Investigation of Strain-Induced Precipitation of Niobium Carbide in Niobium Micro-Alloyed Steels at Elevated Temperatures
Abstract
:1. Introduction
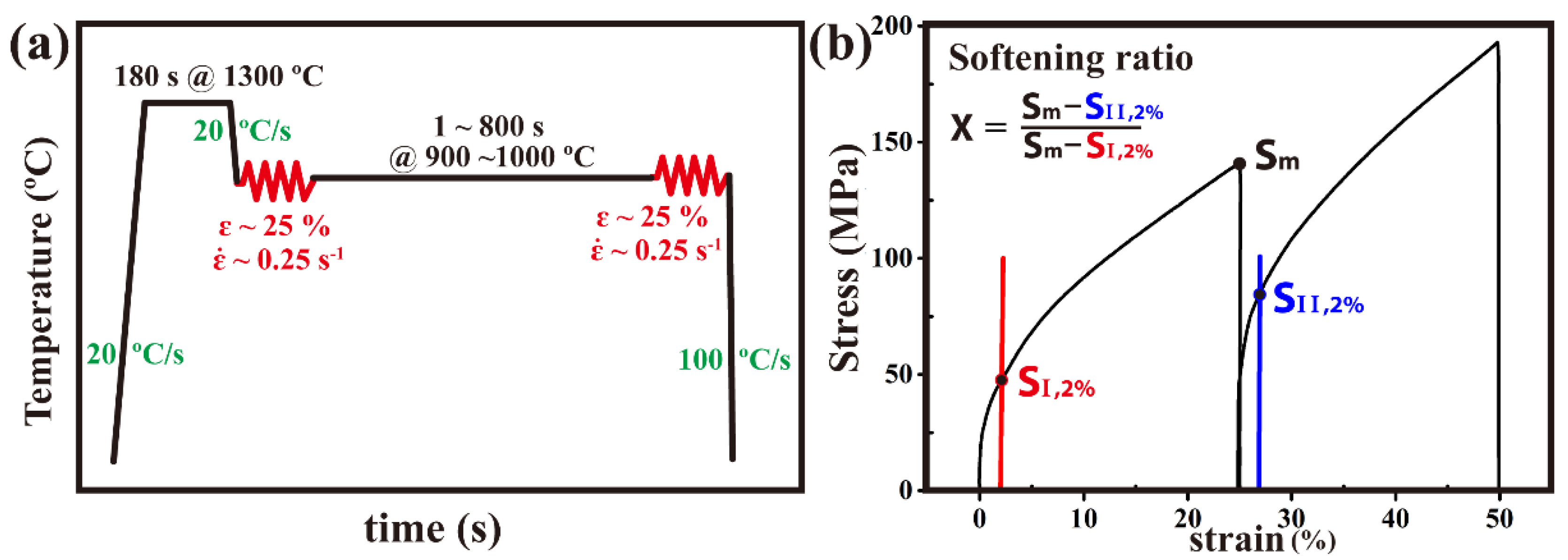
2. Materials and Methods
3. Results and Discussion
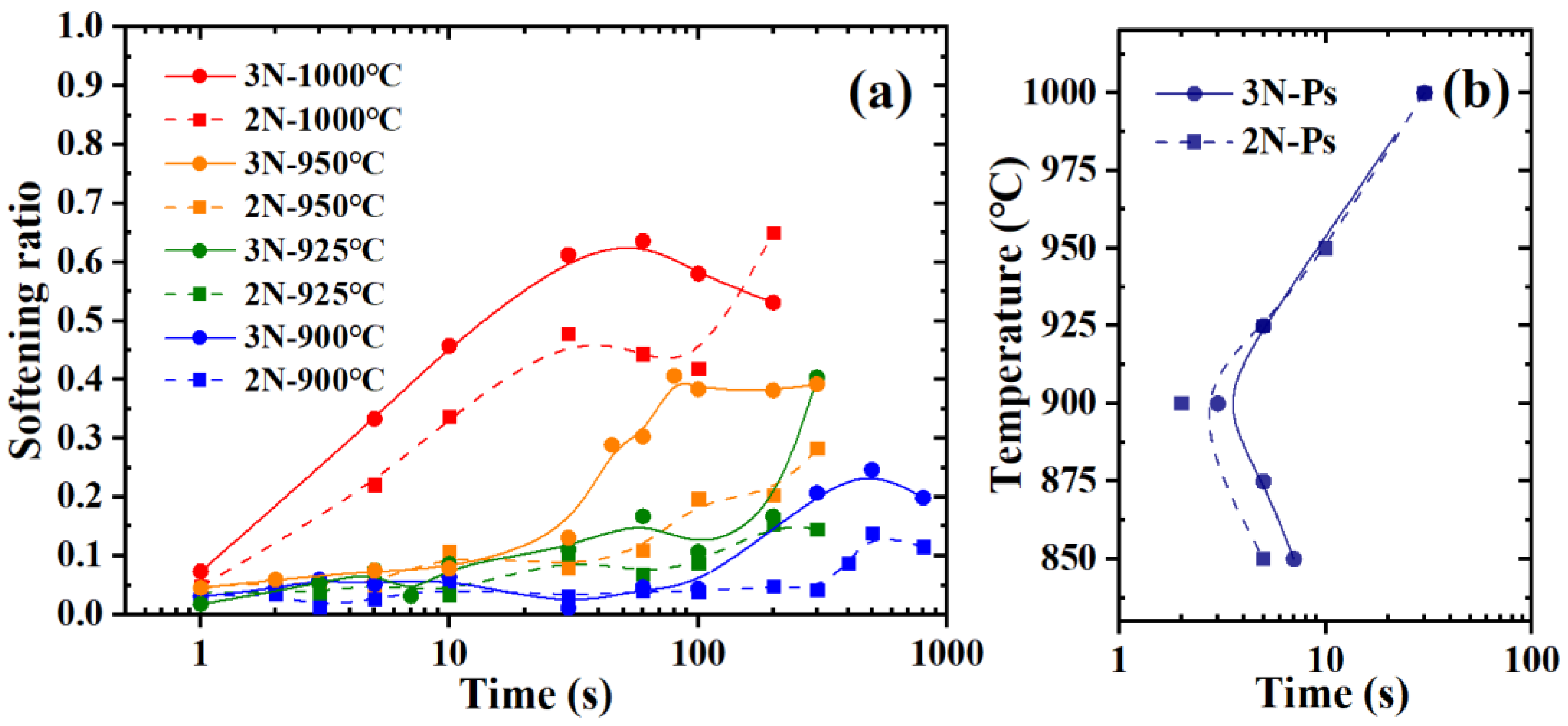
3.1. Softening Ratio Curves and Precipitation-Time-Temperature Curves
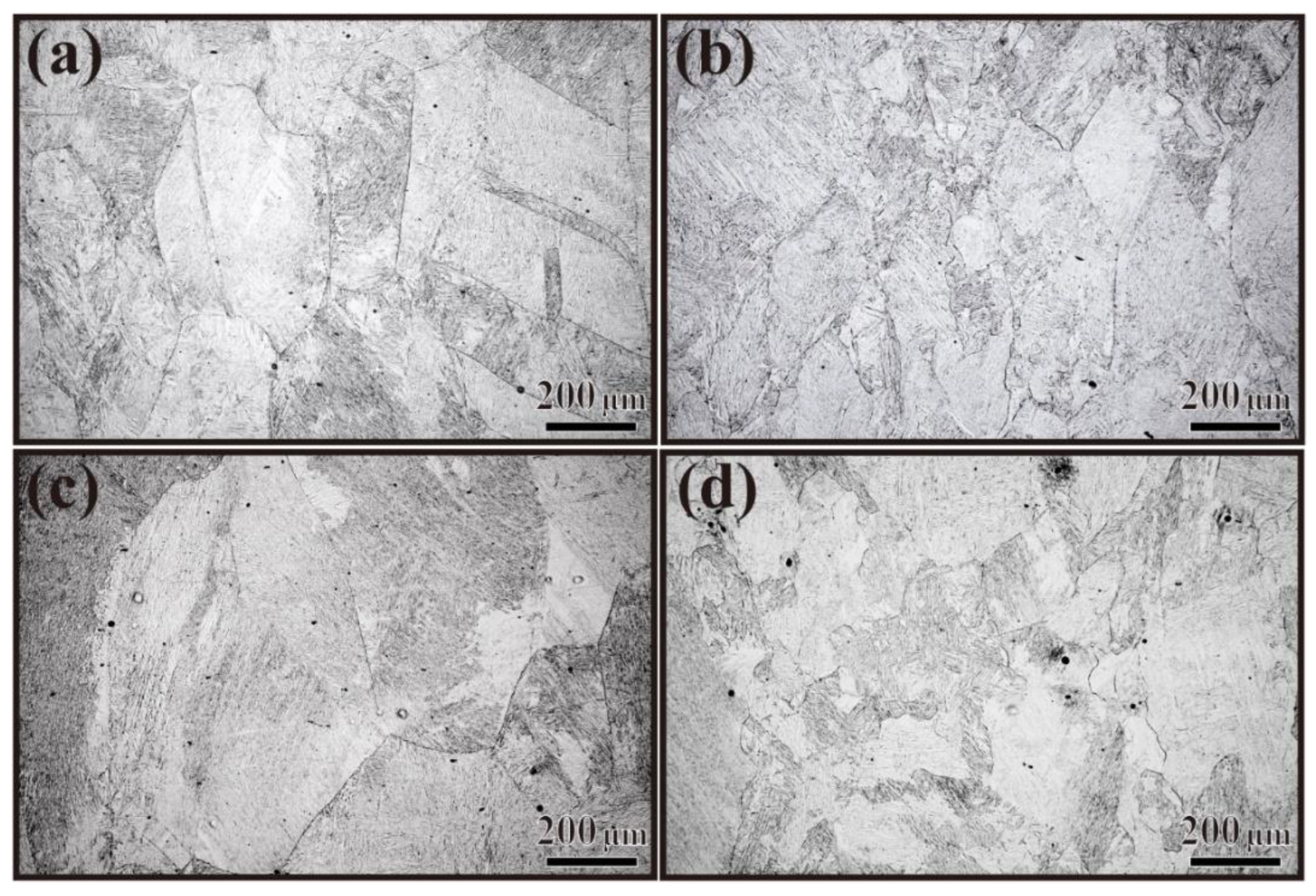
3.2. Prior Austenite Grains
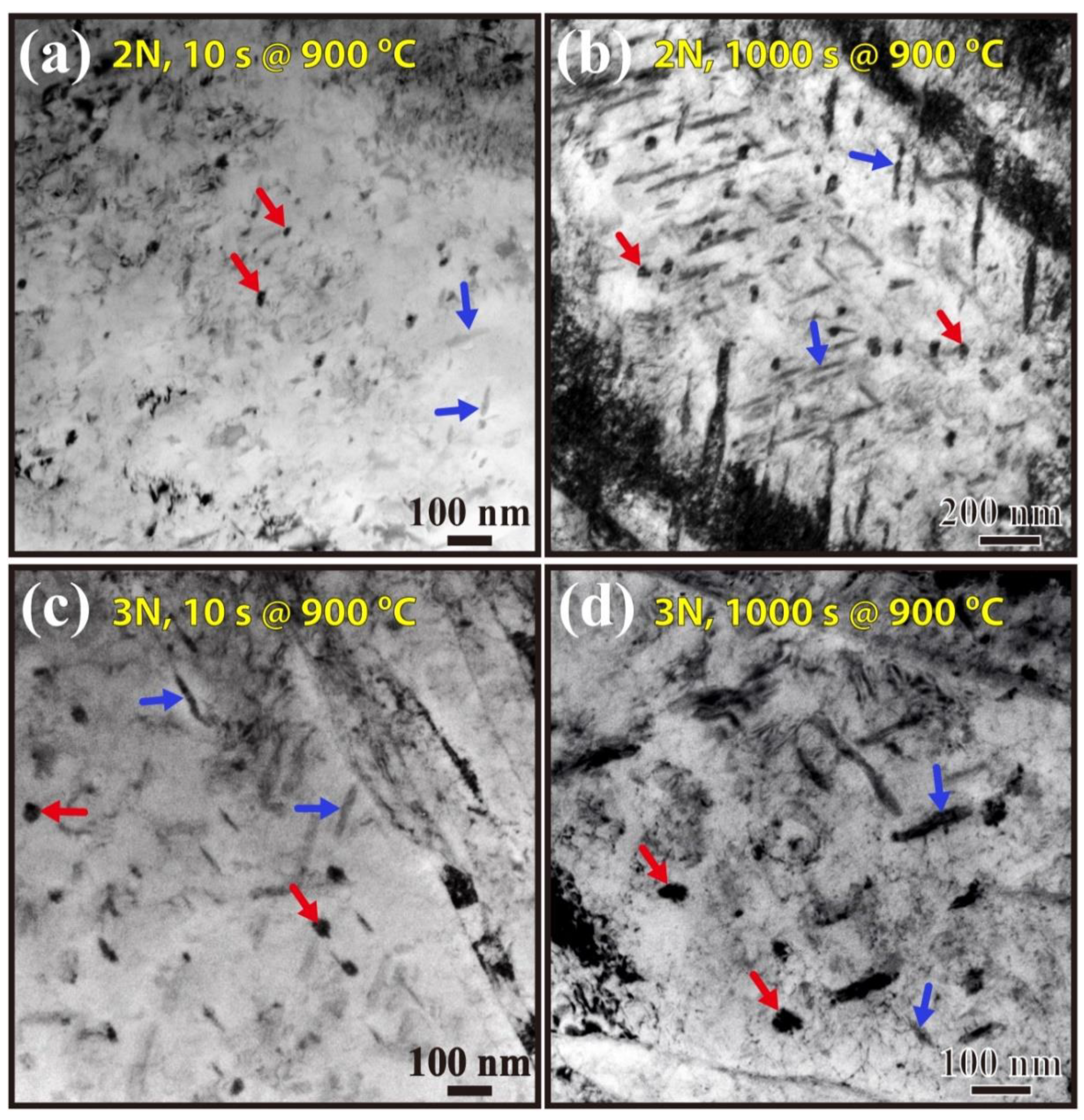
3.3. Precipitate Observation
3.4. Precipitate Statistics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Funakawa, Y.; Shiozaki, T.; Tomita, K.; Yamamoto, T.; Maeda, E. Development of High Strength Hot-rolled Sheet Steel Consisting of Ferrite and Nanometer-sized Carbides. ISIJ Int. 2004, 44, 1945–1951. [Google Scholar] [CrossRef]
- Weng, Y. (Ed.) Ultra-Fine Grained Steels; Springer Science & Business Media: New York, NY, USA, 2009. [Google Scholar]
- Jha, G.; Das, S.; Sinha, S.; Lodh, A.; Haldar, A. Design and development of precipitate strengthened advanced high strength steel for automotive application. Mater. Sci. Eng. A 2013, 561, 394–402. [Google Scholar] [CrossRef]
- Garcia, C.I. High strength low alloyed (HSLA) steels. In Automotive Steels; Woodhead Publishing: Duxford, UK, 2017; pp. 145–167. [Google Scholar]
- Luton, M.J.; Dorvel, R.; Petkovic, R.A. Interaction between deformation, recrystallization and precipitation in niobium steels. Met. Mater. Trans. A 1980, 11, 411–420. [Google Scholar] [CrossRef]
- Bakkaloğlu, A. Effect of processing parameters on the microstructure and properties of an Nb microalloyed steel. Mater. Lett. 2002, 56, 200–209. [Google Scholar] [CrossRef]
- Morrison, W.B. Microalloy steels—The beginning. Mater. Sci. Technol. 2009, 25, 1066–1073. [Google Scholar] [CrossRef]
- DeArdo, A.J.; Hua, M.J.; Cho, K.G.; Garcia, C.I. On strength of microalloyed steels: An interpretive review. Mater. Sci. Technol. 2009, 25, 1074–1082. [Google Scholar] [CrossRef]
- Patel, J.; Wilshire, B. The challenge to produce consistent mechanical properties in Nb-HSLA strip steels. J. Mater. Process. Technol. 2002, 120, 316–321. [Google Scholar] [CrossRef]
- Cochrane, R. Phase transformations in microalloyed high strength low alloy (HSLA) steels. In Phase Transformations in Steels; Woodhead Publishing: Duxford, UK, 2012; pp. 153–212. [Google Scholar] [CrossRef]
- Shao, Y.; Liu, C.; Yan, Z.; Li, H.; Liu, Y. Formation mechanism and control methods of acicular ferrite in HSLA steels: A review. J. Mater. Sci. Technol. 2018, 34, 737–744. [Google Scholar] [CrossRef]
- Dutta, B.; Sellars, C.M. Strengthening of austenite by niobium during hot rolling of microalloyed steel. Mater. Sci. Technol. 1986, 2, 146–153. [Google Scholar] [CrossRef]
- Dutta, B.; Palmiere, E.; Sellars, C. Modelling the kinetics of strain induced precipitation in Nb microalloyed steels. Acta Mater. 2001, 49, 785–794. [Google Scholar] [CrossRef]
- Charleux, M.; Poole, W.J.; Militzer, M.; Deschamps, A. Precipitation behavior and its effect on strengthening of an HSLA-Nb/Ti steel. Met. Mater. Trans. A 2001, 32, 1635–1647. [Google Scholar] [CrossRef]
- Hong, S.; Kang, K.; Park, C. Strain-induced precipitation of NbC in Nb and Nb–Ti microalloyed HSLA steels. Scr. Mater. 2002, 46, 163–168. [Google Scholar] [CrossRef]
- Hin, C.; Bréchet, Y.; Maugis, P.; Soisson, F. Kinetics of heterogeneous dislocation precipitation of NbC in alpha-iron. Acta Mater. 2008, 56, 5535–5543. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Guo, C.; Liu, W.; Yang, Z.; Sun, X.; Zhang, Z.; Jiang, F. Effect of molybdenum addition on the precipitation of carbides in the austenite matrix of titanium micro-alloyed steels. J. Mater. Sci. 2016, 51, 4996–5007. [Google Scholar] [CrossRef]
- Kubota, M.; Ochi, T. Development of anti-coarsening extra-fine steel for carburizing. Nippon Steel Technical Report No. 88. Shinnittetsu Giho 2003, 72–76. [Google Scholar]
- Qiu, Z.-K.; Zhang, P.; Wei, D.-B.; Wei, X.-F.; Chen, X.-H. A study on tribological behavior of double-glow plasma surface alloying W-Mo coating on gear steel. Surf. Coat. Technol. 2015, 278, 92–98. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Wang, H.; Song, Q.; Lu, L.; Wang, W.; Liu, Z. A comparison of the effects of traditional shot peening and micro-shot peening on the scuffing resistance of carburized and quenched gear steel. Wear 2016, 368–369, 253–257. [Google Scholar] [CrossRef]
- AlOgab, K.A.; Matlock, D.K.; Speer, J.G.; Kleebe, H.J. The Effects of Heating Rate on Austenite Grain Growth in a Ti-modified SAE 8620 Steel with Controlled Niobium Additions. ISIJ Int. 2007, 47, 1034–1041. [Google Scholar] [CrossRef]
- Mohrbacher, H. Metallurgical concepts for optimized processing and properties of carburizing steel. Adv. Manuf. 2016, 4, 105–114. [Google Scholar] [CrossRef]
- An, X.; Tian, Y.; Wang, H.; Shen, Y.; Wang, Z. Suppression of Austenite Grain Coarsening by Using Nb–Ti Microalloying in High Temperature Carburizing of a Gear Steel. Adv. Eng. Mater. 2019, 21, 1900132. [Google Scholar] [CrossRef]
- Hillert, M. Inhibition of grain growth by second-phase particles. Acta Met. 1988, 36, 3177–3181. [Google Scholar] [CrossRef]
- Andersen, I.; Grong, Ø. Analytical modelling of grain growth in metals and alloys in the presence of growing and dissolving precipitates—I. Normal grain growth. Acta Metall. Mater. 1995, 43, 2673–2688. [Google Scholar] [CrossRef]
- Manohar, P.A.; Ferry, M.; Chandra, T. Five Decades of the Zener Equation. ISIJ Int. 1998, 38, 913–924. [Google Scholar] [CrossRef]
- Maalekian, M.; Radis, R.; Militzer, M.; Moreau, A.; Poole, W. In situ measurement and modelling of austenite grain growth in a Ti/Nb microalloyed steel. Acta Mater. 2012, 60, 1015–1026. [Google Scholar] [CrossRef]
- Razzak, M.A.; Perez, M.; Sourmail, T.; Cazottes, S.; Frotey, M. Preventing Abnormal Grain Growth of Austenite in Low Alloy Steels. ISIJ Int. 2014, 54, 1927–1934. [Google Scholar] [CrossRef]
- Gladman, T. On the theory of the effect of precipitate particles on grain growth in metals. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1966, 294, 298–309. [Google Scholar] [CrossRef]
- Fernández, J.; Illescas, S.; Guilemany, J. Effect of microalloying elements on the austenitic grain growth in a low carbon HSLA steel. Mater. Lett. 2007, 61, 2389–2392. [Google Scholar] [CrossRef]
- Alogab, K.A.; Matlock, D.K.; Speer, J.G.; Kleebe, H.J. The Influence of Niobium Microalloying on Austenite Grain Coarsening Behavior of Ti-modified SAE 8620 Steel. ISIJ Int. 2007, 47, 307–316. [Google Scholar] [CrossRef]
- Palmiere, E.J.; Garcia, C.I.; De Ardo, A.J. Compositional and microstructural changes which attend reheating and grain coarsening in steels containing niobium. Met. Mater. Trans. A 1994, 25, 277–286. [Google Scholar] [CrossRef]
- Enloe, C.M.; Findley, K.O.; Speer, J.G. Austenite Grain Growth and Precipitate Evolution in a Carburizing Steel with Combined Niobium and Molybdenum Additions. Met. Mater. Trans. A 2015, 46, 5308–5328. [Google Scholar] [CrossRef]
- Saito, G.; Sakaguchi, N.; Ohno, M.; Matsuura, K.; Takeuchi, M.; Sano, T.; Minoguchi, K.; Yamaoka, T. Effects of Fine Precipitates on Austenite Grain Refinement of Micro-alloyed Steel during Cyclic Heat Treatment. ISIJ Int. 2019, 59, 2098–2104. [Google Scholar] [CrossRef]
- Marynowski, P.; Adrian, H.; Głowacki, M. Modeling of the Kinetics of Carbonitride Precipitation Process in High-Strength Low-Alloy Steels Using Cellular Automata Method. J. Mater. Eng. Perform. 2019, 28, 4018–4025. [Google Scholar] [CrossRef]
- Maugis, P.; Gouné, M. Kinetics of vanadium carbonitride precipitation in steel: A computer model. Acta Mater. 2005, 53, 3359–3367. [Google Scholar] [CrossRef]
- Show, B.; Veerababu, R.; Balamuralikrishnan, R.; Malakondaiah, G. Effect of vanadium and titanium modification on the microstructure and mechanical properties of a microalloyed HSLA steel. Mater. Sci. Eng. A 2010, 527, 1595–1604. [Google Scholar] [CrossRef]
- Vervynckt, S.; Verbeken, K.; Thibaux, P.; Houbaert, Y. Recrystallization–precipitation interaction during austenite hot deformation of a Nb microalloyed steel. Mater. Sci. Eng. A 2011, 528, 5519–5528. [Google Scholar] [CrossRef]
- Gong, P.; Palmiere, E.; Rainforth, W. Dissolution and precipitation behaviour in steels microalloyed with niobium during thermomechanical processing. Acta Mater. 2015, 97, 392–403. [Google Scholar] [CrossRef]
- Courtois, E.; Epicier, T.; Scott, C. EELS study of niobium carbo-nitride nano-precipitates in ferrite. Micron 2006, 37, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Dutta, B.; Sellars, C.M. Effect of composition and process variables on Nb (C, N) precipitation in niobium microalloyed austenite. Mater. Sci. Technol. 1987, 3, 197–206. [Google Scholar] [CrossRef]
- Dutta, B.; Valdes, E.; Sellars, C.M. Mechanism and kinetics of strain induced precipitation of Nb (C, N) in austenite. Acta Metall. Mater. 1992, 40, 653–662. [Google Scholar] [CrossRef]
- Karmakar, A.; Biswas, S.; Mukherjee, S.; Chakrabarti, D.; Kumar, V. Effect of composition and thermo-mechanical processing schedule on the microstructure, precipitation and strengthening of Nb-microalloyed steel. Mater. Sci. Eng. A 2017, 690, 158–169. [Google Scholar] [CrossRef]
- Fernández, A.; López, B.; Rodríguez-Ibabe, J. Relationship between the austenite recrystallized fraction and the softening measured from the interrupted torsion test technique. Scr. Mater. 1999, 40, 543–549. [Google Scholar] [CrossRef]
- Miyamoto, G.; Oh, J.C.; Hono, K.; Furuhara, T.; Maki, T. Effect of partitioning of Mn and Si on the growth kinetics of cementite in tempered Fe–0.6 mass% C martensite. Acta Mater. 2007, 55, 5027–5038. [Google Scholar] [CrossRef]
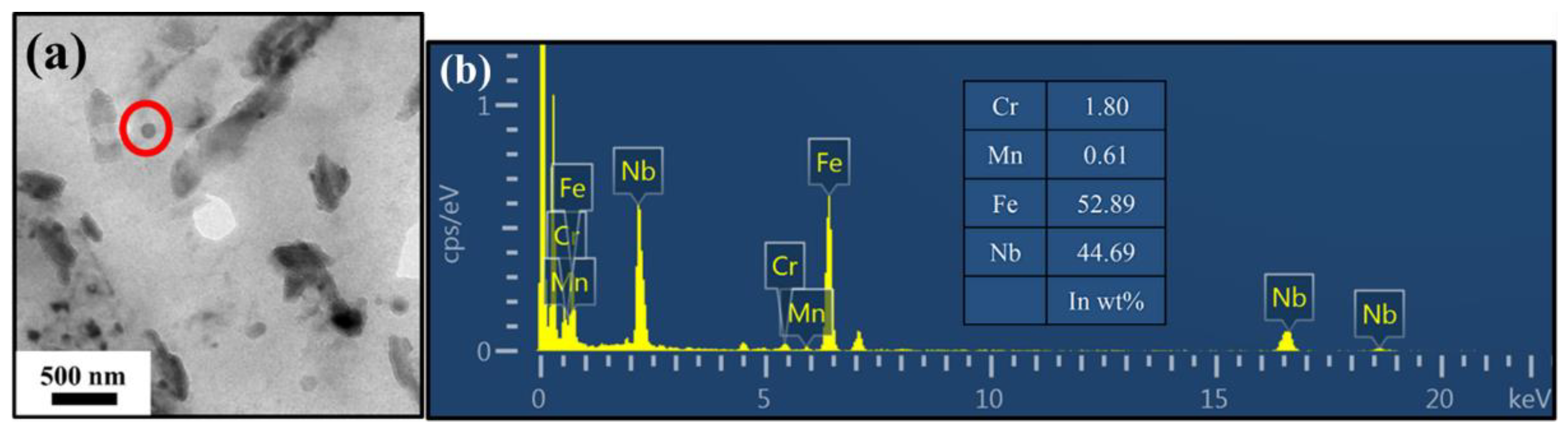
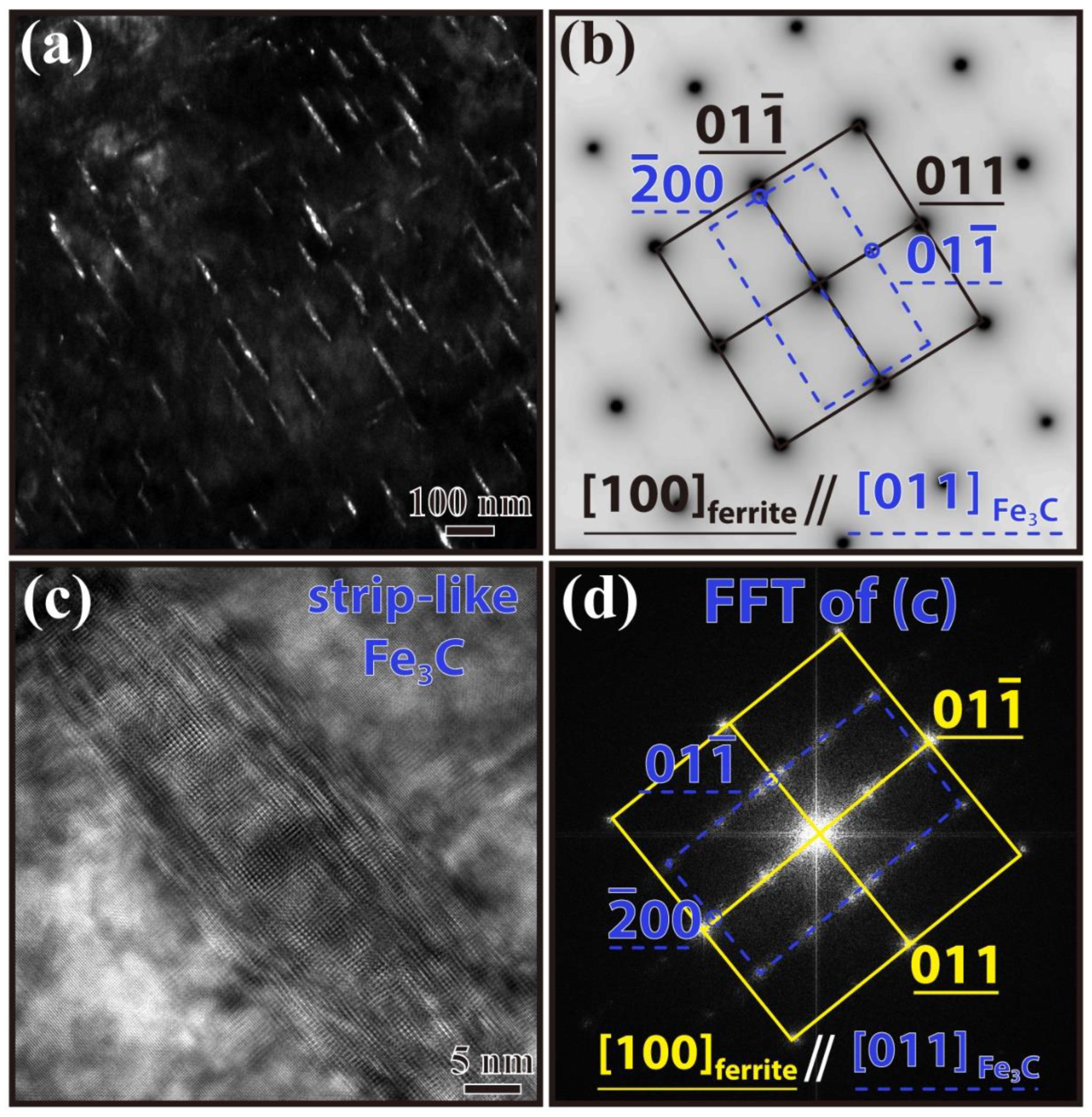
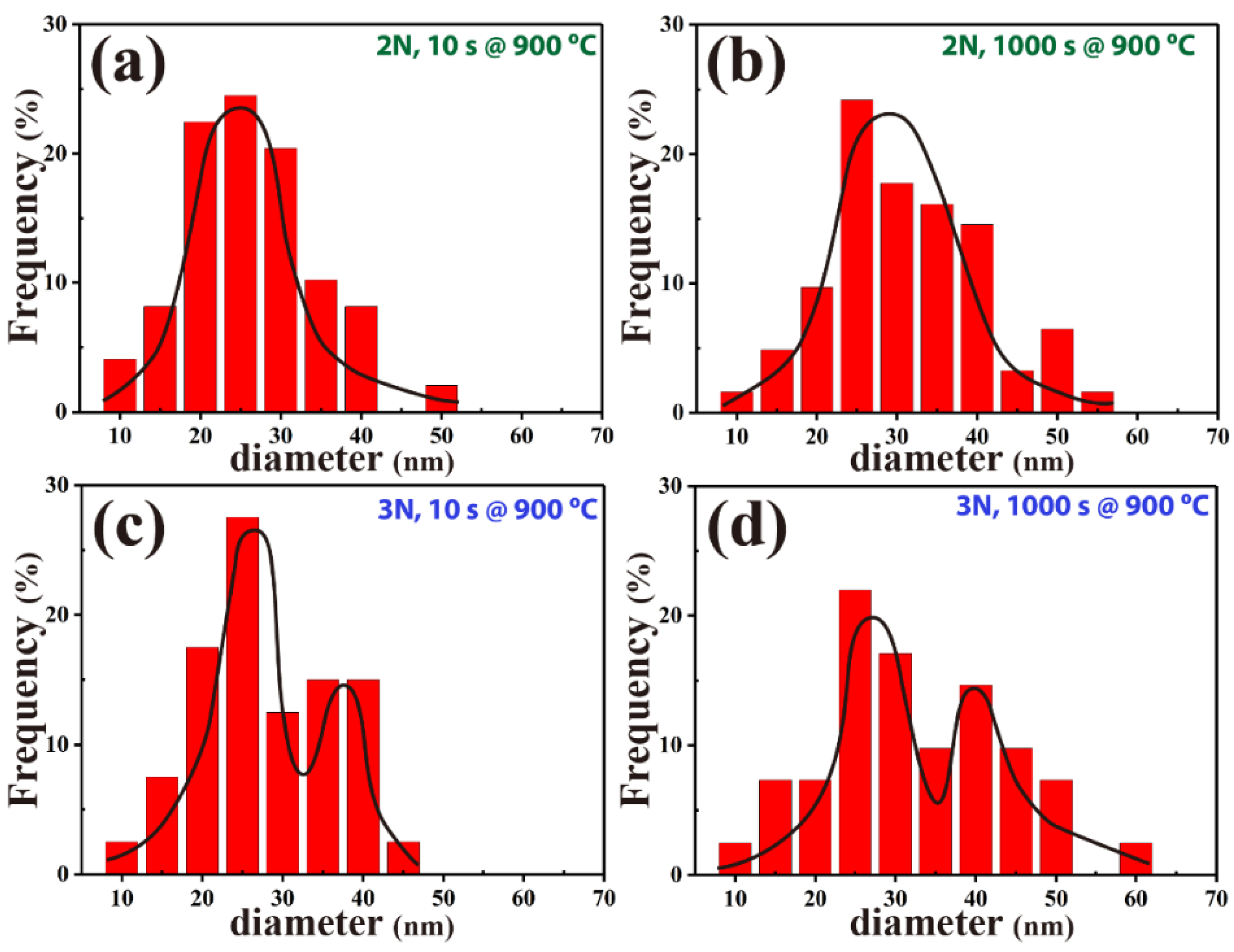
| No. | Fe | C | Cr | Mn | Si | Nb |
|---|---|---|---|---|---|---|
| Steel 2N | Bal. | 0.19 | 1.20 | 0.82 | 0.38 | 0.02 |
| Steel 3N | Bal. | 0.19 | 1.16 | 0.85 | 0.32 | 0.03 |
| Temperature | 2N | 3N | |
|---|---|---|---|
| 1300 °C | stable phase | fully solid solution | fully solid solution |
| amount (in at%) | no precipitation | no precipitation | |
| 900 °C | stable phase | NbC | NbC |
| amount (in at%) | 0.0247 | 0.0372 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsao, T.-C.; Chiu, P.-H.; Tseng, C.-Y.; Tai, C.-L.; Chen, H.-R.; Chung, T.-F.; Chen, C.-Y.; Wang, S.-H.; Tsai, Y.-T.; Yang, J.-R. Investigation of Strain-Induced Precipitation of Niobium Carbide in Niobium Micro-Alloyed Steels at Elevated Temperatures. Metals 2022, 12, 1619. https://doi.org/10.3390/met12101619
Tsao T-C, Chiu P-H, Tseng C-Y, Tai C-L, Chen H-R, Chung T-F, Chen C-Y, Wang S-H, Tsai Y-T, Yang J-R. Investigation of Strain-Induced Precipitation of Niobium Carbide in Niobium Micro-Alloyed Steels at Elevated Temperatures. Metals. 2022; 12(10):1619. https://doi.org/10.3390/met12101619
Chicago/Turabian StyleTsao, Tzu-Ching, Po-Han Chiu, Chien-Yu Tseng, Cheng-Lin Tai, Hsueh-Ren Chen, Tsai-Fu Chung, Chih-Yuan Chen, Shing-Hoa Wang, Yu-Ting Tsai, and Jer-Ren Yang. 2022. "Investigation of Strain-Induced Precipitation of Niobium Carbide in Niobium Micro-Alloyed Steels at Elevated Temperatures" Metals 12, no. 10: 1619. https://doi.org/10.3390/met12101619
APA StyleTsao, T.-C., Chiu, P.-H., Tseng, C.-Y., Tai, C.-L., Chen, H.-R., Chung, T.-F., Chen, C.-Y., Wang, S.-H., Tsai, Y.-T., & Yang, J.-R. (2022). Investigation of Strain-Induced Precipitation of Niobium Carbide in Niobium Micro-Alloyed Steels at Elevated Temperatures. Metals, 12(10), 1619. https://doi.org/10.3390/met12101619







