Effect of Ca and P on the Size and Morphology of Eutectic Mg2Si in High-Purity Al-Mg-Si Alloys
Abstract
:1. Introduction
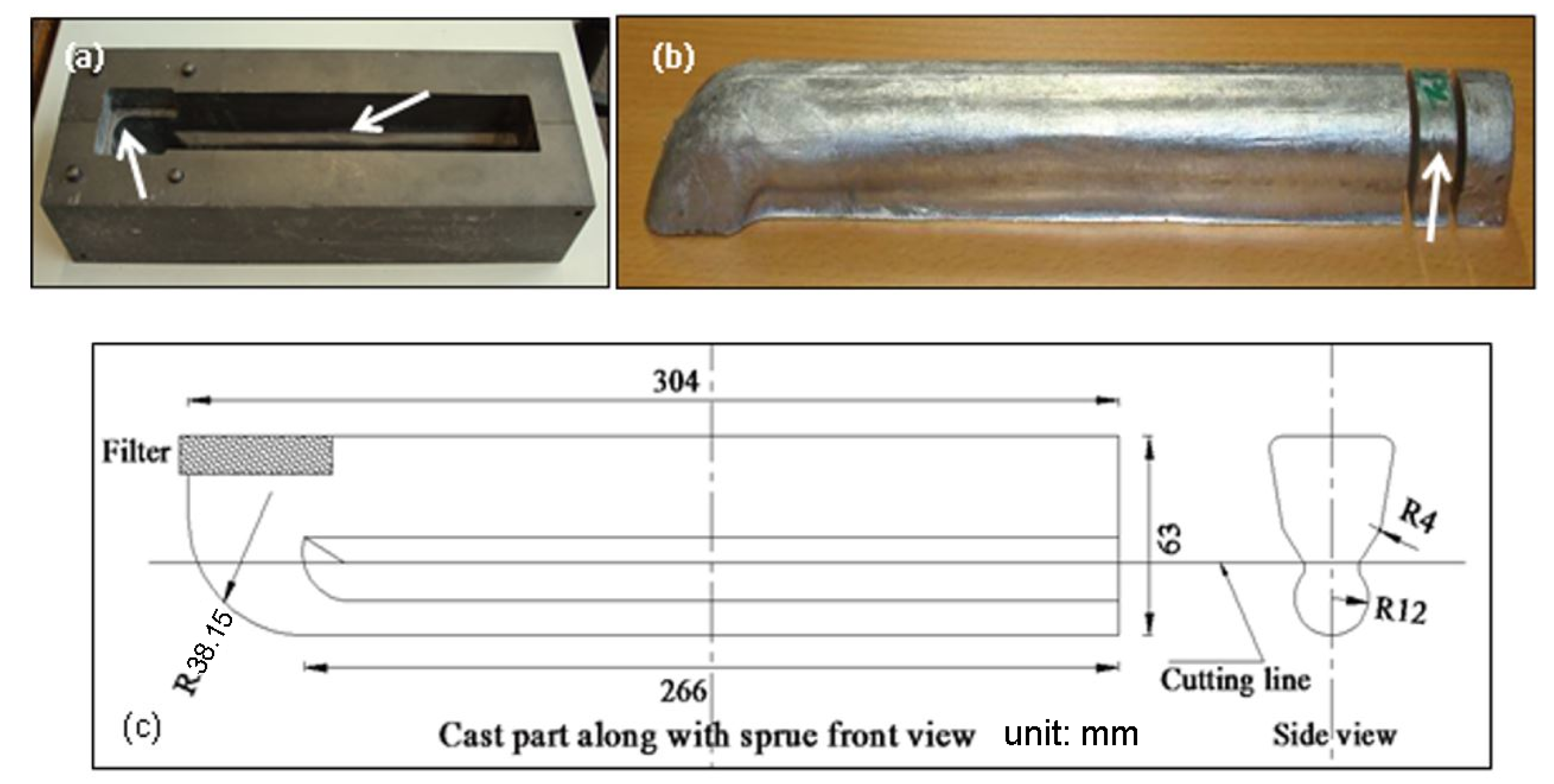
2. Materials and Methods
3. Results
3.1. Scheil Simulation
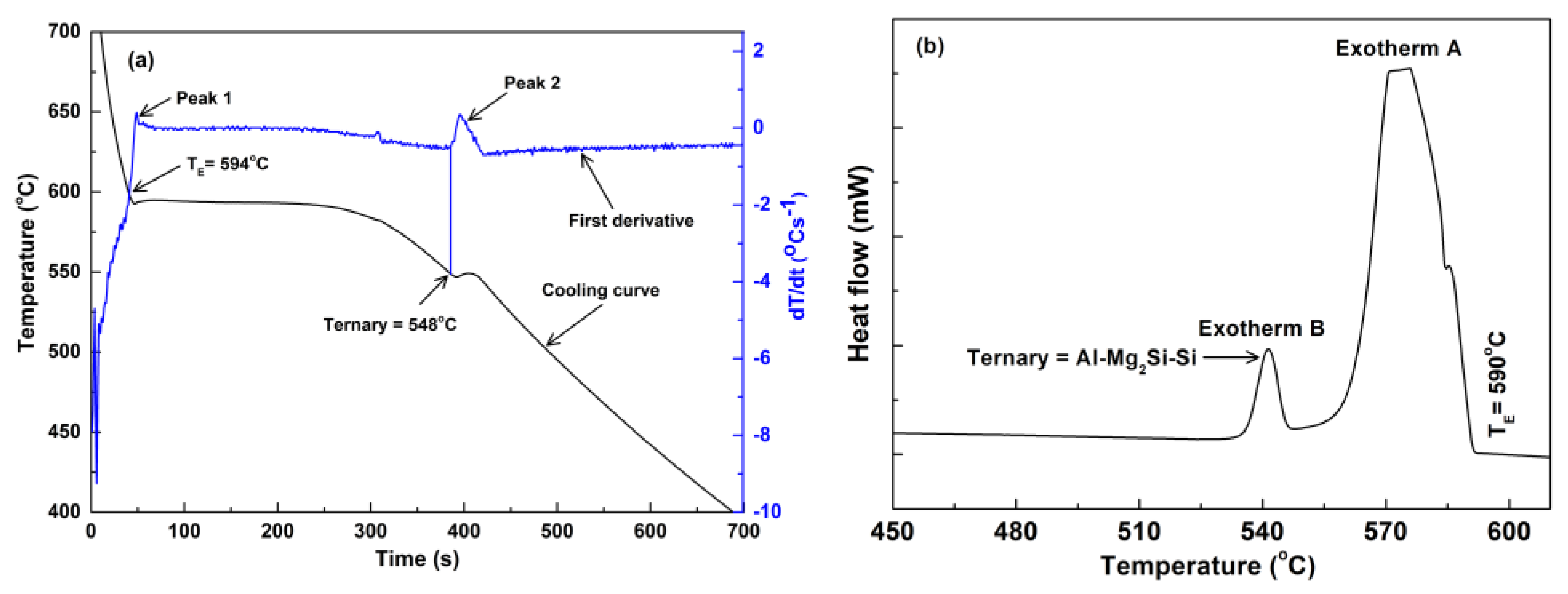
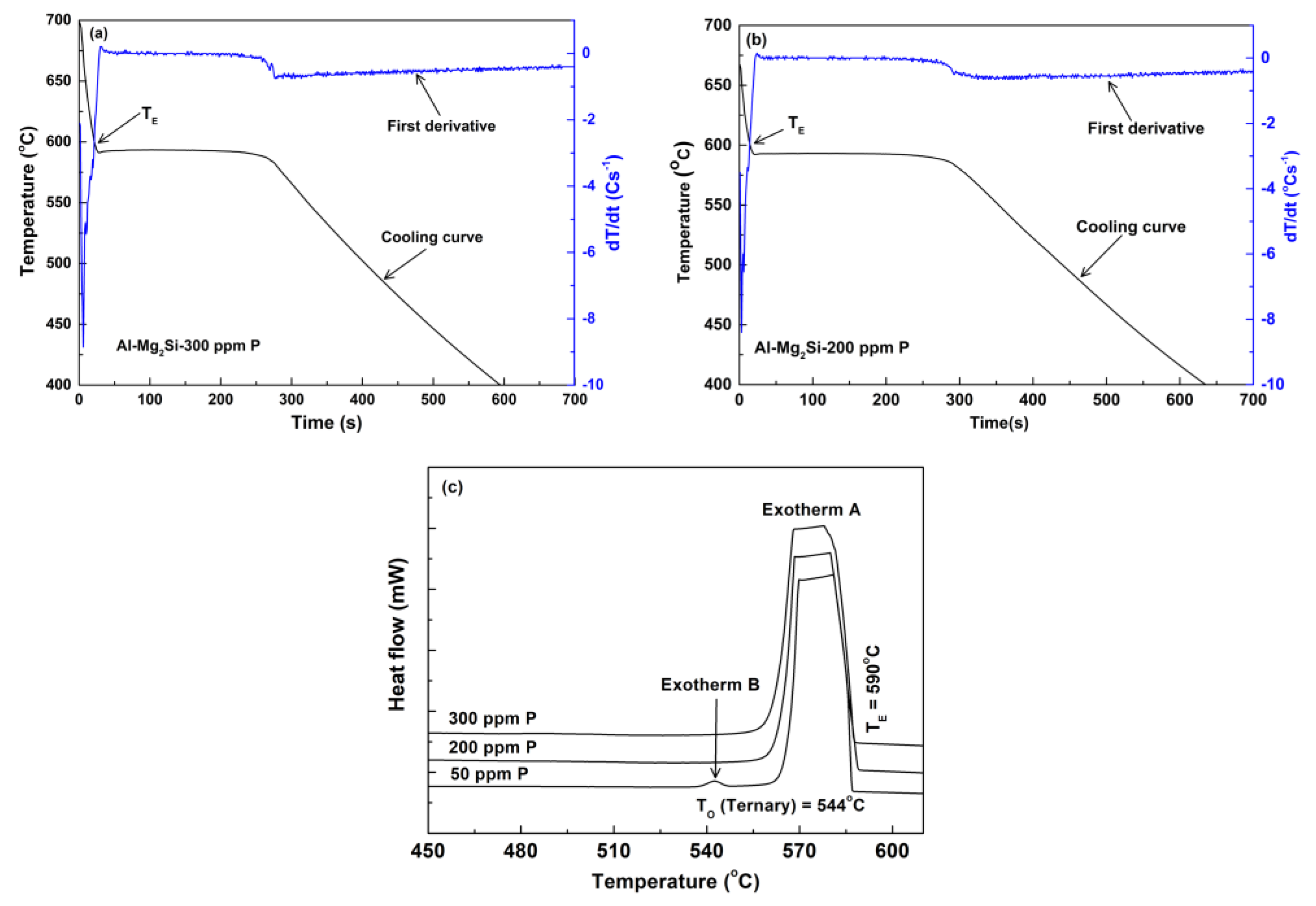
3.2. Thermal Analysis
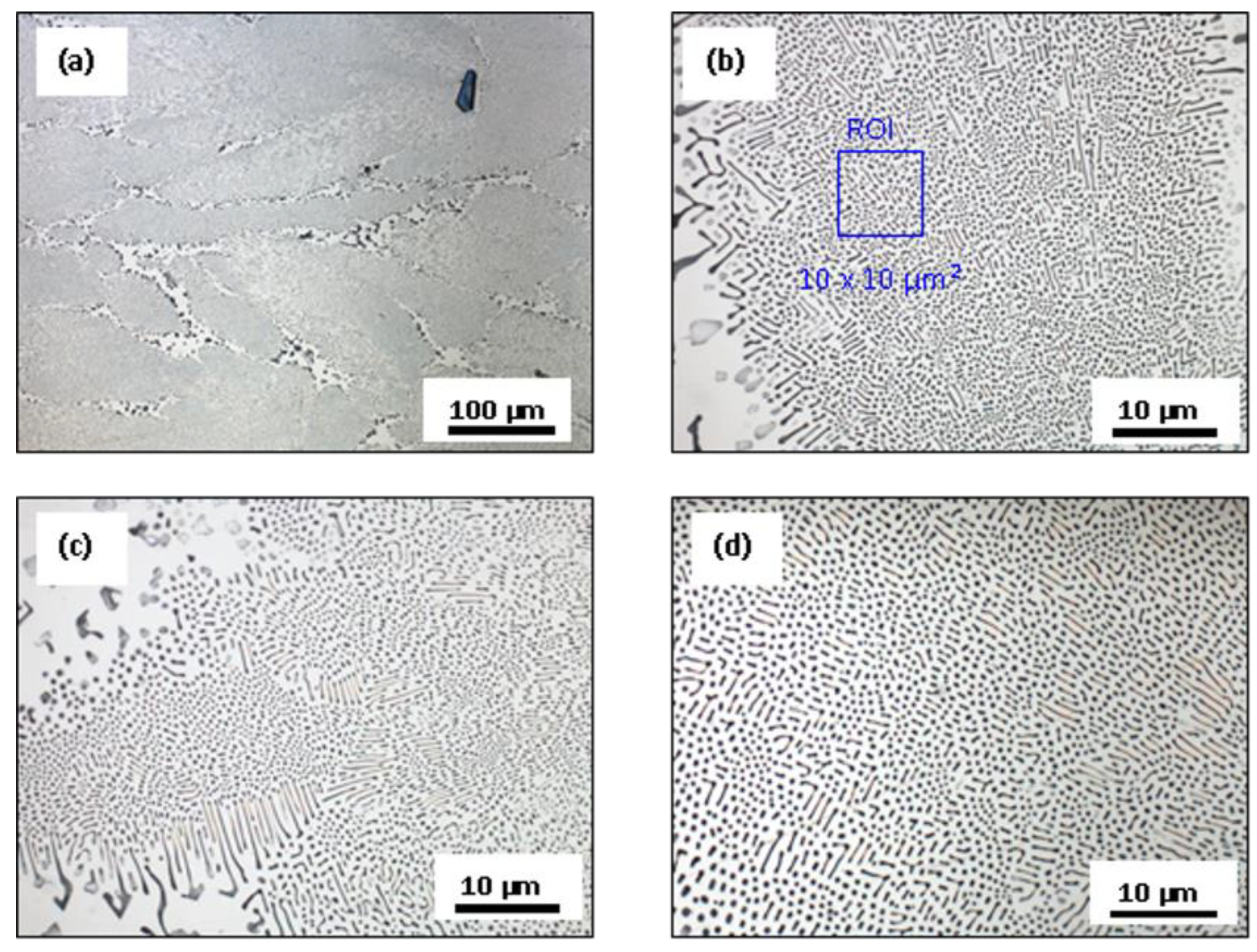
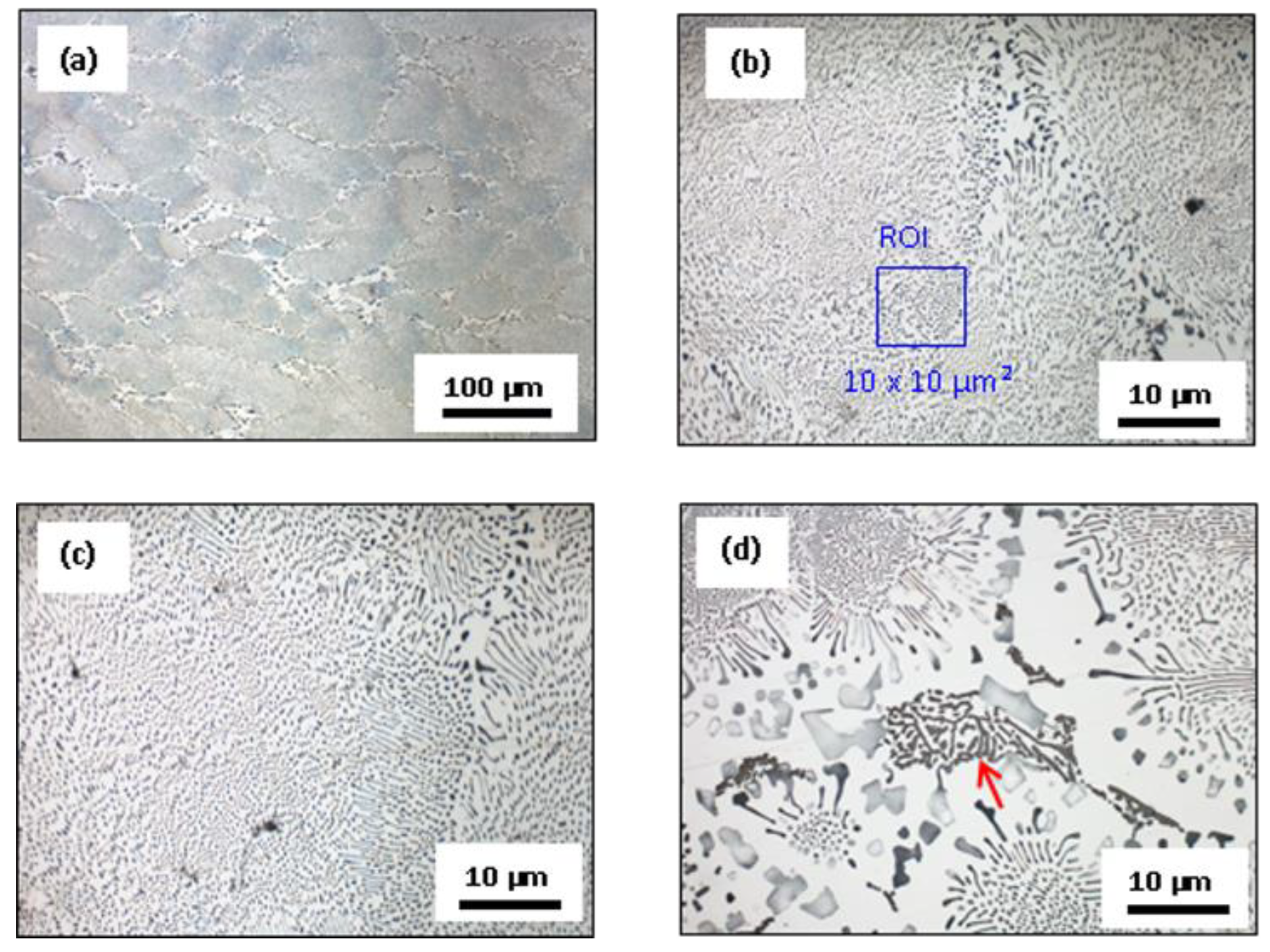
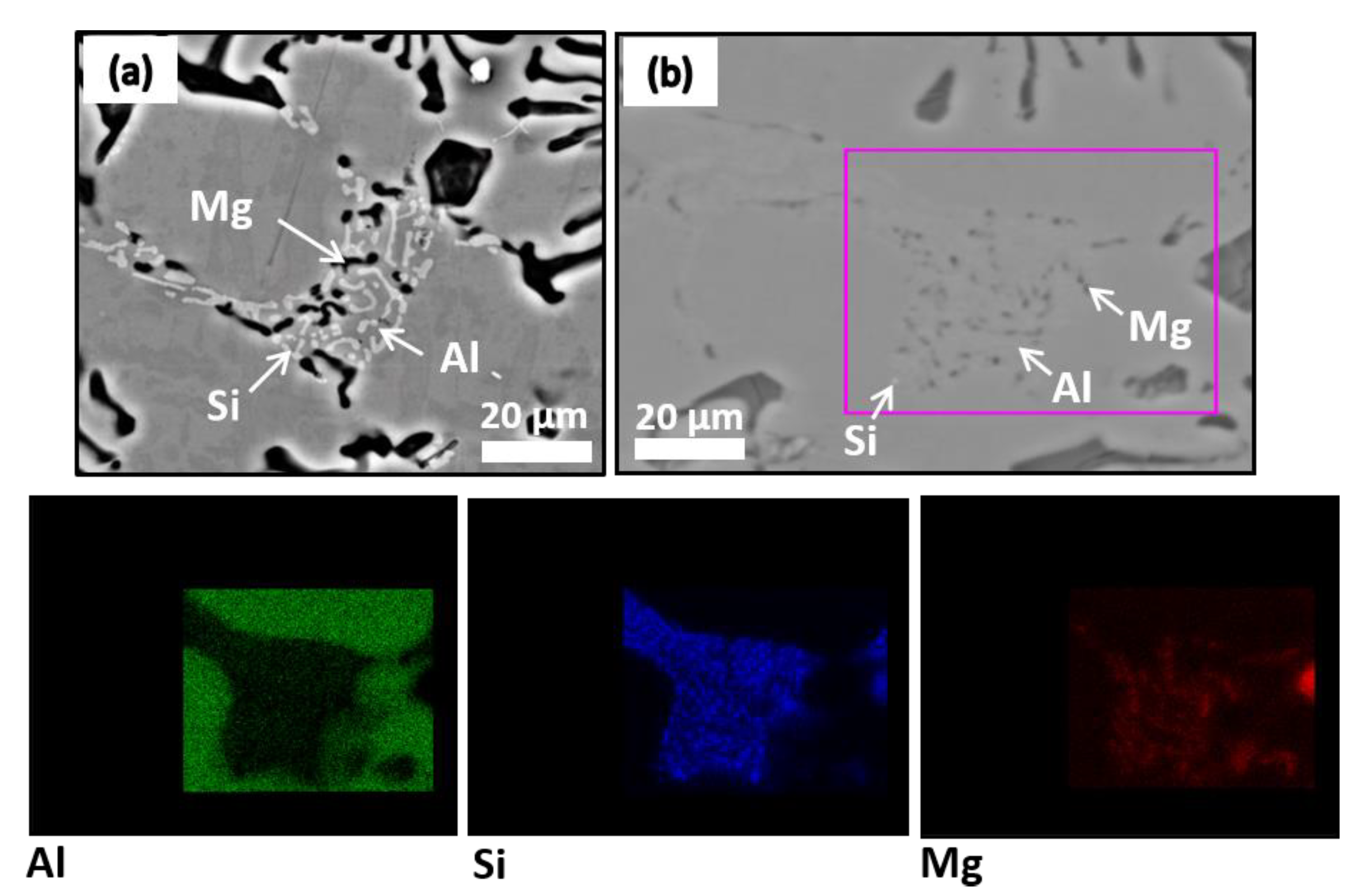
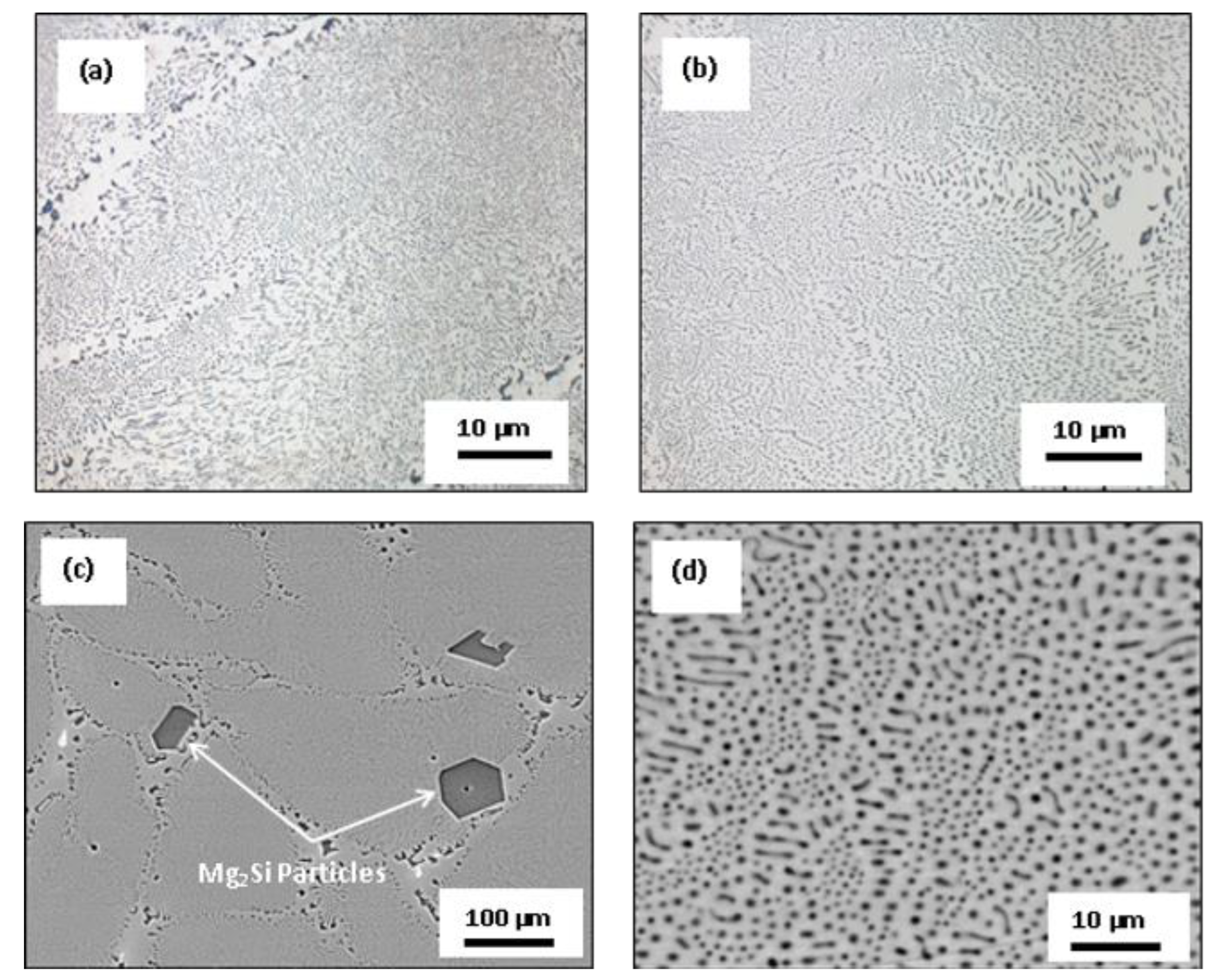
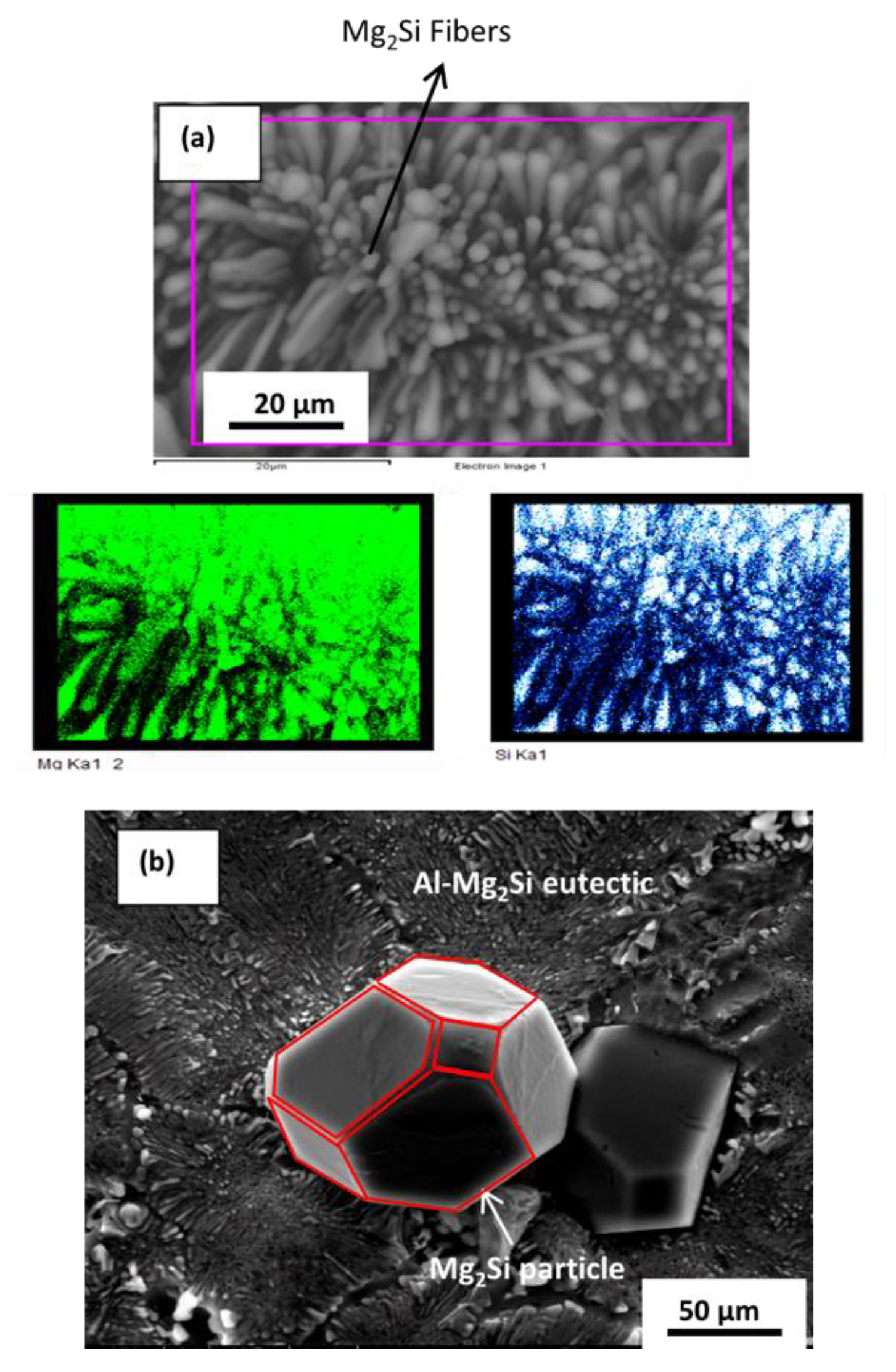
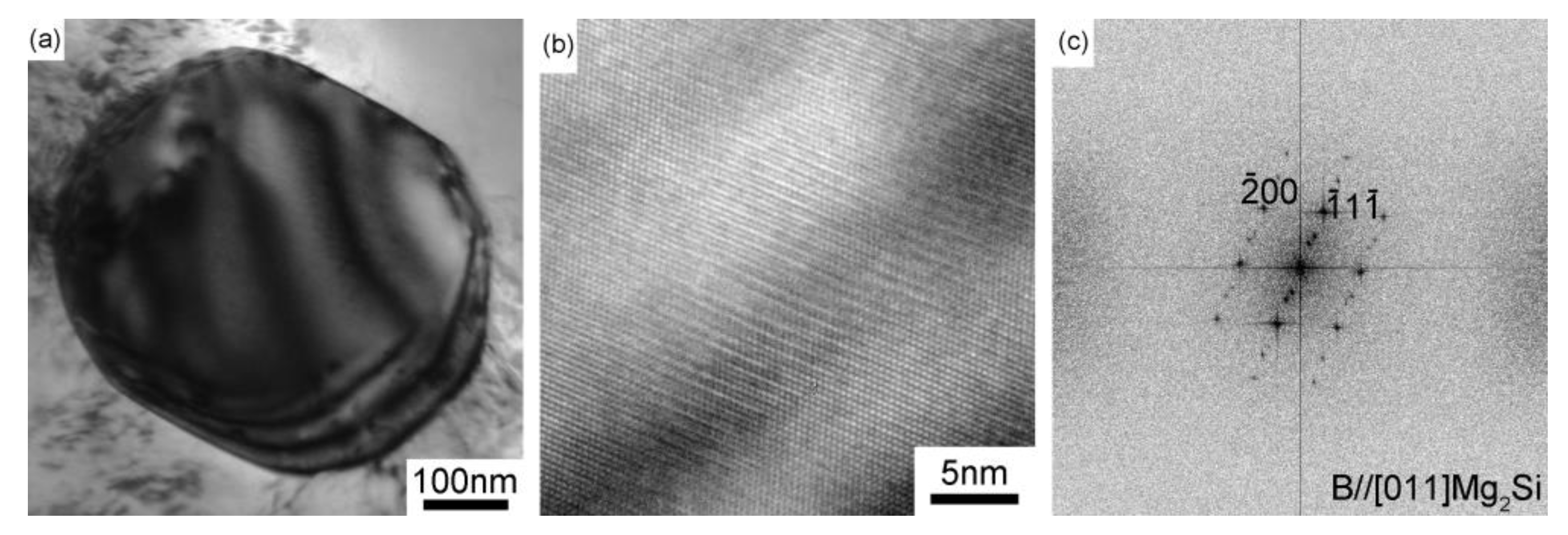
3.3. Microstructural Characterization
4. Discussion
4.1. Microstructure without and with Additions of Ca
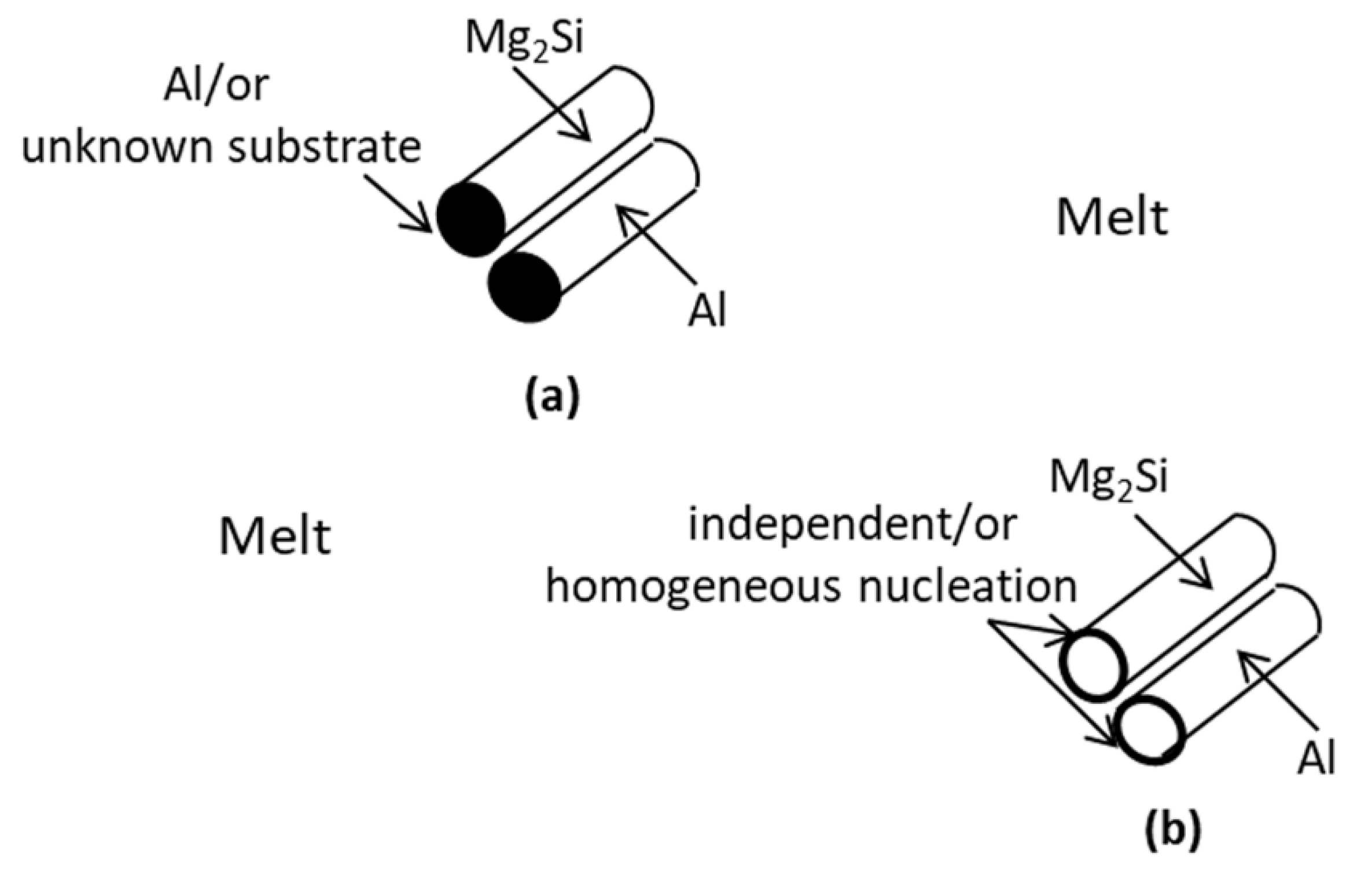
4.1.1. Nucleation Mechanism
4.1.2. Growth Mechanism
4.2. Effects of P on Nucleation and Growth of Ternary Eutectic
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hanson, D.; Gayler, M.L.V. The constitution and age-hardening of the alloys of Al with Mg and Si. J. Inst. Met. 1921, 26, 321–359. [Google Scholar]
- Gwyer, A.G.C.; Phillips, H.W.L. Alloys of Aluminium with Silicon and Iron. J. Inst. Met. 1927, 38, 31–35. [Google Scholar]
- Mondolfo, L.F. Aluminium Alloys: Structure and Properties, 1st ed.; Butterworth and Co.: London, UK, 1976; p. 787. [Google Scholar]
- Davis, J.R. Aluminium and Aluminium Alloys, 4th ed.; ASM Specialty Hand Book; ASM International: Novelty, OH, USA, 1998; p. 59. [Google Scholar]
- Dix, E.H., Jr.; Keller, F.; Graham, R.W. Equilibrium relations in Aluminum-Magnesium-Silicide alloy of high purity. Trans. Am. Inst. Min. Metall. Eng. 1931, 93, 404–420. [Google Scholar]
- Losana, L. The Ternary System: Aluminium-Magnesium-Silicon. Metall. Ital. 1931, 23, 367–382. [Google Scholar]
- Zhang, J.; Fan, Z.; Wang, Y.Q.; Zhou, B.L. Equilibrium pseudobinary Al–Mg2Si phase diagram. Mater. Sci. Technol. 2001, 17, 494–496. [Google Scholar] [CrossRef]
- Barabash, O.M.; Sulgenko, O.V.; Legkaya, T.N.; Korzhova, N.P. Experimental analysis and thermodynamic calculation of the structural regularities in the fusion diagram of the system of alloys Al-Mg-Si. JPE 2001, 22, 5–11. [Google Scholar] [CrossRef]
- Li, S.; Zhao, S.; Pan, M.; Zhao, D.; Chen, X.; Barabash, O.M.; Barabash, R.I. Solidification and structural characteristics of α(Al)-Mg2Si eutectic. Met. Trans. JIM 1997, 38, 553–559. [Google Scholar] [CrossRef]
- Barabash, O.M.; Legkaya, T.N.; Korzhova, N.P.; Sulzhenko, O.V. Thermodynamic Analysis and Experimental Study of Composite Structure Formation in Eutectic Alloys α-Al + Mg2Si. Powder Metall. Met. Ceram. 2000, 39, 458–461. [Google Scholar] [CrossRef]
- Hanemann, H.; Schrader, A. Ternäre Legierung des Aluminiums, Atlas Metallographicus, Band III, Teil2, 1st ed.; Verlag Stahleisen GmbH: Düsseldorf, Germany, 1952; p. 128. [Google Scholar]
- Zhang, J.; Fan, Z.; Wang, Y.Q.; Zhou, B.L. Effect of cooling rate on the microstructure of hypereutectic Al-Mg2Si alloys. J. Mater. Sci. Lett. 2000, 19, 1825–1828. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, Z.; Wang, Y.Q.; Zhou, B.L. Microstructural development of Al–15wt.%Mg2Si in situ composite with mischmetal addition. Mater. Sci. Eng. A 2000, 281, 104–112. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, Z.; Wang, Y.Q.; Zhou, B.L. Microstructure and mechanical properties of in situ Al–Mg2Si composites. J. Mater. Sci. Technol. 2000, 16, 913–918. [Google Scholar] [CrossRef]
- Qin, Q.D.; Zhao, Y.G.; Zhou, W.; Cong, P.J. Effect of phosphorus on microstructure and growth manner of primary Mg2Si crystal in Mg2Si/Al composite. Mater. Sci. Eng. A 2007, 447, 186–191. [Google Scholar] [CrossRef]
- Ben-Hamu, G.; Eliezer, D.; Shin, K.S. The role of Mg2Si on the corrosion behavior of wrought Mg–Zn–Mn alloy. Intermetallic 2008, 16, 860–867. [Google Scholar] [CrossRef]
- Li, C.; Liu, X.; Zhang, G. Heterogeneous nucleating role of TiB2 or AlP/TiB2 coupled compounds on primary Mg2Si in Al–Mg–Si alloys. Mater. Sci. Eng. A 2008, 497, 432–437. [Google Scholar] [CrossRef]
- Pabel, T.; Schumacher, P. Method for the Refining and Structure Modification of AL-MG-SI Alloys. U.S. Patent No. US9279170B2, 8 March 2016. [Google Scholar]
- Pabel, T.; Schumacher, P. Verfahren zur Raffination und Gefügemodifikation von Almgsi-Legierungen. EP Patent No. EP2705171B1, 26 August 2015. [Google Scholar]
- Zarif, M.; McKay, B.; Schumacher, P. Study of Heterogeneous Nucleation of Eutectic Si in High-Purity Al-Si Alloys with Sr Addition. Metall. Mater. Trans. A 2011, 42, 1684–1691. [Google Scholar] [CrossRef]
- Li, J.H.; Zarif, M.Z.; Albu, M.; McKay, B.J.; Hofer, F.; Schumacher, P. Nucleation kinetics of entrained eutectic Si in Al-5Si alloys. Acta Mater. 2014, 72, 80–98. [Google Scholar] [CrossRef]
- Moussa, M.E.; Waly, M.A.; El-Sheikh, A.M. Effect of Ca addition on modification of primary Mg2Si, hardness and wear behavior in Mg2Si hypereutectic alloys. J. Magnes. Alloy 2014, 2, 230–238. [Google Scholar] [CrossRef]
- Shah, A.W.; Ha, S.H.; Kim, B.H.; Yoon, Y.O.; Lim, H.K.; Kim, S.K. Effect of Al2Ca addition and heat treatment on the microstructure modification and tensile properties of hypo-eutectic Al–Mg–Si alloys. Materials 2021, 14, 4588. [Google Scholar] [CrossRef]
- Lotfpour, M.; Bahmani, A.; Mirzadeh, H.; Emamy, M.; Malekan, M.; Kim, W.J.; Taghizadeh, M.; Afsharnaderi, A. Effect of microalloying by Ca on the microstructure and mechanical properties of as-cast and wrought Mg–Mg2Si composites. Mater. Sci. Eng. A 2021, 820, 141574. [Google Scholar] [CrossRef]
- Vander, G.F. ASM Handbook Volume 9: Metallography and Microstructures; ASM International: Novelty, OH, USA, 2004; p. 740. [Google Scholar]
- Wang, H.; Davidson, C.J.; St. John, D.H. Semisolid microstructural evolution of AlSi7Mg alloy during partial remelting. Mater. Sci. Eng. A 2004, 368, 159–167. [Google Scholar] [CrossRef]
- Jung, B.I.; Jung, C.H.; Han, T.K.; Kim, Y.H. Electromagnetic stirring and Sr modification in A356 alloy. J. Mater. Proc. Technol. 2001, 111, 69–73. [Google Scholar] [CrossRef]
- Lu, S.Z.; Hellawell, A. The mechanism of silicon modification in aluminum-silicon alloys: Impurity induced twinning. Met. Trans. A 1987, 18, 1721–1733. [Google Scholar] [CrossRef]
- Grobner, J.; Chumak, I.; Schmid-Fetzer, R. Experimental study of ternary Ca–Mg–Si phase equilibria and thermodynamic assessment of Ca–Si and Ca–Mg–Si systems. Intermetallic 2003, 11, 1065–1074. [Google Scholar] [CrossRef]
- Li, S.P.; Zhao, S.X.; Pan, M.X.; Zhao, D.Q.; Chen, X.C.; Barabash, O.M. Barabash. Eutectic reaction and microstructural characteristics of Al (Li)-Mg2Si alloys. J. Mater. Sci. 2001, 36, 1569–1575. [Google Scholar] [CrossRef]
- Jackson, K.A. Liquid Metal and Solidification. In Growth and Perfection of Crystals; Doremus, R.H., Roberts, B.W., Turnbull, D., Eds.; Wiley and Sons: New York, NY, USA; ASM: Cleveland, OH, USA, 1958; p. 319. [Google Scholar]
- Rosenqvist, T. Principles of Extractive Metallurgy, 2nd ed.; Tapir Academic Press: Trondheim, Norway, 2004; p. 449. [Google Scholar]
- Hellawell, A. The Growth and Structure of Eutectics with Silicon and Germanium. Prog. Mater. Sci. 1970, 15, 3–78. [Google Scholar] [CrossRef]
- Kottcamp, E.H., Jr. ASM Handbook Volume 3: Alloy Phase Diagrams, 2nd ed.; ASM International: Novelty, OH, USA, 1992; p. 2121. [Google Scholar]
- Nogita, K.; McDonald, S.D.; Tsujimoto, K.; Yasuda, K.; Dahle, A.K. Aluminium phosphide as a eutectic grain nucleus in hypoeutectic Al-Si alloys. Microscopy 2004, 53, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.R.; Cantor, B. Heterogeneous nucleation of solidification of Si in Al-Si and Al-Si-P alloys. Acta Metall. Mater. 1995, 43, 3231–3246. [Google Scholar] [CrossRef]
- Crosley, P.B.; Mondolfo, L.F. The Modification of Aluminum-Silicon Alloys. AFS Trans. 1966, 74, 53–64. [Google Scholar]
- Crosley, P.B.; Mondolfo, L.F. The Modification of Aluminum-Silicon Alloys. Mod. Cast. 1966, 49, 89–100. [Google Scholar]
- Schlesinger, M.E. The thermodynamic properties of phosphorus and solid binary phosphides. Chem. Rev. 2002, 102, 4267–4301. [Google Scholar] [CrossRef]
- Brandes, E.A.; Brook, C.B. Smithells Metals Reference Book, 7th ed.; Butterworths-Heinemann: Oxford, UK, 1992; pp. 6–29. [Google Scholar]
- Turnbull, D.; Vonnegut, B. Nucleation Catalysis. Ind. Eng. Chem. 1952, 44, 1292–1298. [Google Scholar] [CrossRef]

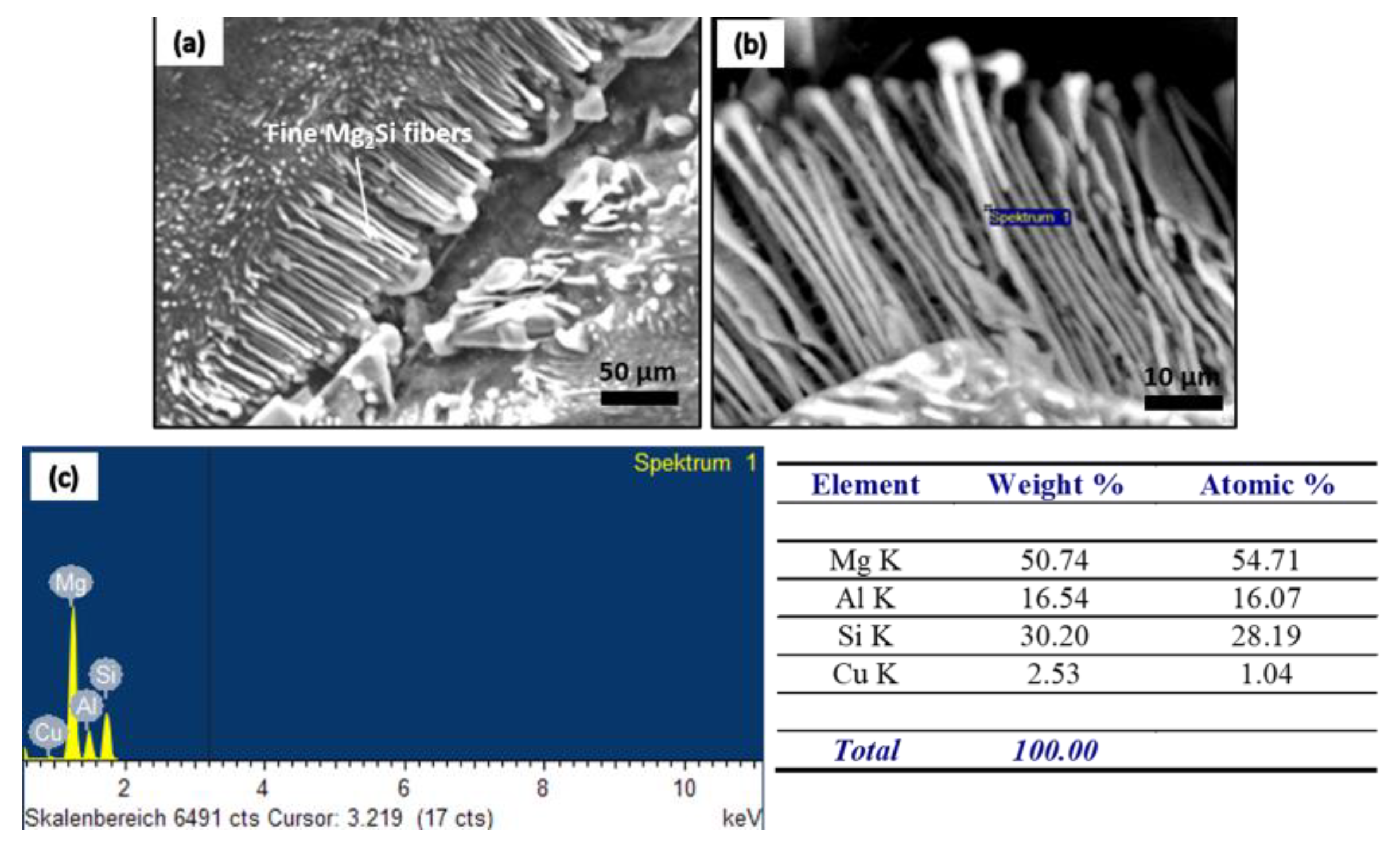
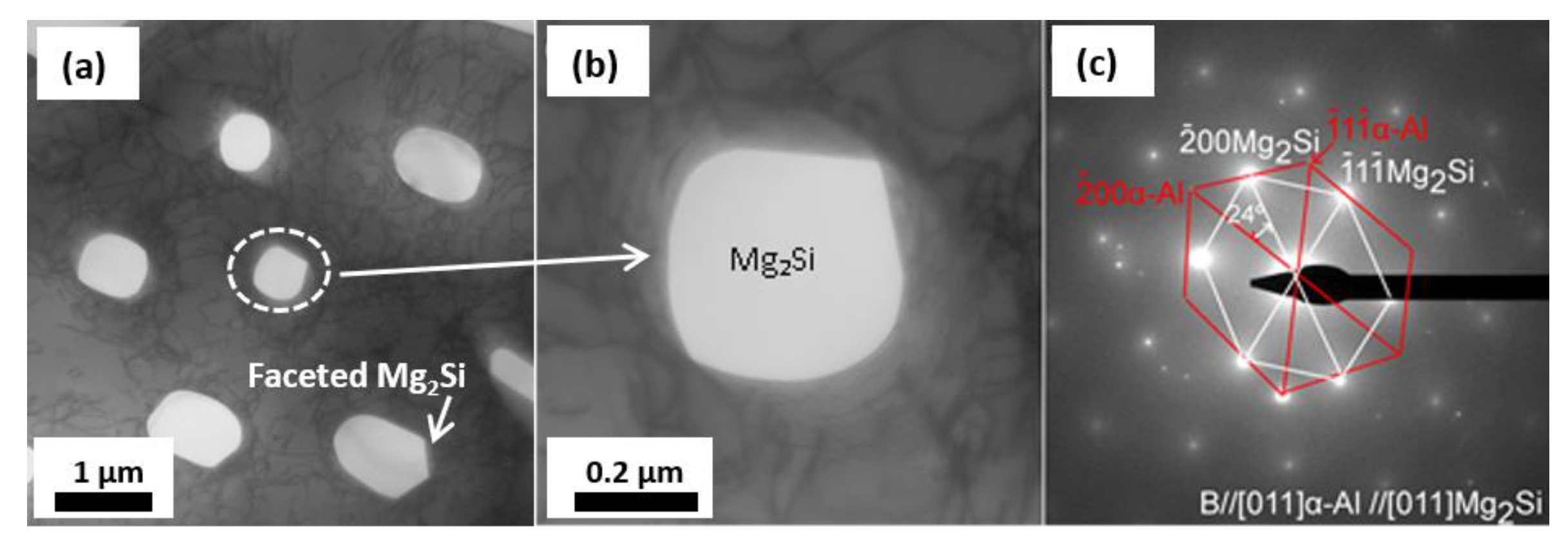
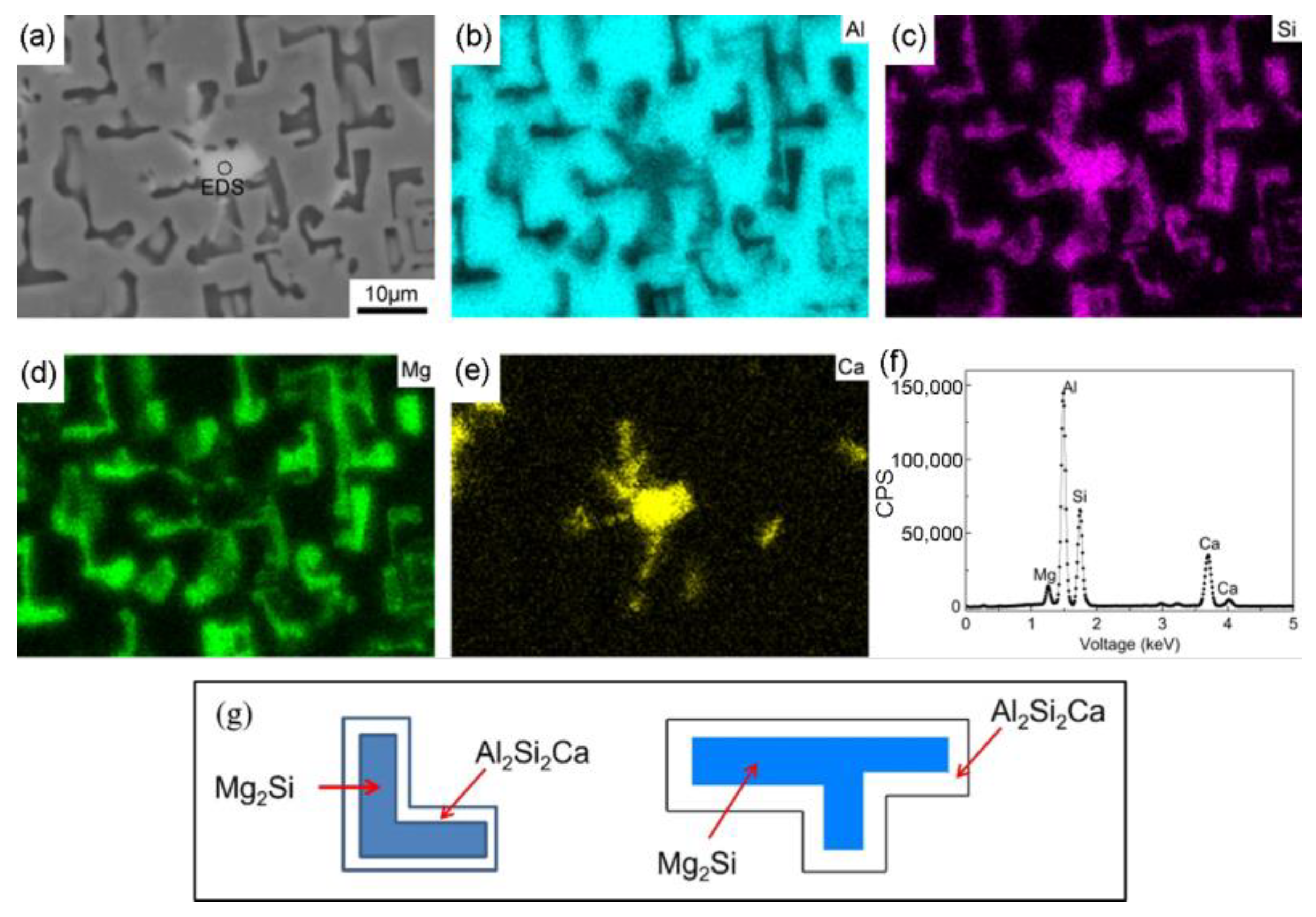
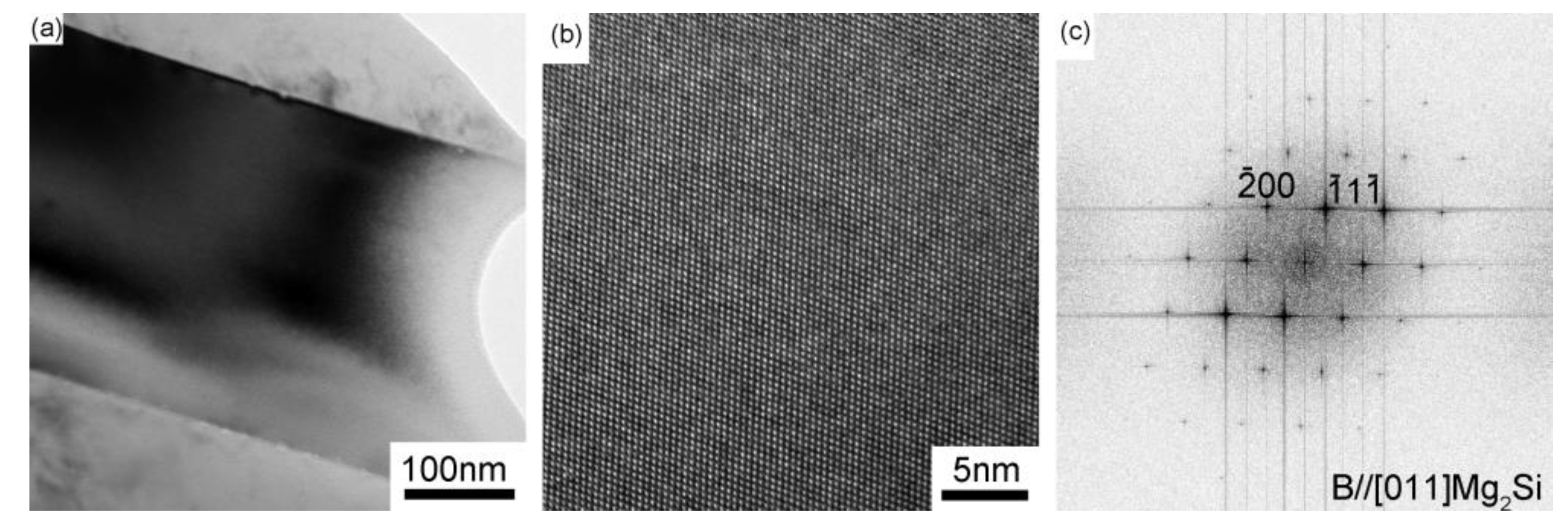
| No. | Mg wt.% | Si wt.% | P (ppm) | Fe (ppm) | Ca (ppm) | Cu wt.% | Cr (ppm) | Ni (ppm) | Ti (ppm) | Al |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8.61 | 4.58 | 0.4 | <5 | 27 | <1 | <3 | <4 | <4 | Bal. |
| 2 | 8.45 | 4.60 | 50 | 35 | 8 | 0.045 | <3 | <4 | <4 | Bal. |
| 3 | 8.64 | 4.54 | 200 | 40 | 6 | 0.273 | <3 | <4 | <4 | Bal. |
| 4 | 8.45 | 4.60 | 300 | 44 | 6 | 0.366 | <3 | <4 | <4 | Bal. |
| 5 | 8.50 | 4.60 | 0.4 | 35 | 500 | <1 | <3 | <4 | <4 | Bal. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarif, M.; Spacil, I.; Pabel, T.; Schumacher, P.; Li, J. Effect of Ca and P on the Size and Morphology of Eutectic Mg2Si in High-Purity Al-Mg-Si Alloys. Metals 2023, 13, 784. https://doi.org/10.3390/met13040784
Zarif M, Spacil I, Pabel T, Schumacher P, Li J. Effect of Ca and P on the Size and Morphology of Eutectic Mg2Si in High-Purity Al-Mg-Si Alloys. Metals. 2023; 13(4):784. https://doi.org/10.3390/met13040784
Chicago/Turabian StyleZarif, Muhammad, Ivo Spacil, Thomas Pabel, Peter Schumacher, and Jiehua Li. 2023. "Effect of Ca and P on the Size and Morphology of Eutectic Mg2Si in High-Purity Al-Mg-Si Alloys" Metals 13, no. 4: 784. https://doi.org/10.3390/met13040784





