Mechanical Properties of Interfaces between Mg and SiC: An Ab Initio Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Interface Models
2.2. DFT Calculation Settings
2.3. Deformation Protocol
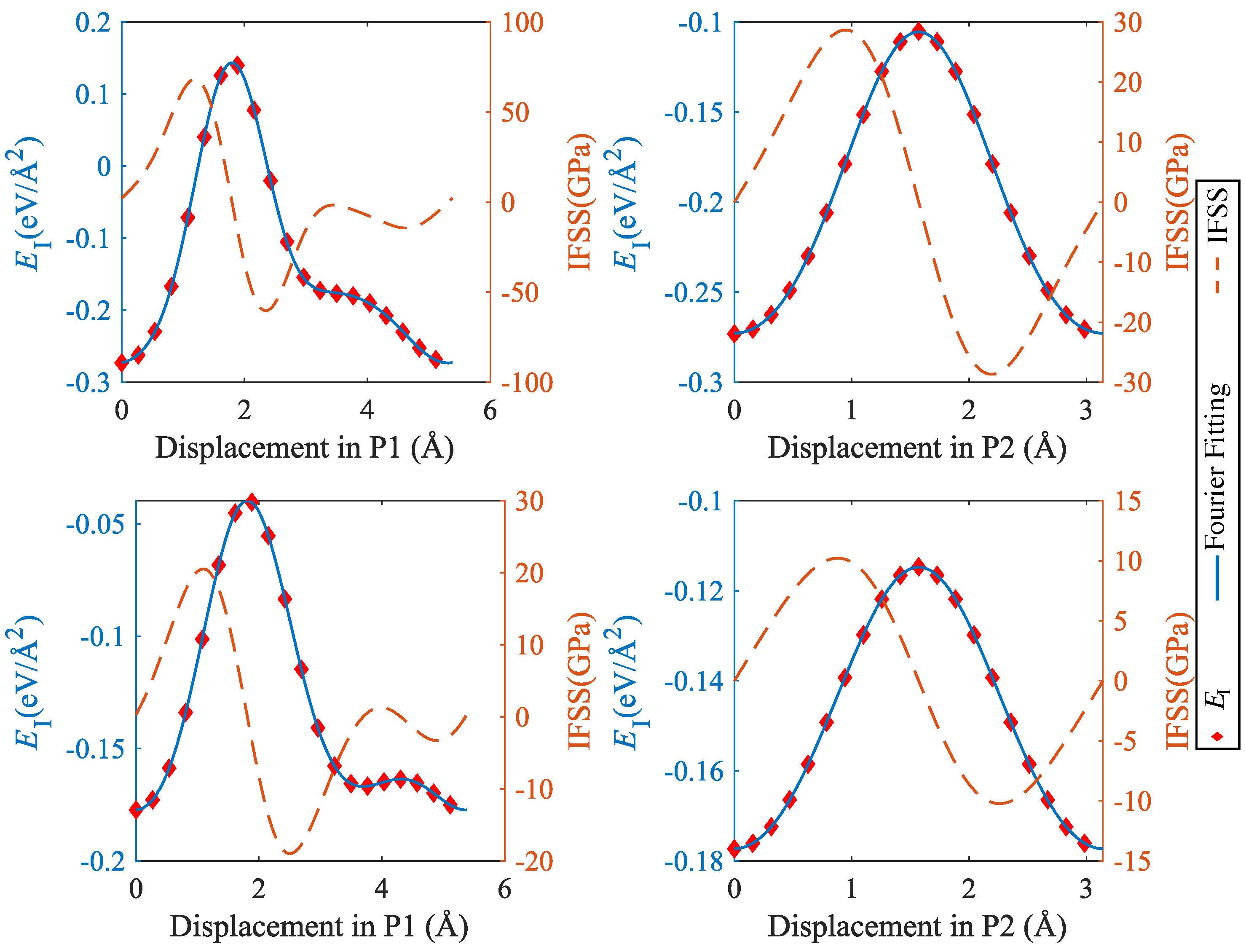
3. Results
3.1. Behavior under Interface-Normal Displacement
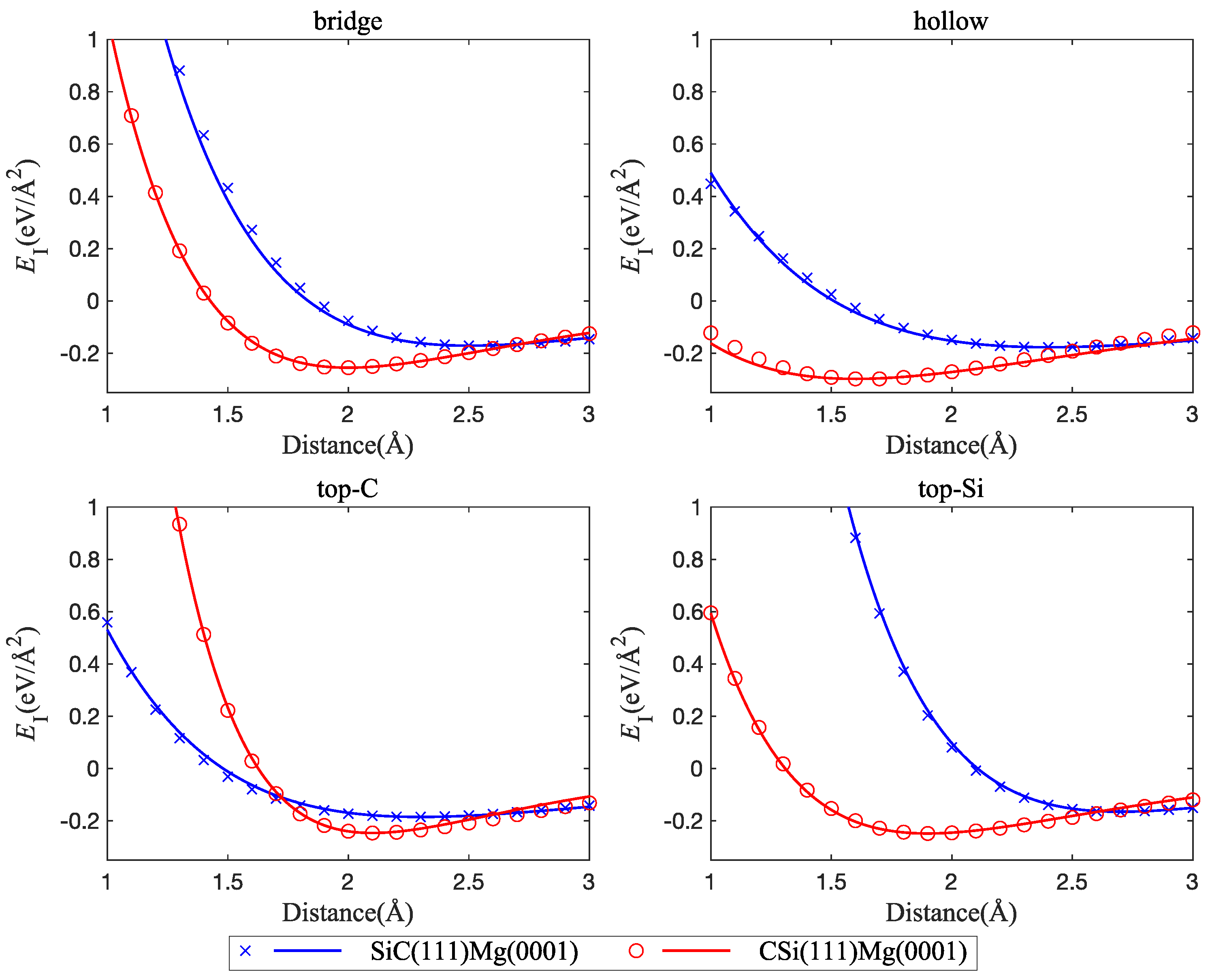
3.1.1. Effects of Polytype and Termination
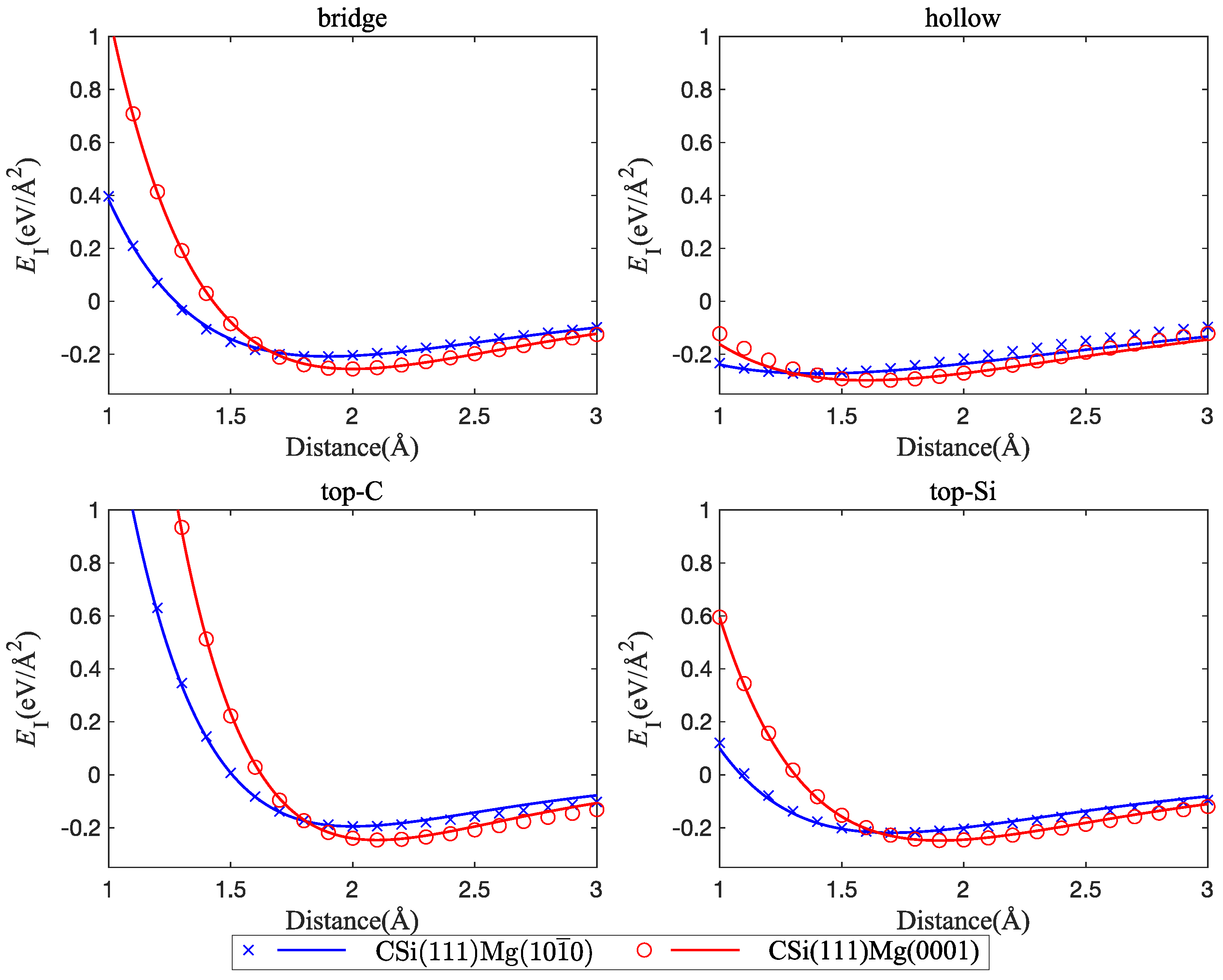
3.1.2. Effect of Mg Orientation
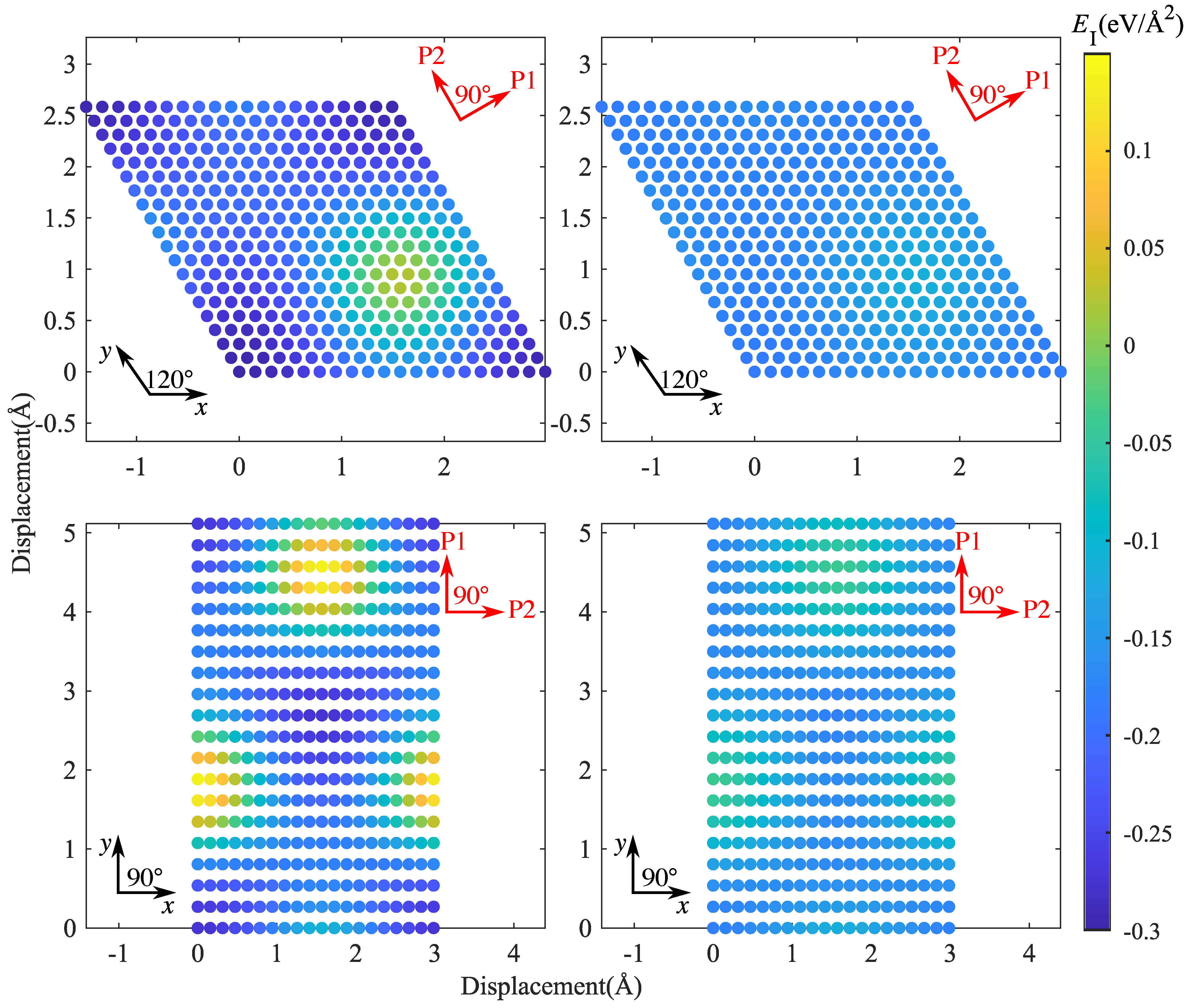
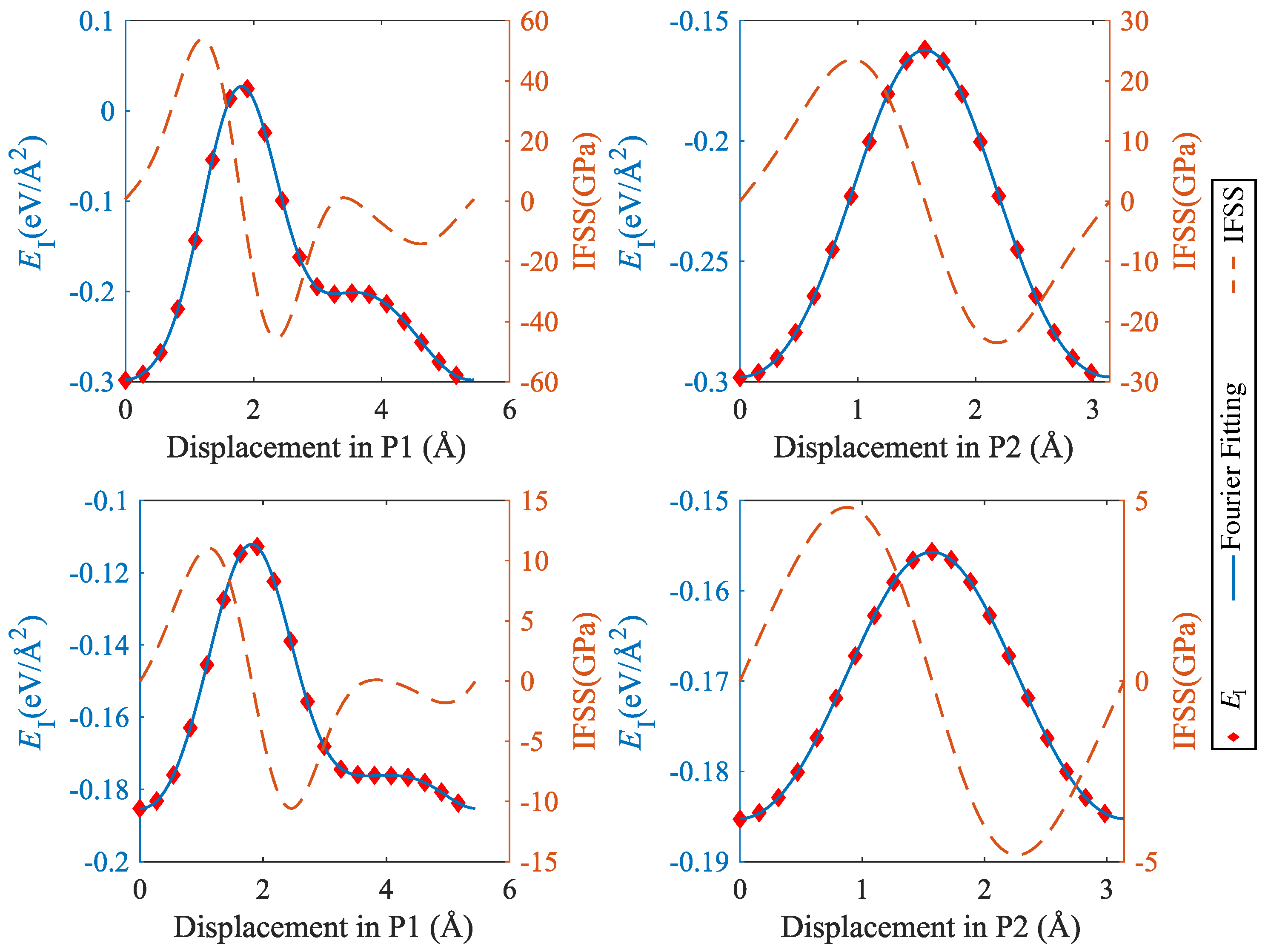
3.2. Interface-Parallel Displacement: Interface Energy Surface and Interface Shear Stress
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ferkel, H.; Mordike, B. Magnesium strengthened by SiC nanoparticles. Mater. Sci. Eng. A 2001, 298, 193–199. [Google Scholar] [CrossRef]
- Mondal, A.; Kumar, S. Impression creep behaviour of magnesium alloy-based hybrid composites in the longitudinal direction. Compos. Sci. Technol. 2008, 68, 3251–3258. [Google Scholar] [CrossRef]
- Ganguly, S.; Mondal, A. Influence of SiC nanoparticles addition on microstructure and creep behavior of squeeze-cast AZ91-Ca-Sb magnesium alloy. Mater. Sci. Eng. A 2018, 718, 377–389. [Google Scholar] [CrossRef]
- Huang, S.J.; Jeng, Y.R.; Semenov, V.; Dai, Y.Z. Particle size effects of silicon carbide on wear behavior of SiC p-reinforced magnesium matrix composites. Tribol. Lett. 2011, 42, 79–87. [Google Scholar] [CrossRef]
- Dey, A.; Pandey, K.M. Magnesium metal matrix composites-a review. Rev. Adv. Mater. Sci. 2015, 42, 58–67. [Google Scholar]
- Seshan, S.; Jayamathy, M.; Kailas, S.; Srivatsan, T.S. The tensile behavior of two magnesium alloys reinforced with silicon carbide particulates. Mater. Sci. Eng. A 2003, 363, 345–351. [Google Scholar] [CrossRef]
- Luo, X.; Zhao, K.; He, X.; Bai, Y.; De Andrade, V.; Zaiser, M.; An, L.; Liu, J. Evading strength and ductility trade-off in an inverse nacre structured magnesium matrix nanocomposite. Acta Mater. 2022, 228, 117730. [Google Scholar] [CrossRef]
- Luo, X.; Liu, J.; Zhang, L.; He, X.; Zhao, K.; An, L. Deformation and failure behavior of heterogeneous Mg/SiC nanocomposite under compression. J. Magnes. Alloys 2022, 10, 3433–3446. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, L.; He, X.; Liu, J.; Zhao, K.; An, L. Heterogeneous magnesium matrix nanocomposites with high bending strength and fracture toughness. J. Alloys Compd. 2021, 855, 157359. [Google Scholar] [CrossRef]
- Nasiri, S.; Yang, G.; Spiecker, E.; Li, Q. An Improved Approach to Manufacture Carbon Nanotube Reinforced Magnesium AZ91 Composites with Increased Strength and Ductility. Metals 2022, 12, 834. [Google Scholar] [CrossRef]
- Fathalian, M.; Postek, E.; Sadowski, T. Mechanical and Electronic Properties of Al (111)/6H-SiC Interfaces: A DFT Study. Molecules 2023, 28, 4345. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, S.; Greff, C.; Wang, K.; Yang, M.; Li, Q.; Moretti, P.; Zaiser, M. Multilayer structures of graphene and Pt nanoparticles: A multiscale computational study. Adv. Eng. Mater. 2020, 22, 2000207. [Google Scholar] [CrossRef]
- Nasiri, S.; Wang, K.; Yang, M.; Guénolé, J.; Li, Q.; Zaiser, M. Atomistic aspects of load transfer and fracture in CNT-reinforced aluminium. Materialia 2022, 22, 101376. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Nardelli, M.B.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens. Matter 2017, 29, 465901. [Google Scholar] [CrossRef] [PubMed]
- Giannozzi, P.; Baseggio, O.; Bonfà, P.; Brunato, D.; Car, R.; Carnimeo, I.; Cavazzoni, C.; De Gironcoli, S.; Delugas, P.; Ferrari Ruffino, F.; et al. Quantum ESPRESSO toward the exascale. J. Chem. Phys. 2020, 152, 154105. [Google Scholar] [CrossRef] [PubMed]
- Prandini, G.; Marrazzo, A.; Castelli, I.E.; Mounet, N.; Marzari, N. Precision and efficiency in solid-state pseudopotential calculations. NPJ Comput. Mater. 2018, 4, 72. [Google Scholar] [CrossRef]
- Lejaeghere, K.; Bihlmayer, G.; Björkman, T.; Blaha, P.; Blügel, S.; Blum, V.; Caliste, D.; Castelli, I.E.; Clark, S.J.; Dal Corso, A.; et al. Reproducibility in density functional theory calculations of solids. Science 2016, 351, aad3000. [Google Scholar] [CrossRef]
- Klimeš, J.; Bowler, D.R.; Michaelides, A. Van der Waals density functionals applied to solids. Phys. Rev. B 2011, 83, 195131. [Google Scholar] [CrossRef]
- Ding, Z.G.; Liu, W.; Li, S.; Zhang, D.L.; Zhao, Y.H.; Lavernia, E.J.; Zhu, Y.T. Contribution of van der Waals forces to the plasticity of magnesium. Acta Mater. 2016, 107, 127–132. [Google Scholar] [CrossRef]
- Muzyk, M.; Pakielaa, Z.; Kurzydlowski, K. Generalized stacking fault energy in magnesium alloys: Density functional theory calculations. Scr. Mater. 2012, 66, 219–222. [Google Scholar] [CrossRef]
- Shang, S.; Wang, W.; Zhou, B.C.; Wang, Y.; Darling, K.; Kecskes, L.; Mathaudhu, S.; Liu, Z. Generalized stacking fault energy, ideal strength and twinnability of dilute Mg-based alloys: A first-principles study of shear deformation. Acta Mater. 2014, 67, 168–180. [Google Scholar] [CrossRef]
- Vitek, V. Intrinsic stacking faults in body-centred cubic crystals. Philos. Mag. 1968, 18, 773–786. [Google Scholar] [CrossRef]
- Tang, J.J.; Yang, X.B.; OuYang, L.; Zhu, M.; Zhao, Y.J. A systematic first-principles study of surface energies, surface relaxation and Friedel oscillation of magnesium surfaces. J. Phys. D Appl. Phys. 2014, 47, 115305. [Google Scholar] [CrossRef]
- Li, Q.; Qiu, S.; Wu, C.; Lau, K.T.; Sun, C.; Jia, B. Computational investigation of MgH2/graphene heterojunctions for hydrogen storage. J. Phys. Chem. C 2021, 125, 2357–2363. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, M.; Zaiser, M.; Yu, S. Enhancing dehydrogenation performance of MgH2/graphene heterojunctions via noble metal intercalation. Int. J. Hydrogen Energy 2023, 48, 16733–16744. [Google Scholar] [CrossRef]
- Han, B.; Jia, Y.; Wang, J.; Xiao, X.; Chen, L.; Sun, L.; Du, Y. The structural, energetic and dehydrogenation properties of pure and Ti-doped Mg (0001)/MgH2(110) interfaces. J. Mater. Chem. A 2023, 11, 26602–26616. [Google Scholar] [CrossRef]
- Ding, Z.; Li, Y.; Yang, H.; Lu, Y.; Tan, J.; Li, J.; Li, Q.; Shaw, L.L.; Pan, F. Tailoring MgH2 for hydrogen storage through nanoengineering and catalysis. J. Magnes. Alloys 2022, 10, 2946–2967. [Google Scholar] [CrossRef]
 Si atoms,
Si atoms,  C atoms,
C atoms,  Mg atoms; left: CSi(0001)Mg(0001), center: CSi(111)Mg(0001), right: CSi(111)Mg(100).
Mg atoms; left: CSi(0001)Mg(0001), center: CSi(111)Mg(0001), right: CSi(111)Mg(100).
 Si atoms,
Si atoms,  C atoms,
C atoms,  Mg atoms; left: CSi(0001)Mg(0001), center: CSi(111)Mg(0001), right: CSi(111)Mg(100).
Mg atoms; left: CSi(0001)Mg(0001), center: CSi(111)Mg(0001), right: CSi(111)Mg(100).




| Structure | Distance | Interface | Interface Separation | |
|---|---|---|---|---|
| (Å) | Energy (eV/Å2) | Stress (GPa) | ||
| Pure Mg | Mg(0001) | 2.7 | −0.060500 | 4.460038 |
| Mg(100) | 0.9 | −0.108209 | 6.484934 | |
| SiC(0001)Mg(0001) | Bridge | 2.6 | −0.169673 | 14.049061 |
| Hollow | 2.4 | −0.174717 | 11.654373 | |
| Top-C | 2.3 | −0.187171 | 13.768239 | |
| Top-Si | 2.7 | −0.161086 | 16.110159 | |
| SiC(111)Mg(0001) | Bridge | 2.5 | −0.171062 | 15.122863 |
| Hollow | 2.4 | −0.177111 | 11.729763 | |
| Top-C | 2.3 | −0.185225 | 13.320760 | |
| Top-Si | 2.7 | −0.165431 | 16.431664 | |
| SiC(111)Mg(100) | Bridge | 2.4 | −0.154134 | 12.344458 |
| Hollow | 2.1 | −0.168706 | 11.051431 | |
| Top-C | 2.0 | −0.177307 | 13.329418 | |
| Top-Si | 2.6 | −0.141755 | 13.083605 | |
| CSi(0001)Mg(0001) | Bridge | 2.0 | −0.237623 | 25.532947 |
| Hollow | 1.7 | −0.269242 | 19.127228 | |
| Top-C | 2.1 | −0.239356 | 30.143036 | |
| Top-Si | 2.0 | −0.222081 | 21.714480 | |
| CSi(111)Mg(0001) | Bridge | 2.0 | −0.255329 | 26.274945 |
| Hollow | 1.6 | −0.298127 | 21.691221 | |
| Top-C | 2.1 | −0.246606 | 30.672501 | |
| Top-Si | 1.9 | −0.248441 | 24.758893 | |
| CSi(111)Mg(100) | Bridge | 1.9 | −0.208181 | 19.599136 |
| Hollow | 1.4 | −0.273121 | 17.279160 | |
| Top-C | 2.0 | −0.195017 | 23.277400 | |
| Top-Si | 1.7 | −0.219156 | 21.113666 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Z.; Nasiri, S.; Yang, M.; Zaiser, M. Mechanical Properties of Interfaces between Mg and SiC: An Ab Initio Study. Metals 2024, 14, 467. https://doi.org/10.3390/met14040467
Yao Z, Nasiri S, Yang M, Zaiser M. Mechanical Properties of Interfaces between Mg and SiC: An Ab Initio Study. Metals. 2024; 14(4):467. https://doi.org/10.3390/met14040467
Chicago/Turabian StyleYao, Zhipeng, Samaneh Nasiri, Mingjun Yang, and Michael Zaiser. 2024. "Mechanical Properties of Interfaces between Mg and SiC: An Ab Initio Study" Metals 14, no. 4: 467. https://doi.org/10.3390/met14040467





