Iron Diffusion in Electron Beam Melt (EBM) γ-TiAl Based Alloy from the Building Platform: Interface Characterization
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
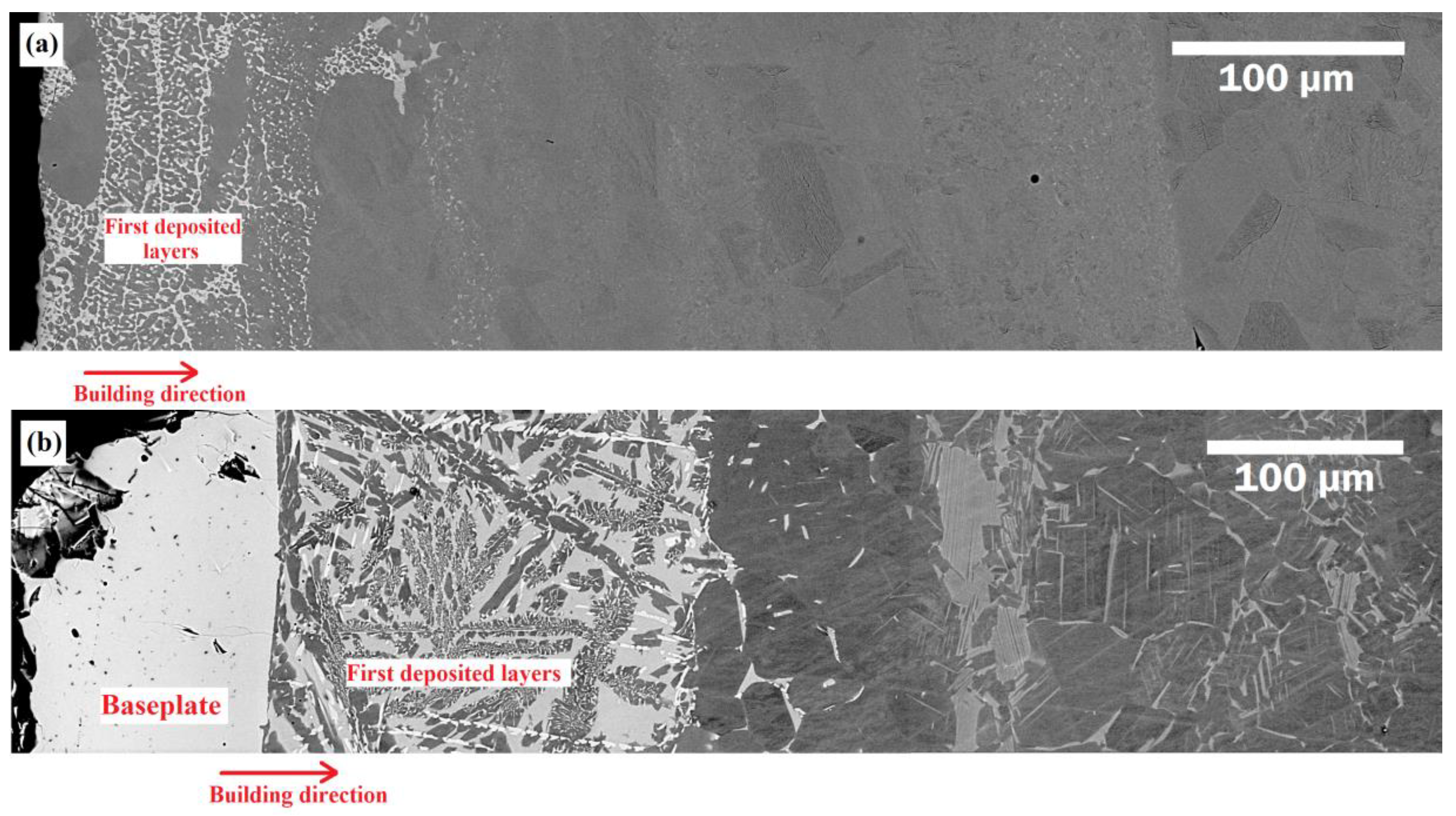
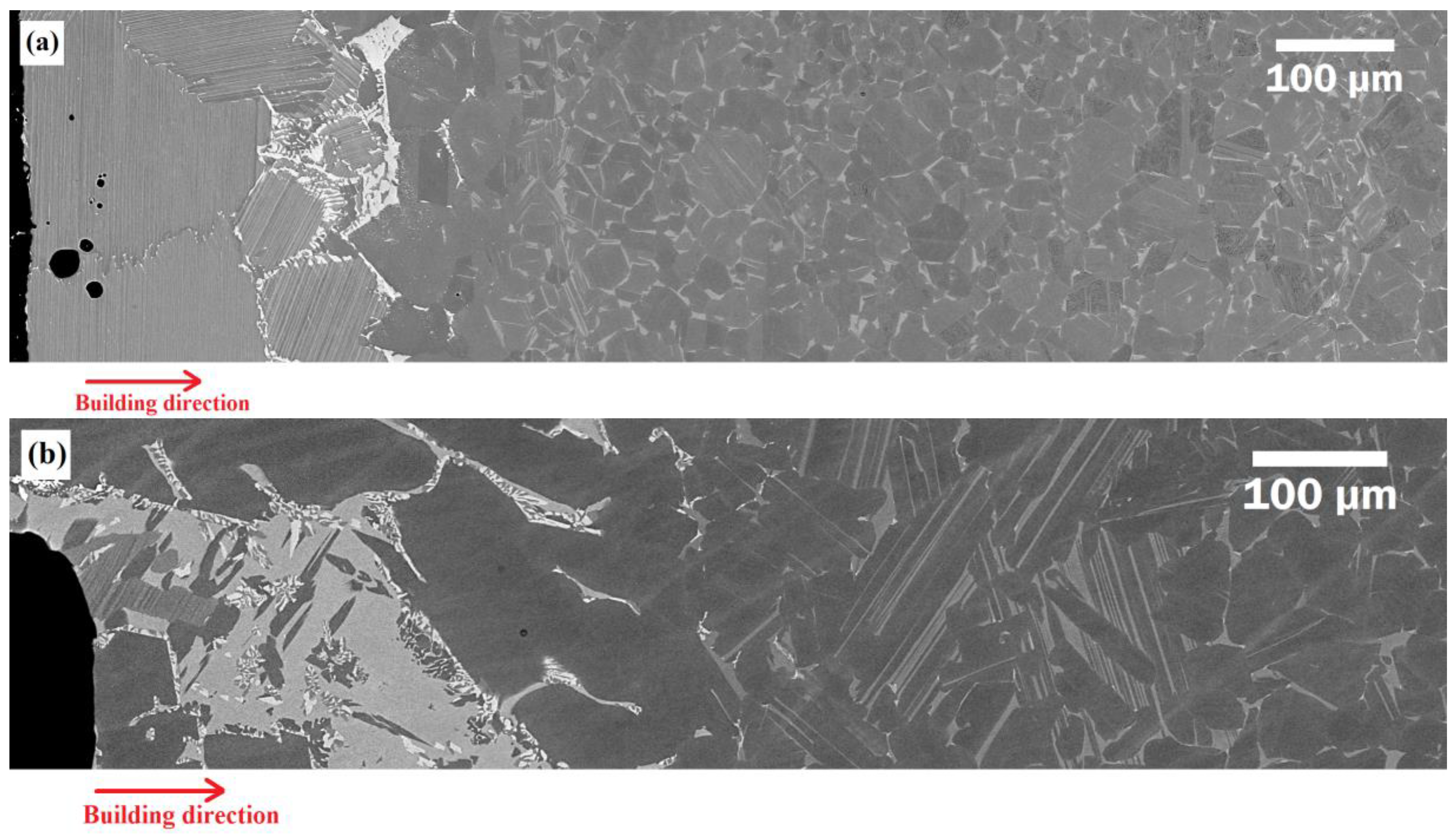
3.1. Microstructural Evolution
3.2. Microhardness Measurement
3.3. XRD Analysis
4. Conclusions
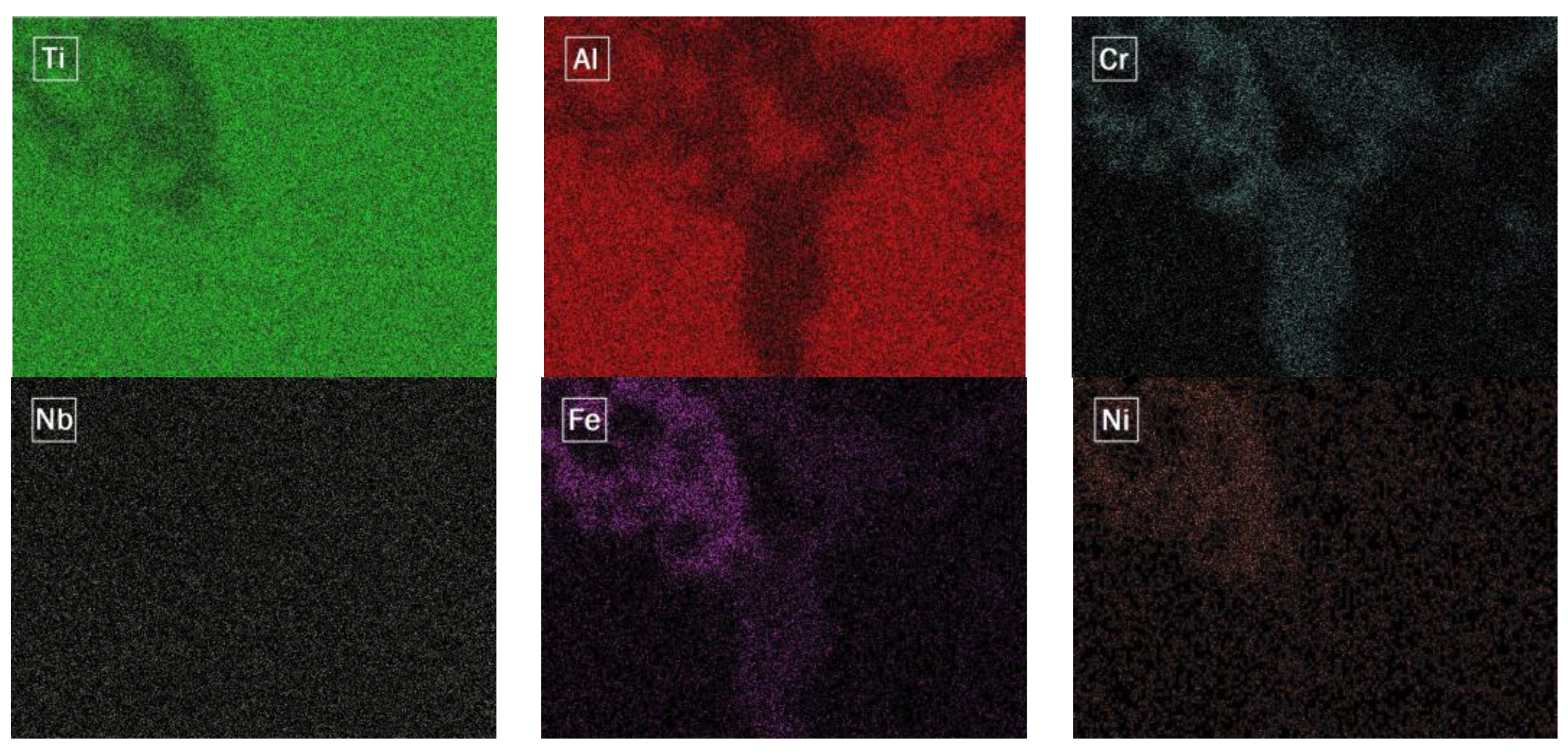
- The diffusion of elements from the baseplate to the densified material led to the formation of some intermetallics and phases containing Ti, Al, Fe, and Ni, whose high hardness may explain the characteristic brittleness of the formed interface.
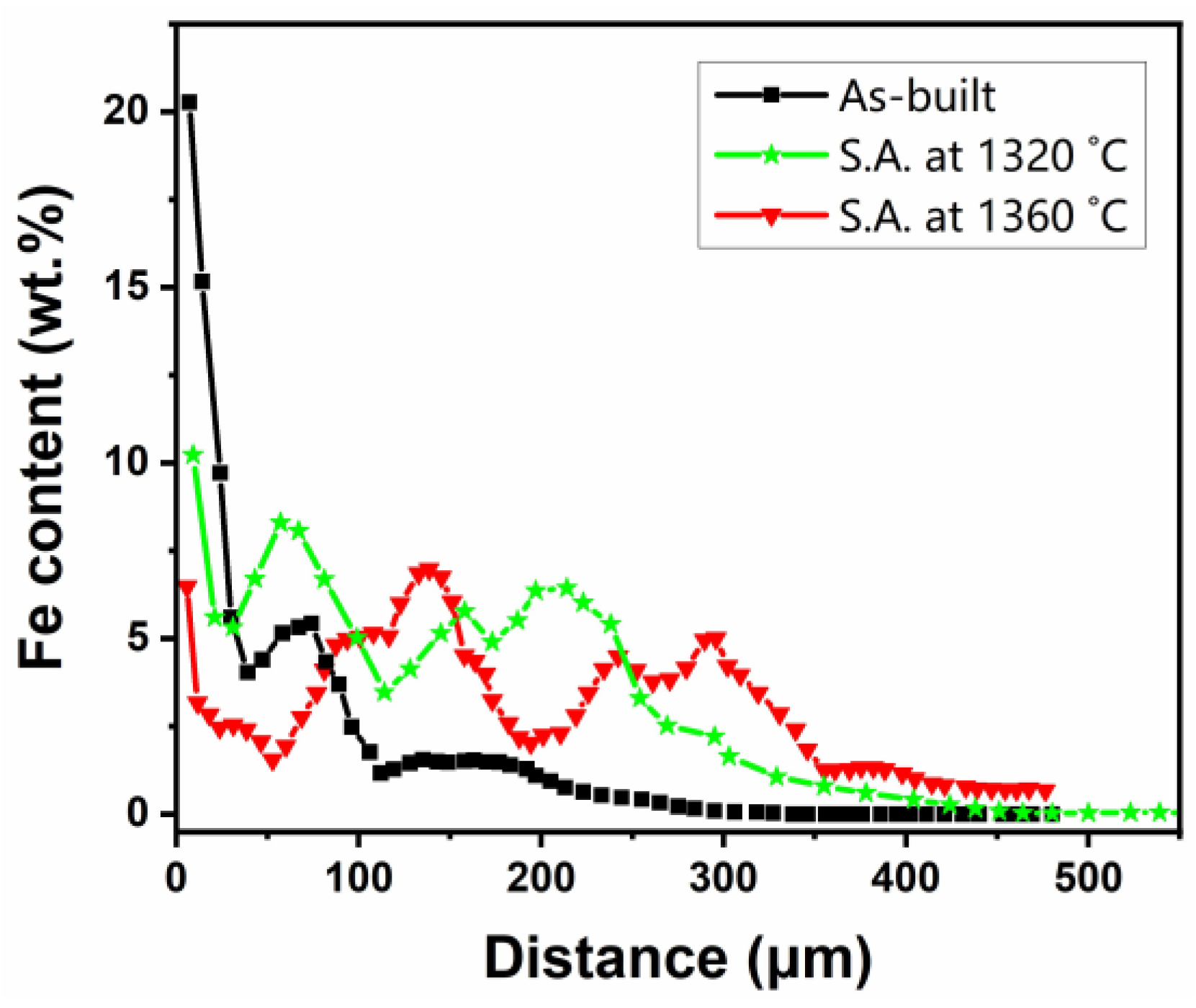
- The diffusion length of Fe in the as-built condition was about 350 μm. However, in the samples solution-annealed at 1320 °C and 1360 °C, it was about 450 μm and >500 μm, respectively.
- Hardness results showed that the matrix of the sample that was solution annealed at the higher temperature had more uniform and higher hardness values.
- XRD patterns confirmed the formation of some compounds close to the interface of the baseplate and the deposited material.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baudana, G.; Biamino, S.; Klöden, B.; Kirchner, A.; Weißgärber, T.; Kieback, B.; Pavese, M.; Ugues, D.; Fino, P.; Badini, C. Electron Beam Melting of Ti-48Al-2Nb-0.7Cr-0.3Si: Feasibility investigation. Intermetallics 2016, 73, 43–49. [Google Scholar] [CrossRef]
- Dzogbewu, T.C.; du Preez, W.B. Additive Manufacturing of Ti-Based Intermetallic Alloys: A Review and Conceptualization of a Next-Generation Machine. Materials 2021, 14, 4317. [Google Scholar] [CrossRef] [PubMed]
- Bewlay, B.P.; Nag, S.; Suzuki, A.; Weimer, M.J. TiAl alloys in commercial aircraft engines. Mater. High Temp. 2016, 33, 549–559. [Google Scholar] [CrossRef]
- Wu, X. Review of alloy and process development of TiAl alloys. Intermetallics 2006, 14, 1114–1122. [Google Scholar] [CrossRef]
- Chen, W.; Li, Z. 11—Additive manufacturing of titanium aluminides. In Additive Manufacturing for the Aerospace Industry; Froes, F., Boyer, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 235–263. [Google Scholar] [CrossRef]
- Clemens, H.; Wallgram, W.; Kremmer, S.; Güther, V.; Otto, A.; Bartels, A. Design of Novel β-Solidifying TiAl Alloys with Adjustable β/B2-Phase Fraction and Excellent Hot-Workability. Adv. Eng. Mater. 2008, 10, 707–713. [Google Scholar] [CrossRef]
- Dahara, M.S.; Tamirisakandalab, S.A.; Lewandowskia, J.J. Fatigue crack growth and fracture behavior of as-cast Ti-43.5AL-4Nb-1Mo-0.1B (TNM) compared to TI-48AL-2Nb-2Cr (4822) (POSTPRINT). Intermetallics 2017, 91, 158–168. [Google Scholar] [CrossRef]
- Fritz Appel, J.D.H.P.; Oehring, M. Gamma Titanium Aluminide Alloys: Science and Technology; John Wiley & Sons: New York, NY, USA, 2011. [Google Scholar]
- Raji, S.A.; Popoola, A.P.I.; Pityana, S.L.; Popoola, O.M. Characteristic effects of alloying elements on β solidifying titanium aluminides: A review. Heliyon 2020, 6, e04463. [Google Scholar] [CrossRef]
- Wimler, D.; Käsznar, K.; Musi, M.; Breuning, C.; Markl, M.; Keckes, J.; Clemens, H.; Körner, C.; Mayer, S. How electron beam melting tailors the Al-sensitive microstructure and mechanical response of a novel process-adapted γ-TiAl based alloy. Mater. Design 2021, 212, 110187. [Google Scholar] [CrossRef]
- Bourell, D.L.; Rosen, D.W.; Leu, M.C. The Roadmap for Additive Manufacturing and Its Impact. 3D Print. Addit. Manuf. 2014, 1, 6–9. [Google Scholar] [CrossRef]
- DebRoy, T.; Wei, H.L.; Zuback, J.S.; Mukherjee, T.; Elmer, J.W.; Milewski, J.O.; Beese, A.M.; Wilson-Heid, A.; De, A.; Zhang, W. Additive manufacturing of metallic components—Process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Galati, M.; Iuliano, L. A literature review of powder-based electron beam melting focusing on numerical simulations. Addit. Manuf. 2018, 19, 1–20. [Google Scholar] [CrossRef]
- Herzog, D.; Seyda, V.; Wycisk, E.; Emmelmann, C. Additive manufacturing of metals. Acta Mater. 2016, 117, 371–392. [Google Scholar] [CrossRef]
- Körner, C. Additive manufacturing of metallic components by selective electron beam melting—A review. Int. Mater. Rev. 2016, 61, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Larsson Morgan, L.U.; Ola, H. Rapid manufacturing with electron beam melting (EBM)—A manufacturing revolution? Solid Free. Fabr. Symp. 2003, 438–443. [Google Scholar]
- Cormier, D.; Harrysson, O.; Mahale, T.; West, H. Freeform Fabrication of Titanium Aluminide via Electron Beam Melting Using Prealloyed and Blended Powders. Res. Lett. Mater. Sci. 2007, 2007, 034737. [Google Scholar] [CrossRef]
- Murr, L.E.; Gaytan, S.M.; Ceylan, A.; Martinez, E.; Martinez, J.L.; Hernandez, D.H.; Machado, B.I.; Ramirez, D.A.; Medina, F.; Collins, S.; et al. Characterization of titanium aluminide alloy components fabricated by additive manufacturing using electron beam melting. Acta Mater. 2010, 58, 1887–1894. [Google Scholar] [CrossRef]
- Schwerdtfeger, J.; Körner, C. Selective electron beam melting of Ti–48Al–2Nb–2Cr: Microstructure and aluminium loss. Intermetallics 2014, 49, 29–35. [Google Scholar] [CrossRef]
- Biamino, S.; Penna, A.; Ackelid, U.; Sabbadini, S.; Tassa, O.; Fino, P.; Pavese, M.; Gennaro, P.; Badini, C. Electron beam melting of Ti–48Al–2Cr–2Nb alloy: Microstructure and mechanical properties investigation. Intermetallics 2011, 19, 776–781. [Google Scholar] [CrossRef]
- Cho, K.; Kawabata, H.; Hayashi, T.; Yasuda, H.Y.; Nakashima, H.; Takeyama, M.; Nakano, T. Peculiar microstructural evolution and tensile properties of β-containing γ-TiAl alloys fabricated by electron beam melting. Addit. Manuf. 2021, 46, 102091. [Google Scholar] [CrossRef]
- Kan, W.; Chen, B.; Jin, C.; Peng, H.; Lin, J. Microstructure and mechanical properties of a high Nb-TiAl alloy fabricated by electron beam melting. Mater. Design 2018, 160, 611–623. [Google Scholar] [CrossRef]
- Galati, M.; Rizza, G.; Salmi, A.; Biamino, S.; Ghibaudo, C.; Fino, P.; Iuliano, L. Residual stress investigation on Ti-48Al-2Cr-2Nb samples produced by Electron Beam Melting process. Procedia CIRP 2021, 99, 336–341. [Google Scholar] [CrossRef]
- Bieske, J.; Franke, M.; Schloffer, M.; Körner, C. Microstructure and properties of TiAl processed via an electron beam powder bed fusion capsule technology. Intermetallics 2020, 126, 106929. [Google Scholar] [CrossRef]
- Mishin, Y.; Herzig, C. Diffusion in the Ti–Al system. Acta Mater. 2000, 48, 589–623. [Google Scholar] [CrossRef]
- Herzig, C.; Przeorski, T.; Friesel, M.; Hisker, F.; Divinski, S. Tracer solute diffusion of Nb, Zr, Cr, Fe, and Ni in γ-TiAl: Effect of preferential site occupation. Intermetallics 2001, 9, 461–472. [Google Scholar] [CrossRef]
- Hao, Y.L.; Xu, D.S.; Cui, Y.Y.; Yang, R.; Li, D. The site occupancies of alloying elements in TiAl and Ti3Al alloys. Acta Mater. 1999, 47, 1129–1139. [Google Scholar] [CrossRef]
- Przeorski, T.; Friesel, M.; Hisker, F.; Divinski, S.V.; Herzig, C. Solute Diffusion in the Intermetallic Compound γ-TiAl. Defect Diffus. Forum 2001, 194–199, 493–498. [Google Scholar] [CrossRef]
- Divinski, S.V.; Herzig, C.; Klinkenberg, C. Tracer diffusion of niobium and titanium in binary and ternary titanium aluminides. J. Phase Equilibria Diffus. 2005, 26, 452–457. [Google Scholar] [CrossRef]
- Ghibaudo, C.; Wartbichler, R.; Marchese, G.; Clemens, H.; Ugues, D.; Biamino, S. Influence of focus offset on the microstructure of an intermetallic γ-TiAl based alloy produced by electron beam powder bed fusion. J. Manuf. Process. 2023, 89, 132–141. [Google Scholar] [CrossRef]
- Brotzu, A.; Felli, F.; Pilone, D. Effect of alloying elements on the behaviour of TiAl-based alloys. Intermetallics 2014, 54, 176–180. [Google Scholar] [CrossRef]
- Shu, S.; Qiu, F.; Tong, C.; Shan, X.; Jiang, Q. Effects of Fe, Co and Ni elements on the ductility of TiAl alloy. J. Alloys Compd. 2014, 617, 302–305. [Google Scholar] [CrossRef]
- Nová, K.; Novák, P.; Arzel, A.; Průša, F. Alloying of Fe-Al-Si Alloys by Nickel and Titanium. Manuf. Technol. J. 2018, 18, 645–649. [Google Scholar] [CrossRef]
- Simões, S.; Tavares, C.J.; Guedes, A. Joining of γ-TiAl Alloy to Ni-Based Superalloy Using Ag-Cu Sputtered Coated Ti Brazing Filler Foil. Metals 2018, 8, 723. [Google Scholar] [CrossRef] [Green Version]
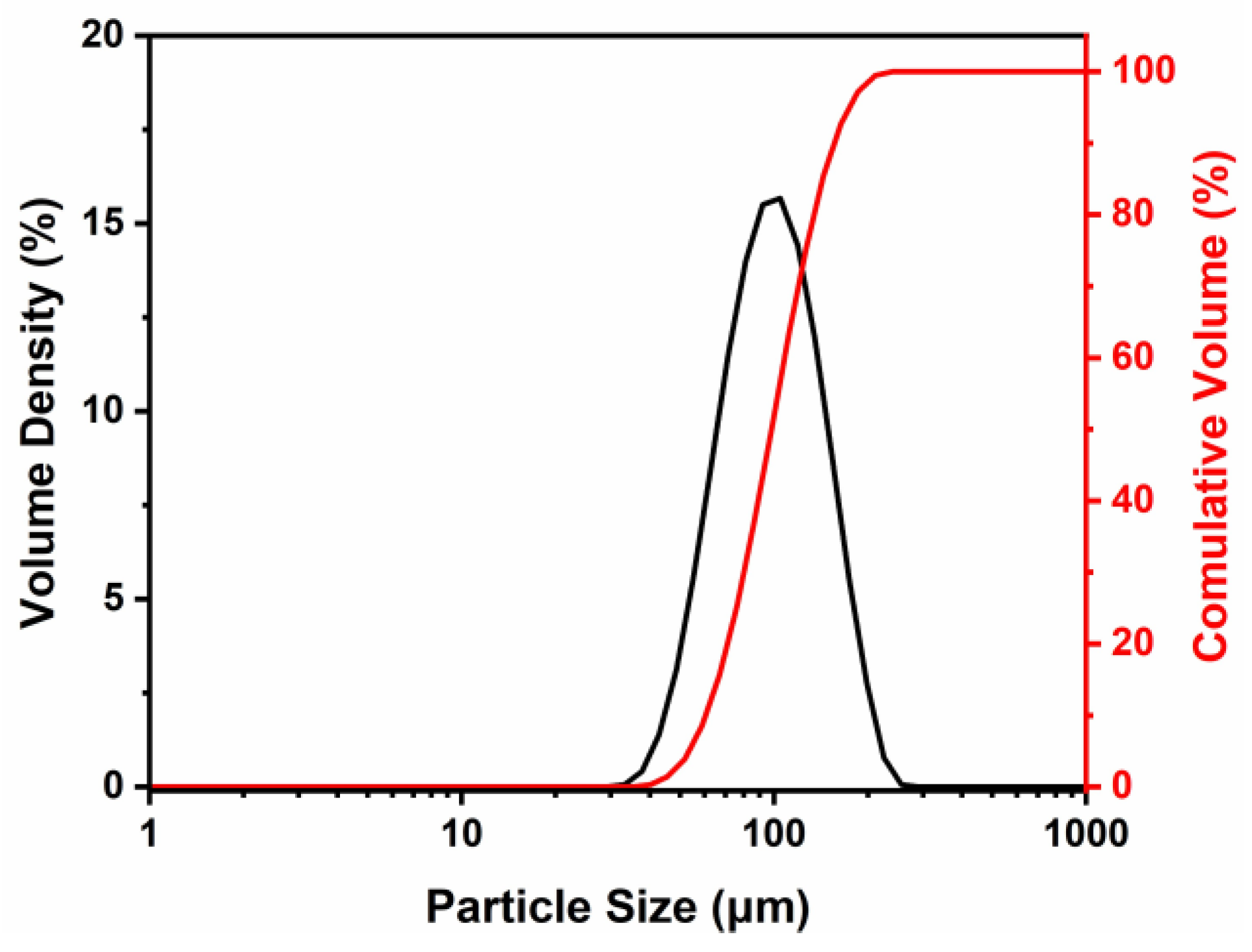





| Element | Ti | Al | Cr | Nb |
|---|---|---|---|---|
| Powder | Bal. | 48.7 | 1.9 | 2.0 |
| Element | Ti | Al | Nb | Cr | Fe | Ni |
|---|---|---|---|---|---|---|
| Area 1 | 46.9 ± 0.23 | 29.2 ± 0.18 | 4.5 ± 0.2 | 7.7 ± 0.14 | 8.2 ± 0.16 | 3.4 ± 0.15 |
| Area 2 | 60.7 ± 0.24 | 20.5 ± 0.15 | 4.5 ± 0.2 | 9.4 ± 0.15 | 4.4 ± 0.14 | 0.4 ± 0.11 |
| Matrix | 59.8 ± 0.23 | 31.5 ± 0.18 | 5.3 ± 0.2 | 2.3 ± 0.1 | 0.9 ± 0.1 | 0.2 ± 0.11 |
| Condition | as-Built | HIPed | Heat Treated at 1320 °C | Heat Treated at 1360 °C |
|---|---|---|---|---|
| Hardness | 321.2 | 431.4 | 440.8 | 435.6 |
| Standard Deviation | 112.7 | 114.8 | 123.4 | 77.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kenevisi, M.S.; Ghibaudo, C.; Bassini, E.; Ugues, D.; Marchese, G.; Biamino, S. Iron Diffusion in Electron Beam Melt (EBM) γ-TiAl Based Alloy from the Building Platform: Interface Characterization. Metals 2023, 13, 772. https://doi.org/10.3390/met13040772
Kenevisi MS, Ghibaudo C, Bassini E, Ugues D, Marchese G, Biamino S. Iron Diffusion in Electron Beam Melt (EBM) γ-TiAl Based Alloy from the Building Platform: Interface Characterization. Metals. 2023; 13(4):772. https://doi.org/10.3390/met13040772
Chicago/Turabian StyleKenevisi, Mohammad Saleh, Cristian Ghibaudo, Emilio Bassini, Daniele Ugues, Giulio Marchese, and Sara Biamino. 2023. "Iron Diffusion in Electron Beam Melt (EBM) γ-TiAl Based Alloy from the Building Platform: Interface Characterization" Metals 13, no. 4: 772. https://doi.org/10.3390/met13040772






