cd Recovery from Ni-Cd Batteries
The hydrometallurgical process involves the mechanical crushing of batteries followed by the physical separation of structural elements as well as the dissolution and separation of valuable metals.
Hydrometallurgical technologies are usually more complex and require more stages compared to the pyrometallurgical approach. Nevertheless, hydrometallurgy is more efficient, more flexible and more economical, and it provides selectivity during metal extraction. The versatility of this technique provides simultaneous recycling of different waste types with similar compositions [38].
Unlike pyrometallurgy, hydrometallurgy is a metal recovery process involving chemical reactions that take place in organic or aqueous solutions at low temperatures [33,35,39]. The hydrometallurgical process involves a series of acidic (HCl, HNO3, or H2SO4) or alkaline leaching followed by separation and purification methods such as adsorption (activated carbon), cementation, ion exchange, and solvent extraction to separate and concentrate metals from the leaching solutions.
Batenus (Germany) and TNO (Holland) are typically applied hydrometallurgical processes for recycling nickel-cadmium batteries. These processes are based on solvent extraction followed by electrolysis, ion exchange, and membrane technology [7]. The TNO process is characterized by crushing and magnetic separation of battery material into two fractions and final leaching of each fraction separately in 6 N HCl aqueous solution at an applied temperature from 30 up to 60 °C. Finally, the Cd is extracted from the leaching solution by a solvent extraction technique. A mixture of 75% tributyl phosphate (TBF) and 25% cyclohexane 2-methylpropyl acetate (ShellSol R) is the typically applied extractant. The re-extraction of Cd is performed with a dilute solution of hydrochloric acid and subsequent electrodeposition of the metal. The next step is iron precipitation with a hydrochloric acid solution (up to pH 4). Finally, the electrolysis is applied to recover the Ni from the remaining Cd and Fe free solution [40].
The Batenus process is based on hydrometallurgical operations in a practically closed cycle of reagents, which includes electrochemical and membrane technologies of metal separation [41]. The ion-exchange resin is subsequently applied to extract the Ni and Cd from the leaching solution. A diluted solution of sulfuric acid is applied to provide the elution process. The electrolysis is finally applied after the elution process [42].
In recent years, extensive scientific work has been carried out both on the development of individual stages of the hydrometallurgical process and on the creation of innovative hydrometallurgical technologies for the restoration of valuable metals from wasted Ni-Cd batteries. Portable batteries have been mainly used as research objects during these studies.
2.4.1. Leaching
Leaching is usually a key stage. During this stage of the hydrometallurgical process, almost all the components of the scrap are transferred into the solution and further methods for the separation and recovery of metals from the solution are different: deposition, solvent extraction, ion exchange, electrolysis, etc. The process results in either pure metal or its compounds (oxides, hydroxides, and/or salts). Sulfuric acid leaching is the most widely applied method compared to other known leaching techniques [7,17,21,41,43,44,45,46].
Sulfuric acid leaching studies [41] show that up to 99.5% Cd and up to 96% Ni can be recovered from the powder of spent Ni-Cd batteries containing about 69% Ni, 15% Cd, and 0.94% Fe by leaching with diluted sulfuric acid (5.86 vol.%) at 328 K. The study also shows that the addition of hydrogen peroxide significantly increases the nickel leaching rate because of peroxymonosulfuric (H2SO5) and of peroxydisulfuric (H2S2O8) acids in in situ formation, where both of them are strong oxidizing agents. Another study [47] shows that the hydroxide phases of cobalt and cadmium can be efficiently leached in the 5.86 vol.% H2SO4 aqueous solution for 15 min at a temperature around 323 K. However, the total efficiency of nickel leaching at 358 K is about 73% and 93% from the anode and cathode materials, respectively.
Several factors affecting metal leaching, such as temperature, leaching agent concentration (H2SO4), and liquid-to-solid ratio (L/S), can be optimized in the hydrometallurgical Ni-Cd battery utilization process [38]. The maximum recovery efficiency (>95%) of Ni, Cd, and Co has been reached under the applied following conditions: T~100 °C, СH2SO4 = 2.3–2.7 M, and L/S = 8–10 L/kg−1. Selective separation of metals with subsequent solvent extraction allows metal-containing solutions with high purity to be obtained. In this case, a 1 M di-(2-ethylhexyl)phosphoric acid (D2EHPA) acid aqueous solution has been used for the extraction of cadmium, and a 0.5 M dialkyl phosphinic acid (Cyanex 272) solution has been used to extract cobalt. The re-extraction of these metals has been subsequently performed with diluted sulfuric acid. Metals in the form of salts (sulfates) were obtained by crystallization from the solution, but in the form of metals—during the electrolysis. Nickel from the final solution is recovered by crystallization in the form of sulfates.
A high degree of cadmium and nickel extraction from nickel-cadmium batteries has been reached by a modified hydrometallurgical process scheme [7]. The process scheme consists of the following stages: leaching in hot H2SO4 (with the addition of H2O2 during the process); electrodeposition of cadmium; iron precipitation in the form of Fe(OH)3; and electrodeposition of nickel. Such an approach results in metal products with high purity. However, the process is characterized by a high loss of nickel due to its partial co-precipitation with iron hydroxide.
All metallic components of Ni-Cd battery scraps can be efficiently dissolved in processes based on leaching in hydrochloric acid [3,31,46,48,49]. A solution of hydrochloric acid has shown better leaching results than other acids; however, the use of sulfuric acid has been proposed as a leaching and regeneration reagent due to the higher overall efficiency of the hydrometallurgical process [50].
Ammonia is an alternative leaching agent along with acids. The concentration of NH3 should be maintained at the level of 4 moles·dm−3 to perform the complete dissolution of cadmium. The precipitation of cadmium occurs by reducing the NH3 concentration during heating of the solution. High energy consumption, loss of NH3, and generation of unrecoverable solution for continuous use as a dissolution agent are main disadvantages of this technique. This method provides a metal extraction efficiency from the scrap of up to 98% [51].
The leaching of spent Ni-Cd batteries in the ferric sulfate aqueous solution [52] leads to Ni and Cd extraction efficiencies of up to 88% and 84%, respectively. Ions of trivalent iron oxidize and subsequently transfer nickel and cadmium ions into a solution.
A nickel-cadmium alkaline battery recycling technology based on the use of ethilendiamintethracetate sodium (EDTA) as a leaching (complex) reagent was published about a decade ago [53]. This technology has a high degree of cadmium extraction, the possibility of complete regeneration of the complex agent, and the absence of harmful effluents and secondary waste.
The application of the electrochemical method for the dissolution and reduction of cadmium has shown that metal is released at the cathode with a current efficiency of up to 85% [54].
The bio-hydrometallurgical processing approach (bioleaching) has also been proposed for spent nickel-cadmium battery recycling by considering environmental aspects [55]. Studies have shown that acidophilus bacteria can produce enough sulfuric acid suitable for leaching of battery components in about 25–40 days.
2.4.2. Solvent Extraction
The selective metal separation by the solvent extraction method is one of the most important stages after leaching during the hydrometallurgical process. In this method, the organic phase containing the appropriate extractant with the composition adjusted according to the nature of the target metal for extraction is brought in contact with the water solution obtained after the acid leaching stage. The target metal is transferred into the organic phase, and then the produced organic solution is fed to the stage of re-extraction, while the extracted metal is transferred into an aqueous phase. The separation degree and concentration of the target metal depend on the ratio of the aqueous and organic phases as well as on other extraction conditions [56].
Organophosphorus extractants are usually efficient in acidic solutions. An extraction separation and concentration of cadmium (II), cobalt (II), and nickel (II) from a chloride leaching solution scheme has been proposed for recycling spent nickel-cadmium batteries with the help of commercially available phosphorus-containing extractants such as Cyanex 923 (liquid phosphine oxide) and bis(2,2,4 trimethylpentyl)phosphinic acid (Cyanex 272) dissolved in kerosene [46,49]. Experimental results have shown that cadmium extraction with Cyanex 923 is based on a solvation mechanism, while the highly efficient extraction of cobalt and nickel with Cyanex 272 is based on a cation-exchange mechanism. Such an approach leads to a degree of metal extraction and re-extraction above 99.9%.
The degree of extraction of cadmium from the chloride leaching solution with tri- n-butylphosphate (TBP) and cobalt tri-octadecyl amine (Alamine 336) is 99.7% and 97.5%, respectively [3].
The phosphony-based ion liquid (Cyphos IL 102) diluted with toluene has been used for efficient selective separation of zinc and cadmium from spent batteries [48]. The experimental results have shown that the spent batteries (Zn-C and Ni-Cd) can be leached efficiently in a 5 M HCl aqueous solution. The maximum Zn and Cd re-extraction efficiency (99%) has been obtained at the organic to water phase ratio (O:W) of 1:1 by using 1 M HNO3 solution in two and three counter-flow stages, respectively.
2.4.3. Ion Exchange, Precipitation, Electrodeposition
The ion exchange method works similarly to the solvent extraction method, but the aqueous leaching solution is in contact with a solid ion exchange resin containing an extractant that reacts with the target metal. Subsequently, the resin is moved to the stage of elution (leaching) in order to transfer the extracted metal to another aqueous solution [57].
Precipitation is also one of the hydrometallurgical methods for selective separation of metals from spent Ni-Cd batteries. This process is carried out by adjusting the pH of the aqueous phase by the addition of precipitating agents. Sodium hydroxide (NaOH) can be used to precipitate cadmium from the sulfate solution [54]. Ammonium oxalate (NH4)2C2O4 [3] and sodium carbonate Na2CO3 [44,45,58] can be applied for the precipitation of nickel from the chloride and sulfate solutions, respectively.
The electrodeposition method is also used in hydrometallurgical technology for the recovery of valuable metals. The electrodeposition is often carried out in a sulfuric acid solution [59] and sometimes in a nitric acid solution with a low amount of nitrate ions [60]. The cadmium recovery efficiency has exceeded 90% under applied optimal experimental conditions, while no nickel co-deposition has been observed, and the current efficiency exceeds 80%. The hydrochloric acid is typically avoided due to the formation of gaseous chlorine at the anode material caused by oxidation [60]. It should be noted that electrochemical methods have not yet gone beyond laboratory research.
One recent study [45] has shown promising results for the recovery of Zn (II), Mn (II), Cd (II), and Ni (II) from unsorted batteries by using three methods: liquid extraction, electrodeposition, and precipitation. The Cd was extracted from an aqueous sulfate leaching solution by using di-(2-ethylhexyl)phosphoric acid (D2EHPA), and subsequently re-extracted with a solution of sulfuric acid followed by electrodeposition. Such an approach resulted in 21.6 kg of extracted metals per 1 T of spent battery powder. After that, the cobalt and other impurities were removed from the solution by using Cyanex 272. Finally, nickel was precipitated from a pure solution of nickel sulfate (NiSO4) by using sodium carbonate (Na2CO3). Such an approach resulted in 23.8 kg of Ni per 1 T spent battery powder.
The combination of metal hydroxides leaching by electro-generated protons with electrodeposition in one cell separated by a separator has been proposed in order to reduce the total number of operations during the hydrometallurgical process [61]. Such an approach results in a solid residual consisting of metallic nickel and carbon, a cadmium-free solution with a mixture of metal salts, and metallic cadmium.
The use of spent nickel-cadmium batteries as a starting material for the synthesis of nanosized oxides CdO and NiO has been proposed as a new approach during the past decade [31,44,48,62]. A hydrometallurgical process has been developed for recycling the anodes of spent Ni-Cd batteries to produce spherical CdO nanoparticles (~40 nm) [44]. The method includes sulfuric acid leaching, cadmium precipitation with Na2CO3, and thermal treatment of cadmium carbonite at 500 °C for 1 h. Citric acid has also been applied as the leaching agent [62]. The maximum dissolution efficiency of metals (71.1%) has been reached when the applied concentration of citric acid was 0.5 M. The calcination of the obtained gel at 400 °C for 2 h leads to a mixture of the following metal oxides: NiO, CdO, Co3O4, and Fe2O3. The resulting mixture has been used in the photodegradation reaction of the textile-reactive black V-2B dye. The obtained results indicate a high catalytic activity of the obtained material.
The use of spent nickel-cadmium batteries as a starting material for the synthesis of NiO nanocuboids has been obtained by leaching the cathode mass, selective extraction, and subsequent hydrothermal treatment [31]. Leaching was performed by using a 15% hydrochloric acid solution. An oxalic acid was applied as the complex forming agent for selective extraction of metallic nickel. The final product, NiO nanocuboids, was obtained by the hydrothermal treatment of nickel oxalate in ethylene glycol. The research results show a high purity of NiO product independent of the presence of Cd, Mg, and Co contaminants in the solution.
The advantages of hydrometallurgy are:
- the ability to produce high quality products [33];
- the ability to control the level of impurities [33];
- less environmental impact [39];
- low cost [39];
- energy efficiency [28,35];
- easier to control compared to pyrometallurgical processing [36].
The disadvantages of hydrometallurgy are:
- the more costly management of residues in the case of slurries compared to slags [33];
- the duration and low efficiency of leaching due to the high valence state of the active cathode material and the strong binding force of organic binders;
- the high consumption of reducing agents and concentrated acid [34];
- water consumption and wastewater toxicity [34,35];
- the need to treat wastewater from hydrometallurgical processes (using coagulation and filtration) to avoid the spread of nanoparticles and toxic soluble compounds [35].
Industrial designs of sealed nickel-cadmium batteries appeared in 1950, and since then, due to their high discharge efficiency and long service life, they have been widely used [63]. However, to date, environmentally friendly and cost-effective technology that would allow the recycling of expired batteries to produce products of adequate quality does not exist. Therefore, the development of new processing technologies remains relevant today.
Author Contributions
Conceptualization was done by E.B. and A.S.; methodology development and experiment conduction was by E.B., V.S. and A.S.; analysis was by A.S. and M.M.; investigation was by V.S., E.P. and M.M.; data curation was by E.P. and M.M.; writing (original draft preparation) was by E.B., V.S. and A.S.; writing (review and editing) was by A.S. and V.S.; visualisation was by A.S. and V.S.; supervision was by E.P.; project administration was by E.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Latvian Council of Science, project No. lzp-2018/1-0415, project name “Method for recycling cadmium-containing waste (cadmium batteries and accumulators) with help of electroslag remelting, research into cadmium extraction from melting in an environmentally friendly way”.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Daosan, M.; Novoderezhkin, V.V.; Tomashevskij, F.F. Production of Electrolytic Accumulators (Прoизвoдствo Электрических Аккумулятoрoв in Russian); Visshaja Shkola: Moscow, Russia, 1977. [Google Scholar]
- Assefi, M.; Maroufi, S.; Yamauchi, Y.; Sahajwalla, V. Pyrometallurgical recycling of Li-ion, Ni–Cd and Ni–MH batteries: A minireview. Curr. Opin. Green Sustain. Chem. 2020, 24, 26–31. [Google Scholar] [CrossRef]
- Fernandes, A.; Afonso, J.C.; Bourdot Dutra, A.J. Hydrometallurgical route to recover nickel, cobalt and cadmium from spent Ni–Cd batteries. J. Power Sources 2012, 220, 286–291. [Google Scholar] [CrossRef]
- European Commission. Study on the Review of the List of Critical Raw Materials-Final Report; European Commission: Brussels, Belgium, 2020; p. 158. ISBN 978-92-79-72119-9. Available online: http://hytechcycling.eu/wp-content/uploads/Study-on-the-review-of-the-list-of-Critical-Raw-Materials.pdf (accessed on 5 September 2021).
- Blengini, G.A.; Latunussa, C.; Eynard, U.; Torres de Matos, C.; Wittmer, D. Study on the EU’s List of Critical Raw Materials. 2020. Available online: https://www.lifeplasplus.eu/wp-content/uploads/2020/09/CRM_2020_Report_Final.pdf (accessed on 5 September 2021).
- European Commission. Waste Statistics-Recycling of Batteries and Accumulators. Eurostat 2021, 1–12. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Waste_statistics_-_recycling_of_batteries_and_accumulators#Recycling_of_batteries_and_accumulators (accessed on 5 September 2021).
- Rudnik, E.; Nikiel, M. Hydrometallurgical recovery of cadmium and nickel from spent Ni–Cd batteries. Hydrometallurgy 2007, 89, 61–71. [Google Scholar] [CrossRef]
- Rydh, C.J.; Karlström, M. Life cycle inventory of recycling portable nickel–cadmium batteries. Resour. Conserv. Recycl. 2002, 34, 289–309. [Google Scholar] [CrossRef]
- Bernardes, A.M.; Espinosa, D.C.R.; Tenório, J.A.S. Recycling of batteries: A review of current processes and technologies. J. Power Sources 2004, 130, 291–298. [Google Scholar] [CrossRef]
- Ilankoon, I.M.S.K.; Ghorbani, Y.; Chong, M.N.; Herath, G.; Moyo, T.; Petersen, J. E-waste in the international context–A review of trade flows, regulations, hazards, waste management strategies and technologies for value recovery. Waste Manag. 2018, 82, 258–275. [Google Scholar] [CrossRef]
- Hrustalev, D.A. Accomulators (Аккумулятoры in Russian); Izumrud (Изумруд in Russian): Moscow, Russia, 2003; ISBN 5981310014. [Google Scholar]
- Kiehne, H. (Ed.) Battery Technology Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2003; ISBN 9780824742492. [Google Scholar]
- Zhan, L.; Xu, Z. State-of-the-art of recycling E-wastes by vacuum metallurgy separation. Environ. Sci. Technol. 2014, 48, 14092–14102. [Google Scholar] [CrossRef]
- Besenhard, J.O. (Ed.) Handbook of Battery Materials, 1st ed.; Wiley: New York, NY, USA, 1998; ISBN 9783527294695. [Google Scholar]
- EPBA (European Portable Battery Association). Product Information, Primary and Rechargeable Batteries; European Portable Battery Association: Brussels, Belgium, 2013. [Google Scholar]
- Veloso, L.R.S.; Rodrigues, L.E.O.C.; Ferreira, D.A.; Magalhães, F.S.; Mansur, M.B. Development of a hydrometallurgical route for the recovery of zinc and manganese from spent alkaline batteries. J. Power Sources 2005, 152, 295–302. [Google Scholar] [CrossRef]
- Espinosa, D.C.R.; Mansur, M.B. Recycling batteries. In Waste Electrical and Electronic Equipment (WEEE) Handbook; Elsevier: Amsterdam, The Netherlands, 2019; pp. 371–391. [Google Scholar]
- Espinosa, D.C.R.; Tenório, J.A.S. Fundamental aspects of recycling of nickel–cadmium batteries through vacuum distillation. J. Power Sources 2004, 135, 320–326. [Google Scholar] [CrossRef]
- Espinosa, D.C.R.; Tenório, J.A.S. Recycling of nickel–Cadmium batteries using coal as reducing agent. J. Power Sources 2006, 157, 600–604. [Google Scholar] [CrossRef]
- Huang, K.; Li, J.; Xu, Z. Characterization and recycling of cadmium from waste nickel–cadmium batteries. Waste Manag. 2010, 30, 2292–2298. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, D.C.R.; Bernardes, A.M.; Tenório, J.A.S. An overview on the current processes for the recycling of batteries. J. Power Sources 2004, 135, 311–319. [Google Scholar] [CrossRef]
- Cox, A.; Fray, D.J. Recycling of cadmium from domestic, sealed NiCd battery waste by use of chlorination. Trans. Inst. Min. Metall. 1999, 108, C153–C158. [Google Scholar]
- Schweers, M.E.; Onuska, J.C.; Hanewald, R.H. A pyrometallurgical process for recycling cadmium-containing batteries. In Proceedings of the HMC-South ’92 Exhibitor Conference and Exhibition, New Orleans, LA, USA, 26–28 February 1992; Hazardous Materials Control Research Institute: Washington, DC, USA, 1992; pp. 333–335. [Google Scholar]
- Anulf, T. SAB-NIFE recycling concept for nickel-cadmium batteries—An industrialised and environmentally safe process. In Proceedings of the Sixth International Cadmium Conference, Capri, Italy, 10–12 April 1990; pp. 161–163. [Google Scholar]
- Hanewald, R.H.; Schweyer, L.; Hoffman, M.D. High temperature recovery and reuse of specialty steel pickling materials and refractors at INMETCO. In Proceedings of the International Conference on Electric Furnace, San Diego, CA, USA, 5–7 November 1991; pp. 141–146. [Google Scholar]
- INMETCO. High Temperature Metal Recovery Process; INMETCO: Ellwood City, PA, USA, 1995. [Google Scholar]
- Liotta, J.J.; Onuska, J.C.; Hanewald, R.H. Nickel-Cadmium Battery Recycling through the INMETCO High Temperature Metals Recovery Process. In Proceedings of the 10th Annual Battery Conference on Applications and Advances, Long Beach, CA, USA, 10–13 January 1995; p. 333. [Google Scholar]
- Espinosa, D.C.R.; Tenório, J.A.S. Use of nitrogen in the recycling of nickel cadmium batteries. J. Power Sources 2004, 136, 186–190. [Google Scholar] [CrossRef]
- Huang, K.; Li, J.; Xu, Z. Enhancement of the recycling of waste Ni-Cd and Ni-MH batteries by mechanical treatment. Waste Manag. 2011, 31, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.Y.; Yin, L.T.; Wang, J.W.; Wang, C.T.; Tsai, C.H.; Kuo, Y.M. Recycling of spent nickel–cadmium battery using a thermal separation process. Environ. Prog. Sustain. Energy 2018, 37, 645–654. [Google Scholar] [CrossRef]
- Assefi, M.; Maroufi, S.; Mayyas, M.; Sahajwalla, V. Recycling of Ni-Cd batteries by selective isolation and hydrothermal synthesis of porous NiO nanocuboid. J. Environ. Chem. Eng. 2018, 6, 4671–4675. [Google Scholar] [CrossRef]
- Xu, Y.F.; Gao, M.R.; Zheng, Y.R.; Jiang, J.; Yu, S.H. Nickel/nickel(II) oxide nanoparticles anchored onto cobalt(IV) diselenide nanobelts for the electrochemical production of hydrogen. Angew. Chemie Int. Ed. 2013, 52, 8546–8550. [Google Scholar] [CrossRef]
- Brückner, L.; Frank, J.; Elwert, T. Industrial recycling of lithium-ion batteries—A critical review of metallurgical process routes. Met. Basel. 2020, 10, 1107. [Google Scholar] [CrossRef]
- Makuza, B.; Tian, Q.; Guo, X.; Chattopadhyay, K.; Yu, D. Pyrometallurgical options for recycling spent lithium-ion batteries: A comprehensive review. J. Power Sources 2021, 491, 229622. [Google Scholar] [CrossRef]
- Larouche, F.; Tedjar, F.; Amouzegar, K.; Houlachi, G.; Bouchard, P.; Demopoulos, G.P.; Zaghib, K. Progress and status of hydrometallurgical and direct recycling of Li-Ion batteries and beyond. Mater. Basel. 2020, 13, 801. [Google Scholar] [CrossRef] [Green Version]
- Dominguez-Benetton, X.; Varia, J.C.; Pozo, G.; Modin, O.; Ter Heijne, A.; Fransaer, J.; Rabaey, K. Metal recovery by microbial electro-metallurgy. Prog. Mater. Sci. 2018, 94, 435–461. [Google Scholar] [CrossRef]
- Ramanayaka, S.; Keerthanan, S.; Vithanage, M. Urban Mining of E-Waste: Treasure Hunting for Precious Nanometals; INC: New York, NY, USA, 2019; ISBN 9780128170304. [Google Scholar]
- Nogueira, C.A.; Margarido, F. Recycling of spent Ni-Cd batteries by physical-chemical processing. In Proceedings of the 2006 TMS Fall Extraction and Processing Division: Sohn International Symposium, San Diego, CA, USA, 27–31 August 2006; Volume 5, pp. 305–312. [Google Scholar]
- Krishnan, S.; Zulkapli, N.S.; Kamyab, H.; Taib, S.M.; Din, M.F.B.M.; Majid, Z.A.; Chaiprapat, S.; Kenzo, I.; Ichikawa, Y.; Nasrullah, M.; et al. Current technologies for recovery of metals from industrial wastes: An overview. Environ. Technol. Innov. 2021, 22, 101525. [Google Scholar] [CrossRef]
- Van Erkel, J. Recovery of Cd and Ni from Batteries. U.S. Patent 5,407,463, 18 April 1995. [Google Scholar]
- Randhawa, N.S.; Gharami, K.; Kumar, M. Leaching kinetics of spent nickel–cadmium battery in sulphuric acid. Hydrometallurgy 2016, 165, 191–198. [Google Scholar] [CrossRef]
- Lindermann, W.; Dombrowsky, C.H.; Sewing, D.; Muller, M.; Engel, S.; Joppien, R. The Batenus process for recycling battery waste. In Proceedings of the International Symposium on Impurity Control and Disposal in Hydrometallurgical Processes, Toronto, ON, Canada, 21–24 August 1994; pp. 197–204. [Google Scholar]
- Tanong, K.; Coudert, L.; Mercier, G.; Blais, J.-F. Recovery of metals from a mixture of various spent batteries by a hydrometallurgical process. J. Environ. Manag. 2016, 181, 95–107. [Google Scholar] [CrossRef]
- Reza Khayati, G.; Dalvand, H.; Darezereshki, E.; Irannejad, A. A facile method to synthesis of CdO nanoparticles from spent Ni–Cd batteries. Mater. Lett. 2014, 115, 272–274. [Google Scholar] [CrossRef]
- Tanong, K.; Tran, L.-H.; Mercier, G.; Blais, J.-F. Recovery of Zn (II), Mn (II), Cd (II) and Ni (II) from the unsorted spent batteries using solvent extraction, electrodeposition and precipitation methods. J. Clean. Prod. 2017, 148, 233–244. [Google Scholar] [CrossRef] [Green Version]
- Reddy, B.R.; Priya, D.N.; Park, K.H. Separation and recovery of cadmium(II), cobalt(II) and nickel(II) from sulphate leach liquors of spent Ni–Cd batteries using phosphorus based extractants. Sep. Purif. Technol. 2006, 50, 161–166. [Google Scholar] [CrossRef]
- Nogueira, C.A.; Margarido, F. Nickel–Cadmium batteries: Effect of electrode phase composition on acid leaching process. Environ. Technol. 2012, 33, 359–366. [Google Scholar] [CrossRef]
- Singh, R.; Mahandra, H.; Gupta, B. Recovery of zinc and cadmium from spent batteries using Cyphos IL 102 via solvent extraction route and synthesis of Zn and Cd oxide nanoparticles. Waste Manag. 2017, 67, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.R.; Priya, D.N. Chloride leaching and solvent extraction of cadmium, cobalt and nickel from spent nickel–Cadmium, batteries using Cyanex 923 and 272. J. Power Sources 2006, 161, 1428–1434. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, J.H.; Thi, L.D.; Qureshi, T.I. Recycling of NiCd batteries by hydrometallurgical process on small scale. J. Chem. Soc. Pak. 2011, 33, 853–857. [Google Scholar]
- Барашев, А.Р.; Карелoв, С.В.; Мамяченкoв, С.В.; Анисимoва, О. Сoвременнoе сoстoяние кoмплекснoй перерабoтки кадмийсoдержащегo втoричнoгo сырья. Металлург 2013, 3, 85–97. [Google Scholar]
- Jadhav, U.U.; Hocheng, H. Removal of nickel and cadmium from battery waste by a chemical method using ferric sulphate. Environ. Technol. 2014, 35, 1263–1268. [Google Scholar] [CrossRef]
- Barashev, A.R.; Karelov, S.V.; Anisimova, O.S.; Mamyachenkov, S.V. Innovative technology for recycling the negative segments of alkaline batteries using recoverable solvent. Metallurgist 2011, 55, 381–385. [Google Scholar] [CrossRef]
- Freitas, M.B.J.G.; Penha, T.R.; Sirtoli, S. Chemical and electrochemical recycling of the negative electrodes from spent Ni–Cd batteries. J. Power Sources 2007, 163, 1114–1119. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, D.; Zhu, N.-W. Bioleaching of spent Ni–Cd batteries by continuous flow system: Effect of hydraulic retention time and process load. J. Hazard. Mater. 2008, 160, 648–654. [Google Scholar] [CrossRef]
- Ritcey, G.M.; Ashbrook, A.W. Solvent Extraction–Principles and Applications to Process Metallurgy; Elsevier: Amsterdam, The Netherlands, 1984; ISBN 0444417702. [Google Scholar]
- Zagorodni, A. Ion Exchange Materials: Properties and Applications, 1st ed.; Elsevier Science: New York, NY, USA, 2006; ISBN 9780080445526. [Google Scholar]
- Bartolozzi, M.; Braccini, G.; Bonvini, S.; Marconi, P.F. Hydrometallurgical recovery process for nickel-cadmium spent batteries. J. Power Sources 1995, 55, 247–250. [Google Scholar] [CrossRef]
- Dolati, A.; Afshar, A.; Ghasemi, H. A kinetic study on the electrodeposition of cadmium with the presence of organic agents in sulfate solutions. Mater. Chem. Phys. 2005, 94, 23–28. [Google Scholar] [CrossRef]
- Mayén-Mondragón, R.; Ibanez, J.G.; Vasquez, R.C.; Baeza, A.; Oropeza, M.T. Electrochemical Recovery of Cadmium from Simulated Waste Nickel–Cadmium Battery Solutions. Water Air Soil Pollut. 2008, 194, 45–55. [Google Scholar] [CrossRef]
- Hazotte, C.; Leclerc, N.; Meux, E.; Lapicque, F. Direct recovery of cadmium and nickel from Ni-Cd spent batteries by electroassisted leaching and electrodeposition in a single-cell process. Hydrometallurgy 2016, 162, 94–103. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Moreira, T.F.M.; Santana, I.L.; Ferreira, S.A.D.; Lelis, M.F.F.; Freitas, M.B.J.G. Sol-gel synthesis, characterization, and catalytic properties of Ni, Cd, Co, and Fe oxides recycled from spent Ni-Cd batteries using citric acid as a leaching agent. Mater. Chem. Phys. 2018, 205, 186–194. [Google Scholar] [CrossRef]
- Varipaev, V.N.; Dasojan, M.A.; Nikolskij, V.A. Chemical Sources of Current (Химические Истoчники Тoка in Russian); Visshaja Shkola (Высшая шкoла in Russian): Moscow, Russia, 1990. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).