Biofilm-Induced Corrosion Inhibition of Q235 Carbon Steel by Tenacibaculum mesophilum D-6 and Bacillus sp. Y-6
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial and Materials
2.2. Electrochemical Measurements
2.3. Surface Analysis and Biofilm Characterization
2.4. Weight Loss and Corrosion Product Analysis
3. Results
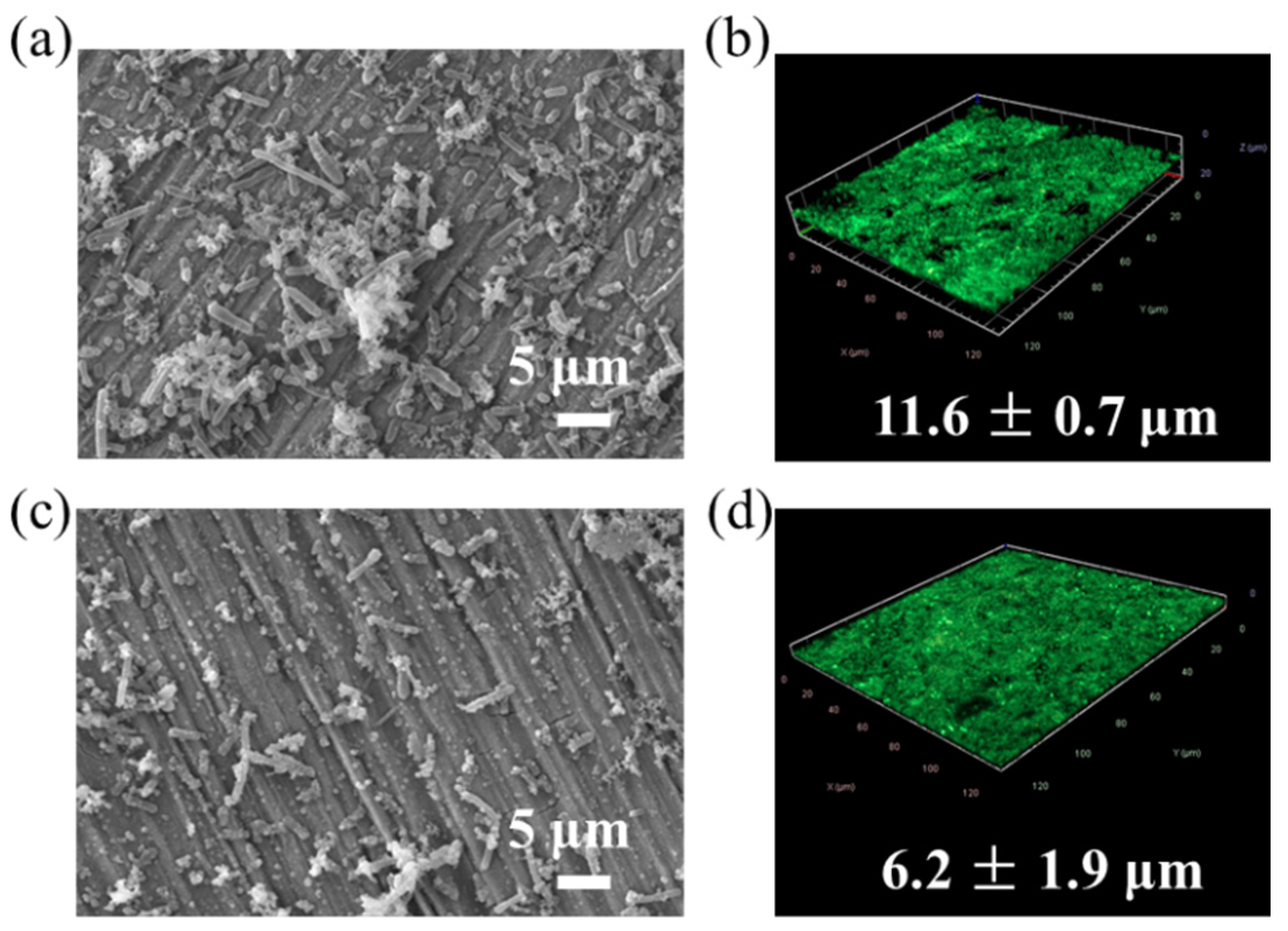
3.1. Biofilm Characterization
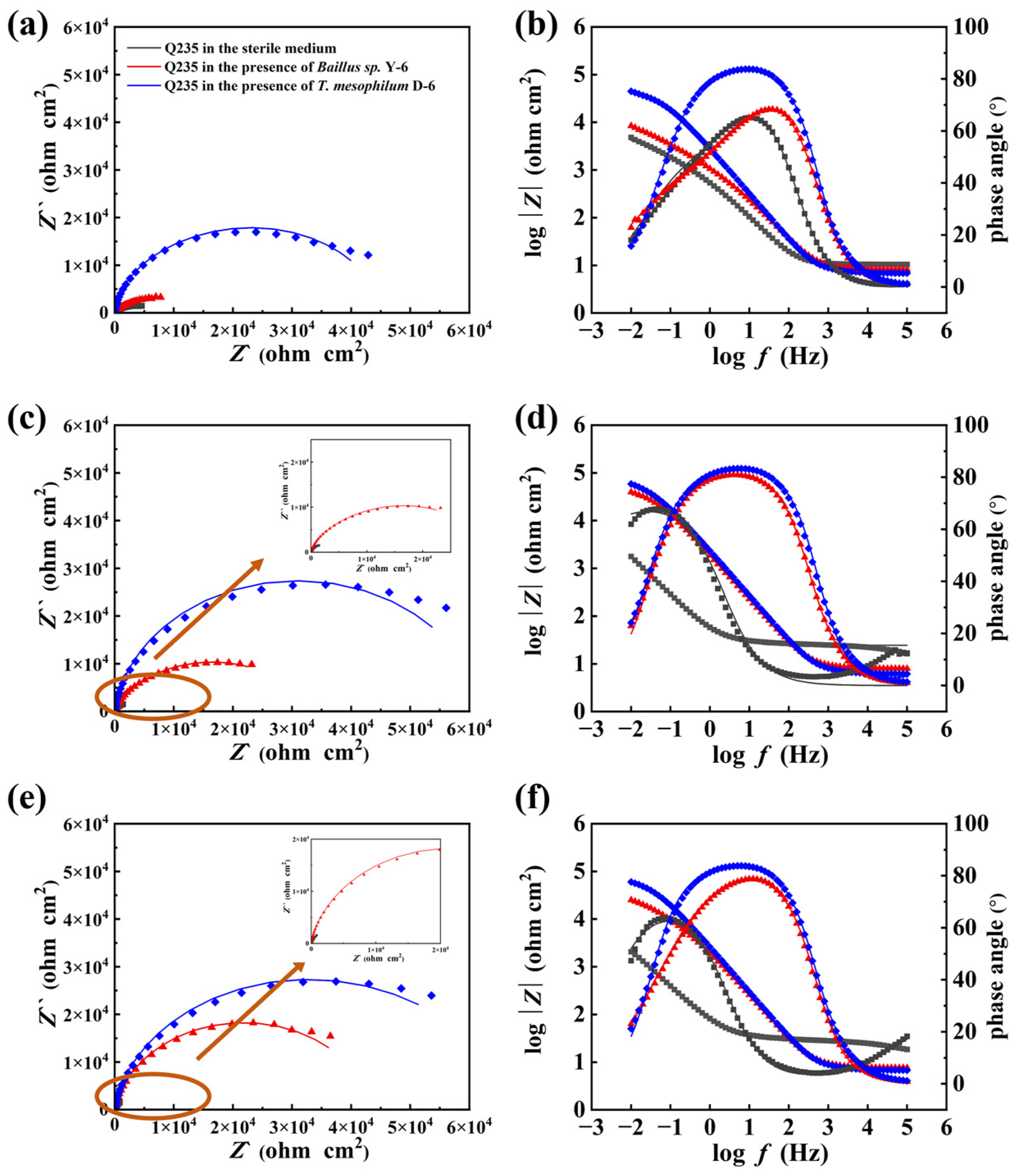
3.2. Electrochemical Tests
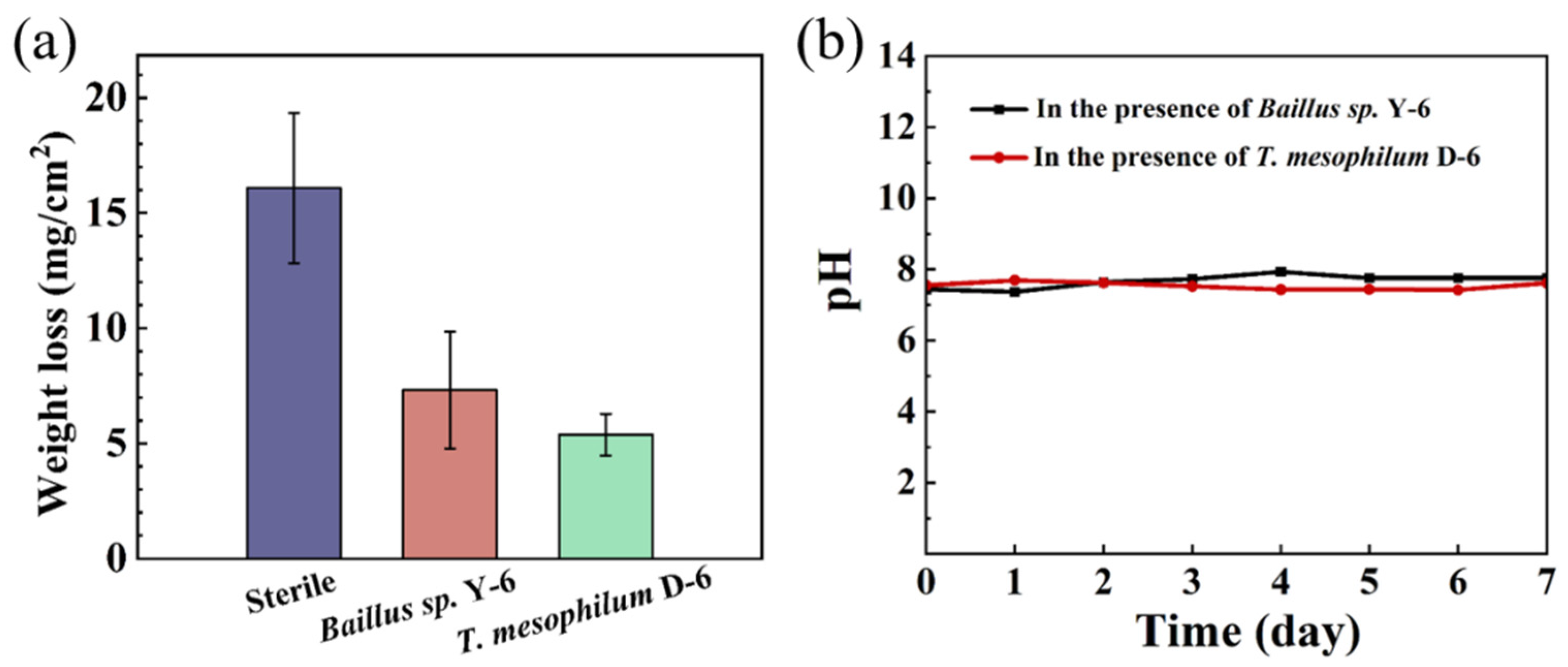
3.3. Weight Loss and Pitting Corrosion
3.4. Corrosion Product Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, Y.; Li, Y.; Wang, F. Corrosion of low carbon steel in atmospheric environments of different chloride content. Corros. Sci. 2009, 51, 997–1006. [Google Scholar] [CrossRef]
- Hou, B.; Li, X.; Ma, X.; Du, C.; Zhang, D.; Zheng, M.; Xu, W.; Lu, D.; Ma, F. The cost of corrosion in China. npj Mater. Degrad. 2017, 1, 4. [Google Scholar] [CrossRef]
- Pedeferri, P. Corrosion Prevention by Coatings. In Corrosion Science and Engineering; Springer International Publishing: Cham, Switzerland, 2018; pp. 327–361. [Google Scholar]
- Hao, X.; Chen, S.; Qin, D.; Zhang, M.; Li, W.; Fan, J.; Wang, C.; Dong, M.; Zhang, J.; Cheng, F.; et al. Antifouling and antibacterial behaviors of capsaicin-based pH responsive smart coatings in marine environments. Mater. Sci. Eng. C 2020, 108, 110361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ju, P.; Pan, M.; Zhang, D.; Huang, Y.; Li, G.; Li, X. Self-healing mechanisms in smart protective coatings: A review. Corros. Sci. 2018, 144, 74–88. [Google Scholar] [CrossRef]
- Yang, Z.; Hao, X.; Chen, S.; Ma, Z.; Wang, W.; Wang, C.; Yue, L.; Sun, H.; Shao, Q.; Murugadoss, V.; et al. Long-term antibacterial stable reduced graphene oxide nanocomposites loaded with cuprous oxide nanoparticles. J. Colloid Interface Sci. 2019, 533, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yang, Z.; Pu, Y.; Dou, W.; Wang, C.; Wang, W.; Hao, X.; Chen, S.; Shao, Q.; Dong, M.; et al. Zinc oxide/vanadium pentoxide heterostructures with enhanced day-night antibacterial activities. J. Colloid Interface Sci. 2019, 547, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Furdek, M.; Vahčič, M.; Ščančar, J.; Milačič, R.; Kniewald, G.; Mikac, N. Organotin compounds in seawater and Mytilus galloprovincialis mussels along the Croatian Adriatic Coast. Mar. Pollut. Bull. 2012, 64, 189–199. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, Z.; Tao, J.; Jia, Z.; Chen, H.; Liu, S.; Jiang, J.; Wang, Z. Spraying Preparation of Eco-Friendly Superhydrophobic Coatings with Ultralow Water Adhesion for Effective Anticorrosion and Antipollution. ACS Appl. Mater. Interfaces 2020, 12, 25484–25493. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, S.J.; Jiang, C.Y. Microbiologically influenced corrosion and mechanisms. Microbiol. China 2017, 44, 1699–1713. [Google Scholar]
- Telegdi, J.; Shaban, A.; Trif, L. 8 - Microbiologically influenced corrosion (MIC). In Trends in Oil and Gas Corrosion Research and Technologies; El-Sherik, A.M., Ed.; Woodhead Publishing: Boston, MA, USA, 2017; pp. 191–214. [Google Scholar]
- Lovley, D.R. Happy together: Microbial communities that hook up to swap electrons. Isme J. 2017, 11, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Dong, H.L.; Reguera, G.; Beyenal, H.; Lu, A.H.; Liu, J.; Yu, H.Q.; Fredrickson, J.K. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 2016, 14, 651–662. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.Y.; Wanger, G.; Leung, K.M.; Yuzvinsky, T.D.; Southam, G.; Yang, J.; Lau, W.M.; Nealson, K.H.; Gorby, Y.A. Electrical transport along bacterial nanowires from Shewanella oneidensis MR-1. Proc. Natl. Acad. Sci. USA 2010, 107, 18127–18131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, J.; Esparza, P.; González, S.; Salvarezza, R.; Arévalo, M.P. The role of Pseudomonas aeruginosa on the localized corrosion of 304 stainless steel. Corros. Sci. 1993, 34, 1531–1540. [Google Scholar] [CrossRef]
- Wang, D.; Liu, J.; Jia, R.; Dou, W.; Kumseranee, S.; Punpruk, S.; Li, X.; Gu, T. Distinguishing two different microbiologically influenced corrosion (MIC) mechanisms using an electron mediator and hydrogen evolution detection. Corros. Sci. 2020, 177, 108993. [Google Scholar] [CrossRef]
- Sowards, J.W.; Mansfield, E. Corrosion of copper and steel alloys in a simulated underground storage-tank sump environment containing acid-producing bacteria. Corros. Sci. 2014, 87, 460–471. [Google Scholar] [CrossRef]
- Huber, B.; Herzog, B.; Drewes, J.E.; Koch, K.; Müller, E. Characterization of sulfur oxidizing bacteria related to biogenic sulfuric acid corrosion in sludge digesters. Bmc Microbiol. 2016, 16, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Li, Y.; Gu, T. Mechanistic modeling of biocorrosion caused by biofilms of sulfate reducing bacteria and acid producing bacteria. Bioelectrochemistry 2016, 110, 52–58. [Google Scholar] [CrossRef]
- Little, B.J.; Lee, J.S. Microbiologically influenced corrosion: An update. Int. Mater. Rev. 2014, 59, 384–393. [Google Scholar] [CrossRef]
- Beech, I.B.; Cheung, C.W.S. Interactions of exopolymers produced by sulphate-reducing bacteria with metal ions. Int. Biodeterior. Biodegrad. 1995, 35, 59–72. [Google Scholar] [CrossRef]
- Javed, M.A.; Neil, W.C.; Stoddart, P.R.; Wade, S.A. Influence of carbon steel grade on the initial attachment of bacteria and microbiologically influenced corrosion. Biofouling 2016, 32, 109–122. [Google Scholar] [CrossRef]
- Javed, M.A.; Stoddart, P.R.; Wade, S.A. Corrosion of carbon steel by sulphate reducing bacteria: Initial attachment and the role of ferrous ions. Corros. Sci. 2015, 93, 48–57. [Google Scholar] [CrossRef]
- Iverson, W.P. Microbial Corrosion of Metals. In Advances in Applied Microbiology; Laskin, A.I., Ed.; Academic Press: Cambridge, MA, USA, 1987; Volume 32, pp. 1–36. [Google Scholar]
- Lou, Y.; Chang, W.; Cui, T.; Wang, J.; Qian, H.; Ma, L.; Hao, X.; Zhang, D. Microbiologically influenced corrosion inhibition mechanisms in corrosion protection: A review. Bioelectrochemistry 2021, 141, 107883. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.; Hermansson, M. Inhibition of metal corrosion by bacteria. Biofouling 1991, 3, 1–11. [Google Scholar] [CrossRef]
- Chongdar, S.; Gunasekaran, G.; Kumar, P. Corrosion inhibition of mild steel by aerobic biofilm. Electrochim. Acta 2005, 50, 4655–4665. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Wang, J.; Yuan, X.; Jiang, X.; Wang, Y.; Zhong, C.; Xu, D.; Gu, T.; Wang, F. Bacterial biofilms as platforms engineered for diverse applications. Biotechnol. Adv. 2022, 57, 107932. [Google Scholar] [CrossRef]
- Stadler, R.; Wei, L.; Fürbeth, W.; Grooters, M.; Kuklinski, A. Influence of bacterial exopolymers on cell adhesion of Desulfovibrio vulgaris on high alloyed steel: Corrosion inhibition by extracellular polymeric substances (EPS). Mater. Corros. 2010, 61, 1008–1016. [Google Scholar] [CrossRef]
- Moradi, M.; Xiao, T.; Song, Z. Investigation of corrosion inhibitory process of marine Vibrio neocaledonicus sp. bacterium for carbon steel. Corros. Sci. 2015, 100, 186–193. [Google Scholar] [CrossRef]
- Liu, H.W.; Gu, T.Y.; Asif, M.; Zhang, G.A.; Liu, H.F. The corrosion behavior and mechanism of carbon steel induced by extracellular polymeric substances of iron-oxidizing bacteria. Corros. Sci. 2017, 114, 102–111. [Google Scholar] [CrossRef]
- Volkland, H.P.; Harms, H.; Knopf, K.; Wanner, O.; Zehnder, A.J.B. Corrosion inhibition of mild steel by bacteria. Biofouling 2000, 15, 287–297. [Google Scholar] [CrossRef]
- Alasvand Zarasvand, K.; Rai, V.R. Microorganisms: Induction and inhibition of corrosion in metals. Int. Biodeterior. Biodegrad. 2014, 87, 66–74. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Mohamad, A.B.; Kadhum, A.A.H.; Shaker, L.M.; Isahak, W.N.R.W.; Takriff, M.S. Experimental and theoretical study on the corrosion inhibition of mild steel by nonanedioic acid derivative in hydrochloric acid solution. Sci. Rep. 2022, 12, 4705. [Google Scholar] [CrossRef] [PubMed]
- Videla, H.A.; Herrera, L.K. Understanding microbial inhibition of corrosion. A comprehensive overview. Int. Biodeterior. Biodegrad. 2009, 63, 896–900. [Google Scholar] [CrossRef]
- Jayaraman, A.; Earthman, J.C.; Wood, T.K. Corrosion inhibition by aerobic biofilms on SAE 1018 steel. Appl. Microbiol. Biotechnol. 1997, 47, 62–68. [Google Scholar] [CrossRef]
- Michael, F.; Georges, M.; John, F. Elliptical Insights: Understanding Statistical Methods through Elliptical Geometry. Stat. Sci. 2013, 28, 1–39. [Google Scholar] [CrossRef]
- Zhou, E.; Zhang, M.; Huang, Y.; Li, H.; Wang, J.; Jiang, G.; Jiang, C.; Xu, D.; Wang, Q.; Wang, F. Accelerated biocorrosion of stainless steel in marine water via extracellular electron transfer encoding gene phzH of Pseudomonas aeruginosa. Water Res. 2022, 220, 118634. [Google Scholar] [CrossRef]
- Moradi, M.; Song, Z.; Xiao, T. Exopolysaccharide produced by Vibrio neocaledonicus sp. as a green corrosion inhibitor: Production and structural characterization. J. Mater. Sci. Technol. 2018, 34, 2447–2457. [Google Scholar] [CrossRef]
- Popova, A.; Sokolova, E.; Raicheva, S.; Christov, M. AC and DC study of the temperature effect on mild steel corrosion in acid media in the presence of benzimidazole derivatives. Corros. Sci. 2003, 45, 33–58. [Google Scholar] [CrossRef]
- Lv, M.; Du, M.; Li, X.; Yue, Y.; Chen, X. Mechanism of microbiologically influenced corrosion of X65 steel in seawater containing sulfate-reducing bacteria and iron-oxidizing bacteria. J. Mater. Res. Technol. 2019, 8, 4066–4078. [Google Scholar] [CrossRef]
- Sheng, X.; Ting, Y.-P.; Pehkonen, S.O. The influence of sulphate-reducing bacteria biofilm on the corrosion of stainless steel AISI 316. Corros. Sci. 2007, 49, 2159–2176. [Google Scholar] [CrossRef]
- Wang, S.; Yin, X.; Zhang, H.; Liu, D.; Du, N. Coupling Effects of pH and Dissolved Oxygen on the Corrosion Behavior and Mechanism of X80 Steel in Acidic Soil Simulated Solution. Materials 2019, 12, 3175. [Google Scholar] [CrossRef] [Green Version]
- Kuklinski, A.; Sand, W. Microbiologically Influenced Corrosion. In Encyclopedia of Applied Electrochemistry; Kreysa, G., Ota, K.-i., Savinell, R.F., Eds.; Springer: New York, NY, USA, 2014; pp. 1276–1290. [Google Scholar]
- Gao, Y.; Zhang, M.; Fan, Y.; Li, Z.; Cristiani, P.; Chen, X.; Xu, D.; Wang, F.; Gu, T. Marine Vibrio spp. protect carbon steel against corrosion through secreting extracellular polymeric substances. npj Mater. Degrad. 2022, 6, 6. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, J.; Yuan, X.; Xu, Y.; Xu, D.; Zhang, D.; Feng, D.; Wang, F. Marine Biofilms with Significant Corrosion Inhibition Performance by Secreting Extracellular Polymeric Substances. ACS Appl. Mater. Interfaces 2021, 13, 47272–47282. [Google Scholar] [CrossRef] [PubMed]




| Fe (Wt %) | O (Wt %) | C (Wt %) | Na (Wt %) | |
|---|---|---|---|---|
| Sterile | 44.7 ± 2.4 | 16.7 ± 7.4 | 15.2 ± 6.9 | 12.4 ± 5.5 |
| Bacillus sp. Y-6 | 67.1 ± 8.5 | 11.7 ± 3.1 | 6.4 ± 1.9 | 7.6 ± 2.1 |
| T. mesophilum D-6 | 85.9 ± 3.3 | 6.5 ± 1.6 | 4.5 ± 1.2 | 3.2 ± 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, X.; Yang, L.; Wang, Y.; Dong, Y.; Xu, D.; Zhang, M. Biofilm-Induced Corrosion Inhibition of Q235 Carbon Steel by Tenacibaculum mesophilum D-6 and Bacillus sp. Y-6. Metals 2023, 13, 649. https://doi.org/10.3390/met13040649
Ruan X, Yang L, Wang Y, Dong Y, Xu D, Zhang M. Biofilm-Induced Corrosion Inhibition of Q235 Carbon Steel by Tenacibaculum mesophilum D-6 and Bacillus sp. Y-6. Metals. 2023; 13(4):649. https://doi.org/10.3390/met13040649
Chicago/Turabian StyleRuan, Xiaoxi, Linlin Yang, Yan Wang, Yizhe Dong, Dake Xu, and Mingxing Zhang. 2023. "Biofilm-Induced Corrosion Inhibition of Q235 Carbon Steel by Tenacibaculum mesophilum D-6 and Bacillus sp. Y-6" Metals 13, no. 4: 649. https://doi.org/10.3390/met13040649
APA StyleRuan, X., Yang, L., Wang, Y., Dong, Y., Xu, D., & Zhang, M. (2023). Biofilm-Induced Corrosion Inhibition of Q235 Carbon Steel by Tenacibaculum mesophilum D-6 and Bacillus sp. Y-6. Metals, 13(4), 649. https://doi.org/10.3390/met13040649







