Exploring the Manufacturing Process of a Renaissance Breach Pike
Abstract
:1. Introduction
2. Materials and Methods
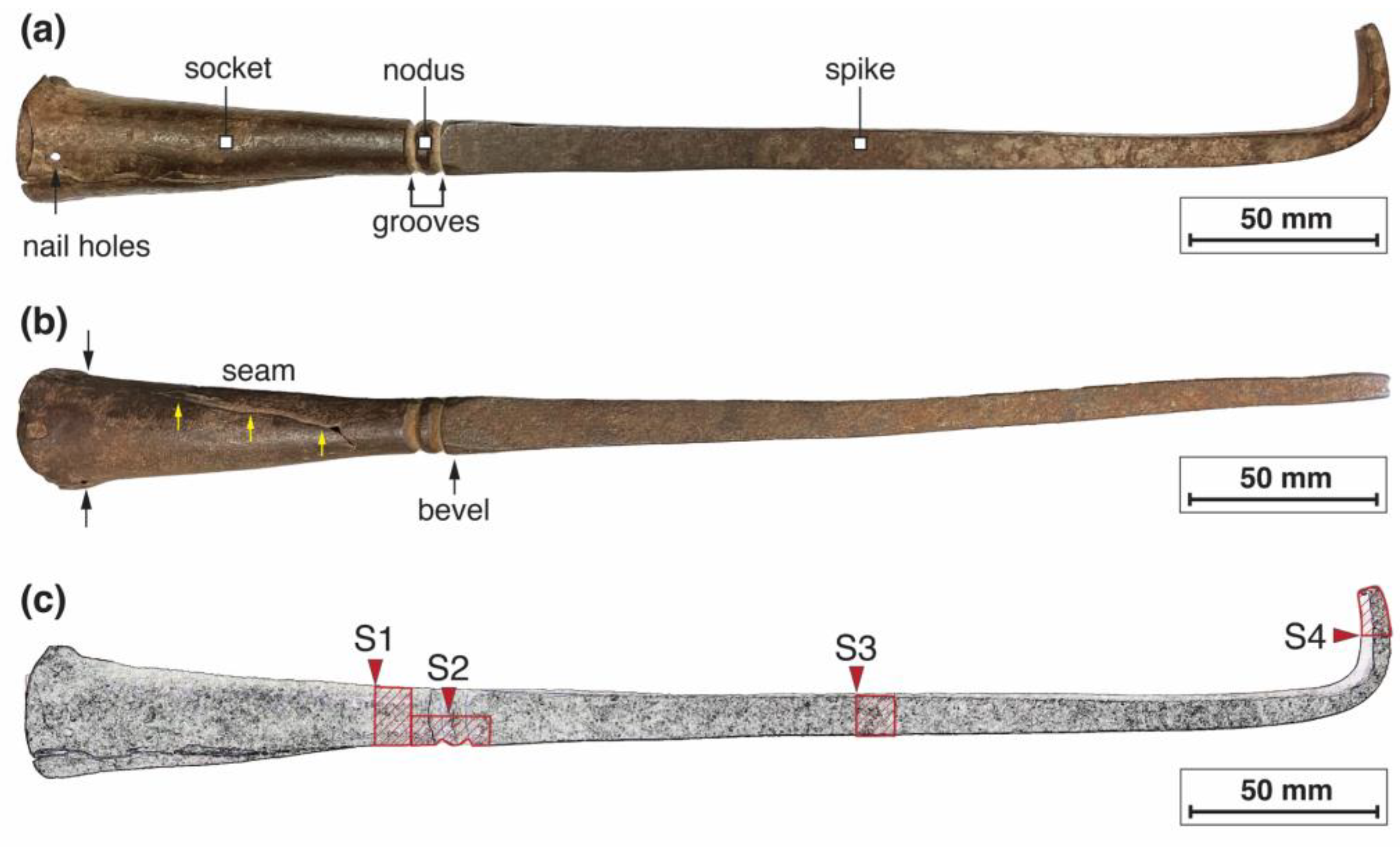
2.1. The Breach Pike: Preliminary Description and Macro-Observations
2.2. Experimental Methods
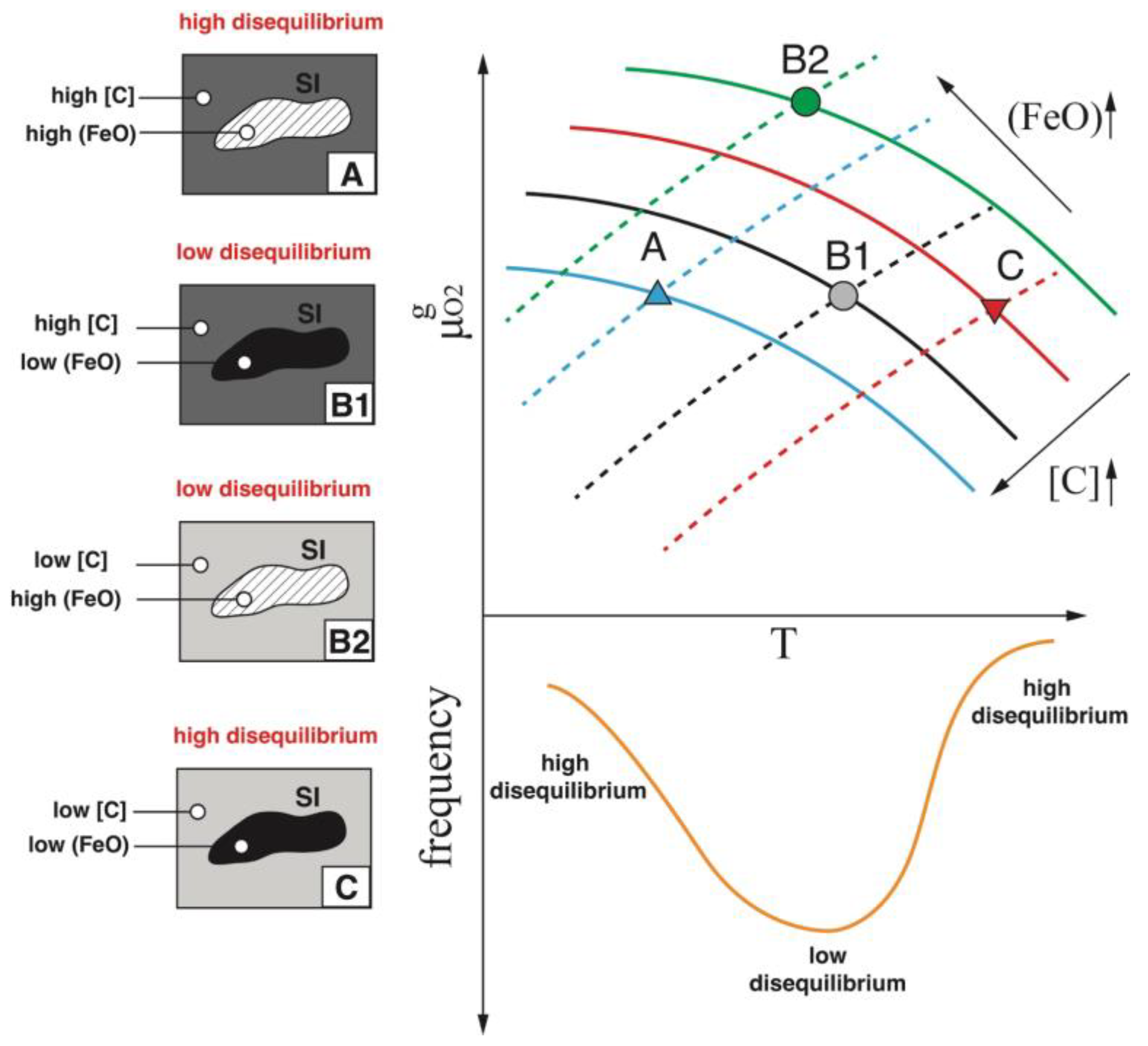
2.3. Slag Inclusion Classification and Data Treatment
2.4. Thermodynamic Modelling of Slag Inclusion–Metal Systems
3. Results and Discussion
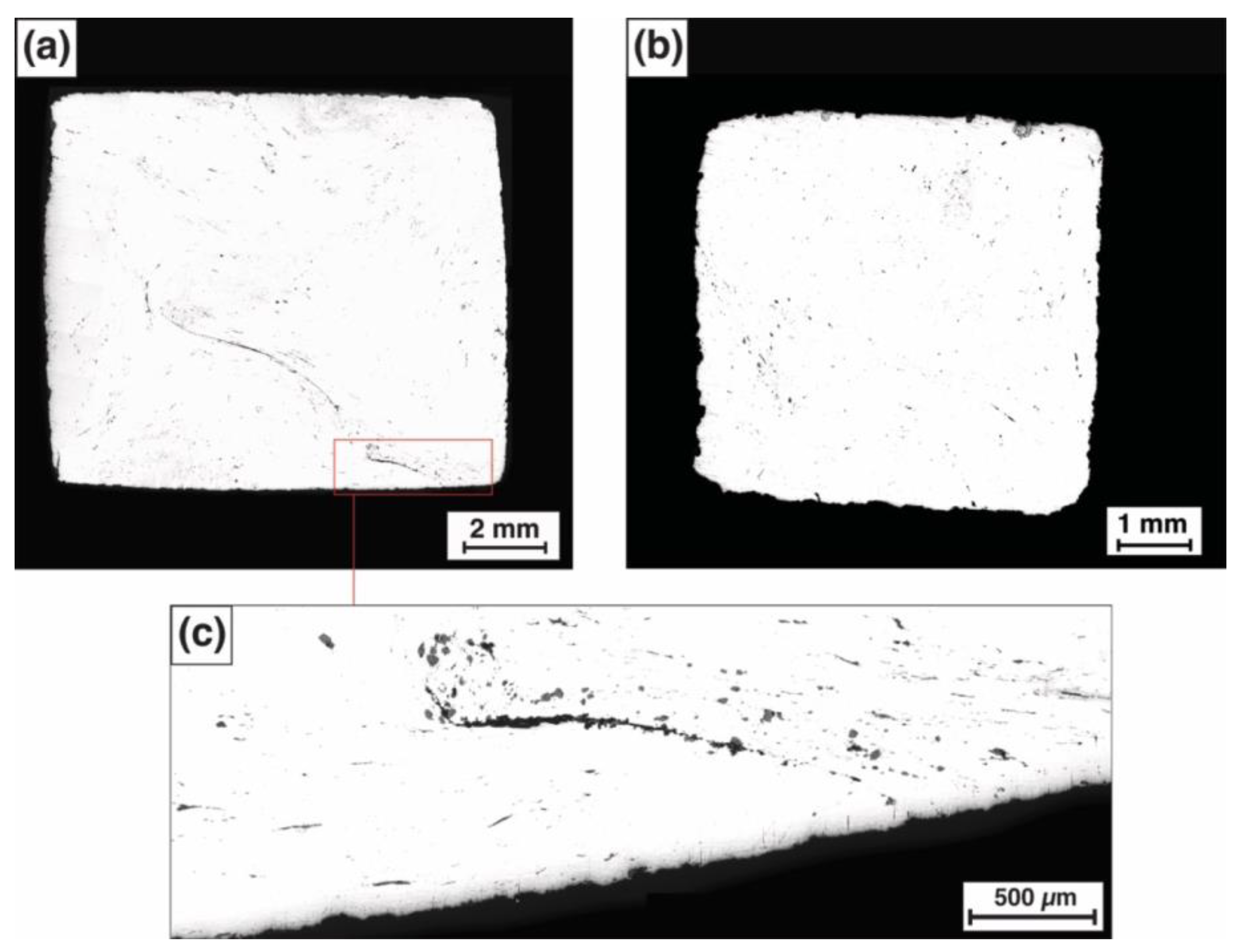
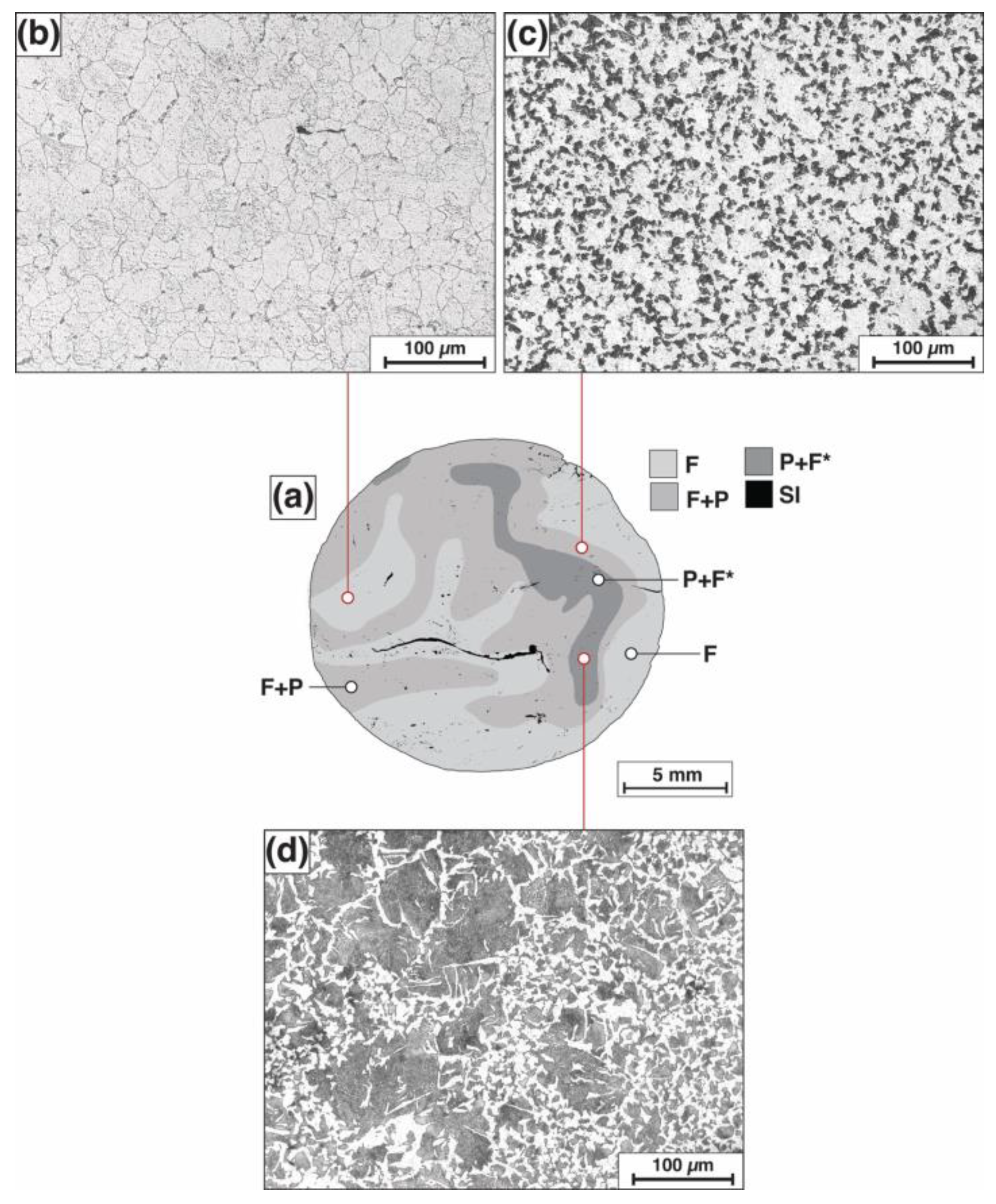
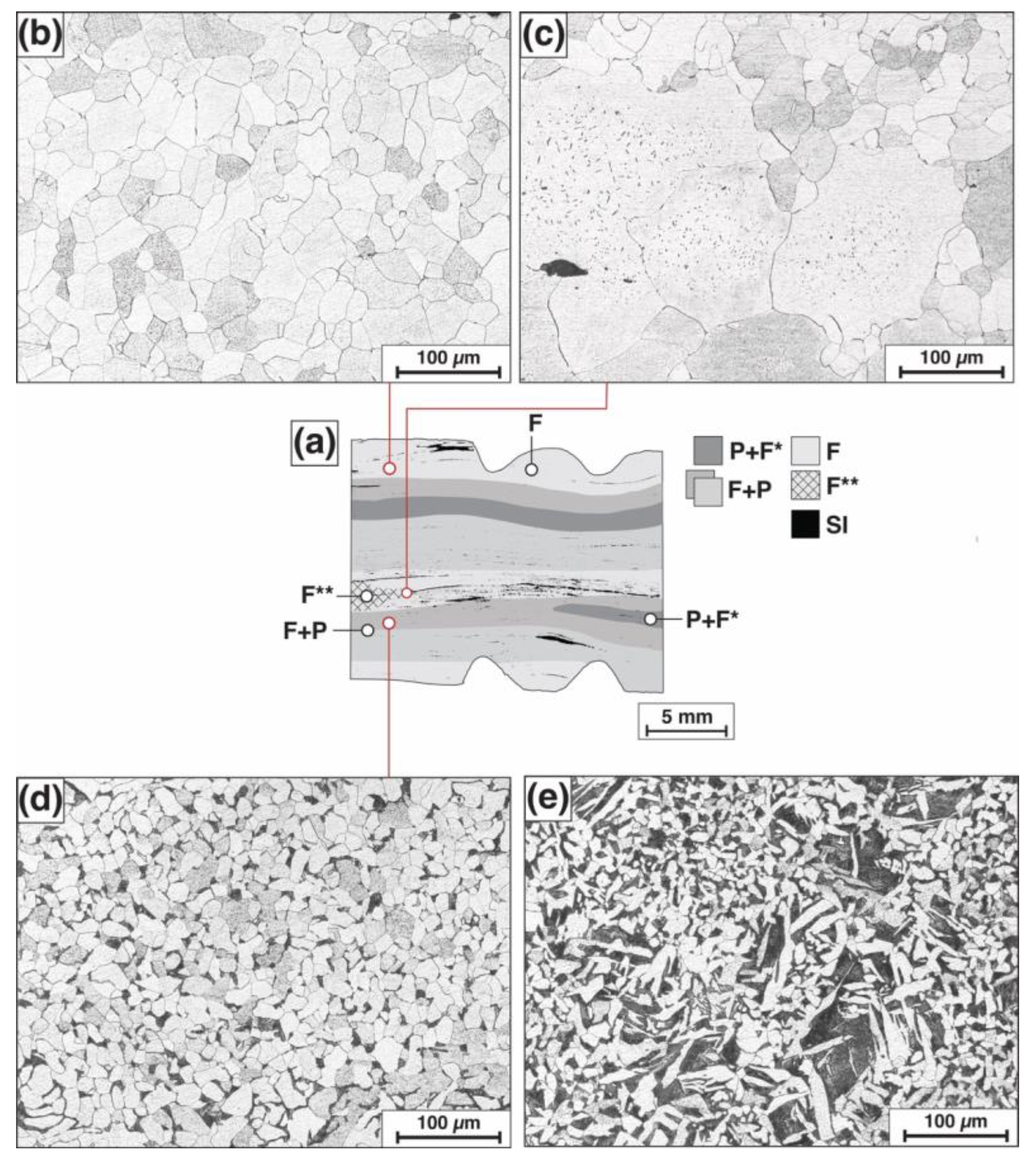
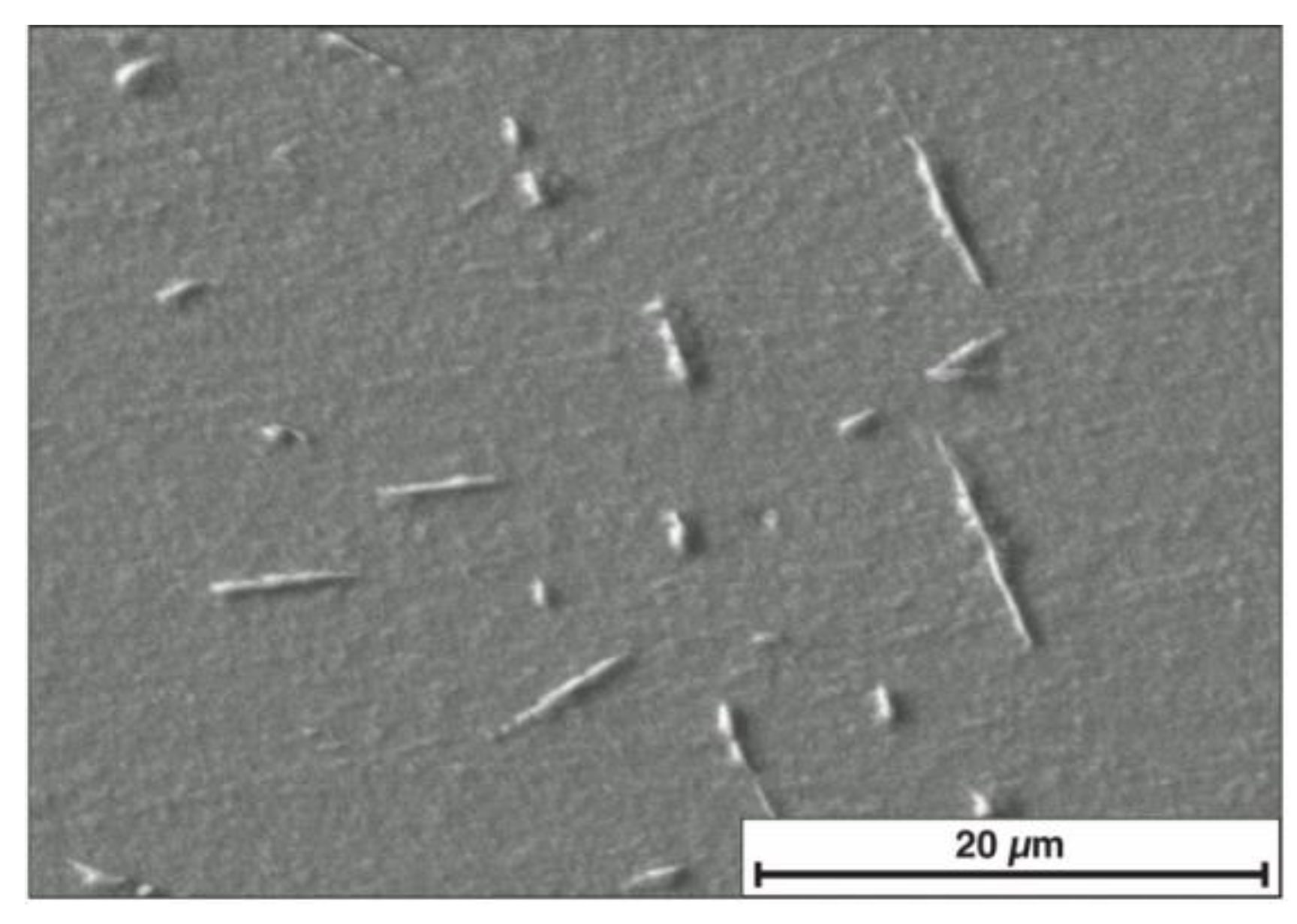
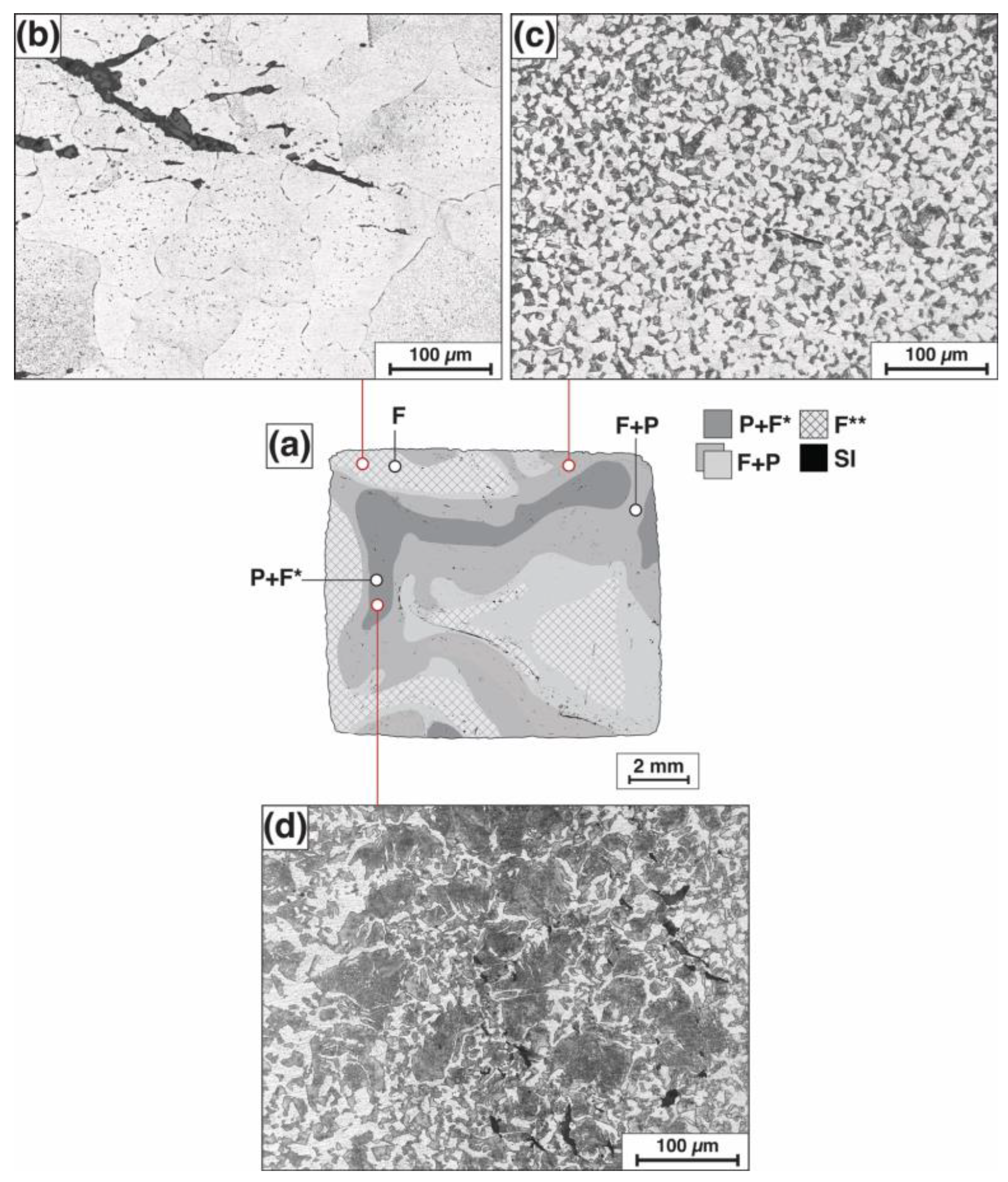
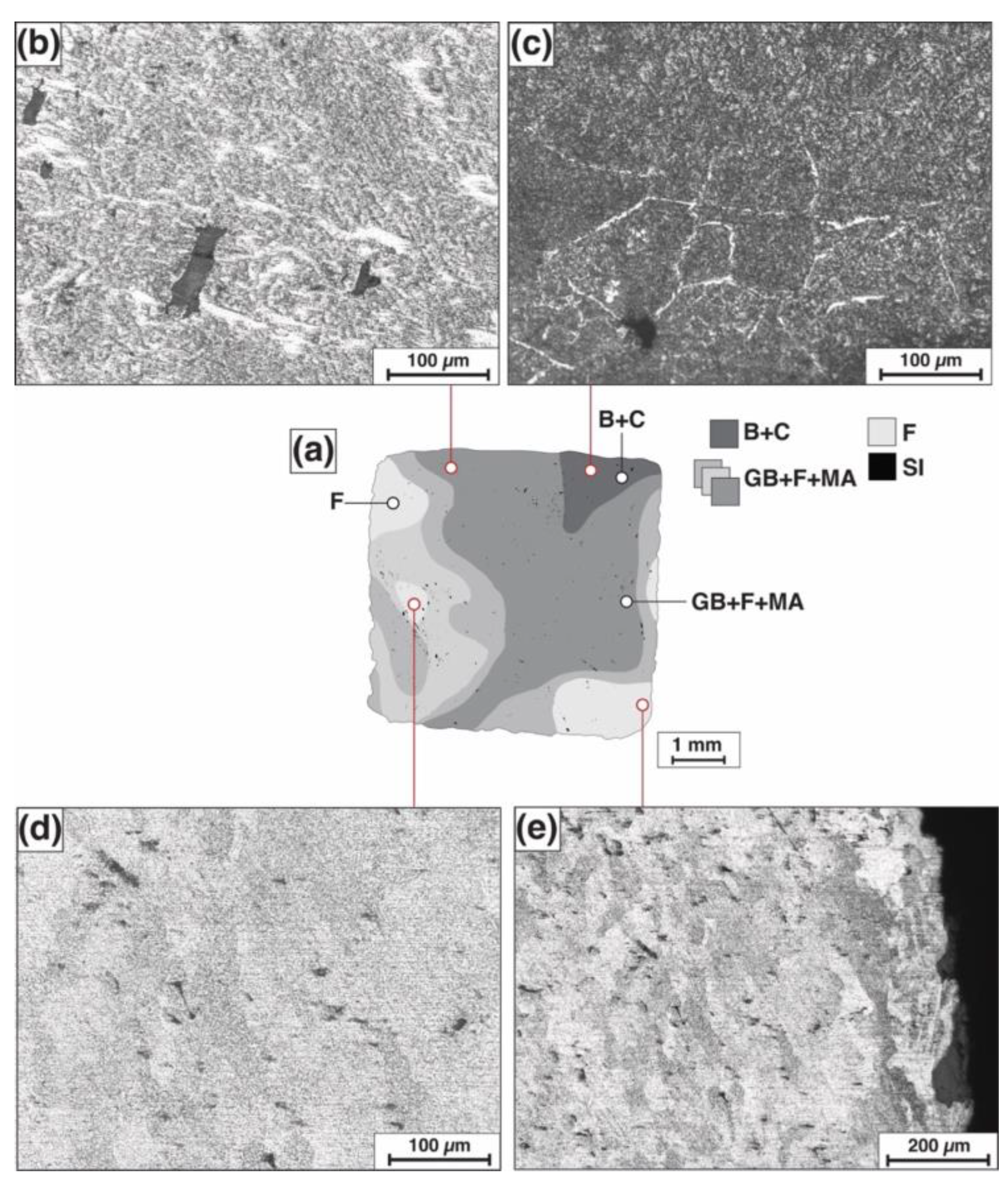
3.1. Metallographic Examination and Microhardness Measurements
3.2. Slag Inclusion Classification
3.3. Analysis of the Steel Technological Origin
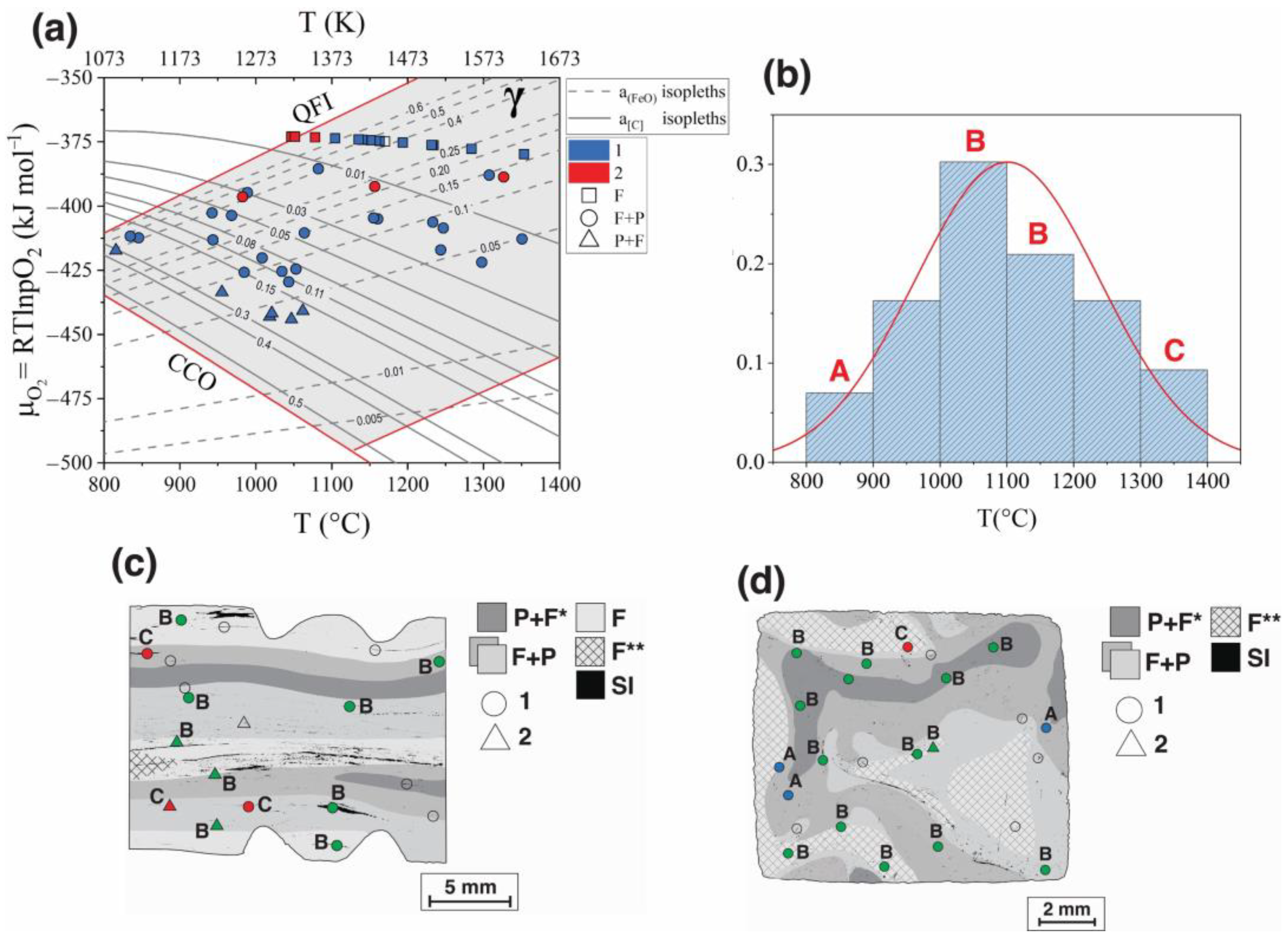
3.4. Thermodynamic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waldman, J. Hafted Weapons in Medieval and Renaissance Europe: The Evolution of European Staff Weapons between 1200 and 1650; Brill: Boston, MA, USA, 2005. [Google Scholar]
- Troso, M. Le Armi in Asta Delle Fanterie Europee (1000–1500); Istituto Geografico De Agostini: Novara, Italy, 1988. [Google Scholar]
- Žákovský, P. Medieval Awl-Pike from Žeranovice in Moravia. Acta Mil. Mediaev. XI 2015, 11, 209–214. [Google Scholar]
- Di Carpegna, N. Le Armi Odescalchi; Museo di Palazzo Venezia: Roma, Italy, 1976. [Google Scholar]
- Hošek, J. Omówienie wyników badań metalograficznych znalezisk z Lubrzy. In Castrum Lubenow. Tragiczne Dzieje Średniowiecznej WIEŻY w Lubrzy Michalak; Muzeum Archeologiczne Środkowego Nadodrza, Pękowice: Świdnica, Poland, 2022. [Google Scholar]
- Merico, P.; Faccoli, M.; Cornacchia, G. Analysis of Ancient Slag Inclusion-Metal Systems as a Method to Disclose Processing Thermo-Chemical Parameters: The Case Study of a Medieval Lombard Steel Bar from Northern Italy. Metall. Mater. Trans. B 2023, 54, 1408–1421. [Google Scholar] [CrossRef]
- Merico, P.; Faccoli, M.; Gotti, R.; Cornacchia, G. Archeometallurgical Study of a Renaissance Spearhead from Northern Italy. Metall. Mater. Trans. B 2023, 54, 82–100. [Google Scholar] [CrossRef]
- Cornacchia, G.; Faccoli, M.; Roberti, R. Metallurgical Investigation of a Steel Miner’s Chisel From Ponte Val Gabbia III Site. JOM 2015, 67, 260–271. [Google Scholar] [CrossRef]
- Tonelli, G.; Faccoli, M.; Gotti, R.; Roberti, R.; Cornacchia, G. Historical and Metallurgical Characterization of a “Falchion” Sword Manufactured in Caino (Brescia, Italy) in the Early 17th Century A.D. JOM 2016, 68, 2233–2249. [Google Scholar] [CrossRef]
- Bérard, E.; Dillmann, P.; Renaudeau, O.; Verna, C.; Toureille, V. Fabrication of a Suit of Armour at the End of Middle Ages: An Extensive Archaeometallurgical Characterization of the Armour of Laval. J. Cult. Herit. 2022, 53, 88–99. [Google Scholar] [CrossRef]
- Bérard, E.; Dillmann, P.; Disser, A.; Vega, E.; Verna, C.; Toureille, V. The Medieval Bombards of Meaux: Manufacturing Processes and Supply of the Metal. J. Archaeol. Sci. Rep. 2022, 41, 103307. [Google Scholar] [CrossRef]
- Bai, X.; Allègre, H.; Gosselin, M.; Dillmann, P.; Lopez, M.; Téreygeold, F.; Detallea, V. Impact of laser-induced breakdown spectroscopy implementation for the quantification of carbon content distribution in archaeological ferrous metals. Spectrochim. Acta Part B 2020, 172, 105964. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- L’Héritier, M.; Azéma, A.; Syvilay, D.; Delqué-Kolic, E.; Beck, L.; Guillot, I.; Bernard, M.; Dillmann, P. Notre-Dame de Paris: The First Iron Lady? Archaeometallurgical Study and Dating of the Parisian Cathedral Iron Reinforcements. PLoS ONE 2023, 18, e0280945. [Google Scholar] [CrossRef] [PubMed]
- Disser, A.; Dillmann, P.; Bourgain, C.; L’Héritier, M.; Vega, E.; Bauvais, S.; Leroy, M. Iron Reinforcements in Beauvais and Metz Cathedrals: From Bloomery or Finery? The Use of Logistic Regression for Differentiating Smelting Processes. J. Archaeol. Sci. 2014, 42, 315–333. [Google Scholar] [CrossRef]
- Dillmann, P.; L’Héritier, M. Slag Inclusion Analyses for Studying Ferrous Alloys Employed in French Medieval Buildings: Supply of Materials and Diffusion of Smelting Processes. J. Archaeol. Sci. 2007, 34, 1810–1823. [Google Scholar] [CrossRef]
- Charlton, M.F.; Blakelock, E.; Martinón-Torres, M.; Young, T. Investigating the Production Provenance of Iron Artifacts with Multivariate Methods. J. Archaeol. Sci. 2012, 39, 2280–2293. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Buchwald, V.F. Iron, Steel and Cast Iron before Bessemer: The Slag-Analytical Method and the Role of Carbon and Phosphorus; The Royal Danish Academy of Sciences and Letters: Copenhagen, Denmark, 2008; pp. 183–188. [Google Scholar]
- Buchwald, V.F.; Wivel, H. Slag Analysis as a Method for the Characterization and Provenancing of Ancient Iron Objects. Mater. Charact. 1998, 40, 73–96. [Google Scholar] [CrossRef]
- Tesser, E.; Silva, C.; Artigas, A.; Monsalve, A. Article Effect of Carbon Content and Intercritical Annealing on Microstructure and Mechanical Tensile Properties in FeCMnSiCr Trip-Assisted Steels. Metals 2021, 11, 1546. [Google Scholar] [CrossRef]
- De-Castro, D.; Eres-Castellanos, A.; Vivas, J.; Caballero, F.G.; San-Martín, D.; Capdevila, C. Morphological and Crystallographic Features of Granular and Lath-like Bainite in a Low Carbon Microalloyed Steel. Mater. Charact. 2022, 184, 111703. [Google Scholar] [CrossRef]
- Żabiński, G.; Gramacki, J.; Gramacki, A.; Stepanov, I.S.; Woźniak, M. Old Wine into New Wineskins? “Legacy Data” in Research on Roman Period East Germanic Iron Smelting. PLoS ONE 2023, 18, e0289771. [Google Scholar] [CrossRef] [PubMed]
- Ghiorso, M.S.; Gualda, G.A.R. An H2O–CO2 Mixed Fluid Saturation Model Compatible with Rhyolite-MELTS. Contrib. Mineral. Petrol. 2015, 169, 1–30. [Google Scholar] [CrossRef]
- Gualda, G.A.R.; Ghiorso, M.S.; Lemons, R.V.; Carley, T.L. Rhyolite-MELTS: A Modified Calibration of MELTS Optimized for Silica-Rich, Fluid-Bearing Magmatic Systems. J. Petrol. 2012, 53, 875–890. [Google Scholar] [CrossRef]




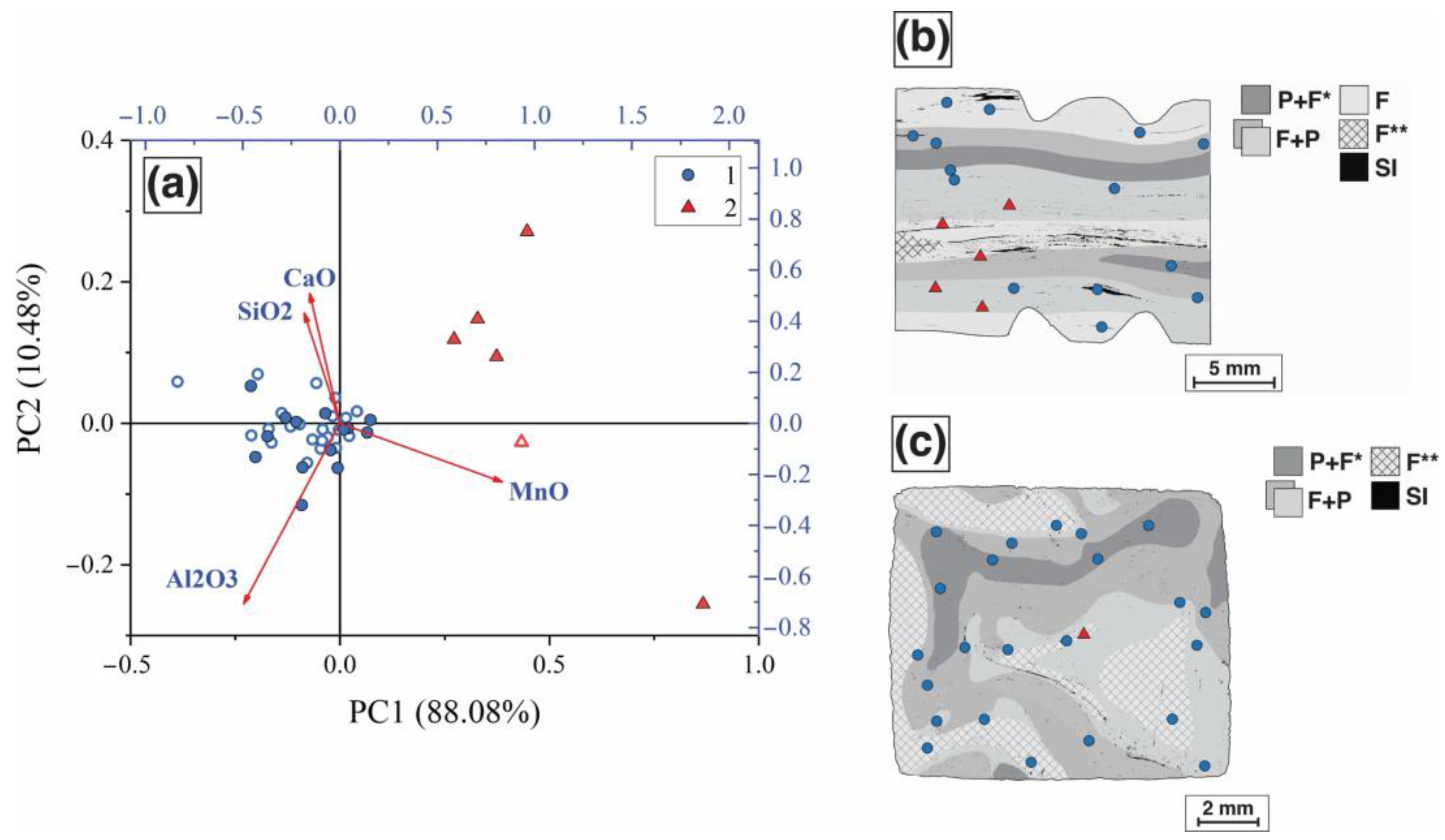
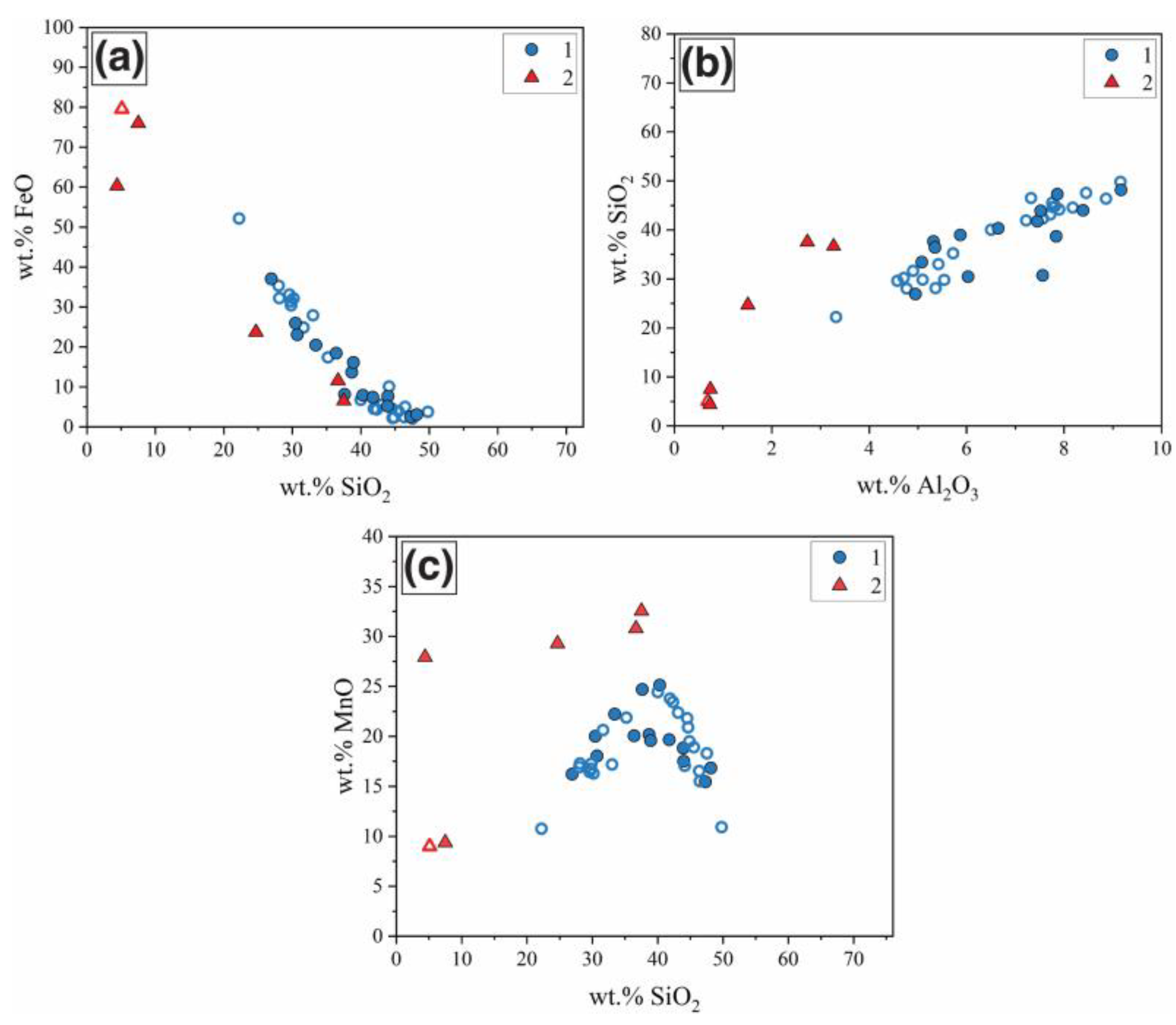


| Group | Na2O | MgO | Al2O3 | SiO2 | K2O | CaO | TiO2 | MnO | FeO | SO2 | P2O5 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.07 | 4.03 | 6.96 | 36.84 | 2.08 | 11.93 | 0.88 | 18.87 | 17.75 | 0.30 | 0.27 |
| 2 | 0.00 | 4.24 | 1.15 | 12.18 | 0.42 | 4.15 | 0.49 | 22.88 | 53.82 | 0.02 | 0.67 |
| Na2O | MgO | Al2O3 | SiO2 | K2O | CaO | TiO2 | MnO | FeO | SO2 | P2O5 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 0.00 | 2.60 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 19.46 | 77.93 | 0.00 | 0.00 |
| B | 1.04 | 2.61 | 6.84 | 36.77 | 2.23 | 12.64 | 0.00 | 16.13 | 20.51 | 0.00 | 1.22 |
| C | 2.44 | 5.53 | 7.82 | 44.89 | 2.29 | 14.51 | 0.73 | 19.50 | 2.31 | 0.00 | 0.00 |
| Parameter | Value | |
|---|---|---|
| Intercept | β0 | 5.22 |
| MgO | βMg | 0.13 |
| Al2O3 | βAl | −0.95 |
| SiO2 | βSi | 0.007 |
| P2O5 | βP | 0.16 |
| K2O | βK | −0.84 |
| CaO | βCa | 0.088 |
| MnO | βMn | 0.018 |
| Group | MgO** | Al2O3** | SiO2** | K2O** | CaO** | MnO** | P2O5** |
|---|---|---|---|---|---|---|---|
| 1 | 4.89 | 8.47 | 44.79 | 2.53 | 14.50 | 22.94 | 0.33 |
| Metal Microstructure | T (°C) | μO2 (kJ/mol) |
|---|---|---|
| F + F** | 1118 | −374 |
| F + P | 1084 | −411 |
| P + F* | 1095 | −442 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merico, P.; Faccoli, M.; Gotti, R.; Cornacchia, G. Exploring the Manufacturing Process of a Renaissance Breach Pike. Metals 2024, 14, 41. https://doi.org/10.3390/met14010041
Merico P, Faccoli M, Gotti R, Cornacchia G. Exploring the Manufacturing Process of a Renaissance Breach Pike. Metals. 2024; 14(1):41. https://doi.org/10.3390/met14010041
Chicago/Turabian StyleMerico, Paolomarco, Michela Faccoli, Roberto Gotti, and Giovanna Cornacchia. 2024. "Exploring the Manufacturing Process of a Renaissance Breach Pike" Metals 14, no. 1: 41. https://doi.org/10.3390/met14010041
APA StyleMerico, P., Faccoli, M., Gotti, R., & Cornacchia, G. (2024). Exploring the Manufacturing Process of a Renaissance Breach Pike. Metals, 14(1), 41. https://doi.org/10.3390/met14010041






