Effect of Carbon Addition on Direct Reduction Behavior of Low Quality Magnetite Ore by Reducing Gas Atmosphere
Abstract
:1. Introduction
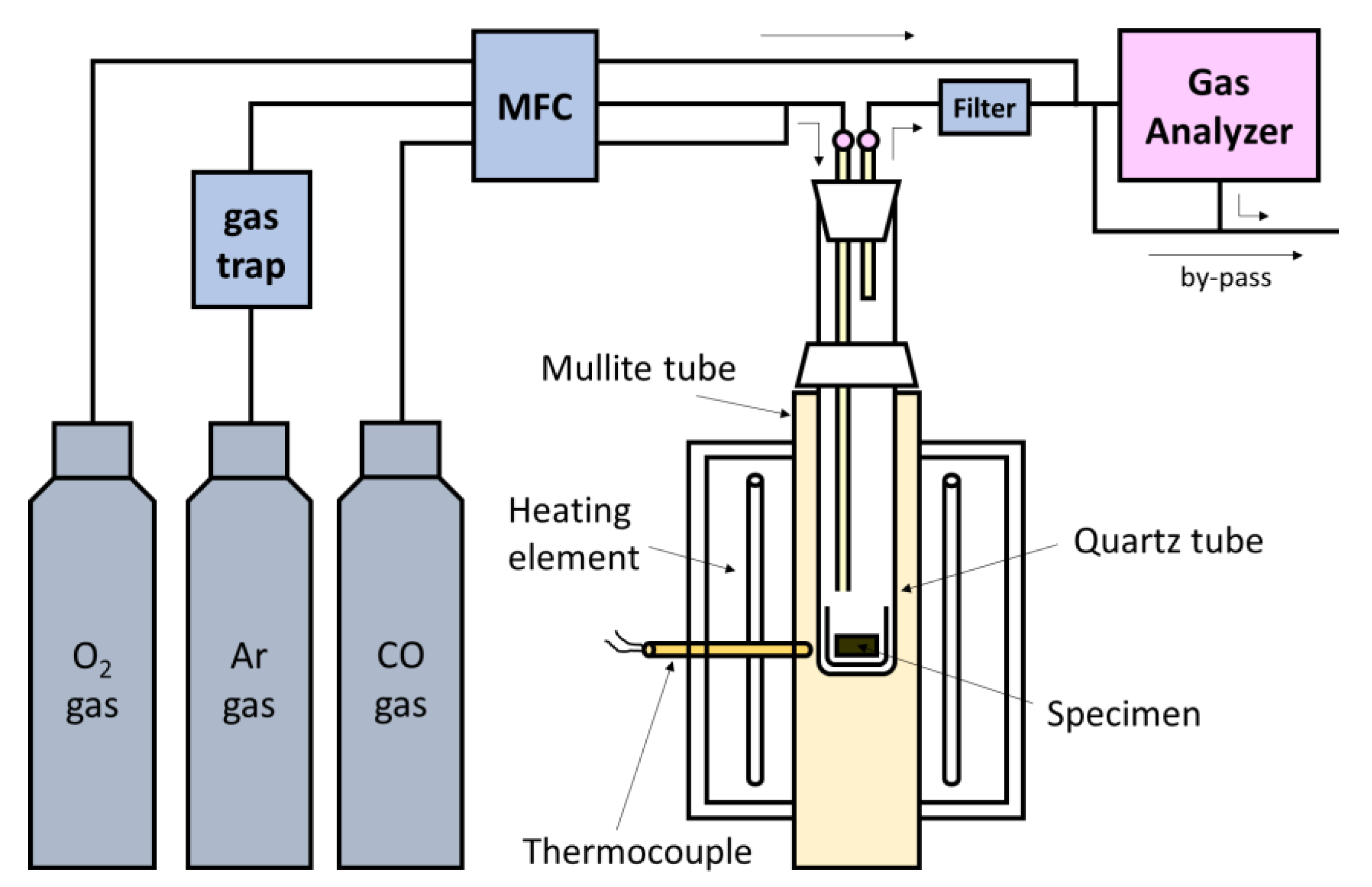
2. Materials and Methods
2.1. Sample Preparation
2.2. Experimental Procedure
3. Results and Discussion
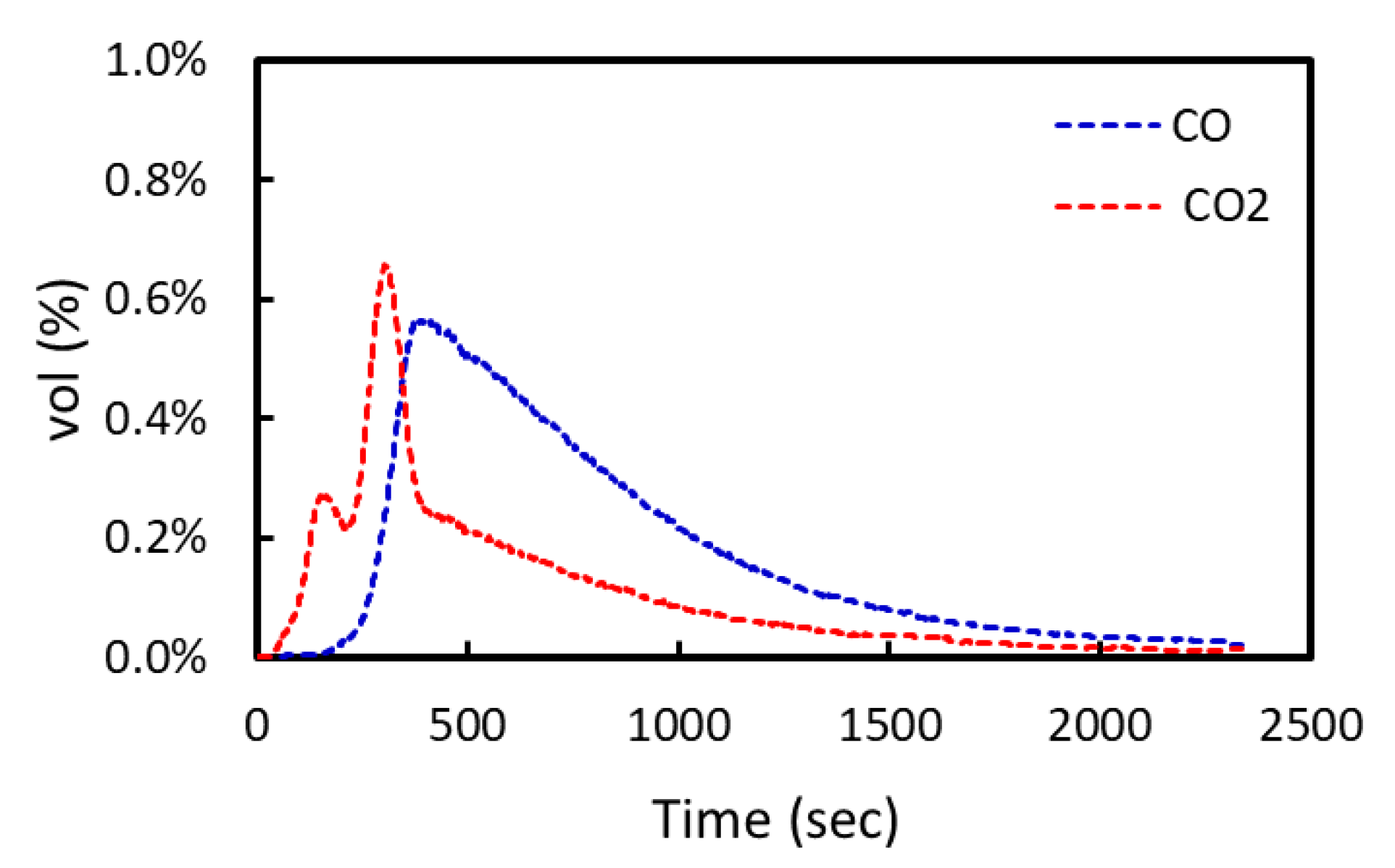
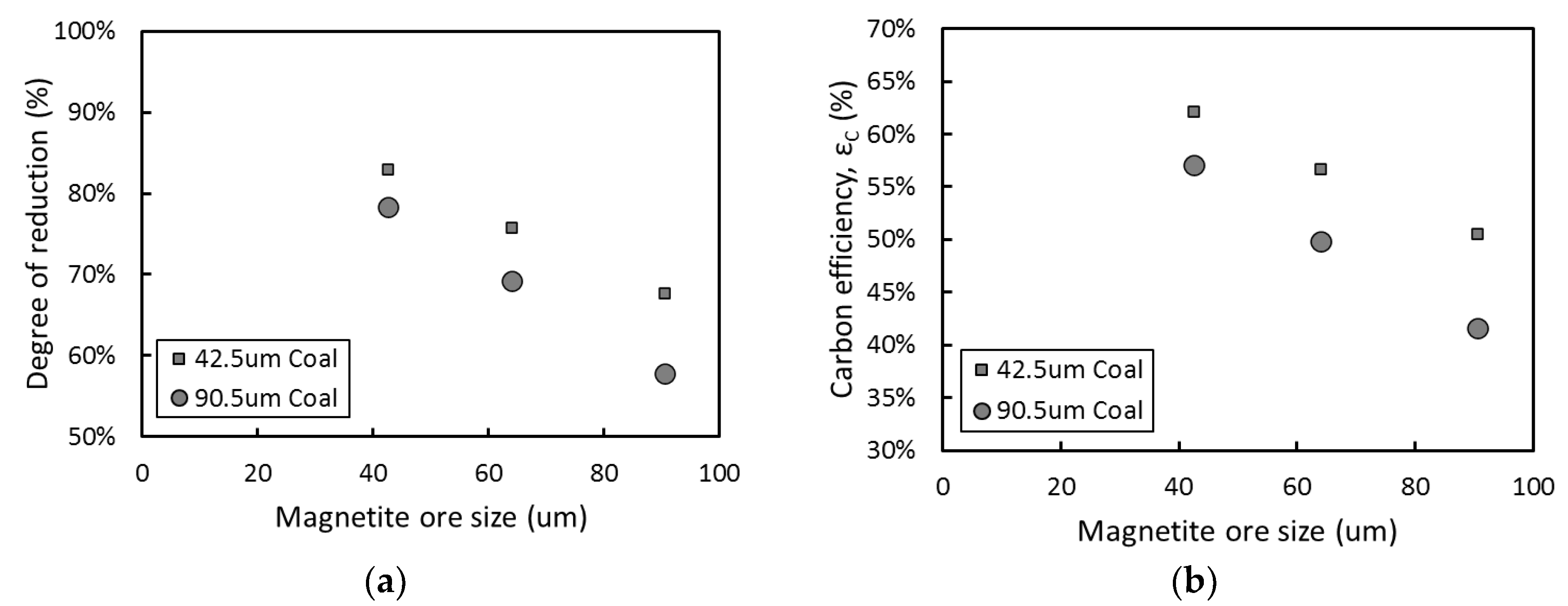
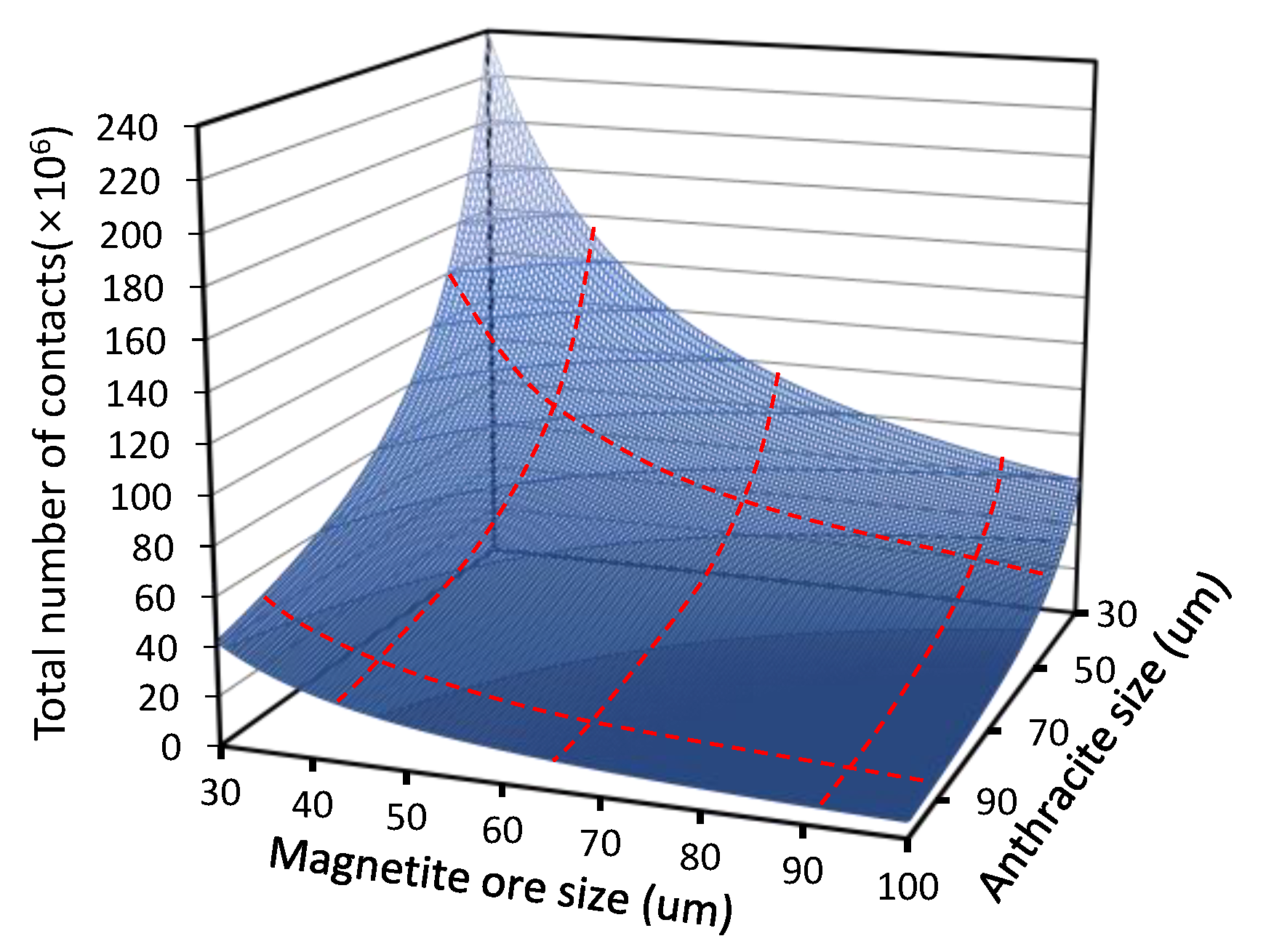
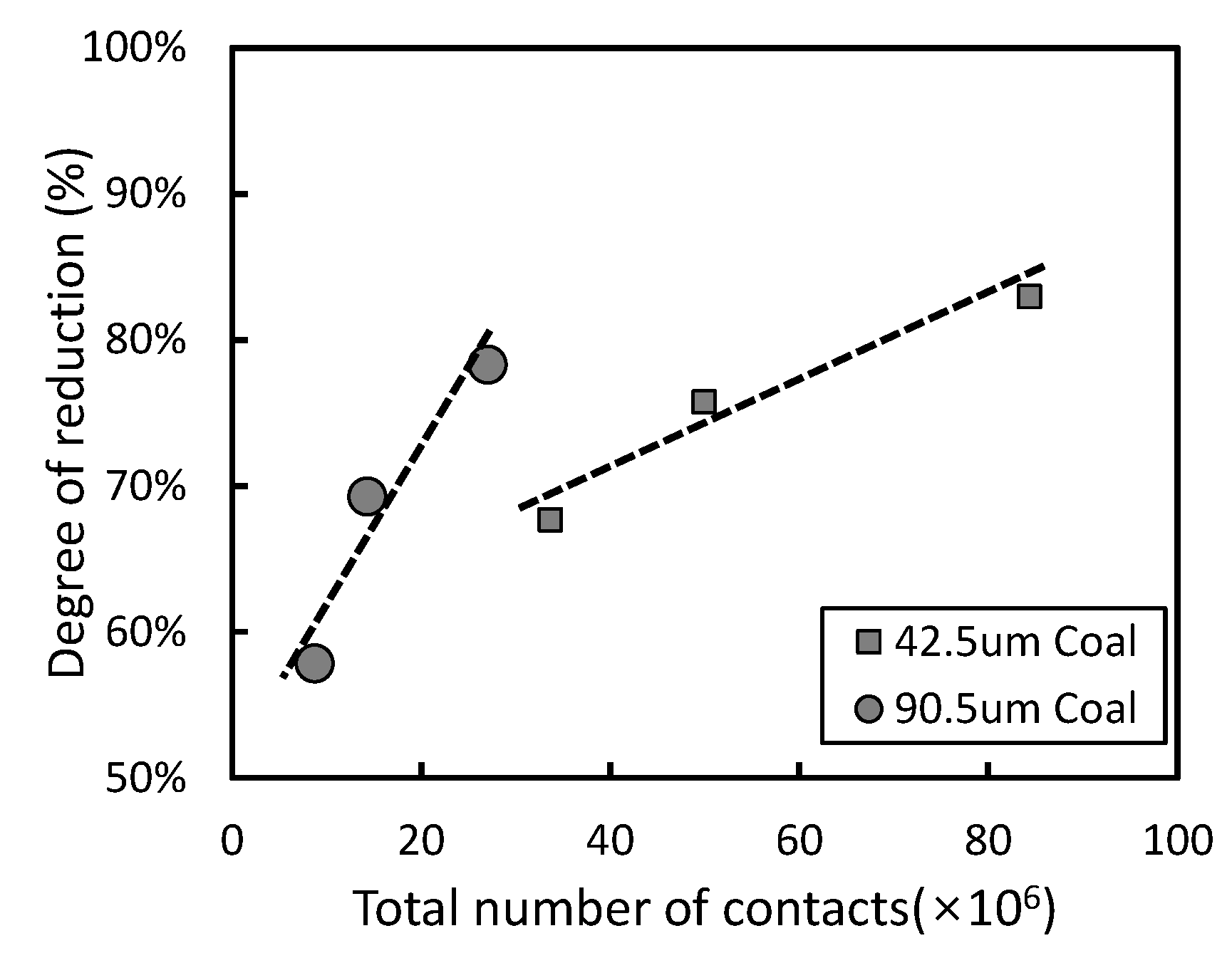
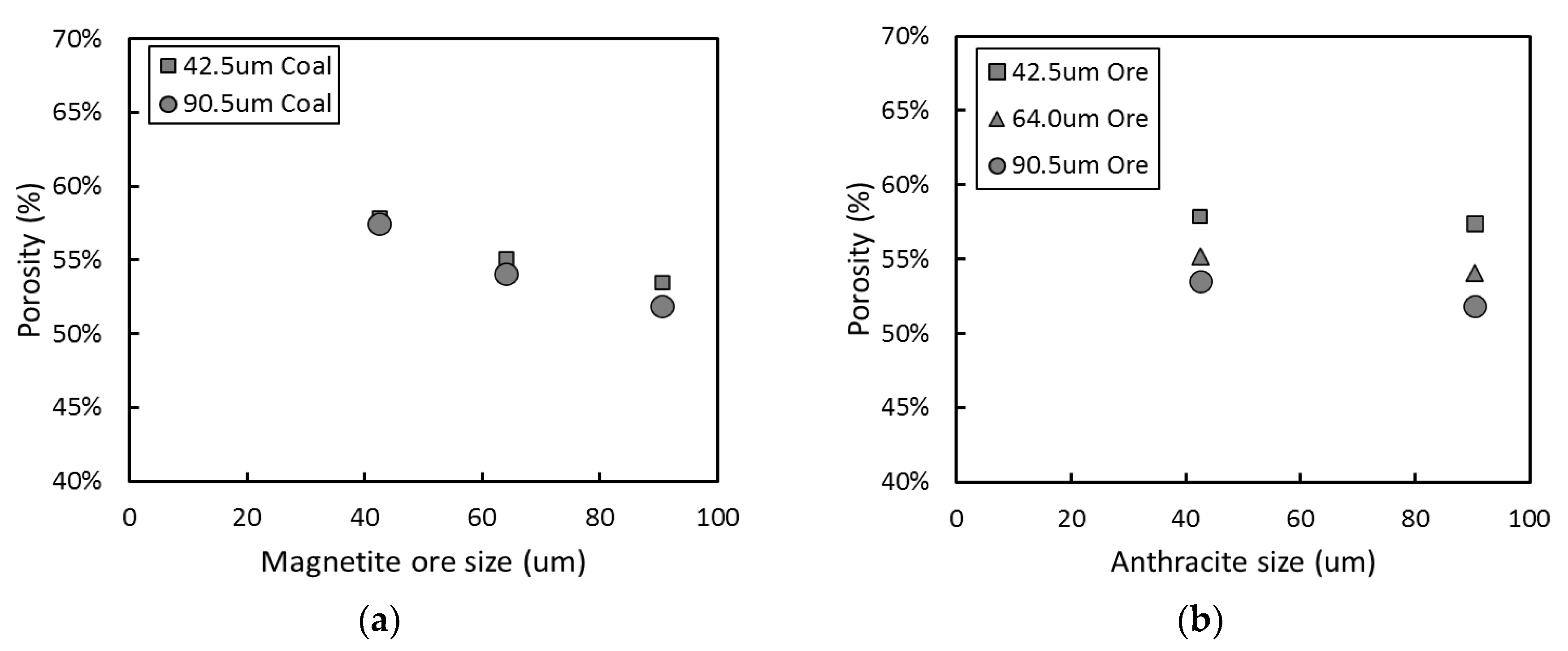
3.1. Effect of Particle Size in Carbon-Based Reduction
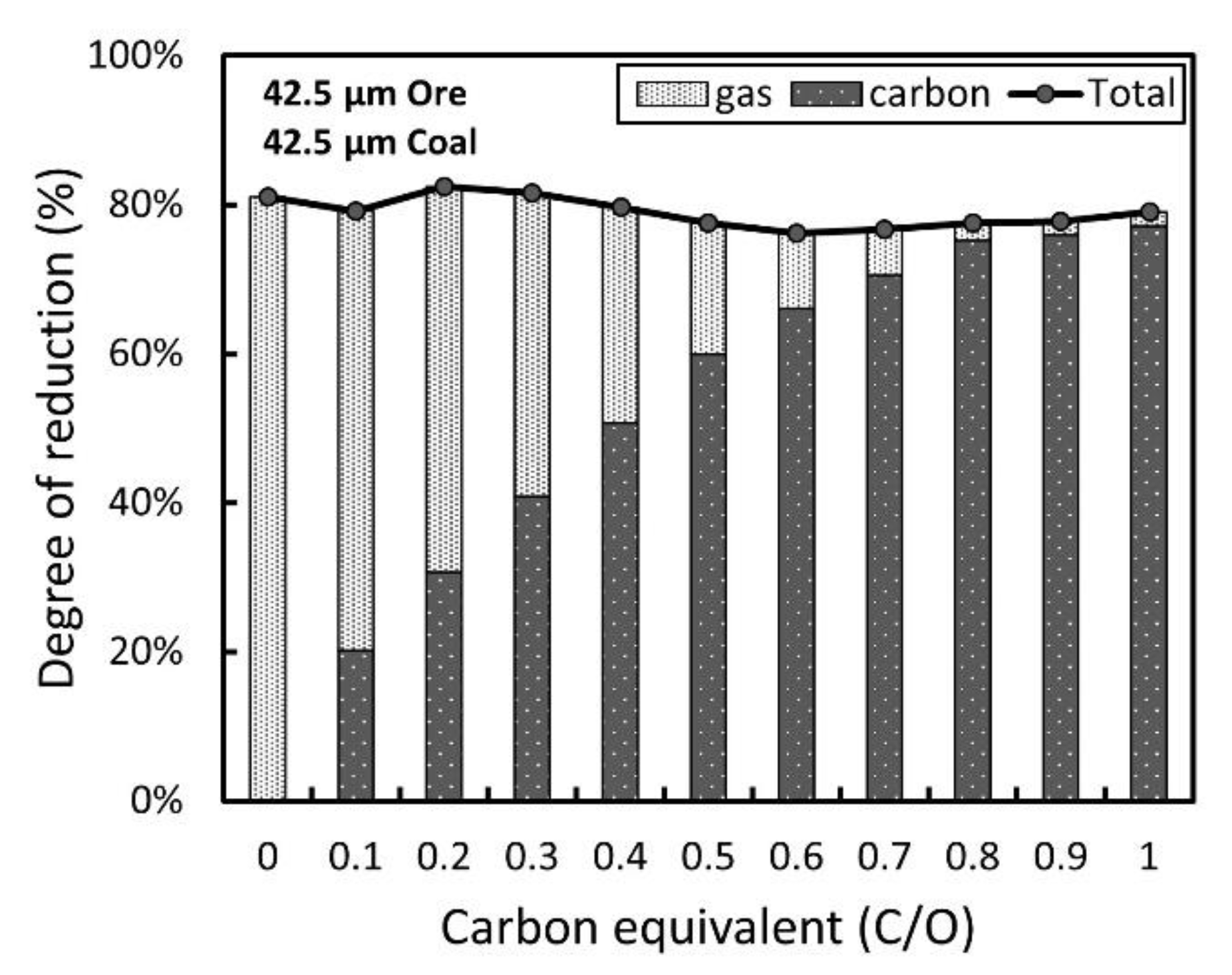
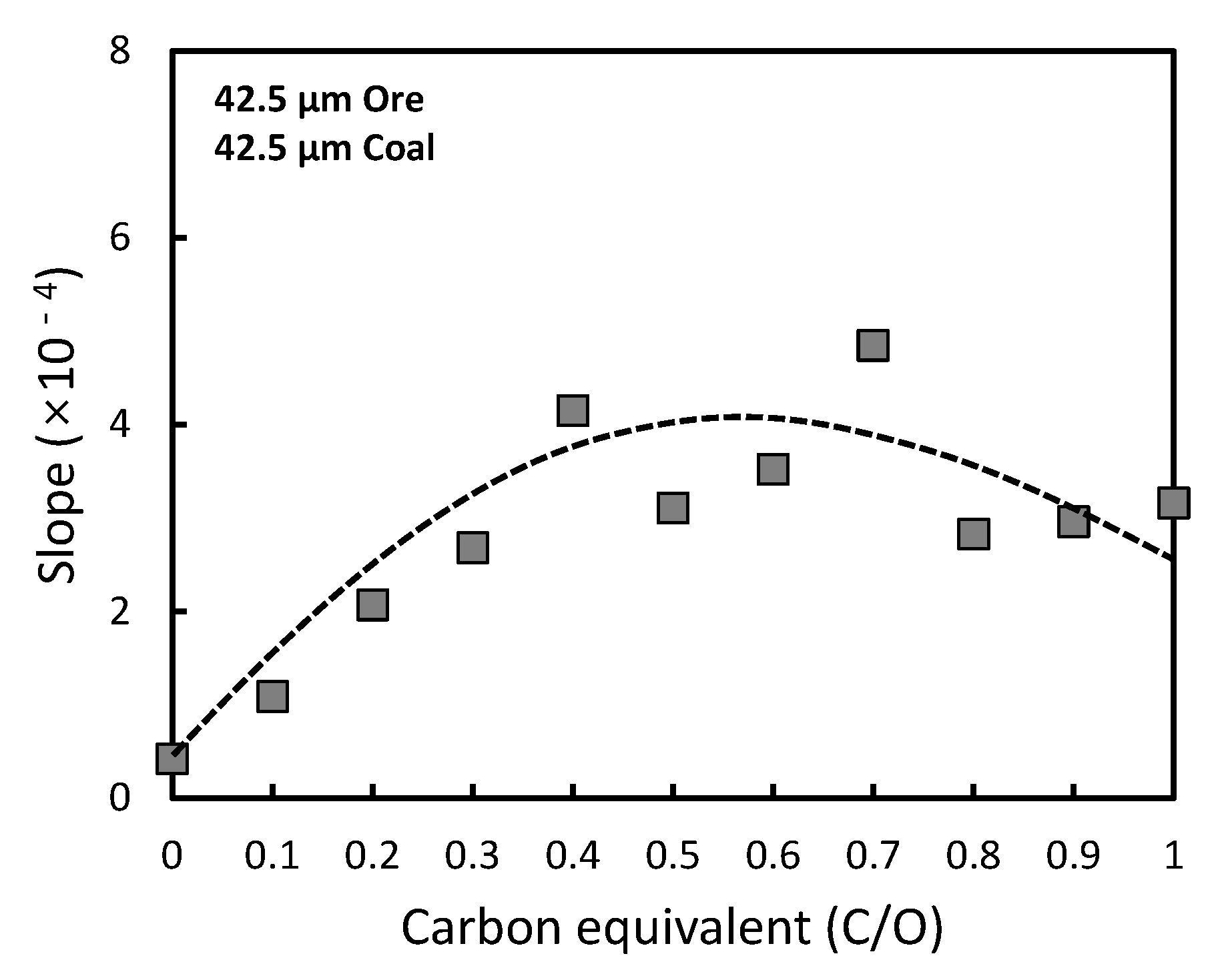
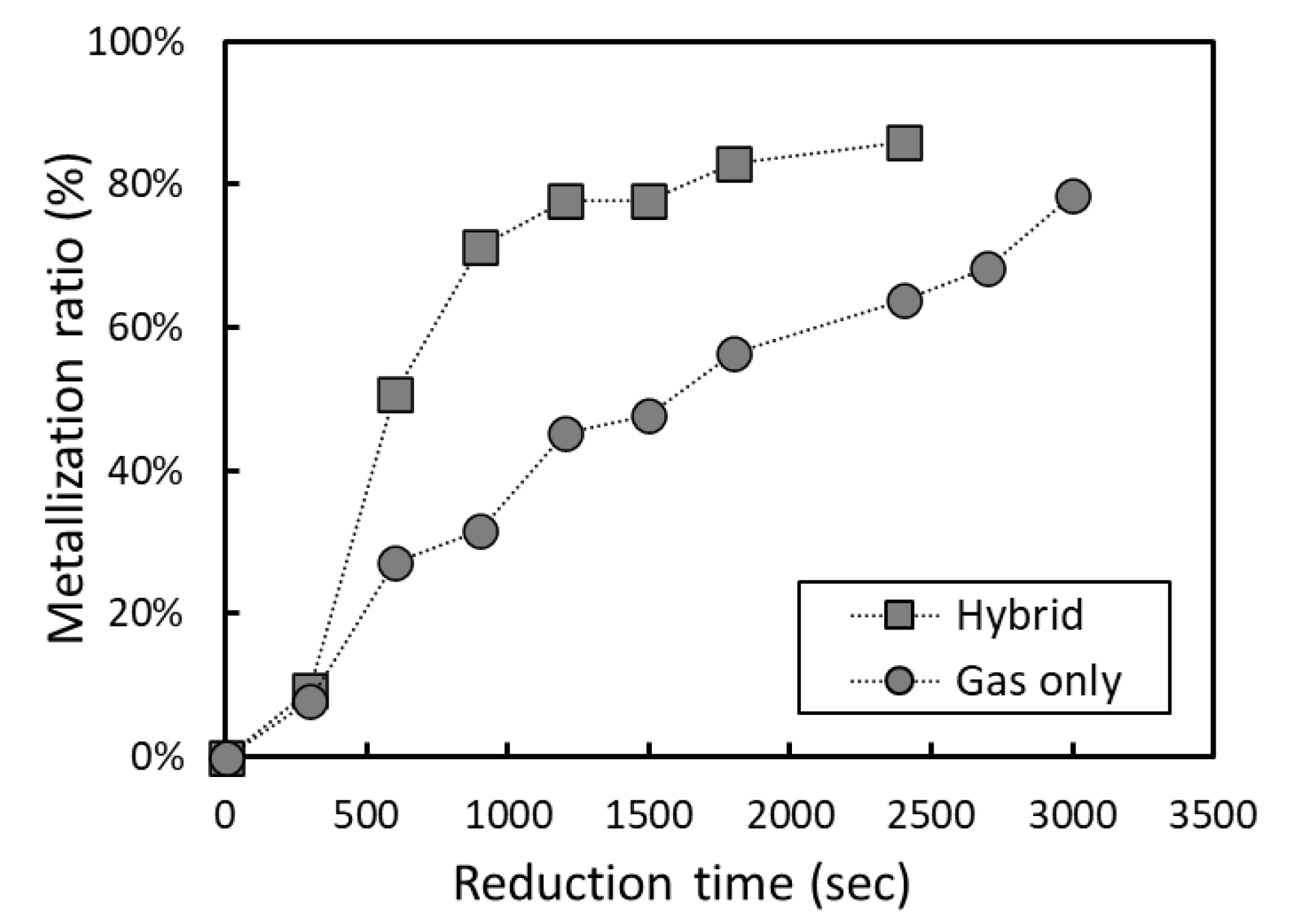
3.2. Optimization of Carbon-Based Reduction Prior to Gas Reduction
3.3. Kinetics of Reduction of Iron Ore by CO Gas
4. Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morales, R.D.; Rodriguez, H.; Conejo, A.N. A Mathematical Simulator for the EAF Steelmaking Process Using Direct Reduced Iron. ISIJ Int. 2001, 41, 426–436. [Google Scholar] [CrossRef]
- Hornby, S.; Madias, J.; Torre, F. Myths and realities of charging DRI/HBI in electric arc furnaces. In Proceedings of the AISTech 2015 Proceedings, Cleveland, OH, USA, 4–7 May 2015; pp. 1895–1905. [Google Scholar]
- Elkader, M.A.; Fathy, A.; Eissa, M.; Shama, S. Effect of Direct Reduced Iron Proportion in Metallic Charge on Technological Parameters of EAF Steelmaking Process. Int. J. Sci. Res. 2016, 5, 2016–2024. [Google Scholar]
- Erwee, M.W.; Pistorius, P.C. Nitrogen in SL/RN direct reduced iron: Origin and effect on nitrogen control in EAF steelmaking. Ironmak. Steelmak. 2012, 39, 336–341. [Google Scholar] [CrossRef] [Green Version]
- Heo, J.H.; Park, J.H. Effect of direct reduced iron (DRI) on dephosphorization of molten steel by electric arc furnace slag. Met. Mater. B 2018, 49B, 3381–3389. [Google Scholar] [CrossRef] [Green Version]
- Matsui, T.; Ishikawa, N.; Hara, Y.; Takeda, K. Influence of Gangue Composition on Melting Behavior of Coal-Reduced Iron Mixture. ISIJ Int. 2004, 44, 2105–2111. [Google Scholar] [CrossRef]
- Norio, O.; Tanaka, T. Estimation of the Average Number of Contacts between Randomly Mixed Solid Particles. Ind. Eng. Chem. Fundam. 1980, 19, 338–340. [Google Scholar]
- Yun, T.S.; Cho, C.W. Kinetics of Gaseous Reduction of Wüstite Pellets. Korean J. Met. Mater. 1963, 1, 169–179. [Google Scholar]
- Sohn, I.; Fruehan, R.J. The reduction of iron oxides by volatiles in a rotary hearth furnace process: Part I. The role and kinetics of volatile reduction. Met. Mater. Trans. B 2005, 36, 605–612. [Google Scholar] [CrossRef]
- Hosokai, S.; Matsui, K.; Okinaka, N.; Ohno, K.; Shimizu, M.; Akiyama, T. Kinetics study on the reduction reaction of biomass-tar-infiltrated iron ore. Energy Fuels 2012, 26, 7274–7279. [Google Scholar] [CrossRef]
- Guo, D.; Hu, M.; Pu, C.; Xiao, B.; Hu, Z.; Liu, S.; Wang, X.; Zhu, X. Kinetics and mechanisms of direct reduction of iron ore-biomass composite pellets with hydrogen gas. Int. J. Hydrog. Energy 2015, 40, 4733–4740. [Google Scholar] [CrossRef]
- Turkdogan, E.T.; Vinters, J.V. Gaseous reduction of iron oxides: Part I. Reduction of hematite in hydrogen. Met. Trans. 1971, 2, 3175–3188. [Google Scholar]


| Magnetite ore | t-Fe | Fe3O4 | Fe2O3 | MgO | SiO2 | CaO |
| 43.5% | 53.7% | 6.6% | 15.1% | 17.7% | 4.3% | |
| Anthracite | F.C. | V.M. | Ash | |||
| 58.9 | 6.5% | 34.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, S.; Kang, Y. Effect of Carbon Addition on Direct Reduction Behavior of Low Quality Magnetite Ore by Reducing Gas Atmosphere. Metals 2021, 11, 1404. https://doi.org/10.3390/met11091404
Song S, Kang Y. Effect of Carbon Addition on Direct Reduction Behavior of Low Quality Magnetite Ore by Reducing Gas Atmosphere. Metals. 2021; 11(9):1404. https://doi.org/10.3390/met11091404
Chicago/Turabian StyleSong, Seongrim, and Youngjo Kang. 2021. "Effect of Carbon Addition on Direct Reduction Behavior of Low Quality Magnetite Ore by Reducing Gas Atmosphere" Metals 11, no. 9: 1404. https://doi.org/10.3390/met11091404
APA StyleSong, S., & Kang, Y. (2021). Effect of Carbon Addition on Direct Reduction Behavior of Low Quality Magnetite Ore by Reducing Gas Atmosphere. Metals, 11(9), 1404. https://doi.org/10.3390/met11091404






