Dynamics of Oxidation of Reduced Forms of CO2 under Electrochemical and Open-Сircuit Conditions on Polycrystalline Pt in H2CO3
Abstract
1. Introduction
- (1).
- Whether the features of electrochemical oxidation of rCO2 into carbon dioxide saturated aqueous H2CO3 solution without electrolyte additives can be detected?
- (2).
- Is there any correlation between the electrocatalytic oxidation and open-circuit oxidation of rCO2?
- (3).
- Does the oxidation of rCO2 under electrocatalytic and open-circuit conditions proceed as an oscillatory reaction, and, if so, what useful physicochemical information can be derived from this process?
2. Materials and Methods
2.1. Electrocatalytic Measurements under Potentiodynamic Conditions
2.2. Catalytic Measurements under Open-Circuit Conditions
2.3. Electrocatalytic Measurements under Galvanostatic Conditions
2.4. Other Measurements
2.5. Quantum-Chemical Calculations
3. Results
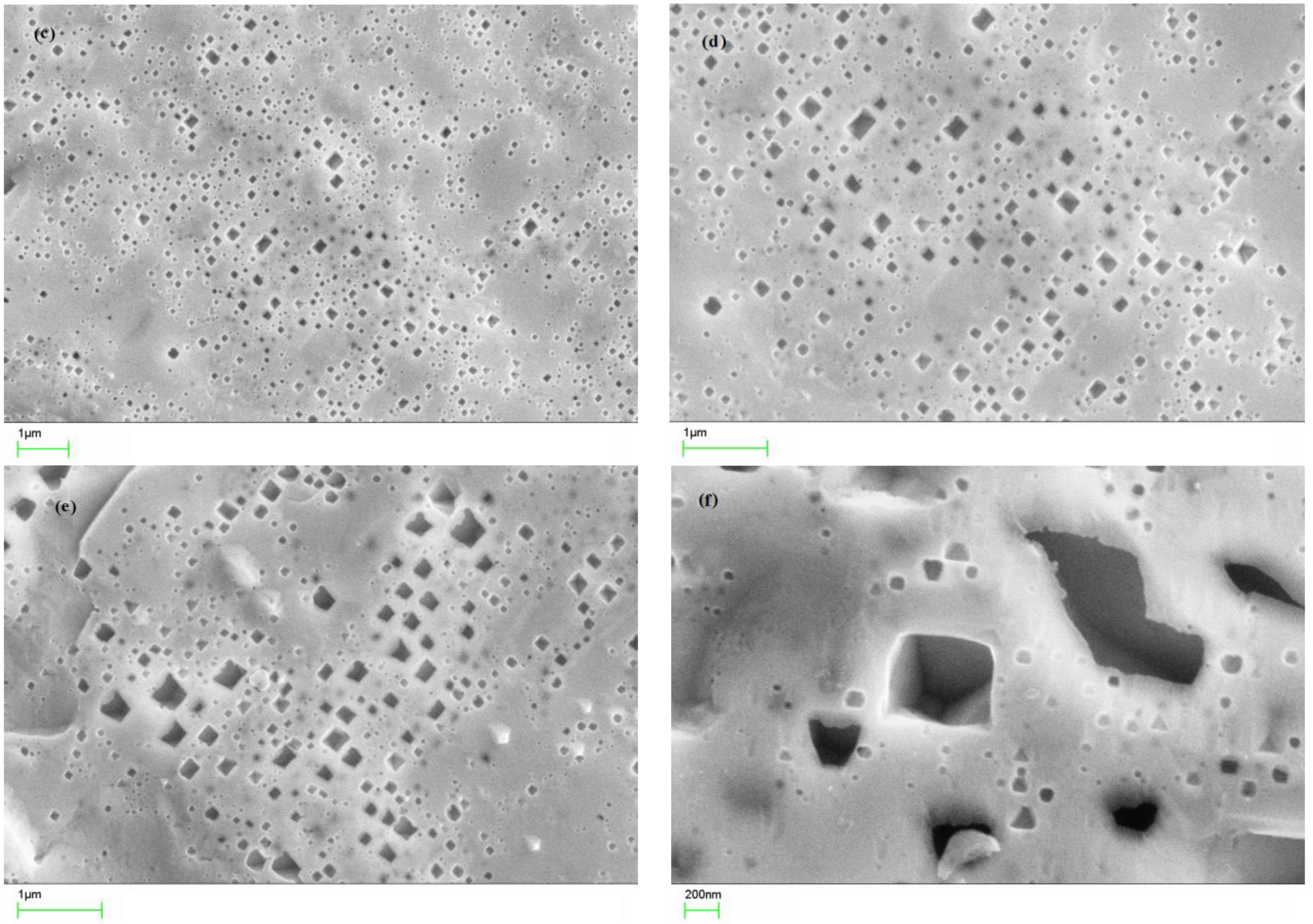
3.1. Morphology of pcPt Electrode Surface
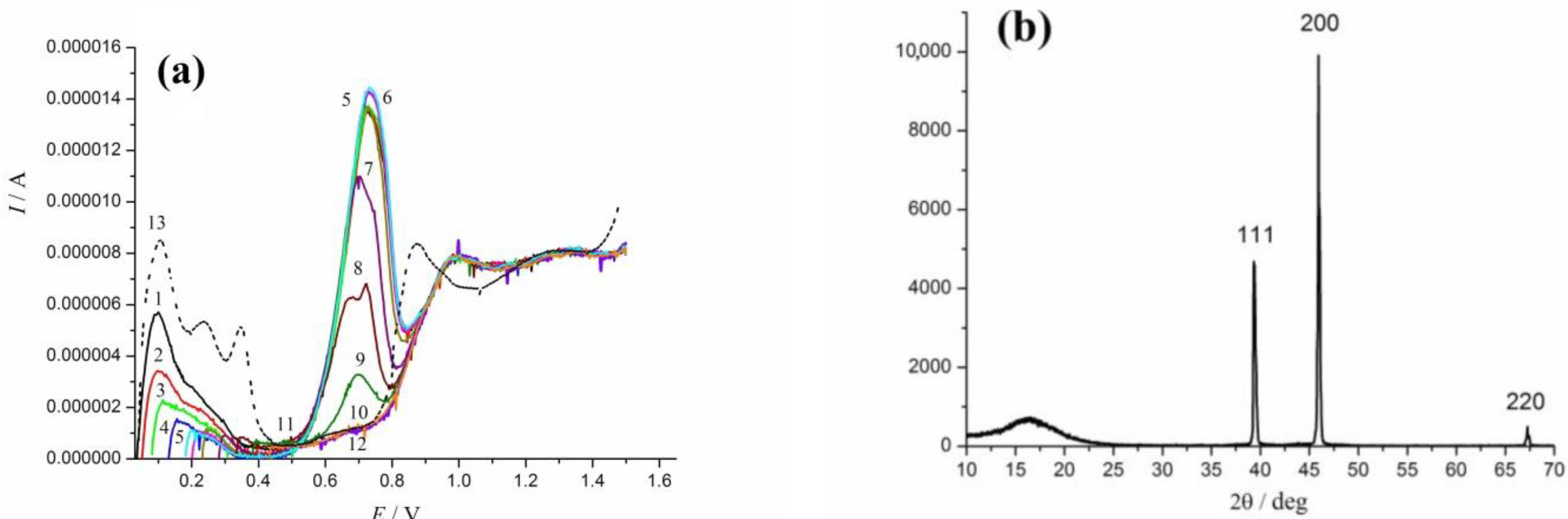
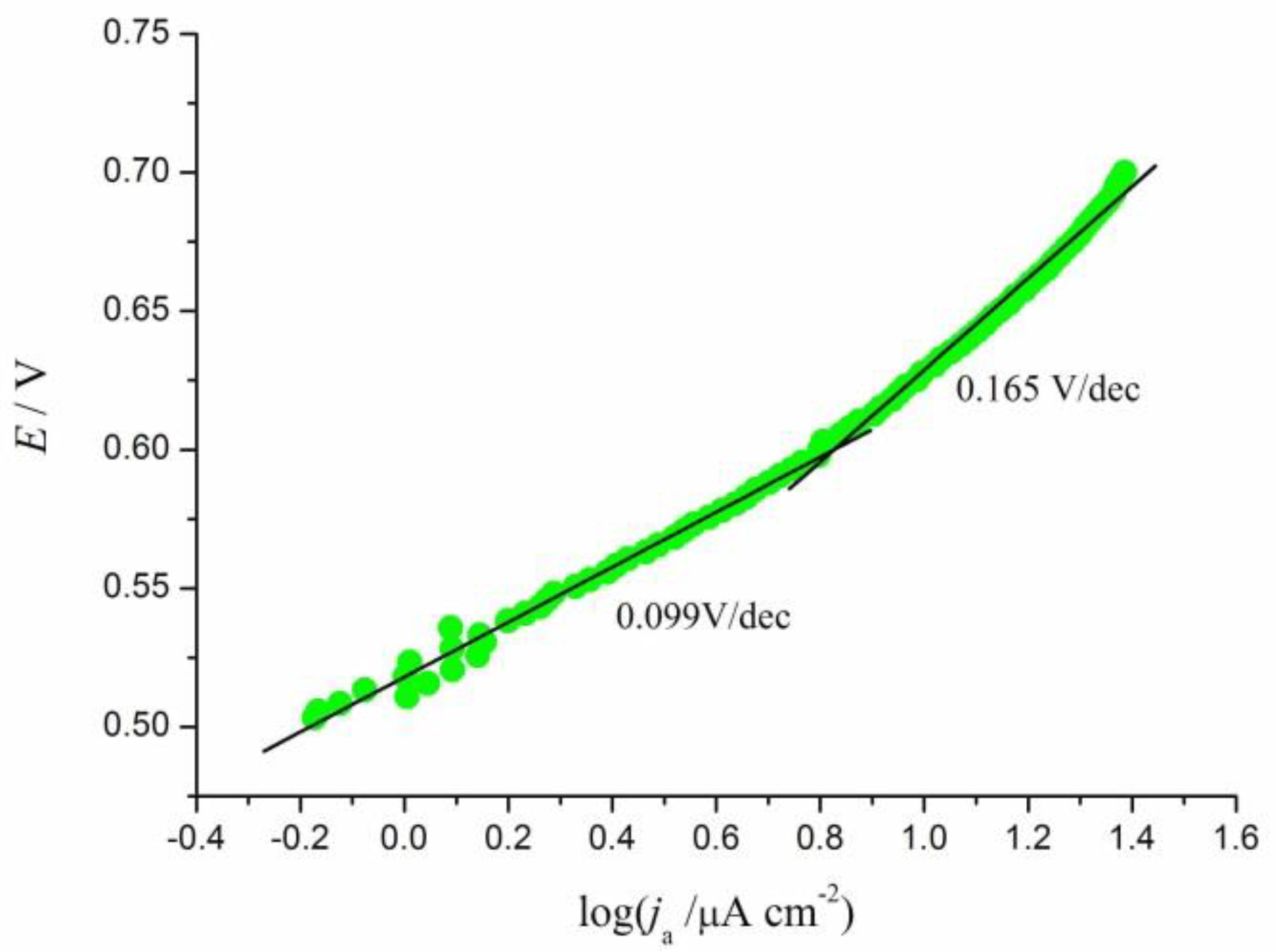
3.2. Electrochemical Oxidation of rCO2 under Potentiodynamic Conditions
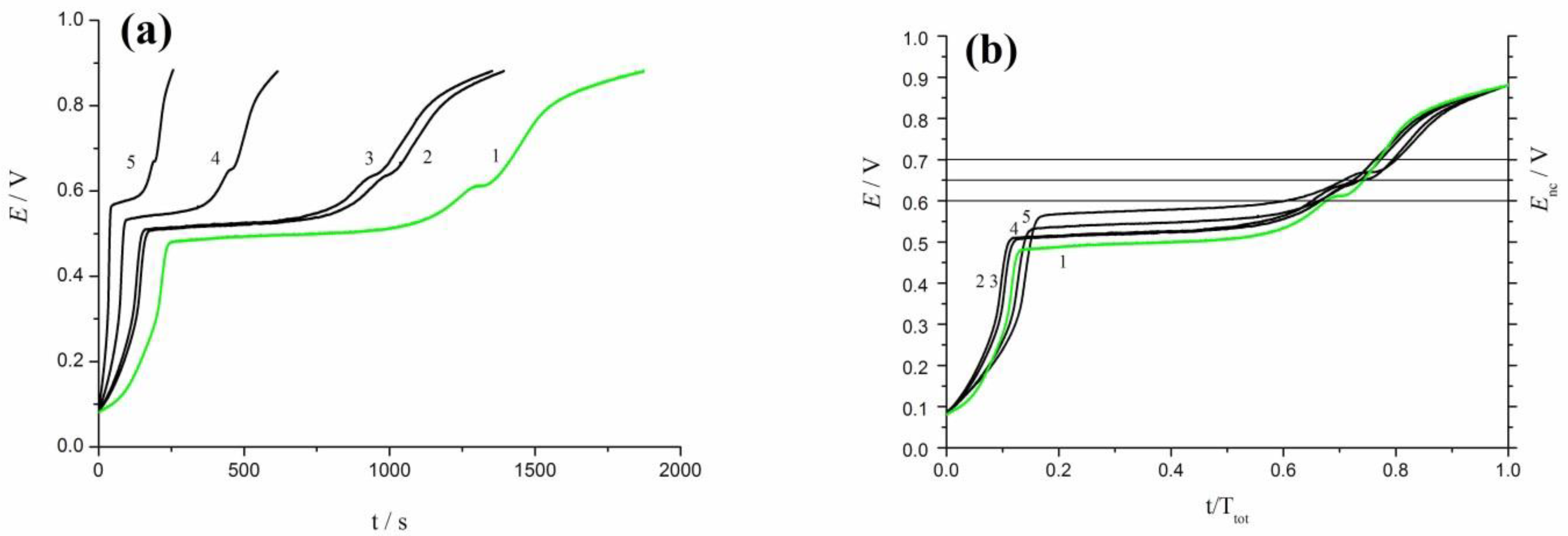
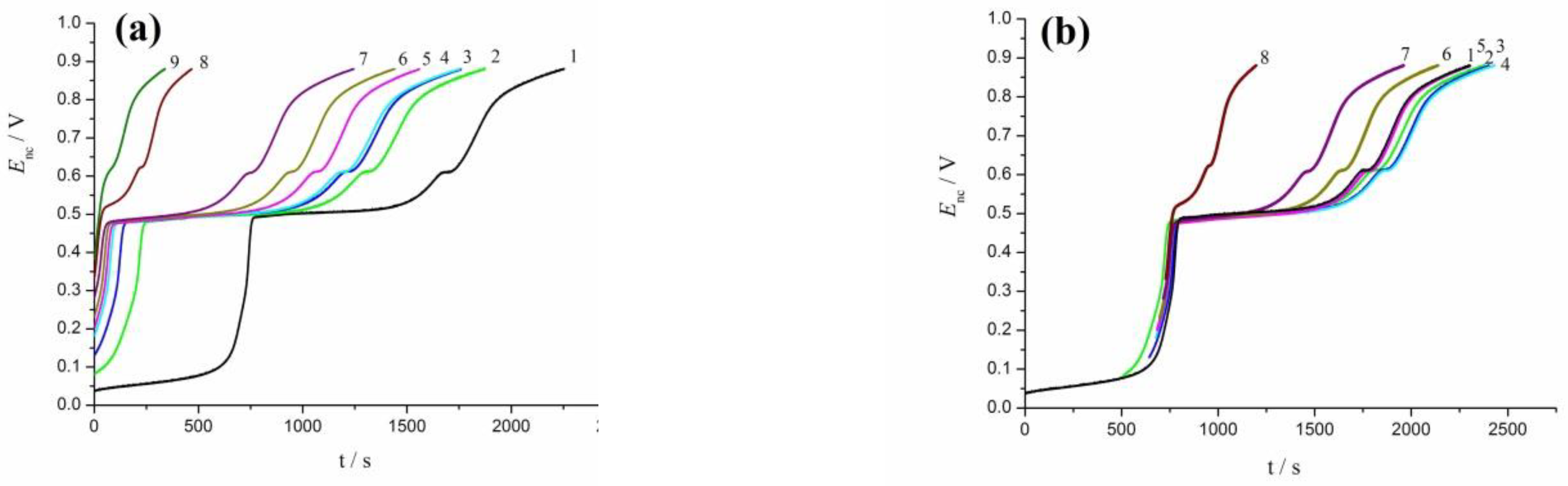
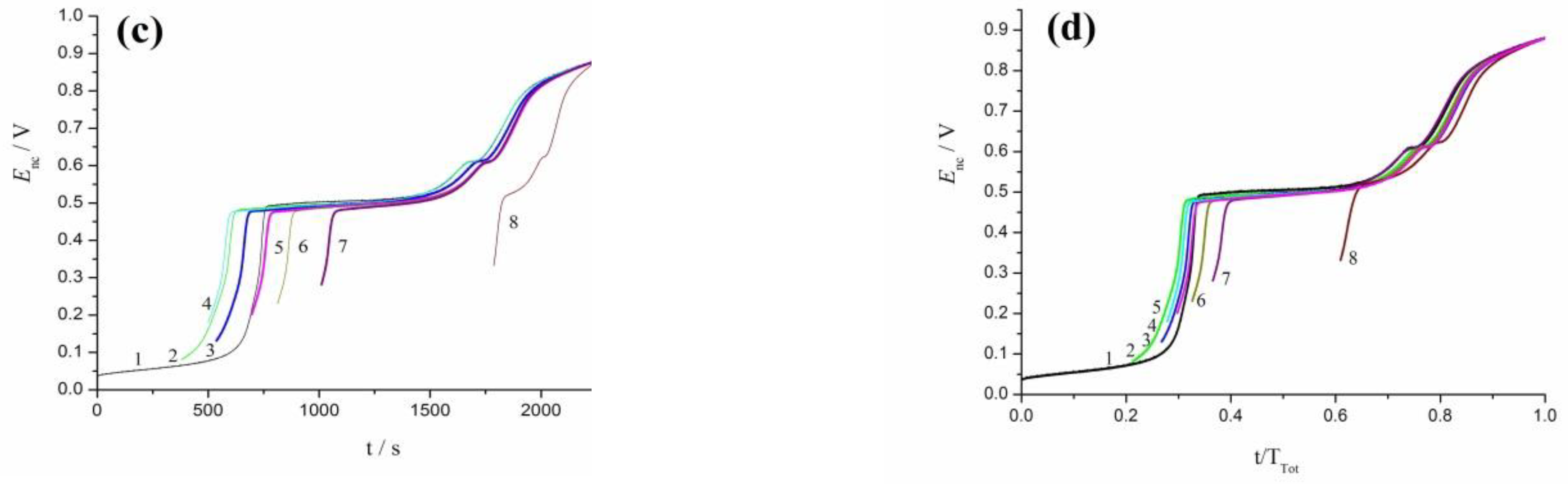
3.3. Oxidation of rCO2 under Galvanostatic and Open-Circuit Conditions
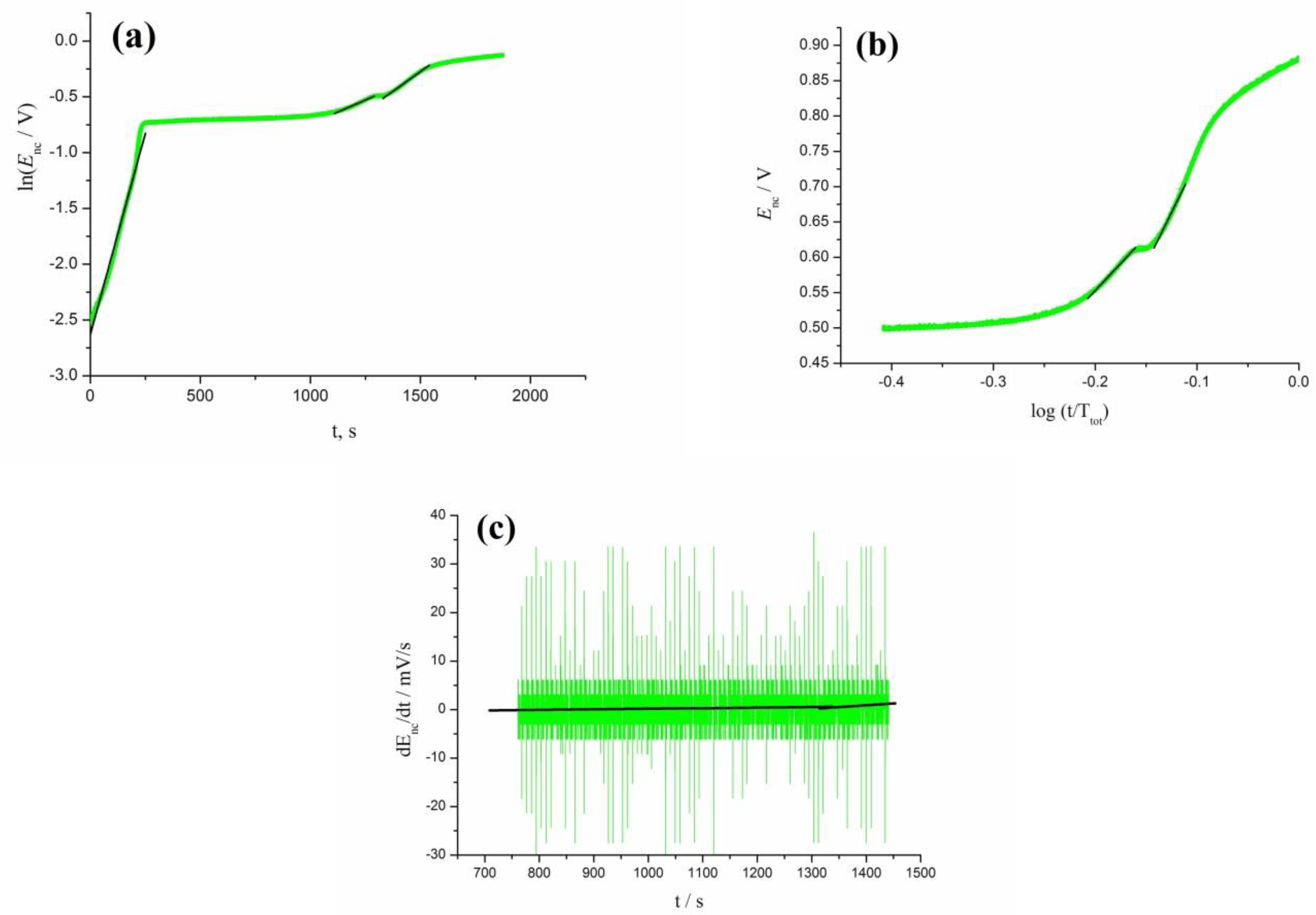
3.4. Oscillation Effects in Oxidation of rCO2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hori, Y. Electrochemical CO2 Reduction on Metal Electrodes. Modern Aspects of Electrochemistry; Springer Inc.: New York, NY, USA, 2008; pp. 89–189. [Google Scholar]
- Gómez-Marín, A.M.; Feliu, J.M. Oxygen reduction at platinum electrodes: The interplay between surface and surroundings properties. Curr. Opin. Electrochem. 2018, 9, 166–172. [Google Scholar] [CrossRef]
- Briega-Martos, V.; Herrero, E.; Feliu, J.M. Pt(hkl) surface charge and reactivity. Curr. Opin. Electrochem. 2019, 17, 97–105. [Google Scholar] [CrossRef]
- Malara, A.; Leonardi, S.G.; Bonavita, A.; Fazio, E.; Stelitano, S.; Neri, G.; Neri, F.; Santangelo, S. Origin of the different behavior of some platinum decorated nanocarbons towards the electrochemical oxidation of hydrogen peroxide. Mater. Chem. Phys. 2016, 184, 269–278. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Q.; Jiang, H.; Wang, X. Pt modified carbon fiber microelectrode for electrochemically catalytic reduction of hydrogen peroxide and its application in living cell H2O2 detection. J. Electroanal. Chem. 2016, 781, 233–237. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, W.; Zuo, X.-Q.; Cai, J.; Chen, Y.-X. The kinetics of the oxidation and reduction of H2O2 at a Pt electrode: A differential electrochemical mass spectrometric study. Electrochem. Commun. 2016, 73, 38–41. [Google Scholar] [CrossRef]
- Sitta, E.; Da Silva, K.N.; Feliu, J.M. Hydrogen Peroxide Oxidation/Reduction Reaction on Platinum Surfaces. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Wandelt, K., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 5.2, pp. 682–689. [Google Scholar]
- Gómez-Marín, A.M.; Feliu, J.M. Oxygen Reduction on Platinum Single Crystal Electrodes. In Encyclopedia of Interfacial Chemistry: Surface Science Electrochemistry; Wandelt, K., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 5.2, pp. 820–830. [Google Scholar]
- Conway, B.E. Some theoretical problems in electrocatalysis with charge transfer. J. Molec. Catal. 1989, 54, 353–369. [Google Scholar] [CrossRef]
- Podlovchenko, B.I.; Gladysheva, T.D. Adsorption and electrooxidation of carbon monoxide on platinized platinum at low anodic potentials. Russ. J. Electrochem. 1998, 34, 256–262. [Google Scholar]
- Smolin, A.V.; Podlovchenko, B.I. Electrochemical behavior of carbon monoxide on electrolytic deposits of palladium in sulfuric acid solutions. Russ. J. Electrochem. 1998, 34, 263–270. [Google Scholar]
- Podlovchenko, B.I.; Gladysheva, T.D.; Smolin, A.V. Transients of current at the adsorption of carbon monoxide on platinum and palladium electrodes. Mendeleev Commun. 1999, 3, 97–99. [Google Scholar] [CrossRef]
- Podlovchenko, B.I. Interrelation between transients of current and open-circuit potential during adsorption of neutral particles. Russ. J. Electrochem. 2000, 36, 821–824. [Google Scholar]
- Frumkin, A.N.; Petrii, O.A.; Damaskin, B.B. Potentials of zero charge. In Comprehensive Treatise of Electrochemistry; Bocris, J.O.M., Conway, B.E., Yeager, E., Eds.; Plenum Press: New York, NY, USA; London, UK, 1980; Volume 1, pp. 221–289. [Google Scholar]
- Frumkin, A.N.; Petrii, O.A. The Dependence of Free Surface Energy of Platinum Electrode on Solution Potential and Composition. Dokl. Akad. Nauk Sssr 1971, 196, 1387–1390. [Google Scholar]
- Manzhos, R.A.; Podlovchenko, B.I.; Maksimov, Y.M. Interaction of HCO-substances with adsorbed oxygen on platinum electrodes open-circuit transient reactions of HCOOH and CO. Electrochim. Acta 2005, 50, 4807–4813. [Google Scholar]
- Manzhos, R.A.; Podlovchenko, B.I.; Maksimov, Y.M. Kinetics and mechanism of interaction between methanol and adsorbed oxygen on a smooth polycrystalline platinum electrode: Transients of the open-circuit potential. Russ. J. Electrochem. 2006, 42, 1061–1066. [Google Scholar]
- Manzhos, R.A.; Podlovchenko, B.I.; Maksimov, Y.M. Special features of methanol interaction with adsorbed oxygen at platinized platinum electrode: Transients of the open-circuit potential. Russ. J. Electrochem. 2007, 43, 1268–1272. [Google Scholar] [CrossRef]
- Podlovchenko, B.I.; Smolin, A.V.; Maksimov, Y.M. Formaldehyde interaction with adsorbed oxygen on open-circuit platinum electrodes in aqueous sulfuric acid solutions. Russ. J. Electrochem. 2009, 45, 246–251. [Google Scholar]
- Smolin, A.V.; Podlovchenko, B.I. The Effect of Hydrocarbon Chain Length in Normal Aliphatic Alcohols on Their Reaction with Oxygen Adsorbed on Polycrystalline Platinum Electrode. Russ. J. Electrochem. 2010, 46, 367–373. [Google Scholar] [CrossRef]
- Sakong, S.; Gross, A. Methanol Oxidation on Pt(111) from First-Principles in Heterogeneous and Electrocatalysis. Electrocatalysis 2017, 8, 577–586. [Google Scholar] [CrossRef]
- Malkhandi, S.; Bauer, P.R.; Bonnefont, A.; Krischer, K. Mechanistic aspects of oscillations during CO electrooxidation on Pt in the presence of anions: Experiments and simulations. Catal. Today 2013, 202, 144–153. [Google Scholar] [CrossRef]
- Orlik, M. Self-Organization in Electrochemical Systems I-II; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Machado, E.G.; Varela, H. Kinetic Instabilities in Electrocatalysis. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Wandelt, K., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 5.2, pp. 701–718. [Google Scholar]
- Makeev, A.G.; Slinko, M.M.; Luss, D. Mathematical modeling of oscillating CO oxidation on Pt-group metals at near atmospheric pressure: Activity of metallic and oxidized surfaces. Appl. Catal. A Gen. 2019, 571, 127–136. [Google Scholar] [CrossRef]
- Kim, M.; Bertram, M.; Pollmann, M.; Von Oertzen, A.; Mikhailov, A.S.; Rotermund, H.H.; Ertl, G. Controlling chemical turbulence by global delayed feedback: Pattern formation in catalytic CO oxidation on Pt(110). Science 2001, 292, 1357–1360. [Google Scholar] [CrossRef] [PubMed]
- Kuzmin, V.I.; Tytik, D.L.; Gadzaov, A.F.; Abaturov, M.A.; Belashchenko, S.A.; Busev, D.K.; Kasatkin, V.E.; Smolin, A.V.; Tsetlin, V.V. Discreteness and Continuity in the Properties of Physical-Chemical Systems; Kuzmin, V.I., Tytik, D.L., Gadzaov, A.F., Eds.; FIZMATLIT: Moscow, Russia, 2014; p. 176. Available online: https://www.rfbr.ru/rffi/ru/books/o_1915369 (accessed on 31 December 2020).
- Petrii, O.A. The progress in understanding the mechanisms of methanol and formic acid electrooxidation on platinum group metals (Review). Russ. J. Electrochem. 2019, 55, 1–33. [Google Scholar] [CrossRef]
- Camara, G.A.; Iwasita, T. Parallel pathways of ethanol oxidation: The effect of ethanol concentration. J. Electroanal. Chem. 2005, 578, 315–321. [Google Scholar] [CrossRef]
- Bunkin, N.F.; Ninham, B.W.; Babenko, V.A.; Suyazov, N.V.; Sychev, A.A. Role of Dissolved Gas in Optical Breakdown of Water: Differences between Effects Due to Helium and Other Gases. J. Phys. Chem. B 2010, 114, 7743–7752. [Google Scholar] [CrossRef] [PubMed]
- Giner, J. Electrochemical reduction of co2 on platinum electrodes in acid solutions. Electrochim. Acta 1963, 8, 857–865. [Google Scholar] [CrossRef]
- Breiter, M.W. On the nature of reduced carbon dioxide. Electrochim. Acta 1967, 12, 1213–1218. [Google Scholar] [CrossRef]
- Vassiliev, Y.B.; Bagotzky, V.S.; Osetrova, N.V.; Mikhailova, A.A. Electroreduction of carbon dioxide part iii. adsorption and reduction of co2, on platlnum metals. J. Electroanal. Chem. Interfacial Electrochem. 1985, 189, 311–324. [Google Scholar] [CrossRef]
- Łukaszewski, M.; Siwek, H.; Czerwiński, A. Electrosorption of carbon dioxide on platinum group metals and alloys—A review. J. Solid. State Electrochem. 2009, 13, 813–827. [Google Scholar] [CrossRef]
- Podlovchenko, B.I.; Stenin, V.F.; Ekibaeva, A.A. On the influence of the solution composition upon the carbon dioxide chemisorption on a platinized platinum electrode. Sov. Electrochem. 1968, 4, 1374–1378. [Google Scholar]
- Vasina, S.Y.; Petry, O.A. Perchlorate anions adsorption on platinum and rhodium electrodes. Sov. Electrochem. 1970, 6, 242–246. [Google Scholar]
- Bunkin, N.F.; Yurchenko, S.O.; Suyazov, N.V.; Shkirin, A.V. Structure of the nanobubble clusters of dissolved air in liquid media. J. Biol. Phys. 2012, 38, 121–152. [Google Scholar] [CrossRef]
- Heinze, J. Ultramicroelectrodes in Electrochemistry. Angew. Chem. Int. Ed. Engl. 1993, 32, 1268–1288. [Google Scholar] [CrossRef]
- Wilhelm, E.; Battino, R.; Wilcock, R.J. Low-Pressure Solubility of Gases in Liquid Water. Chemical. Rev. 1977, 77, 219–262. [Google Scholar] [CrossRef]
- Palmer, D.A.; Eldik, R.V. The chemistry of metal carbonato and carbon dioxide complexes. Chem. Rev. 1983, 83, 651–731. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. J. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Stevens, W.J.; Krauss, M.; Basch, H.; Jasien, P.G. Relativistic compact effective potentials and efficient, shared-exponent basis sets for the third-, fourth-, and fifth-row atoms. Can. J. Chem. 1992, 70, 612–630. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.J.; Koseki, S.; Matsunaga, K.A.; Nguyen, S.S.; Windus, T.L.; et al. General atomic and molecular electronic structure system. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Granovsky, A.A. Firefly, Version 8. Available online: www http://classic.chem.msu.su/gran/firefly/index.html (accessed on 31 December 2020).
- Glendening, E.D.; Badenhoop, J.K.; Reed, A.E.; Carpenter, J.E.; Bohmann, J.A.; Morales, C.M.; Weinhold, F. NBO 5.9; Theoretical Chemistry Institute, University of Wisconsin: Madison, WI, USA, 2012. [Google Scholar]
- Rodes, A.; Pastor, E.; Iwasita, T. Structural effects on CO2 reduction at Pt single-crystal electrodes. Part 2. Pt(111) and vicinal surfaces in the [Oī1] zone. J. Electroanal. Chem. 1994, 373, 167–175. [Google Scholar] [CrossRef]
- Iwasita, T.; Nart, F.C.; Lopez, B.; Vielstich, W. On the study of adsorbed species at platinum from methanol, formic acid and reduced carbon dioxide via in situ ft-ir spectroscopy. Electrochim. Acta 1992, 37, 2361–2367. [Google Scholar] [CrossRef]
- Markovic, N.M.; Ross, P.N. Surface science studies of model fuel cell electrocatalysts. Surf. Sci. Rep. 2002, 45, 117–229. [Google Scholar] [CrossRef]
- Mayrhofer, K.J.J.; Arenz, M.; Blizanak, B.; Stamenkovich, V.; Ross, P.N.; Markovic, N.M. CO surface electrochemistry on Pt-nanoparticles: A selective review. Electrochim. Acta 2005, 50, 5144–5154. [Google Scholar] [CrossRef]
- Cuesta, A. The oxidation of adsorbed CO on Pt(100) electrodes in the pre-peak region. Electrocatalysis 2010, 1, 7–18. [Google Scholar] [CrossRef]
- Cuesta, A. Electrooxidation of C1 organic molecules on Pt electrodes. Curr. Opin. Electrochem. 2017, 4, 32–38. [Google Scholar] [CrossRef]
- Brimaud, S.; Pronier, S.; Contanceau, C.; Leger, J.M. New findings on CO electrooxidation at platinum nanoparticle surfaces. Electrochem. Commun. 2008, 10, 1703–1707. [Google Scholar] [CrossRef]
- Urchada, P.; Baranton, S.; Coutanceau, C.; Jerkiewicz, G. Electro-oxidation of COchem on Pt Nanosurfaces: Solution of the Peak Multiplicity Puzzle. Langmuir 2012, 28, 3658–3663. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jusus, Z.; Behm, R.J.; Abruna, H.D. New Insights into the Mechanism and Kinetics of Adsorbed CO Electrooxidation on Platinum: Online Mass Spectrometry and Kinetic Monte Carlo Simulation Studies. J. Phys. Chem. C 2012, 116, 11040–11053. [Google Scholar] [CrossRef]
- Wang, H.; Abruna, H.D. Origin of Multi-peaks in the Potentiodynamic Oxidation of CO Adlayers on Pt and Ru-modified Pt Electrodes. J. Phys. Chem. Lett. 2015, 6, 1899–1906. [Google Scholar] [CrossRef]
- Farias, M.J.S.; Buso-Rogero, C.; Vidal-Iglesias, F.J.; Solla-Gullon, J.; Camara, G.A.; Feliu, J.M. Mobility and Oxidation of Adsorbed CO on Shape-Controlled Pt Nanoparticles in Acidic Medium. Langmuir 2017, 33, 865–871. [Google Scholar] [CrossRef]
- Arevalo, M.C.; Gomis-Bas, C.; Hahn, F.; Beden, B.; Arevalo, A.; Arvia, A.J. A contribution to the mechanism of “reduced” co2 adsorbates electro-oxidation from combined spectroelectrochemical and voltammetric data. Electrochim. Acta 1994, 39, 793–799. [Google Scholar] [CrossRef]
- Tokarz, W.; Siwek, H.; Piela, P.; Czerwiński, A. Electro-oxidation of methanol on Pt-Rh alloys. Electrochim. Acta 2007, 52, 5565–5573. [Google Scholar] [CrossRef]
- Łukaszewski, M.; Czerwiński, A. Comparative EQCM study on electrooxidation of carbon oxides adsorption products on noble metals and their alloys. Polycrystalline Pd-based systems. J. Electroanal. Chem. 2007, 606, 117–133. [Google Scholar]
- Behm, R.J.; Thiel, P.A.; Norton, P.R.; Ertl, G. The interaction of CO and Pt(100). I. Mechanism of adsorption and Pt phase transition. J. Chem. Phys. 1983, 78, 7437–7447. [Google Scholar] [CrossRef]
- Yang, S.; Noguchi, H.; Uosaki, K. Broader energy distribution of CO adsorbed at polycrystalline Pt electrode in comparison with that at Pt(111) electrode in H2SO4 solution confirmed by potential dependent IR/visible double resonance sum frequency generation spectroscopy. Electrochim. Acta 2017, 235, 280–286. [Google Scholar] [CrossRef]
- Lykianycheva, V.I.; Bagotskii, V.S. Oxygen adsorption on smooth degassed platinum in electrolyte solutions. Dokl. Akad. Nauk SSSR 1964, 155, 160–163. [Google Scholar]
- Tikhomirova, V.I.; Lukyanychev, V.I.; Bagotsky, V.S. Oxygen-hydrogen peroxide equilibrium on outgassed platinum in the presence of oxygen traces. Sov. Electrochem. 1965, 1, 645–650. [Google Scholar]
- Johnson, M. Correlations of Cycles in Weather, Solar Activity, Geomagnetic Values and Planetary Configurations; Phillips and Van Orden: San Fransisco, CA, USA, 1944; p. 149. [Google Scholar]
- Martins, M.E. Electrochemical behaviour of carbon dioxide on platinum and rhodium. J. Argent. Chem. Soc. 2005, 93, 143–153. [Google Scholar]
- Baruzzi, A.M.; Leiva, E.P.M.; Giordano, M.C. The influence of solution composition on the kinetiks of “reduced” co2, electrooxidation at p0lycrystalllne platinum. J. Electroanal. Chem. Interfacial Electrochem. 1985, 189, 257–269. [Google Scholar] [CrossRef]
- Baruzzi, A.M.; Leiva, E.P.M.; Giordano, M.C. Complex kinetic behaviour of “reduced” CO2 electro-oxidation at Pt electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1983, 158, 103–114. [Google Scholar]
- Bagotskii, V.S.; Nekrasov, L.N.; Shumilova, N.A. Electrochemical Reduction of Oxygen. Russ. Chem. Rev. (Uspekhi Khimii) 1965, 34, 717–730. [Google Scholar] [CrossRef]
- Noel, J.-M.; Latus, A.; Lagrost, C.; Volanschi, E.; Hapiot, P. Evidence for OH Radical Production during Electrocatalysis of Oxygen Reduction on Pt Surfaces: Consequences and Application. J. Am. Chem. Soc. 2012, 134, 2835–2841. [Google Scholar] [CrossRef]
- Chen, J.; Fang, L.; Luo, S.; Liu, Y.; Chen, S. Electrocatalytic O2 Reduction on Pt: Multiple Roles of Oxygenated Adsorbates, Nature of Active Sites and Origin of Overpotential. J. Phys. Chem. C 2017, 121, 6209–6217. [Google Scholar] [CrossRef]
- Gomez-Marín, A.M.; Feliu, J.M.; Ticianelli, E. Oxygen Reduction on Platinum Surfaces in Acid Media: Experimental Evidence of a CECE/DISP Initial Reaction Path. ACS Catal. 2019, 9, 2238–2251. [Google Scholar]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Damjanovic, A.; Brusic, V. Electrode kinetics of oxygen reduction on oxide-free platinum electrodes. Electrochim. Acta 1967, 12, 615–628. [Google Scholar] [CrossRef]
- Jinnouchi, R.; Kodama, K.; Hatanaka, T.; Morimoto, Y. First principles based mean field model for oxygen reduction reaction. Phys. Chem. Chem. Phys. 2011, 13, 21070–21083. [Google Scholar] [CrossRef]
- Hansen, H.A.; Viswanathan, V.; Nørskov, J.K. Unifying Kinetic and Thermodynamic Analysis of 2 e− and 4 e− Reduction of Oxygen on Metal Surfaces. J. Phys. Chem. C 2014, 118, 6706–6718. [Google Scholar] [CrossRef]
- Staszak-Jirkovský, J.; Ahlberg, E.; Panas, I.; Schiffrin, D.J. The bifurcation point of the oxygen reduction reaction on Au–Pd nanoalloys. Faraday Discuss. 2016, 188, 257–278. [Google Scholar] [CrossRef]
- Gómez-Marín, A.M.; Rizo, R.; Feliu, J.M. Oxygen reduction reaction at Pt single crystals: A critical overview. Catal. Sci. Technol. 2014, 4, 1685–1698. [Google Scholar] [CrossRef]
- Ruvinskiy, P.S.; Bonnefont, A.; Pham-Huu, C.; Savinova, E.R. Using Ordered Carbon Nanomaterials for Shedding Light on the Mechanism of the Cathodic Oxygen Reduction Reaction. Langmuir 2011, 27, 9018–9027. [Google Scholar] [CrossRef]
- Gomez-Marin, A.M.; Feliu, J.M. New Insights into the Oxygen Reduction ReactionMechanism on Pt(111): A Detailed Electrochemical Study. Chemsuschem 2013, 6, 1091–1100. [Google Scholar] [CrossRef]
- Schumb, W.C.; Satterfield, C.N.; Wentworth, R.L. Hydrogen Peroxide; Reinold Publishing Corp.: New York, NY, USA; Chapman & Nall, Ltd.: London, UK, 1956; p. 579. [Google Scholar]
- Schmidt, V.M.; Rodriguez, J.L.; Pastor, E. The Influence of H2O2 on the Adsorption and Oxidation of CO on Pt Electrodes in Sulfuric Acid Solution. J. Electrochem. Soc. 2001, 148, A293–A298. [Google Scholar] [CrossRef]
- Martinez, S.; Zinola, F.; Planes, G.; Guillén-Villafuerte, O.; Rodríguez, J.L.; Pastor, E. The influence of hydrogen peroxide on carbon monoxide electrooxidation at Pt/C and Pt:Ru/C electrodes. J. Solid State Electrochem. 2007, 11, 1521–1529. [Google Scholar] [CrossRef]
- Briega-Martos, V.; Herrero, E.; Feliu, J.M. The inhibition of hydrogen peroxide reduction at low potentials on Pt(111): Hydrogen adsorption or interfacial charge? Electrochem. Commun. 2017, 85, 32–35. [Google Scholar] [CrossRef][Green Version]
- Takahashi, M.; Chiba, K.; Li, P. Free-Radical Generation from Collapsing Microbubbles in the Absence of a Dynamic Stimulus. J. Phys. Chem. B. 2007, 111, 1343–1347. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.; Bruskov, V.; Astashev, M.; Chernikov, A.; Yaguzhinsky, L.; Zakharov, S. Oxygen Dependent Auto-Oscillations of Water Luminescence Triggered by the 1264 nm Radiation. J. Phys. Chem. B 2011, 115, 7693–7698. [Google Scholar] [CrossRef] [PubMed]
- Bunkin, N.F.; Shkirin, A.V.; Ignatiev, P.S.; Chaikov, L.L.; Burkhanov, I.S.; Starosvetskij, A.V. Nanobubble clusters of dissolve gas in aqueous solutions of electrolyte. I. Experimental proof. J. Chem. Phys. 2012, 137, 054706-1–054706-10. [Google Scholar]
- Bunkin, N.F.; Suyazov, N.V.; Shkirin, A.V.; Ignatiev, P.S.; Indukaev, K.V. Nanoscale structure of dissolved air bubbles in water as studied by measuring the elements of the scattering matrix. J. Chem. Phys. 2009, 130, 134308-1–134308-12. [Google Scholar] [CrossRef]
- Ushikubo, F.Y.; Enari, M.; Furukawa, T.; Nakagawa, R.; Makino, Y.; Kawagoe, Y.; Oshita, S. Zeta-potential of micro-and/or nano-bubbles in water formed by certain types of gases. Ifac Proc. Vol. 2010, 43, 283–288. [Google Scholar] [CrossRef]
- Buchachenko, A. Magneto–Biology and Medicine; Nova Science Publishers: New York, NY, USA, 2014; p. 225. [Google Scholar]
- Roberts, J.G.; Voinov, M.A.; Schmidt, A.C.; Smirnova, T.I.; Sombers, L.A. The Hydroxyl Radical is a Critical Intermediate in the Voltammetric Detection of Hydrogen Peroxide. J. Am. Chem. Soc. 2016, 138, 2516–2519. [Google Scholar] [CrossRef]
- Villegas, I.; Weaver, M.J. Carbon monoxide adlayer structures on platinum (111) electrodes: A synergy between insitu scanning tunneling microscopy and infrared spectroscopy. J. Chem. Phys. 1994, 101, 1648–1660. [Google Scholar] [CrossRef]
- Inukai, J.; Tryk, D.A.; Abe, T.; Wakisaka, M.; Uchida, H.; Watanabe, M. Direct STM Elucidation of the Effects of Atomic-Level Structure on Pt(111) Electrodes for Dissolved CO Oxidation. J. Am. Chem. Soc. 2013, 135, 1476–1490. [Google Scholar] [CrossRef] [PubMed]
- Podlovchenko, B.I.; Gladysheva, T.D.; Kolyadko, E.A. Experimental check-up of the relationship between transients of current and open circuit potential for strong adsorption of neutral species and ions on a hydrogen electrode. J. Electroanal. Chem. 2003, 552, 85–96. [Google Scholar] [CrossRef]
- Petrii, O.A. Zero Charge Potentials of Platinum Metals and Electron Work Functions (Review). Russ. J. Electrochem. 2013, 49, 401–422. [Google Scholar] [CrossRef]
- Martínez-Hincapié, R.; Berná, A.; Rodes, A.; Climent, V.; Feliu, J.M. Surface Acid−Base Properties of Anion-Adsorbed Species at Pt(111) Electrode Surfaces in Contact with CO2-Containing Perchloric Acid Solutions. J. Phys. Chem. C 2016, 120, 16191–16199. [Google Scholar] [CrossRef]
- Huber, K.P.; Herzberg, G. Molecular Spectra and Molecule Structure. IV. Constants of Diatomic Molecules; Van Nostrand Renhold Comp.: New York, NY, USA; Cincinnati, OH, USA; Atlanta, GA, USA; Dallas, TX, USA; San Francisco, CA, USA; London, UK; Toronto, ON, Canada; Melbourne, Australia, 1979. [Google Scholar]
- Thiel, P.A.; Behm, R.J.; Norton, P.R.; Ertl, G. The interaction of CO and Pt(100). II. Energetic and kinetic parameters. J. Chem. Phys. 1983, 78, 7448–7458. [Google Scholar] [CrossRef]
- Noguchi, H.; Okada, T.; Uosaki, K. SFG study on potential-dependent structure of water at Pt electrode/electrolyte solution interface. Electrochim. Acta 2008, 53, 6841–6844. [Google Scholar] [CrossRef]
- Vassilyev, Y.B.; Khazova, O.A.; Nikolaeva, N.N. Kinetics and mechanism of glucose electrooxidation on different electrode-catalysts. Part, I. Adsorption and oxidation on platinum. J. Electroanal. Chem. Interfacial Electrochem. 1985, 196, 105–125. [Google Scholar] [CrossRef]
- Vassilyev, Y.B.; Khazova, O.A.; Nikolaeva, N.N. Kinetics and mechanism of glucose electrooxidation on different electrode-catalysts part II. effect of the nature of the electrode and the electrooxidation mechanism. J. Electroanal. Chem. Interfacial Electrochem. 1985, 196, 127–144. [Google Scholar] [CrossRef]
- Lavrik, N.L.; Bazhin, N.M. On the Impossibility of OH Radical Formation from the Hydroxyl Ion in Water. Biophysics 2011, 56, 535–536. [Google Scholar] [CrossRef]
- Solans-Monfort, X.; Branchadell, V.; Sodupe, M.; Sierka, M.; Sauer, J. Electron hole formation in acidic zeolite catalysts. J. Chem. Phys. 2004, 121, 6034–6040. [Google Scholar] [CrossRef]
- Chen, H.; Handoko, A.D.; Xiao, J.; Feng, X.; Fan, Y.; Wang, T.; Legut, D.; Seh, Z.W.; Zhang, Q. Catalytic effect on CO2 electroreduction by hydroxyl terminated two-dimensional MXenes. ACS Appl. Mater. Interfaces 2019, 11, 36571–36579. [Google Scholar] [CrossRef] [PubMed]
- Handoko, A.D.; Khoo, K.H.; Tan, T.L.; Jin, H.; Seh, Z.W. Establishing new scaling relations on two-dimensional MXenes for CO2 electroreduction. J. Mater. Chem. A 2018, 6, 21885–21890. [Google Scholar] [CrossRef]
- Handoko, A.D.; Wei, F.; Yeo, B.S.; Seh, Z.W. Understanding heterogeneous electrocatalytic carbon dioxide reduction through operando techniques. Nat. Catal. 2018, 1, 922–934. [Google Scholar] [CrossRef]
- Handoko, A.D.; Steinmann, S.N.; Seh, Z.W. Theory-guided materials design: Two-dimensional MXenes in electro- and photocatalysis. Nanoscale Horiz. 2019, 4, 809–827. [Google Scholar] [CrossRef]






| E, V | Eps | |||||
|---|---|---|---|---|---|---|
| 0.03 | 171.45 | 74.06 | 194.38 | 97.39 | 1.00 | 1.99 |
| 0.08 | 131.75 | 34.96 | 196.09 | 96.79 | 0.77 | 1.56 |
| 0.13 | 91.97 | 17.25 | 204.88 | 74.72 | 0.54 | 1.48 |
| 0.18 | 73.94 | 9.51 | 209.26 | 64.43 | 0.43 | 1.40 |
| 0.23 | 48.84 | 6.69 | 192.86 | 42.15 | 0.29 | 1.33 |
| 0.28 | 32.08 | 3.57 | 147.50 | 28.51 | 0.19 | 0.98 |
| 0.33 | 13.21 | 1.19 | 88.85 | 12.02 | 0.08 | 0.59 |
| 0.38 | 1.10 | 0.03 | 27.78 | 1.07 | 0.01 | 0.26 |
| Structure | Pt13 | CO | OH |
| Pt13-CO | −0.03 | 0.03 | – |
| Pt13-(CO)2 | −0.02 | 0.01 | – |
| Pt13-(CO)3 | −0.12 | 0.04 | – |
| Pt13-(CO)4 | −0.20 | 0.05 | – |
| r-OH-Pt13-CO | 0.38 | 0.03 | −0.41 |
| r-OH-Pt13-(CO)2 | 0.36 | 0.02 | −0.40 |
| r-OH-Pt13-(CO)3 | 0.25 | 0.05 | −0.40 |
| r-OH-Pt13-(CO)4 | 0.18 | 0.05 | −0.38 |
| a-OH-Pt13-CO | −0.43 | −0.07 | −0.50 |
| a-OH-Pt13-(CO)2 | −0.38 | −0.06 | −0.50 |
| a-OH-Pt13-(CO)3 | −0.33 | −0.06 | −0.49 |
| a-OH-Pt13-(CO)4 | −0.35 | −0.04 | −0.49 |
| Structure | Pt | (Pt3) rCOH | OH |
| rOH-Pt13-(rCOH) | 0.67 | −0.214 | −0.457 |
| aOH-Pt13-(rCOH), | −0.257 | −0.747 | −0.483 |
| Structure | Pt | (Pt1)rCOOH | OH |
| rOH-Pt13-(rCOOH) | 0.561 | −0.147 | −0.415 |
| aOH-Pt13-(rCOOH), | 0.005 | −0.28 | −0.484 |
| Numder of the Structure | Structure | ΔE, kcal/mol rOH | ΔG, kcal/mol rOH | ΔE, kcal/mol aOH | ΔG, kcal/mol aOH |
|---|---|---|---|---|---|
| 0 |  | 0.0 | 0.0 | 0.0 | 0.0 |
| 1 | 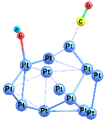 | −40.9 | −28.0 | −41.3 | −29.0 |
| 1-ts | 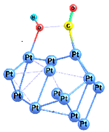 | −25.1 | −12.1 | −20.9 | −7.6 |
| 2-1 | 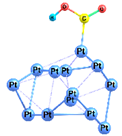 | −35.7 | −22.1 | −44.2 | −30.7 |
| 2-2 | 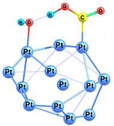 | −52.5 | −39.9 | −44.4 | −30.9 |
| 2-ts | 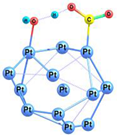 | −52.5 | −39.6 | −42.9 | −31.4 |
| 3 | 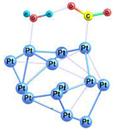 | −54.5 | −40.3 | −44.0 | −30.6 |
| Numder of the Structure | ΔE, kcal/mol rOH | ΔG, kcal/mol rOH | ΔE, kcal/mol aOH | ΔG, kcal/mol aOH |
|---|---|---|---|---|
| 0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 1 | −44.3 | −31.8 | −40.5 | −27.6 |
| 1-ts | −26.5 | −13.0 | −21.7 | −7.6 |
| 2-1 | −41.7 | −28.0 | −36.4 | −22.7 |
| 2-2 | −41.4 | −28.0 | −37.1 | −24.3 |
| 2-ts | −41.5 | −28.3 | −34.0 | −20.7 |
| 3 | −43.4 | −29.3 | −36.8 | −25.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smolin, A.V.; Mikhailov, М.N.; Gadzaov, A.F.; Kustov, L.M. Dynamics of Oxidation of Reduced Forms of CO2 under Electrochemical and Open-Сircuit Conditions on Polycrystalline Pt in H2CO3. Metals 2021, 11, 274. https://doi.org/10.3390/met11020274
Smolin AV, Mikhailov МN, Gadzaov AF, Kustov LM. Dynamics of Oxidation of Reduced Forms of CO2 under Electrochemical and Open-Сircuit Conditions on Polycrystalline Pt in H2CO3. Metals. 2021; 11(2):274. https://doi.org/10.3390/met11020274
Chicago/Turabian StyleSmolin, Alexander V., Мikhail N. Mikhailov, Aleksey F. Gadzaov, and Leonid M. Kustov. 2021. "Dynamics of Oxidation of Reduced Forms of CO2 under Electrochemical and Open-Сircuit Conditions on Polycrystalline Pt in H2CO3" Metals 11, no. 2: 274. https://doi.org/10.3390/met11020274
APA StyleSmolin, A. V., Mikhailov, М. N., Gadzaov, A. F., & Kustov, L. M. (2021). Dynamics of Oxidation of Reduced Forms of CO2 under Electrochemical and Open-Сircuit Conditions on Polycrystalline Pt in H2CO3. Metals, 11(2), 274. https://doi.org/10.3390/met11020274






