Effect of CaO-MgO-FeO-SiO2-xNa2O Slag System on Converter Dephosphorization
Abstract
1. Introduction
2. Experimental Method
2.1. Laboratory Experiment Scheme
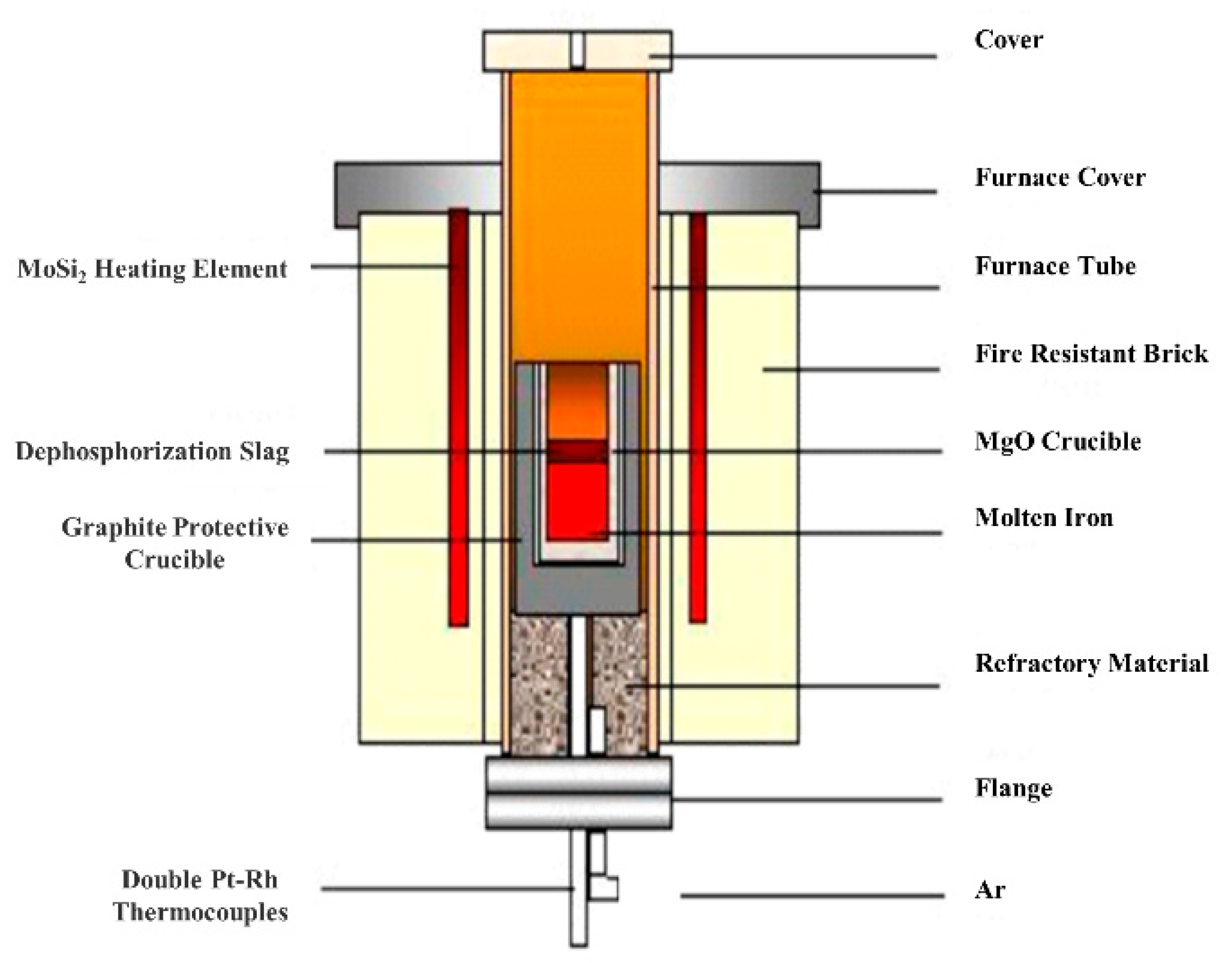
2.1.1. High Temperature Dephosphorization Experimental Scheme
2.1.2. Melting Point Testing Scheme
2.1.3. Viscosity Calculation Method
2.2. Industrial Program
2.3. Analysis Method
2.3.1. Analysis Method of Molten Iron Sample
2.3.2. Analysis Method of Slag Sample
3. Experimental Results
3.1. Laboratory Dephosphorization Experimental Results
3.2. Industrial Results
4. Analysis and Discussion
4.1. Effect of Na2O on Dephosphorization Reaction
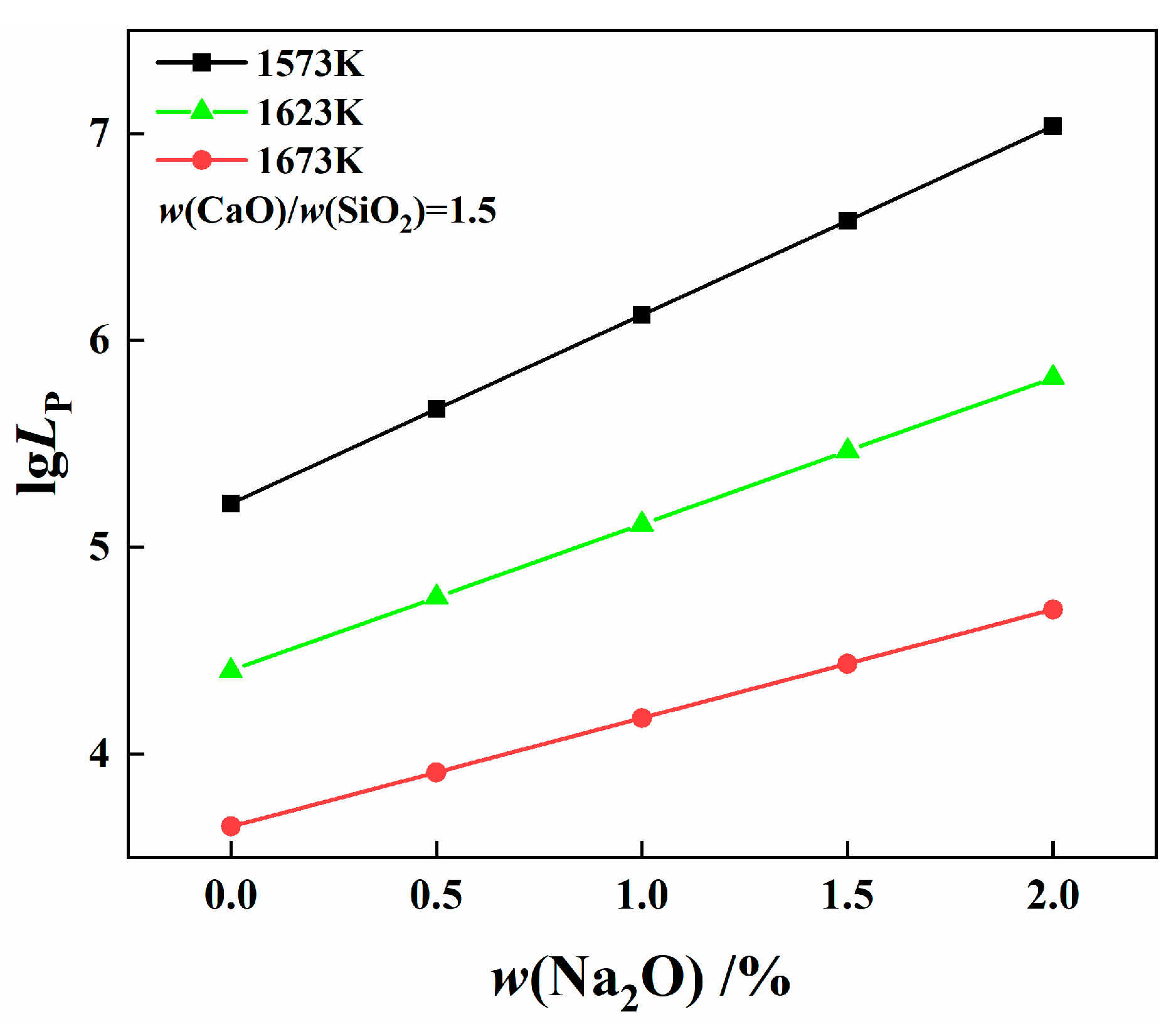
4.2. Effect of Na2O on Phosphorus Distribution Ratio
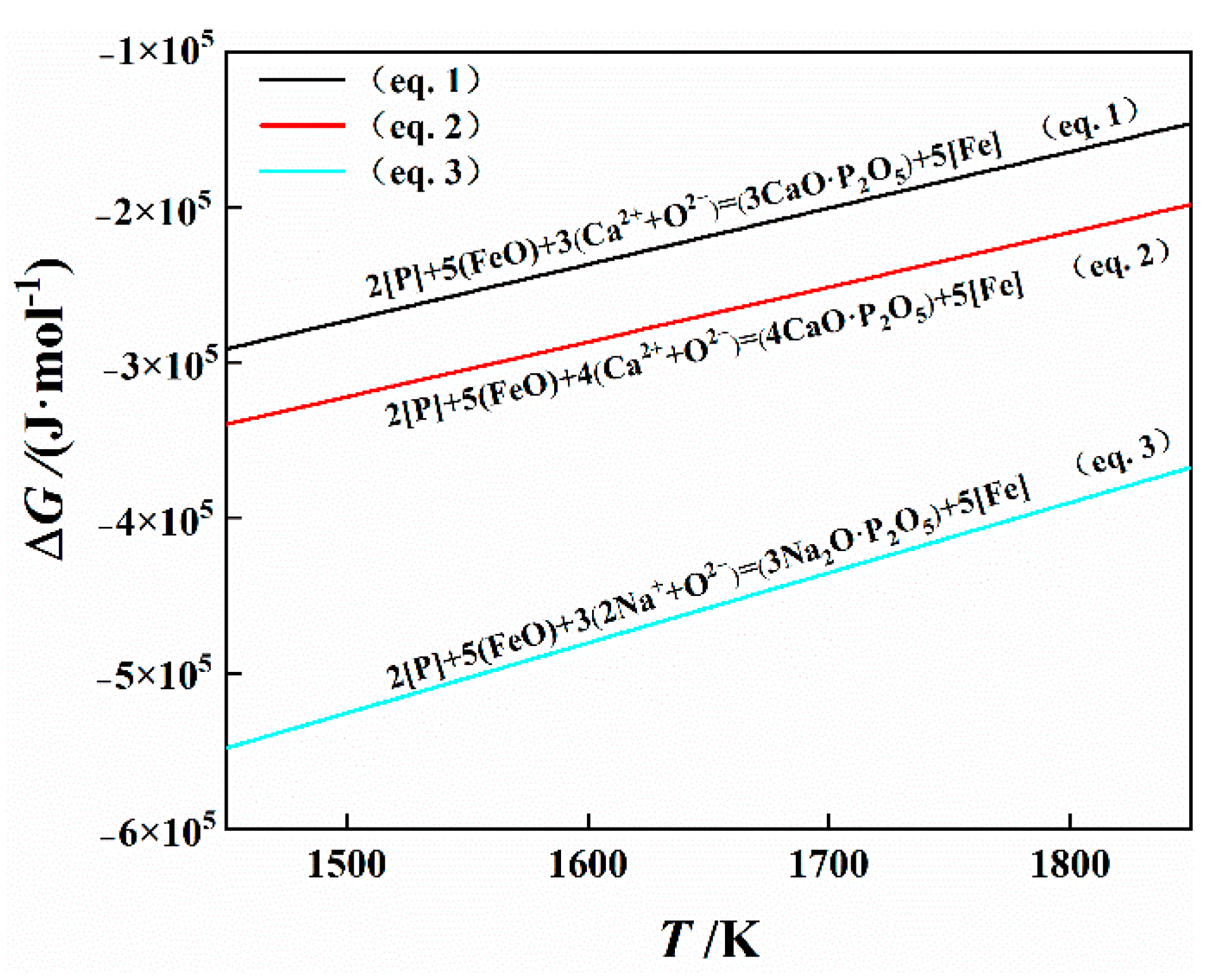
4.2.1. Establishment of Phosphorus Distribution Ratio Model
- (1)
- Ions: Ca2+, Fe2+, O2-, Mn2+, Mg2+, Na+;
- (2)
- Simple molecules: SiO2, Al2O3, P2O5;
- (3)
- Complex molecule: CaO·SiO2, 2CaO·SiO2, 3CaO·SiO2, 3CaO·2SiO2, MgO·SiO2, 2MgO·SiO2, CaO·Al2O3, CaO·2Al2O3, CaO·6Al2O3, 3CaO·Al2O3, 12CaO·7Al2O3, 3Al2O3·2SiO2, 2FeO·SiO2, MnO·SiO2, 2MnO·SiO2, Na2O·Al2O3, FeO·Al2O3, MnO·Al2O3, MgO·Al2O3, 2CaO·P2O5, 3CaO·P2O5, 4CaO·P2O5, 3FeO·P2O5, 4FeO·P2O5, 3MnO·P2O5, 2MgO·P2O5, 3MgO·P2O5, 3Na2O·P2O5, CaO·MgO·SiO2, CaO·MgO·2SiO2, 2CaO·MgO·2SiO2, 3CaO·MgO·2SiO2, CaO·Al2O3·2SiO2, 2CaO·Al2O3·SiO2, Na2O·Al2O3·2SiO2, Na2O·Al2O3·6SiO2.
4.2.2. Effect of Na2O on Phosphorus Distribution Ratio
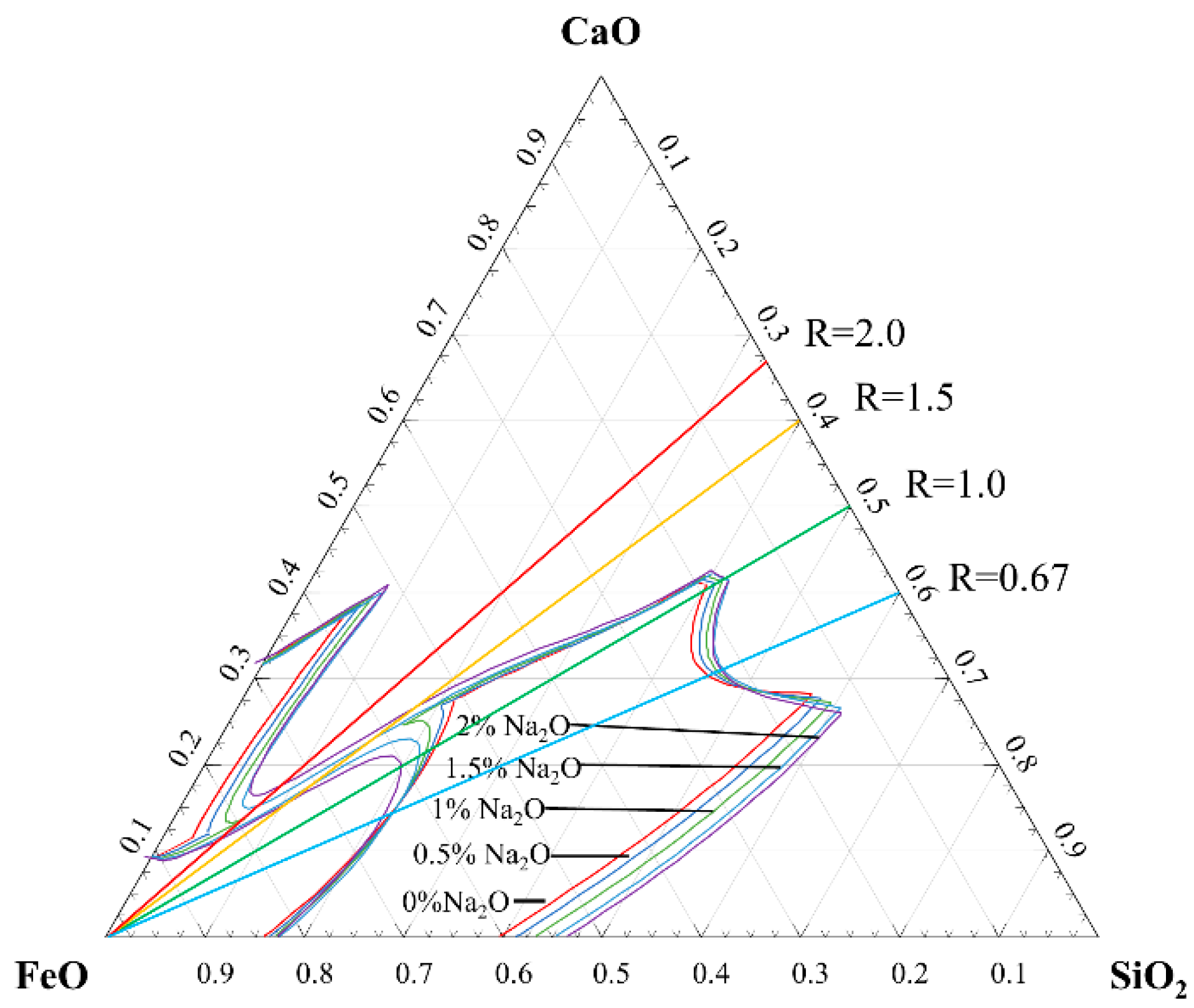
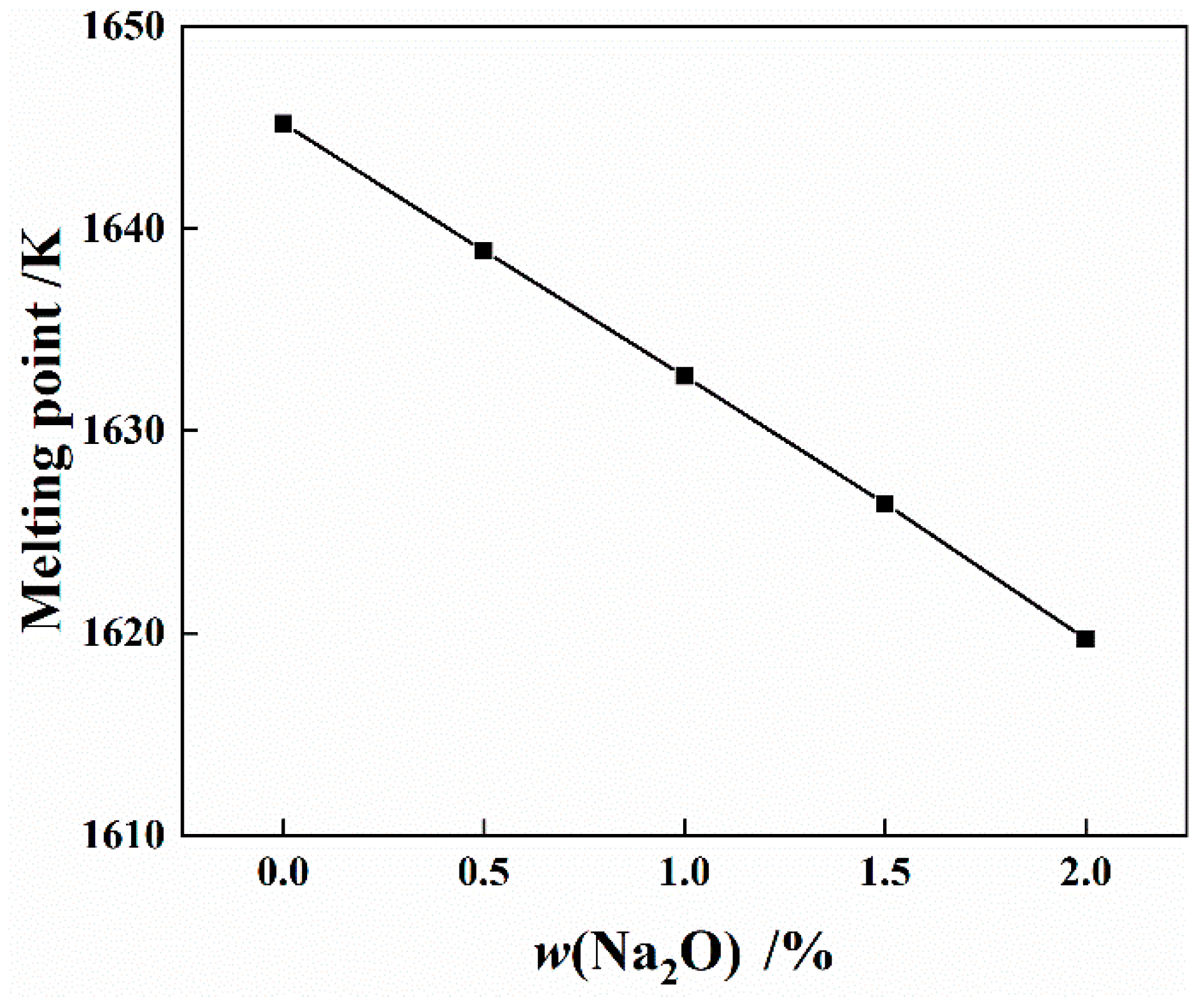
4.3. Effect of Na2O on Physicochemical and Chemical Properties of Slag System
4.3.1. Effect of Na2O on Melting Point of Slag
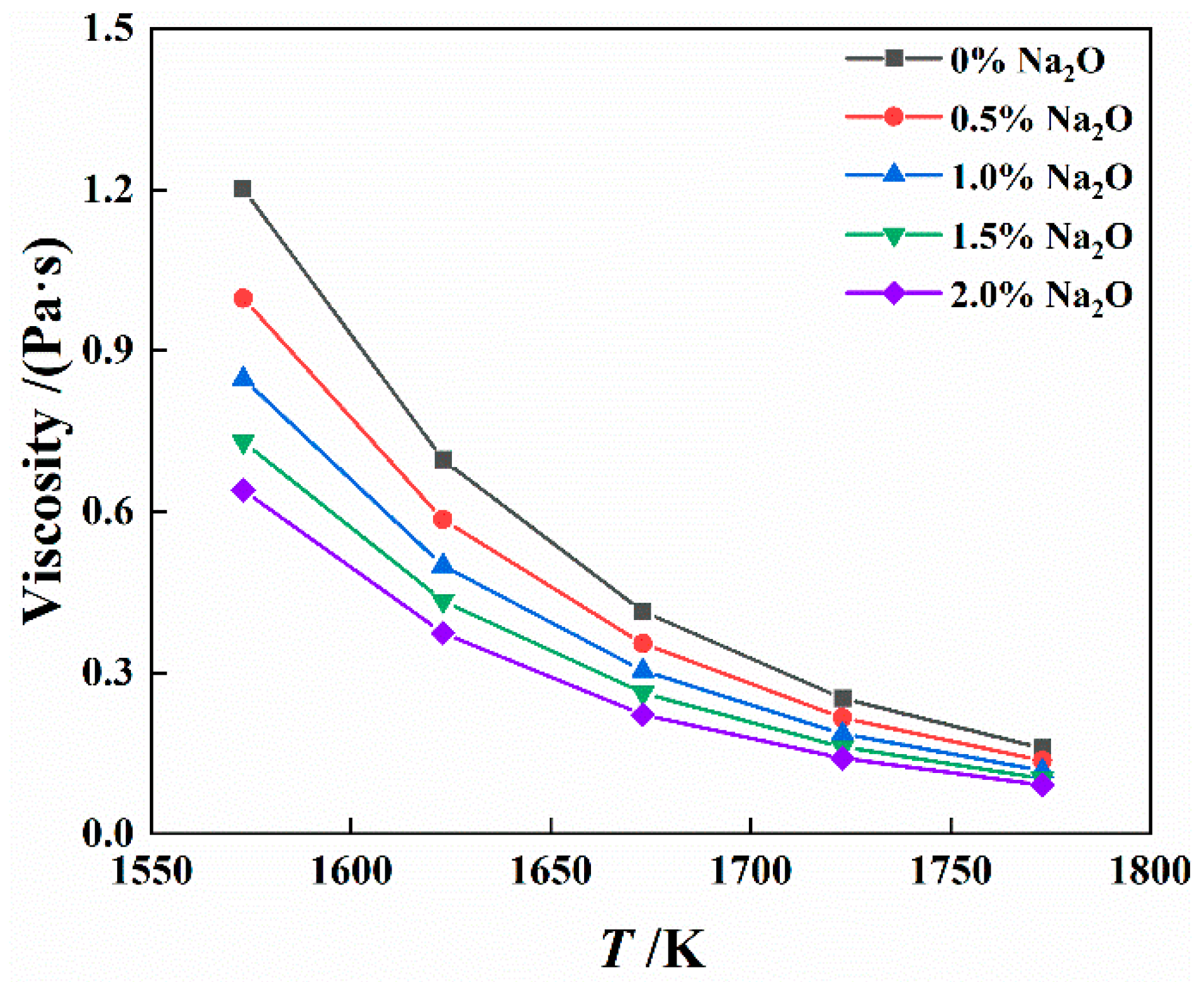
4.3.2. Effect of Na2O on Viscosity of Slag
5. Conclusions
- (1)
- With the increase of w(Na2O), the dephosphorization rate increases, and the Ca, Si, O, and P elements in the dephosphorization slag are distributed in the same area, mainly in the form of phosphate minerals, such as Ca2SiO4·0.05Ca3(PO4)2 and 6Ca2SiO4·Ca3(PO4)2. After adding Na2O, part of the Na will replace the Ca in the phosphorus-containing phase, forming a Ca2SiO4·Ca2Na2(PO4)2 phase.
- (2)
- After adding sodium-containing slagging material, the average dephosphorization rate of blowing for 6 min and at the end point can reach 62.39% and 72.03%, which are 19.62% and 9.89% higher, respectively, than the corresponding values of the conventional heats. The average final slag basicity of the test heats is 0.19% lower than that of the conventional heats, while the average w(P2O5) of the final slag increases by 0.36%, and the average T.Fe decreases by 0.69%. The average slagging materials consumption of the test heats is 35.93 kg/t, which is 7.24 kg/t lower than that of the conventional heats.
- (3)
- Through thermodynamic calculation, we found that with the increase of w(Na2O), the phosphorus distribution ratio between the slag and the molten iron increases significantly, the area of the liquid phase zone of the slag system increases continuously, and the viscosity decreases continuously.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Messier, R.W.; Li, L. Separating effects of phosphorus and sulfur in weld cracking of austenitic stainless steels for technological and economic benefits. J. Adv. Mater. 2001, 33, 3–13. [Google Scholar]
- Ogawa, Y.; Yano, M.; Kitamura, S.; Hirata, H. Development of the continuous dephosphorization and decarburization process using BOF. Tetsu-Hagané 2001, 87, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Zhu, G.S.; Li, H.B.; Lv, Y.C. Investigation on “slag remaining + double-slag” BOF steelmaking technology. Chin. Metall. 2013, 23, 40–46. [Google Scholar]
- Gao, S.Y.; Tian, Z.H.; Zhu, L.X.; Zhao, C.L.; Yan, Z.H.; Luo, B.G.; Wei, G. Study on the dephosphorization of hot metal in converter. Chin. J. Eng. 2011, 33, 63–67. [Google Scholar]
- Kang, F.; Lu, Z.X.; Jiang, X.F.; Zhong, Z.M. Research and development of BRP technology at Baosteel. Steel 2005, 3, 25–28. [Google Scholar]
- Zhou, C.G.; Hu, J.Z.; Ai, L.Q.; Wang, S.H.; Yang, H.Z.; Chen, H. Research progress and prospect of dephosphorization in steelmaking converter. J. Iron Steel Res. 2021, 33, 183–195. [Google Scholar]
- Liu, J.; Li, G.Q.; Zhu, C.Y.; Wang, C.A. Research progress in treatment of high phosphorus iron ore and dephosphorization of high phosphorus hot metal. J. Mater. Metall. 2007, 23, 173–179. [Google Scholar]
- Pak, J.J.; Fruehan, R.J. The effect of Na2O on dephosphorization by CaO-based steelmaking slags. Metall. Trans. B 1991, 22, 39–46. [Google Scholar] [CrossRef]
- Pak, J.J.; Fruehan, R.J. Soda slag system for hot metal dephosphorization. Metall. Trans. B 1986, 17, 797–804. [Google Scholar] [CrossRef]
- Li, G.Q.; Hamano, T.; Tsukihashi, F. The effect of Na2O and Al2O3 on dephosphorization of molten steel by high basicity MgO saturated CaO-FeOx-SiO2 slag. ISIJ Int. 2005, 45, 12–18. [Google Scholar] [CrossRef]
- Diao, J. Effect of Al2O3 and Na2O on dephosphorization of high phosphorus hot metal. J. Iron Steel Res. 2013, 25, 9–13. [Google Scholar]
- Du, C.M.; Lv, N.N.; Su, C.; Liu, W.M.; Yang, J.X.; Wang, H.C. Distribution of P2O5 between solid solution and liquid phase in dephosphorization slag of CaO-SiO2-FeO-P2O5-Na2O system. J. Iron Steel Res. Int. 2019, 26, 1162–1170. [Google Scholar] [CrossRef]
- Wrampelmeyer, J.C.; Dimitrov, S.; Janke, D. Dephosphorization equilibria between pure molten iron and CaO-saturated FeOn-CaO-SiO2 and FeOn-CaO-Al2O3 slags. Steel Res. 1989, 60, 539. [Google Scholar] [CrossRef]
- Xing, W.; Wu, L.; Li, S.Q.; Yao, L.; Liu, R.Z. Experimental study on the melting temperature characteristic of the CaO-FeO-Al2O3-SiO2 slag system. Chin. J. Eng. 2014, 36, 603–607. [Google Scholar]
- Zhang, H.Y.; Jiang, Z.H.; Wang, W.Z.; Liu, X.; Gu, W.B.; Wang, J. Effect of BaO and Na2O on equilibrium phosphorus content in LF refining liquid steel. Spec. Steel 2002, 1, 14–16. [Google Scholar]
- Taposhe, G.I.A.; Khajavi, L.T. Removal of phosphorus from Si-Fe alloy by CaO-Al2O3-SiO2-Na2O slag refining. J. Miner. Met. Mater. Soc. 2021, 73, 729–735. [Google Scholar] [CrossRef]
- Li, F.S.; Li, X.P.; Yang, S.F.; Zhang, Y.L. Distribution ratios of phosphorus between CaO-FeO-SiO2-Al2O3/Na2O/TiO2 slags and carbon-saturated iron. Metall. Mater. Trans. B 2017, 48, 2367–2378. [Google Scholar] [CrossRef]
- Li, F.S.; Li, X.P.; Zhang, Y.L.; Gao, M. Phosphate capacities of CaO-FeO-SiO2-Al2O3/Na2O/TiO2 slags. High Temp. Mater. Process. 2019, 38, 50–59. [Google Scholar] [CrossRef]
- Li, X.P.; Gao, J.T.; Zhang, Y.L.; Xing, L.L.; Zou, L. Distribution behavior of phosphorus between CaO-FeO-SiO2-Al2O3/Na2O/TiO2 slags and carbon-saturated iron. Steel 2017, 52, 18–23. [Google Scholar] [CrossRef]
- Zhou, Z.Q.; Zhu, Z.Z.; Ding, Y.C.; Zhou, S.N. Effect of Na2O on dephosphorization for high phosphorus Si-Mn alloys. Steel 2017, 52, 13–17. [Google Scholar]
- Xuan, X.Y.; Shi, Z.; Qi, X.; Cai, J.W. Study on dephosphorization of high-phosphorus hot metal in CaO-SiO2-FeO-Na2O-Al2O3 slag system. Min. Metall. 2015, 24, 51–54. [Google Scholar]
- Moriya, T.; Fujii, M. Dephosphorization and desulfurization of molten pig iron by Na2CO3. Trans. Iron Steel Inst. Jpn. 1981, 21, 732–741. [Google Scholar] [CrossRef]
- Maddocks, W.R.; Turkdogan, E.T. The effect of sodium oxide additions to steelmaking slags dephosphorization of steel by soda-slags. J. Iron Steel Inst. 1952, 171, 128–136. [Google Scholar]
- Oelsen, W. The dephosphorization and desulfurization of high-carbon iron melts with the production of water-soluble high phosphorus slags. Arch Eisenhuttenw 1965, 36, 861–871. [Google Scholar]
- Ito, K.; Sang, N. Phosphorus distribution between basic slags and carbon-saturated iron at hot-metal temperatures. Trans. Iron Steel Inst. Jpn. 1985, 25, 355–362. [Google Scholar] [CrossRef]
- Marukawa, K.; Shirota, Y.; Anezaki, S.; Hirahara, H. Refining process of molten iron by sodium carbonate. Tetsu-Hagané 1981, 67, 323. [Google Scholar] [CrossRef]
- Du, C.M.; Gao, X.; Ueda, S.; Kitamura, S.Y. Effect of Na2O addition on phosphorus dissolution from steelmaking slag with high P2O5 content. J. Sustain. Metall. 2017, 3, 671–682. [Google Scholar] [CrossRef]
- Yu, K.; Zhang, Y.L.; Li, F.S.; Gao, M. Effect of Al2O3/TiO2/Na2O on enrichment of phosphorus in P-bearing steelmaking slag. J. Iron Steel Res. Int. 2019, 26, 796–805. [Google Scholar] [CrossRef]
- Wang, D.Z.; Bao, Y.P.; Wang, M. Study on the enrichment behavior of phosphorus in high phosphorus converter slag. Chin. J. Eng. 2018, 40, 65–72. [Google Scholar]
- He, S.; Liu, Y.Q.; Lin, L.; Hou, Z.X.; Huang, F.; Hu, Y.B. Effect of Na2O on the enrichment behavior of phosphorus in the slag system CaO-SiO2-Fe2O3-P2O5. Iron Steel Vanadium Titan. 2022, 43, 140–145. [Google Scholar]
- Lin, L.; Bao, Y.P.; Jiang, W.; Wu, Q.F. Effect of Na2O on phosphorus enrichment and separation in P-bearing steelmaking slag. ISIJ Int. 2015, 55, 552–558. [Google Scholar] [CrossRef]
- Zhang, J. Computational Thermodynamics of Metallurgical Melts and Solutions; Metallurgical Industry Press: Beijing, China, 2007. [Google Scholar]
- Zhang, K.H.; Zhang, Y.L.; Li, F.S.; Wu, T. A thermodynamic model for calculation of sulfur distribution ratio between CaO-SiO2-Al2O3-Na2O-TiO2-(MgO) slag and carbon saturated hot metal. J. Iron Steel Res. 2018, 30, 265–272. [Google Scholar]
- Sun, J.L.; Liu, C.J.; Jiang, M.F. Thermodynamic model of dephosphorization of CaO-SiO2-FeO-Al2O3-Na2O-TiO2-P2O5 slag system. Iron Steel Vanadium Titan. 2021, 42, 146–151+178. [Google Scholar]
- Gong, W.; Jiang, Z.H.; Zhan, D.P. Intelligent information technology application association: The model of mass action concentration for slag system of CaO-SiO2-A12O3-MgO and its application. In Proceedings of the 2011 AASRI Conference on Artificial Intelligence and Industry Application (AASRI-AIIA 2011 V4), Male, Maldives, 23–24 May 2011; American Applied Sciences Research Institute: Miramar, FL, USA, 2011; p. 4. [Google Scholar]
- Yang, X.M.; Duan, J.P.; Shi, C.B.; Zhang, M.; Zhang, Y.L.; Wang, J.C. A thermodynamic model of phosphorus distribution ratio between CaO-SiO2-MgO-FeO-Fe2O3-MnO-Al2O3-P2O5 slags and molten steel during a top-bottom combined blown converter steelmaking process based on the ion and molecule coexistence theory. Metall. Mater. Trans. B 2011, 42, 738–770. [Google Scholar] [CrossRef]
- Li, P.C.; Zhang, J.L. Retraction: A prediction model of phosphorus distribution between CaO-SiO2-MgO-FeO-Fe2O3-P2O5 slags and liquid iron. ISIJ Int. 2014, 54, 756–765. [Google Scholar] [CrossRef]
- Zhang, J. Coexistence theory of slag structure and its application to calculation of oxidizing capability of slag melts. J. Iron Steel Res. Int. 2003, 10, 1–10. [Google Scholar]
- Yang, X.M.; Li, J.Y.; Zhang, M.; Chai, G.M.; Zhang, J. Prediction model of sulfide capacity for CaO-FeO-Fe2O3-Al2O3-P2O5 slags in a large variation range of oxygen potential based on the ion and molecule coexistence theory. Metall. Mater. Trans. B 2014, 45, 2118–2137. [Google Scholar] [CrossRef]
- Yang, X.M.; Li, J.Y.; Zhang, M.; Zhang, J. Prediction model of sulphur distribution ratio between CaO-FeO-Fe2O3-Al2O3-P2O5 slags and liquid iron over large variation range of oxygen potential during secondary refining process of molten steel based on ion and molecule coexistence theory. Ironmak. Steelmak. 2016, 43, 39–55. [Google Scholar] [CrossRef]
- Sun, Y.H.; Zhao, C.L.; Luo, L.; Zhao, Z.C. Calculation and analysis of slag phosphate capacity and prediction model in 300 t top and bottom-blowing converter. Chin. J. Eng. 2016, 38, 83–89. [Google Scholar]
- Jeoungkiu, I.M.; Morita, K.; Sano, N. Phosphorus distribution ratios between CaO-SiO2-FetO slags and carbon-saturated iron at 1573 K. ISIJ Int. 1996, 36, 517–521. [Google Scholar]
- Basu, S.; Lahiri, A.K.; Seetharaman, S. Phosphorus partition between liquid steel and CaO-SiO2-P2O5-MgO slag containing low FeO. Metall. Mater. Trans. B 2007, 38, 357. [Google Scholar] [CrossRef]
- Li, J.; Yue, K.X. Study on dephosphorizing flux containing CaO-Fe2O3-CaF2-Al2O3-Na2CO3 for hot metal pretreatment. J. Anhui Univ. Technol. (Nat. Sci. Ed.) 2003, 20, 177–180. [Google Scholar]
- Niekerk, W.H.V.; Dippenaar, R.J. Thermodynamic aspects of Na2O and CaF2 containing lime-based slags used for the desulphurization of hot-metal. ISIJ Int. 1993, 33, 59–65. [Google Scholar] [CrossRef]
- Takahira, N.; Hanao, M.; Tsukaguchi, Y. Viscosity and solidification temperature of SiO2-CaO-Na2O melts for fluorine free mould flux. ISIJ Int. 2013, 53, 818–822. [Google Scholar] [CrossRef]
- Sukenaga, S.; Saito, N.; Kawakami, K.; Nakashima, K. Viscosities of CaO-SiO2-Al2O3-(R2O or RO) melts. ISIJ Int. 2006, 46, 352–358. [Google Scholar] [CrossRef]
- Chen, C.Y.; Jiang, Z.H.; Li, Y.; Sun, M.; Chen, K.; Wang, Q.; Li, H.B. Effect of Na2O and Rb2O on inclusion removal in C96V saw wire steels using low-basicity LF (Ladle Furnace) refining slags. Metals 2018, 8, 691. [Google Scholar] [CrossRef]
- Kim, H.; Kim, W.H.; Park, J.H.; Min, D.J. A study on the effect of Na2O on the viscosity for ironmaking slags. Steel Res. Int. 2010, 81, 17–24. [Google Scholar] [CrossRef]

| C | Si | Mn | P | S |
|---|---|---|---|---|
| 3.08 | 0.03 | 0.08 | 0.135 | 0.034 |
| Heats | Compositions | R | |||||
|---|---|---|---|---|---|---|---|
| CaO | Fe2O3 | MgO | MnO | SiO2 | Na2CO3 | ||
| 1 | 31.6 | 33.3 | 9 | 5 | 21.1 | 0 | 1.50 |
| 2 | 29.6 | 33.3 | 9 | 5 | 19.7 | 3.42 | 1.50 |
| 3 | 27.6 | 33.3 | 9 | 5 | 18.4 | 6.84 | 1.50 |
| 4 | 25.6 | 33.3 | 9 | 5 | 17.1 | 10.26 | 1.50 |
| Heats | Compositions/% | Dephosphorization Rate/% | lgLP/% | ||||
|---|---|---|---|---|---|---|---|
| C | Si | Mn | P | S | |||
| No. 1 | 2.80 | 0.02 | 0.04 | 0.038 | 0.030 | 71.85 | 5.05 |
| No. 2 | 2.67 | 0.01 | 0.05 | 0.032 | 0.030 | 76.30 | 5.24 |
| No. 3 | 2.20 | 0.02 | 0.05 | 0.030 | 0.030 | 77.78 | 5.31 |
| No. 4 | 1.80 | 0.02 | 0.04 | 0.029 | 0.032 | 78.52 | 5.36 |
| Heats | CaO | T.Fe | MgO | MnO | SiO2 | Na2O | P2O5 | R |
|---|---|---|---|---|---|---|---|---|
| No. 1 | 34.76 | 19.42 | 7.18 | 4.34 | 23.24 | 0.00 | 1.63 | 1.50 |
| No. 2 | 33.56 | 17.41 | 8.74 | 3.44 | 22.67 | 0.94 | 1.76 | 1.48 |
| No. 3 | 37.83 | 14.13 | 9.28 | 3.50 | 26.32 | 2.42 | 1.83 | 1.40 |
| No. 4 | 36.31 | 16.02 | 10.05 | 3.60 | 24.32 | 2.98 | 1.92 | 1.49 |
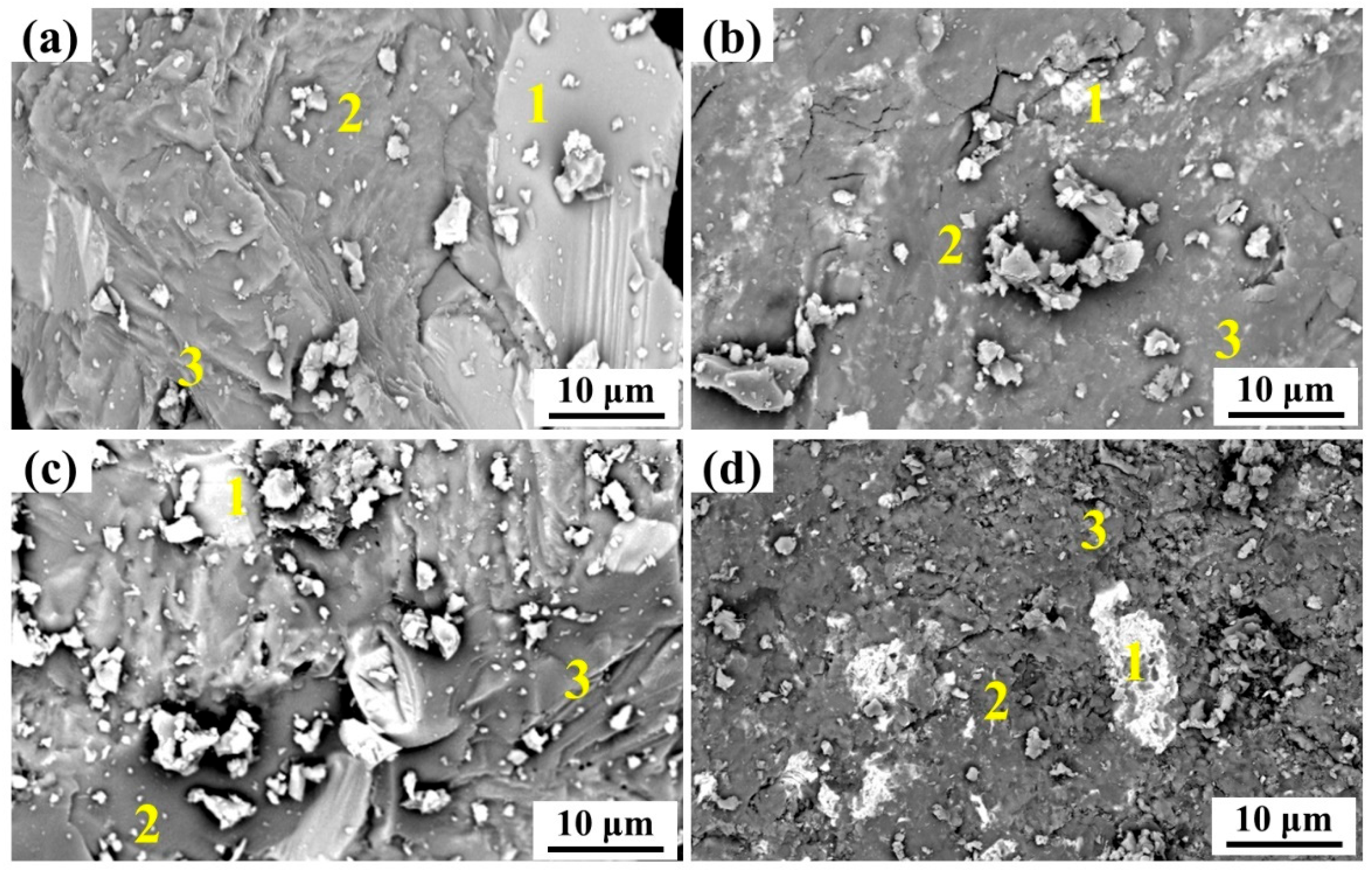
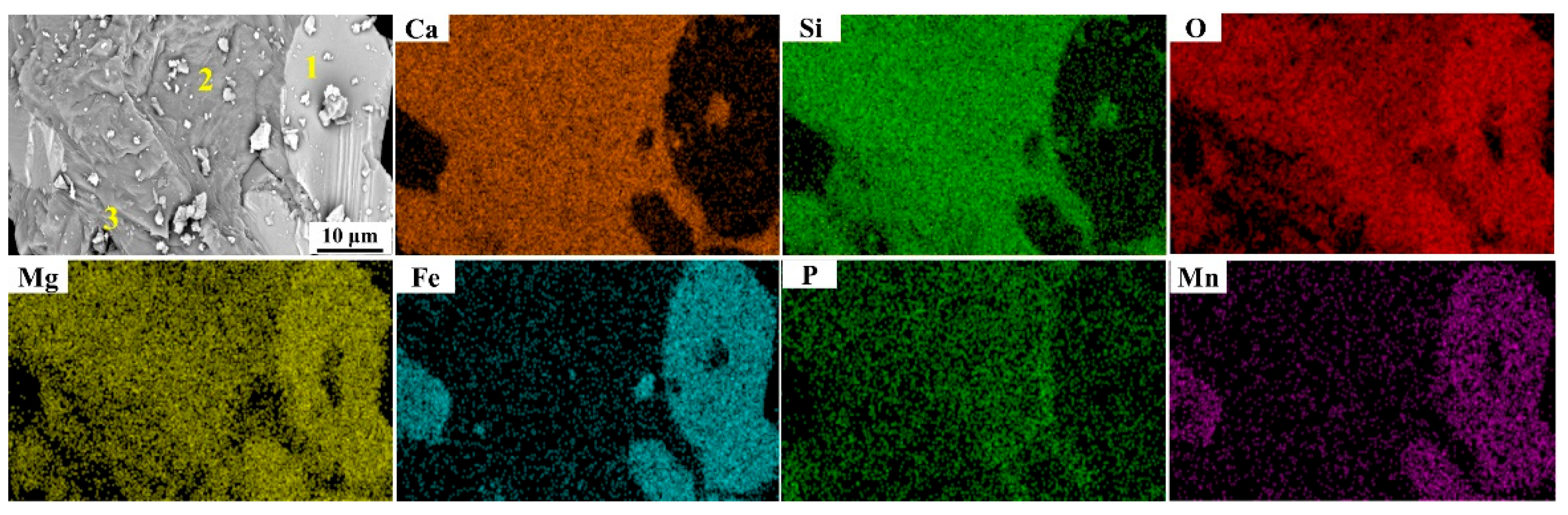
| Heats | Areas | Ca | Si | O | Mg | Fe | P | Mn | Na |
|---|---|---|---|---|---|---|---|---|---|
| No. 1 | 1 | 0.71 | 0.28 | 27.79 | 15.38 | 45.23 | 0 | 10.61 | 0 |
| 2 | 38.50 | 13.57 | 38.02 | 4.97 | 2.94 | 0.93 | 1.06 | 0 | |
| 3 | 42.47 | 17.61 | 29.38 | 5.32 | 3.98 | 0.04 | 1.20 | 0 | |
| No. 2 | 1 | 3.72 | 0.38 | 16.49 | 23.23 | 46.89 | 0 | 9.20 | 0.09 |
| 2 | 45.06 | 15.75 | 32.25 | 2.32 | 1.18 | 1.15 | 1.43 | 1.01 | |
| 3 | 35.57 | 16.34 | 36.33 | 5.77 | 4.17 | 0.08 | 1.57 | 0.17 | |
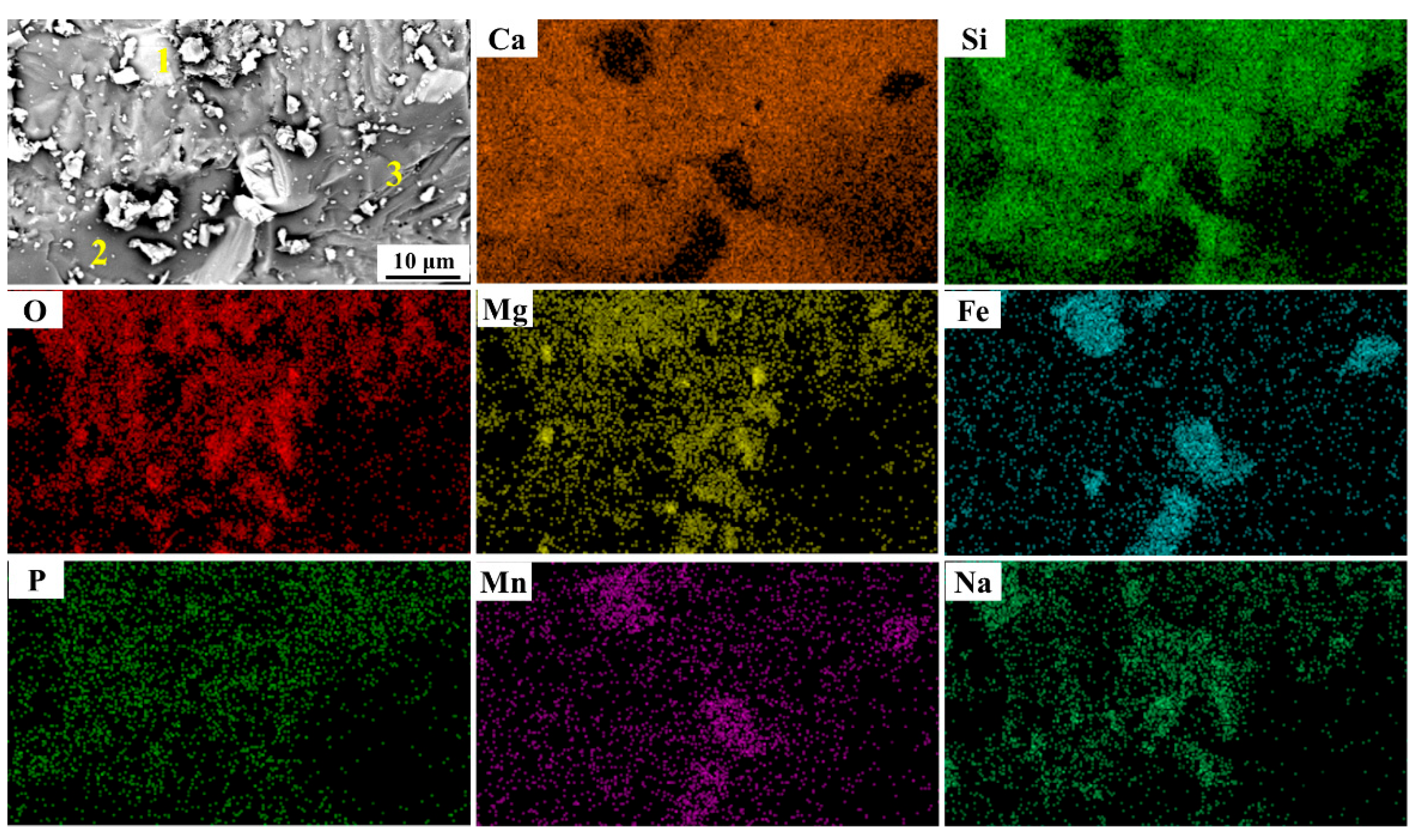
| No. 3 | 1 | 3.39 | 1.58 | 14.37 | 11.81 | 51.17 | 0 | 17.20 | 0.48 |
| 2 | 39.23 | 13.78 | 36.26 | 0.95 | 4.98 | 1.95 | 1.11 | 1.75 | |
| 3 | 36.73 | 17.57 | 37.22 | 2.57 | 3.57 | 0.07 | 1.95 | 0.32 | |
| No. 4 | 1 | 3.30 | 1.21 | 28.23 | 9.97 | 46.05 | 0 | 10.91 | 0.33 |
| 2 | 36.62 | 16.66 | 35.20 | 2.56 | 2.19 | 2.15 | 1.88 | 2.75 | |
| 3 | 36.51 | 20.24 | 30.79 | 4.37 | 5.74 | 0.12 | 1.64 | 0.59 |
| Heats | P Mass Fraction/% | Dephosphorization Rate/% | ||
|---|---|---|---|---|
| 6 min | End Point | 6 min | End Point | |
| Conventional | 0.060 | 0.040 | 42.77 | 62.14 |
| Test | 0.041 | 0.030 | 62.39 | 72.03 |
| Heats | CaO | T.Fe | MgO | MnO | SiO2 | Al2O3 | P2O5 | Na2O | R |
|---|---|---|---|---|---|---|---|---|---|
| Conventional | 43.81 | 15.62 | 9.60 | 5.04 | 17.54 | 2.79 | 2.47 | <0.01 | 2.50 |
| Test | 41.69 | 14.93 | 9.55 | 5.38 | 18.00 | 2.82 | 2.83 | 0.16 | 2.31 |
| Heats | Lime | Magnesium Oxide Ball | Dolomite | Sodium-Containing Slag | Total Slag Consumption | Iron and Steel Consumption |
|---|---|---|---|---|---|---|
| Conventional | 32.31 | 8.19 | 2.67 | — | 43.17 | 1053.60 |
| Test | 26.15 | 8.24 | — | 1.54 | 35.93 | 1052.23 |
| Dephosphorization Reactions | ||
|---|---|---|
| 2[P] + 5(FeO) = (P2O5) + 5[Fe] | −122,412 + 312.522 T | |
| 2[P] + 5(FeO) + 2(Ca2+ + O2−) = (2CaO·P2O5) + 5[Fe] | −606,784 + 285.953 T | |
| 2[P] + 5(FeO) + 3(Ca2+ + O2−) = (3CaO·P2O5) + 5[Fe] | −816,975.125 + 362.419 T | |
| 2[P] + 5(FeO) + 4(Ca2+ + O2−) = (4CaO·P2O5) + 5[Fe] | −851,492.98 + 352.822 T | |
| 2[P] + 5(FeO) + 3(Fe2+ + O2−) = (3FeO·P2O5) + 5[Fe] | −552,816 + 405.23 T | |
| 2[P] + 5(FeO) + 4(Fe2+ + O2−) = (4FeO·P2O5) + 5[Fe] | −504,243 + 359.889 T | |
| 2[P] + 5(FeO) + 3(Mn2+ + O2−) = (3MnO·P2O5) + 5[Fe] | −648,833.411 + 414.571 T | |
| 2[P] + 5(FeO) + 2(Mg2+ + O2−) = (2MgO·P2O5) + 5[Fe] | 45,957 − 26.835 T | |
| 2[P] + 5(FeO) + 3(Mg2+ + O2−) = (3MgO·P2O5) + 5[Fe] | −609,127.5 + 349.366 T | |
| 2[P] + 5(FeO) + 3(2Na2+ + O2−) = (3Na2O·P2O5) + 5[Fe] | −1,202,452 + 451.222 T |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, B.; Zhan, D.; Jiang, Z.; Yang, Y. Effect of CaO-MgO-FeO-SiO2-xNa2O Slag System on Converter Dephosphorization. Metals 2023, 13, 844. https://doi.org/10.3390/met13050844
Geng B, Zhan D, Jiang Z, Yang Y. Effect of CaO-MgO-FeO-SiO2-xNa2O Slag System on Converter Dephosphorization. Metals. 2023; 13(5):844. https://doi.org/10.3390/met13050844
Chicago/Turabian StyleGeng, Bin, Dongping Zhan, Zhouhua Jiang, and Yongkun Yang. 2023. "Effect of CaO-MgO-FeO-SiO2-xNa2O Slag System on Converter Dephosphorization" Metals 13, no. 5: 844. https://doi.org/10.3390/met13050844
APA StyleGeng, B., Zhan, D., Jiang, Z., & Yang, Y. (2023). Effect of CaO-MgO-FeO-SiO2-xNa2O Slag System on Converter Dephosphorization. Metals, 13(5), 844. https://doi.org/10.3390/met13050844







