Quantitative Microstructure of Multiphase Al-Zn-Si-(Mg) Coatings and Their Effects on Sacrificial Protection for Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Accelerated Corrosion Test
- A configuration in which, prior to the corrosion test, coated plates of 100 × 200 mm2 surface area were scratched, until the steel was revealed, by a tool of 2 and 3 mm width, and the corrosion test was carried out until the appearance of the red rust;
- A configuration in which the plates of 100 × 50 mm2 with only 1 mm scratches were exposed for 1 week to examine the initial corrosion mechanisms and formation of corrosion products.
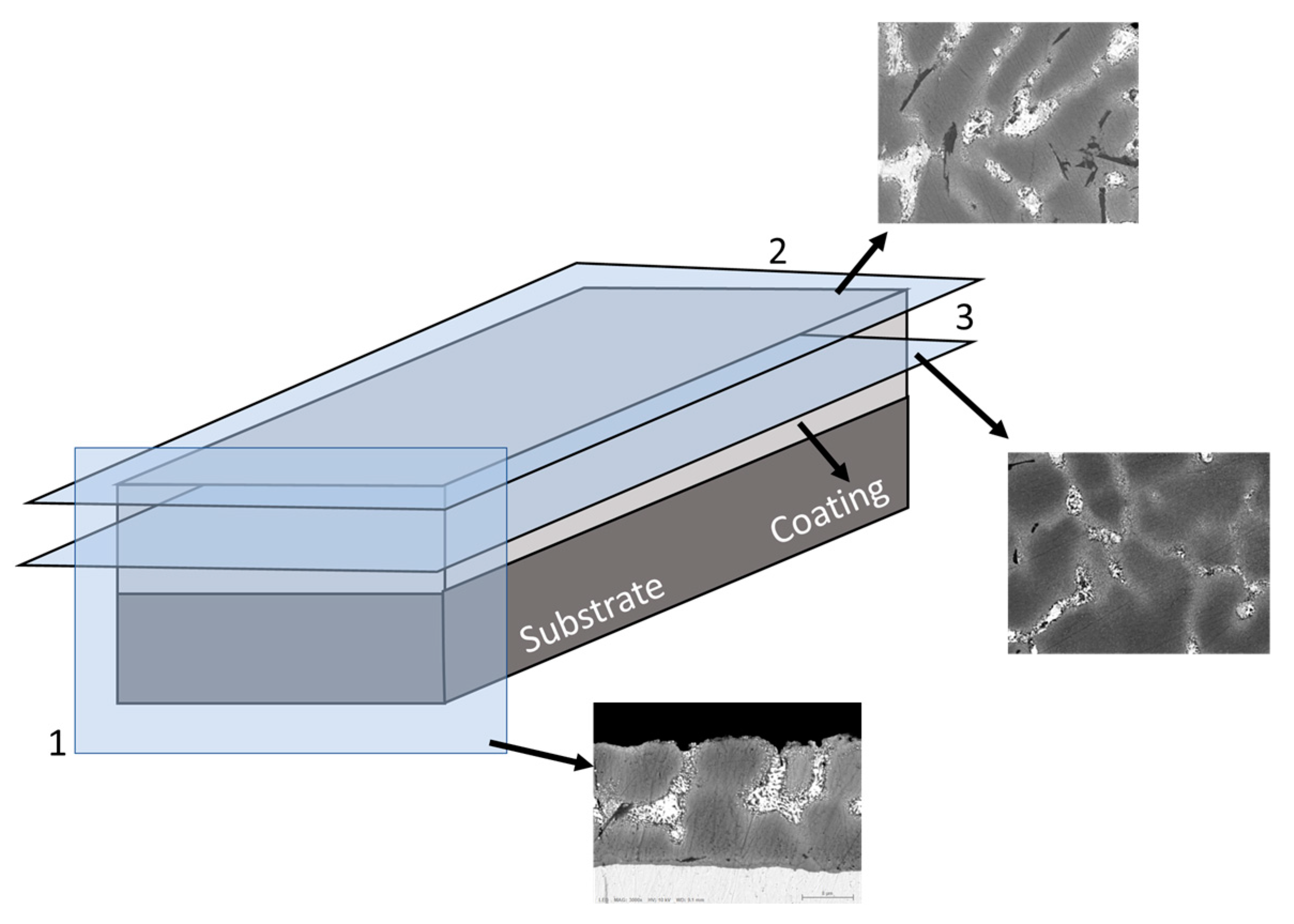
2.3. Microstructure and Corrosion Products Analyses
2.4. Quantitative Microstructure Description Using Aphelion Software
2.4.1. Phase Composition Set Up
2.4.2. Automatic Phase Attribution and Microstructure Analysis
3. Results and Discussion
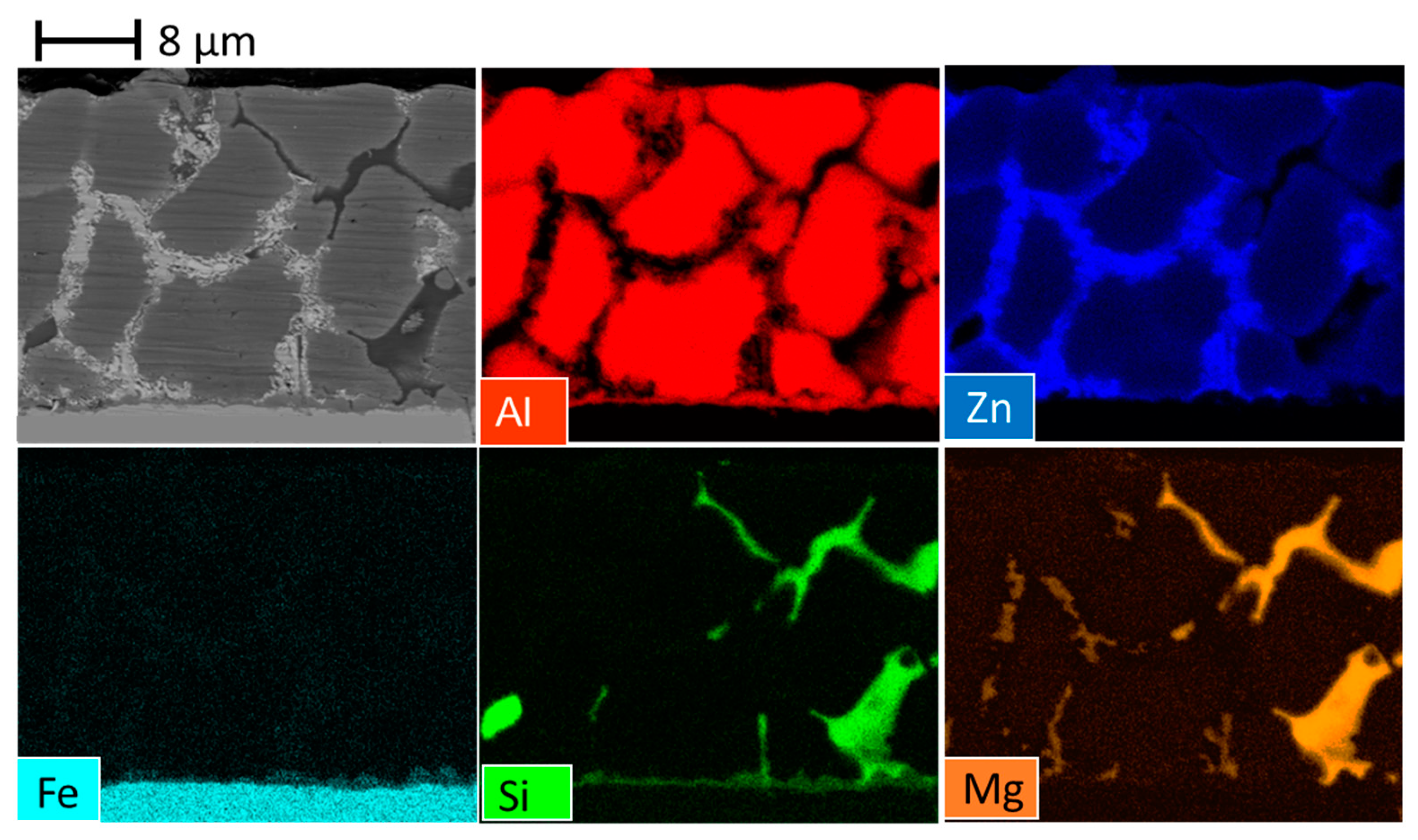
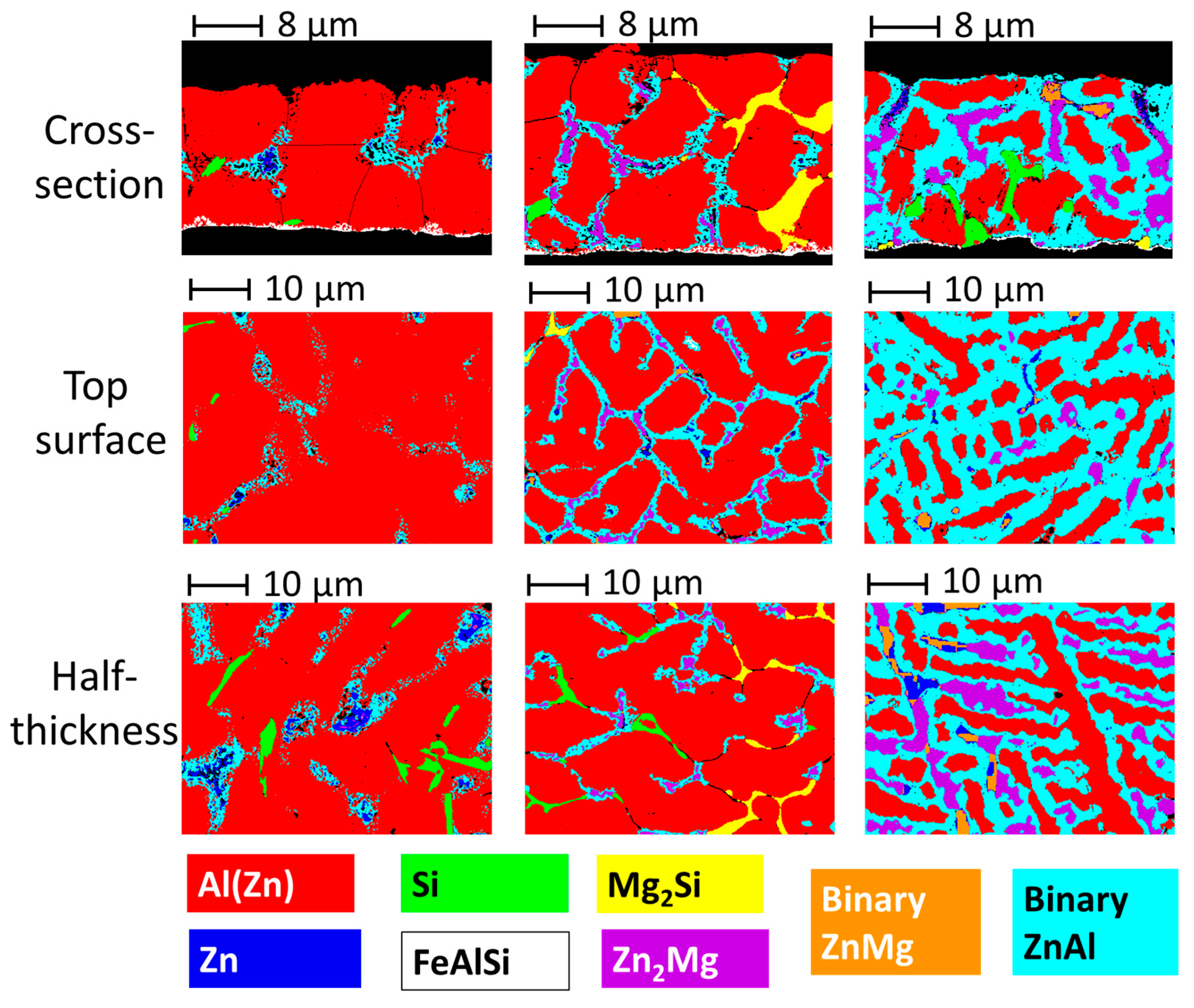
3.1. Quantitative Microstructures of Three Coatings
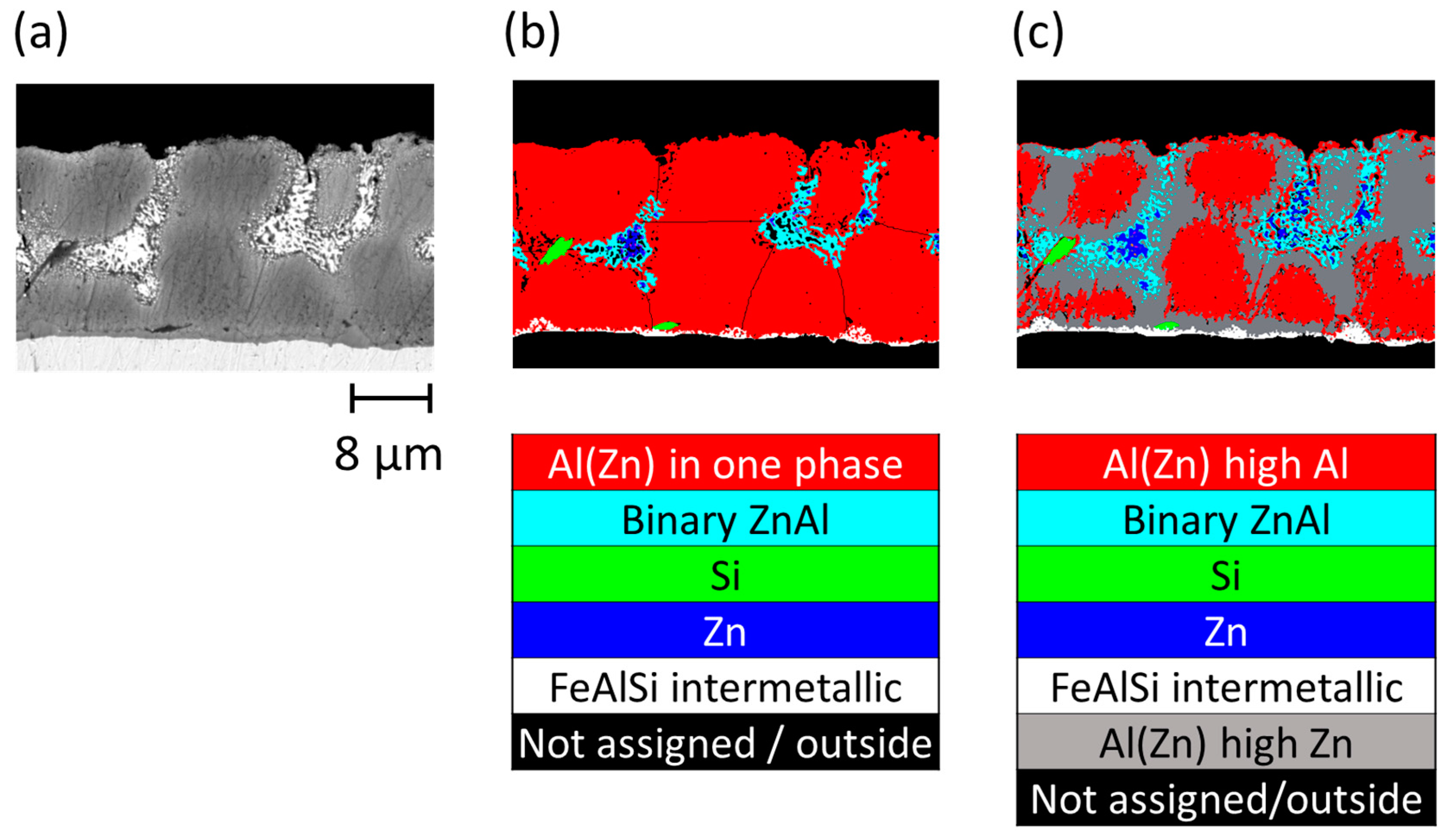
3.2. Al-Rich Dendrites on AZ
3.3. Accelerated Corrosion Tests
3.4. Use of Quantitative Microstructure Data to Understand Corrosion of AlZnSi(Mg) Coatings
3.4.1. Sacrificial Capacity of Different Phases Composing the Coatings Versus Steel
3.4.2. Percolation Approach to Sacrificial Protection by Quaternary AlZnSi(Mg) Coatings
4. Conclusions
- All the coatings contain a big fraction of Al(Zn) dendrites. For the AZ coating, a gradient of aluminum composition was observed on this phase, varying from 70 wt.% in the center to 40 wt.% at the edges. For the AZM and L-AZM, with the presence of Mg, the composition was more homogeneous, around 60 wt.%.
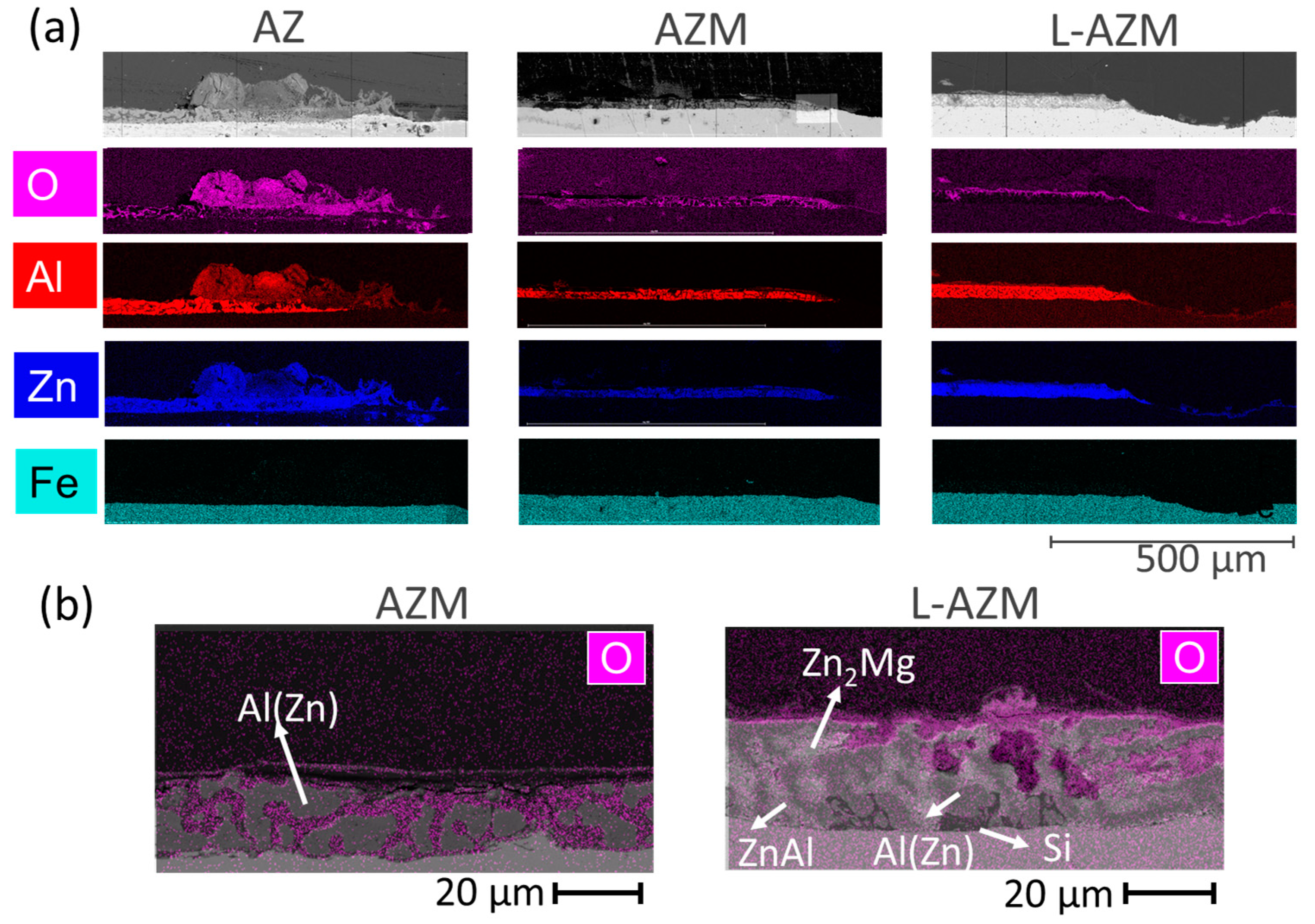
- The interdendritic phases for the AZ coating are as follows: binary ZnAl, pure Si needles, and pure Zn phase.
- The addition of Mg to the AZ coating leads to the formation of new interdendritic phases, Zn2Mg and Mg2Si, as well as an increase in the overall quantity of sacrificial phases.
- The reduction in the amount of Al results in the formation of a binary Zn-Mg phase, as well as almost complete suppression of the formation of Mg2Si, with a significant increase in the amount of interdendritic phases, especially the binary ZnAl.
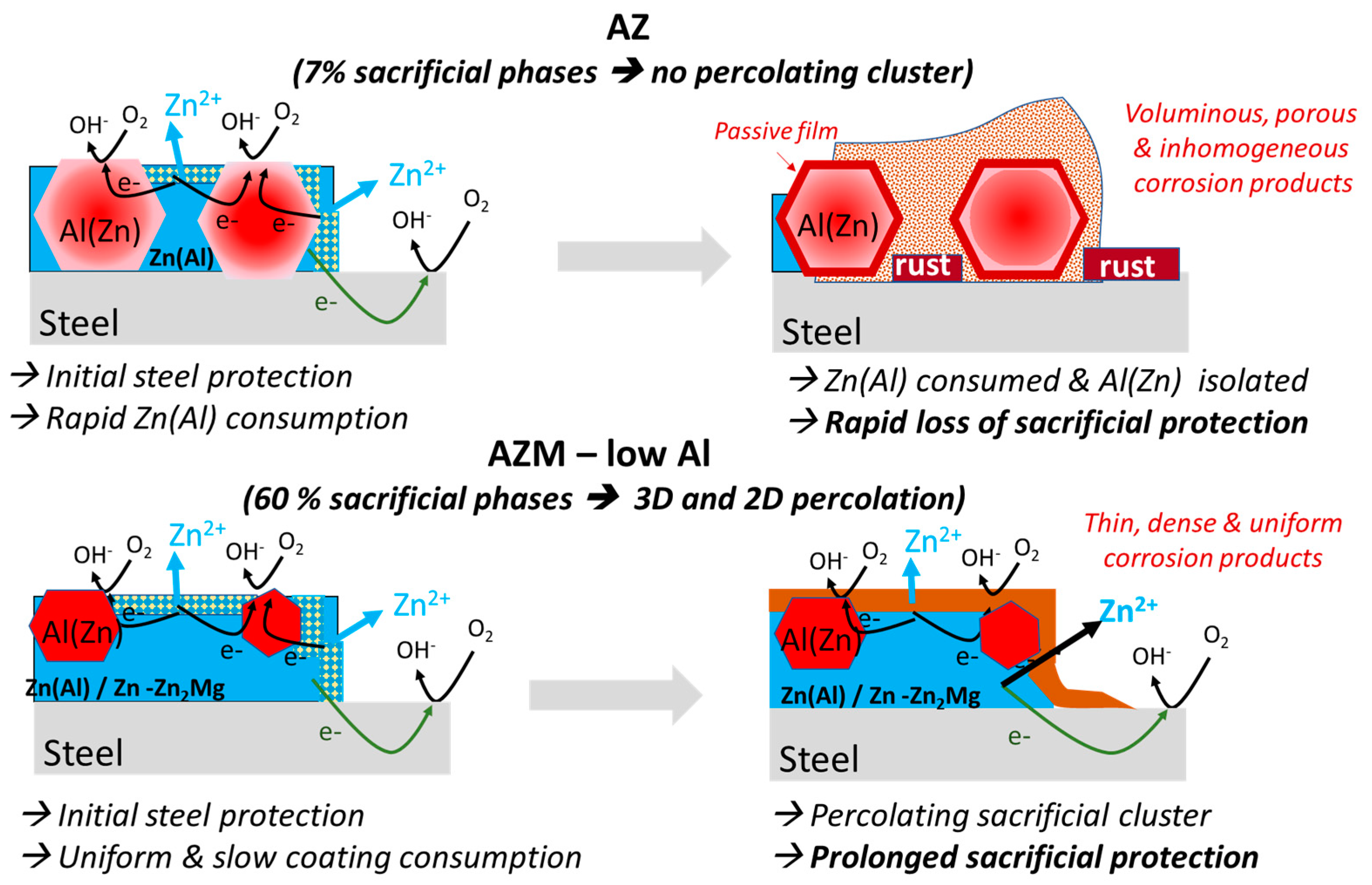
- Quantitative analysis suggests that the interdendritic phases form a 3D percolating cluster in the AZM and L-AZM coatings, but not in AZ. Percolation of sacrificial phases should improve the sacrificial capacity of the coating as it shares the anodic current more homogeneously, reducing the corrosion rate. From a percolation approach, the L-AZM coating is expected to demonstrate the best performance for the steel protection.
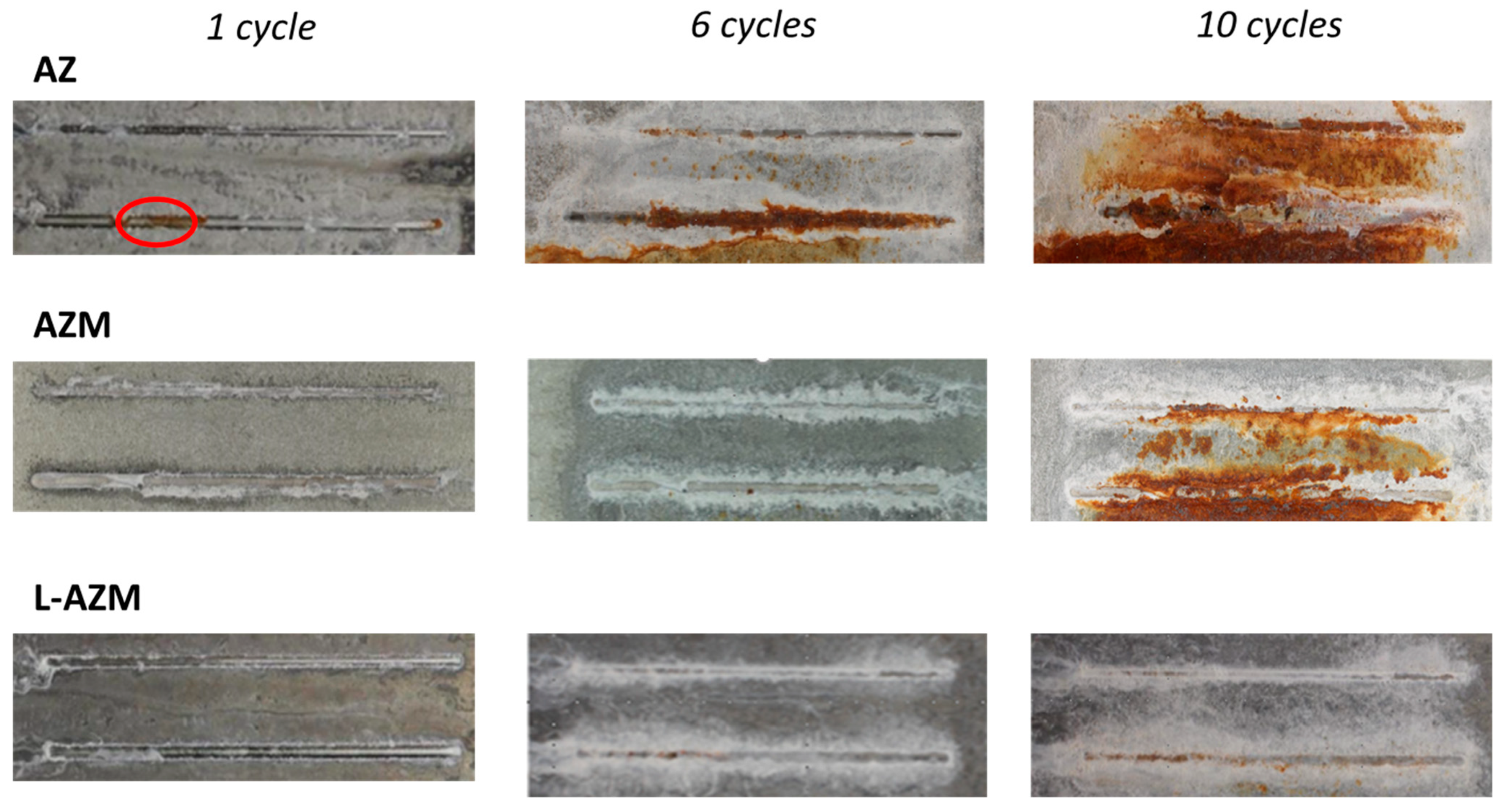
- Accelerated corrosion tests of the samples with scratches until the steel substrate confirmed the predictions from the percolation model. L-AZM has the best sacrificial protection of scratches, while AZ has the worst. The AZM took a longer time to begin the formation of red rust in comparison to AZ; however, once it was formed, it propagated quickly.
- Combined SEM-BSE and EDX analyses of the surface and the cross-sections near the scratch confirmed the most homogeneous deposition of corrosion products in the scratch for the sample L-AZM and the lowest for the AZM. Furthermore, voluminous corrosion products were formed on the coating for the AZ sample.
- Corrosion of the AZ coating occurred not only in the interdendritic zones, but the dendrites were also partially corroded. A strong gradient of composition was found inside the dendritic phase before corrosion, with a Zn fraction of 40% at the borders and 20% in the center. The dendritic skeleton that remained after corrosion had the composition of the central part, indicating that the corroded zones were the areas with a higher amount of Zn. Thus, the dendritic phase should be considered in two phases: more and less rich in Al.
- Percolation, though it has the beneficial effect of reducing the anodic current, can facilitate the formation of corrosion pits until the substrate when there are not enough sacrificial phases and no formation of protective corrosion products. This increases the cathodic zones, changing the cathodic to anodic areas ratio and thus further increasing the corrosion rate of the coating. The formation of these corrosion paths was confirmed by the cross-sectional view. A more detailed analysis of the role and nature of the corrosion products will be presented in further publications.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koch, G. 1—Cost of corrosion. In Trends in Oil and Gas Corrosion Research and Technologies; El-Sherik, A.M., Ed.; Woodhead Publishing Series in Energy; Woodhead Publishing: Boston, FL, USA, 2017; pp. 3–30. [Google Scholar] [CrossRef]
- Bierwagen, G.P. Reflections on corrosion control by organic coatings. Prog. Org. Coat. 1996, 28, 43–48. [Google Scholar] [CrossRef]
- Spennemann, D.H.R. Stanislas Sorel and the origins of cold galvanising. Trans. IMF 2021, 99, 115–120. [Google Scholar] [CrossRef]
- Marder, A.; Goodwin, F. The Metallurgy of Zinc Coated Steels; Elsevier: Cambridge, MA, USA, 2023. [Google Scholar]
- Borzillo, A.R.; Crowley, J.E.; Horton, J.B. Ferrous Metal Article Coated with an Aluminum Zinc Alloy. U.S. Patent US3343930A, 26 September 1967. [Google Scholar]
- Moreira, A.R.; Panossian, Z.; Camargo, P.; Moreira, M.F.; da Silva, I.; de Carvalho, J.R. Zn/55Al coating microstructure and corrosion mechanism. Corros. Sci. 2006, 48, 564–576. [Google Scholar] [CrossRef]
- Monnoyer, M.; Corlu, B.; Amorim, T.M.; Dosdat, L.; Dieu, C. Interest of new generation Zn-Al3.7-Mg3.0 coatings for the industry and construction market. In Proceedings of the 10th International Conference on Zinc and Zinc Alloy Coated Steel Sheet (Galvatech 2015), Toronto, ON, Canada, 31 May–4 June 2015; Association for Iron & Steel Technology: Warrendale, PA, USA, 2015; pp. 231–238. [Google Scholar]
- Luba, M.; Mikołajczyk, T.; Mikolajczyk, T.; Pierozynski, B. Comparison of the Corrosion Resistance of Commercial coated steel and hot-dip Zn Coatings under Changing Environmental Conditions. Int. J. Electrochem. Sci. 2019, 14, 10306–10317. [Google Scholar] [CrossRef]
- Tsujimura, T.; Komatsu, A.; Andoh, A. Influence of Mg content in coating layer and coating structure on corrosion resistance of hot-dip Zn–Al–Mg–Si alloy coated steel sheet. In Proceedings of the Galvatech ’01, International Conference on Zinc and Zinc Alloy Coated Steel, Brussels, Belgium, 26–28 June 2001; pp. 145–152. [Google Scholar]
- Wang, S.; Zhou, W.; Chen, S.; Ma, R.; Du, A.; Zhao, X.; Fan, Y.; Li, G. Study on corrosion behavior II of hot-dipped Zn-6Al-3Mg alloy coating: Surface oxide layer and ternary eutectic corrosion. J. Alloys Compd. 2024, 1008, 176534. [Google Scholar] [CrossRef]
- Tanaka, S.; Honda, K.; Takahashi, A.; Morimoto, Y.; Kurosaki, M.; Shindo, H.; Nishimura, K.; Sugiyama, M. The performance of Zn–Al–Mg–Si hot-dip galvanized steel sheet. In Proceedings of the Galvatech ’01, International Conference on Zinc and Zinc Alloy Coated Steel, Brussels, Belgium, 26–28 June 2001; pp. 153–160. [Google Scholar]
- Morimoto, Y.; Honda, K.; Nishimura, K.; Tanaka, S.; Takahashi, A.; Scindo, H.; Kurosaki, M. Excellent corrosion-resistant Zn–Al–Mg–Si-Si alloy hot-dip galvanized steel sheet “Super Dyma”. Nippon Steel Tech. Rep. 2003, 87, 24–26. [Google Scholar]
- Sohn, I.-R.; Kim, T.-C.; Ju, G.-I.; Kim, M.-S.; Kim, J.-S. Development of PosMAC® Steel and Its Application Properties. Korean J. Met. Mater. 2021, 59, 613–623. [Google Scholar] [CrossRef]
- Wallinder, I.O.; Leygraf, C. A Critical Review on Corrosion and Runoff from Zinc and Zinc-Based Alloys in Atmospheric Environments. Corrosion 2017, 73, 1060–1077. [Google Scholar] [CrossRef]
- Ding, Z.-L.; Yan, B.-H.; Jin, X.-Y.; Wang, X.-J.; Zhang, J.; Jiang, S.-M.; Zhang, Q.-F. Effect of magnesium on the microstructure and corrosion resistance of 55% Al-Zn coating. In Proceedings of the 12th International Conference on Zinc and Zinc Alloy Coated Steel Sheet (Galvatech 2021), Virtual Conference, 22–24 June 2021; pp. 164–171. [Google Scholar]
- Li, S.; Gao, B.; Tu, G.; Hu, L.; Sun, S.; Zhu, G.; Yin, S. Effects of magnesium on the microstructure and corrosion resistance of Zn–55Al–1.6Si coating. Constr. Build. Mater. 2014, 71, 124–131. [Google Scholar] [CrossRef]
- Nicard, C.; Allély, C.; Volovitch, P. Effect of Zn and Mg alloying on microstructure and anticorrosion mechanisms of Al-Si based coatings for high strength steel. Corros. Sci. 2018, 146, 192–201. [Google Scholar] [CrossRef]
- Panossian, Z.; Mariaca, L.; Morcillo, M.; Flores, S.; Rocha, J.; Peña, J.; Herrera, F.; Corvo, F.; Sanchez, M.; Rincon, O.; et al. Steel cathodic protection afforded by zinc, aluminium and zinc/aluminium alloy coatings in the atmosphere. Surf. Coat. Technol. 2005, 190, 244–248. [Google Scholar] [CrossRef]
- Lee, S.; Chang, L.-M.; Skibniewski, M. Automated recognition of surface defects using digital color image processing. Autom. Constr. 2006, 15, 540–549. [Google Scholar] [CrossRef]
- Chen, P.-H.; Yang, Y.-C.; Chang, L.-M. Automated bridge coating defect recognition using adaptive ellipse approach. Autom. Constr. 2009, 18, 632–643. [Google Scholar] [CrossRef]
- Khan, A.; Ali, S.S.A.; Anwer, A.; Adil, S.H.; Meriaudeau, F. Subsea Pipeline Corrosion Estimation by Restoring and Enhancing Degraded Underwater Images. IEEE Access 2018, 6, 40585–40601. [Google Scholar] [CrossRef]
- Chen, P.-H.; Chang, L.-M. Artificial intelligence application to bridge painting assessment. Autom. Constr. 2003, 12, 431–445. [Google Scholar] [CrossRef]
- Lee, S.; Chang, L.-M.; Chen, P.-H. Performance Comparison of Bridge Coating Defect Recognition Methods. Corrosion 2005, 61, 12–20. [Google Scholar] [CrossRef]
- Enikeev, M.; Maleeva, M. Analysis of Corrosion Process Development on Metals by Means of Computer Vision. Eng. J. 2017, 21, 183–192. [Google Scholar] [CrossRef]
- Alkanhal, A. Image processing techniques applied for pitting corrosion analysis. Int. J. Res. Eng. Technol. 2014, 3, 385–391. [Google Scholar] [CrossRef]
- Petricca, L.; Moss, T.; Figueroa, G.; Broen, S. Corrosion Detection Using A.I: A Comparison of Standard Computer Vision Techniques and Deep Learning Model. In Computer Science & Information Technology (CS & IT); Academy & Industry Research Collaboration Center (AIRCC): Horten, Norway, 2016; pp. 91–99. [Google Scholar] [CrossRef]
- Wint, N.; Cooze, N.; Searle, J.R.; Sullivan, J.H.; Williams, G.; McMurray, H.N.; Luckeneder, G.; Riener, C. The Effect of Microstructural Refinement on the Localized Corrosion of Model Zn-Al-Mg Alloy Coatings on Steel. J. Electrochem. Soc. 2019, 166, C3147–C3158. [Google Scholar] [CrossRef]
- Sullivan, J.; Cooze, N.; Gallagher, C.; Lewis, T.; Prosek, T.; Thierry, D. In situ monitoring of corrosion mechanisms and phosphate inhibitor surface deposition during corrosion of zinc–magnesium–aluminium (ZMA) alloys using novel time-lapse microscopy. Faraday Discuss. 2015, 180, 361–379. [Google Scholar] [CrossRef]
- Malla, A.D.; Sullivan, J.H.; Penney, D.J.; Goldsworthy, M.; Britton, D.; Williams, G.; Goodwin, F.; Cardoso, A.P. Mechanistic investigation on the influence of coating weights on the corrosion behaviour of hot-dip-galvanised Zn-Mg-Al coatings. NPJ Mater. Degrad. 2024, 8, 78. [Google Scholar] [CrossRef]
- Mopon, M.; Mol, A.; Garcia, S.J. Effect of delayed inhibitor supply on AA2024-T3 intermetallic activity: A local in situ analysis with reflected microscopy. Corros. Sci. 2024, 230, 111910. [Google Scholar] [CrossRef]
- Shkirskiy, V.; Kanoufi, F. Reflective microscopy for mechanistic insights in corrosion research. Curr. Opin. Electrochem. 2023, 39, 101259. [Google Scholar] [CrossRef]
- Denissen, P.J.; Homborg, A.M.; Garcia, S.J. Interpreting Electrochemical Noise and Monitoring Local Corrosion by Means of Highly Resolved Spatiotemporal Real-Time Optics. J. Electrochem. Soc. 2019, 166, C3275–C3283. [Google Scholar] [CrossRef]
- Raffin, F.; Makogon, A.; Kanoufi, F.; Echouard, J.; Shkirskiy, V.; Volovitch, P. Initial cathodic reactivity of intermetallic particles in 7175 aluminum alloy buried under 6 µm thick anodized oxide layer revealed by in-situ reflective microscopy. Electrochim. Acta 2024, 492, 144155. [Google Scholar] [CrossRef]
- Chen, C.-L.; Thomson, R. The combined use of EBSD and EDX analyses for the identification of complex intermetallic phases in multicomponent Al–Si piston alloys. J. Alloys Compd. 2009, 490, 293–300. [Google Scholar] [CrossRef]
- Haberlah, D.; Owen, M.; Botha, P.W.; Gottlieb, P. Sem-EDS-Based Protocol for Subsurface Drilling Mineral Identification and Petrological Classification. In Proceedings of the 10th International Congress for Applied Mineralogy (ICAM), Trondheim, Norway, 1–5 August 2011; Broekmans, M.A.T.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 265–273. [Google Scholar] [CrossRef]
- Müller, M.; Stiefel, M.; Bachmann, B.-I.; Britz, D.; Mücklich, F. Overview: Machine Learning for Segmentation and Classification of Complex Steel Microstructures. Metals 2024, 14, 553. [Google Scholar] [CrossRef]
- Stauffer, D.; Aharony, A. Introduction to Percolation Theory, 2nd ed.; Transferred to Digital Print; Taylor & Francis: London, UK, 2003. [Google Scholar]
- Kim, S.; Choi, S.; Oh, E.; Byun, J.; Kim, H.; Lee, B.; Lee, S.; Hong, Y. Revisit to three-dimensional percolation theory: Accurate analysis for highly stretchable conductive composite materials. Sci. Rep. 2016, 6, 34632. [Google Scholar] [CrossRef]
- Thommerel, E.; Valmalette, J.; Musso, J.; Villain, S.; Gavarri, J.; Spada, D. Relations between microstructure, electrical percolation and corrosion in metal—Insulator composites. Mater. Sci. Eng. A 2002, 328, 67–79. [Google Scholar] [CrossRef]
- Zhao, F.; Ling, Y.; Hu, Y.; Wang, W.; Bai, Y.; Yang, Z.; Zhang, Z. The role of secondary phase precipitates in zircaloy corrosion: From point defects to local percolation. Corros. Sci. 2024, 229, 111856. [Google Scholar] [CrossRef]
- Lehockey, E.; Brennenstuhl, A.; Thompson, I. On the relationship between grain boundary connectivity, coincident site lattice boundaries, and intergranular stress corrosion cracking. Corros. Sci. 2004, 46, 2383–2404. [Google Scholar] [CrossRef]
- Sieradzki, K.; Newman, R.C. A Percolation Model for Passivation in Stainless Steels. J. Electrochem. Soc. 1986, 133, 1979–1980. [Google Scholar] [CrossRef]
- McCafferty, E. Graph theory and the passivity of binary alloys. Corros. Sci. 2000, 42, 1993–2011. [Google Scholar] [CrossRef]
- VDA 621-415; Testing of Corrosion Protection of Vehicle Paint by Alternating Cycles Test. Ascott Analytical: Berlin, Germany, 1982. Available online: https://www.ascott-analytical.com/test-standards/vda-621-415/ (accessed on 4 February 2024).
- Popova, K.; Prošek, T. Mechanism of Zinc Corrosion in Accelerated Corrosion Tests. Mater. Corros. 2025, 56, 581–588. [Google Scholar] [CrossRef]
- Eggert, F. Effect of the Silicon Drift Detector on EDAX Standardless Quant Methods. Microsc. Today 2020, 28, 34–39. [Google Scholar] [CrossRef]
- Maffre Gervais Gauthier, G.P.; Bruno, L.; Julien, R.; Damien, E.; Mélanie, F.; Arthur, D.; Hugo. ‘Aphelion SDK’, ADCIS. Available online: https://www.adcis.net/en/aphelion-sdk/ (accessed on 19 September 2024).
- Selverian, J.H.; Marder, A.R.; Notis, M.R. The effects of silicon on the reaction between solid iron and liquid 55 wt pct Al−Zn baths. Met. Trans. A 1989, 20, 543–555. [Google Scholar] [CrossRef]
- Yadav, A.; Katayama, H.; Noda, K.; Masuda, H.; Nishikata, A.; Tsuru, T. Effect of Al on the galvanic ability of Zn–Al coating under thin layer of electrolyte. Electrochim. Acta 2006, 52, 2411–2422. [Google Scholar] [CrossRef]
- Prosek, T.; Persson, D.; Stoulil, J.; Thierry, D. Composition of corrosion products formed on Zn–Mg, Zn-Al and Zn–Al–Mg in model atmospheric conditions. Corros. Sci. 2014, 86, 231–238. [Google Scholar] [CrossRef]
- Kim, S.O.; Yang, W.S.; Kim, S.J. Effects of the Combined Addition of Zn and Mg on Corrosion Behaviors of Electropainted AlSi-Based Metallic Coatings Used for Hot-Stamping Steel Sheets. Materials 2020, 13, 3379. [Google Scholar] [CrossRef]
- Rosalbino, F.; Carlini, R.; Parodi, R.; Zanicchi, G.; Scavino, G. Investigation of passivity and its breakdown on Fe3Al–Si and Fe3Al–Ge intermetallics in chloride-containing solution. Corros. Sci. 2014, 85, 394–400. [Google Scholar] [CrossRef]
- Birbilis, N.; Buchheit, R.G. Electrochemical Characteristics of Intermetallic Phases in Aluminum Alloys. J. Electrochem. Soc. 2005, 152, B140–B151. [Google Scholar] [CrossRef]
- Li, J.; Dang, J. A Summary of Corrosion Properties of Al-Rich Solid Solution and Secondary Phase Particles in Al Alloys. Metals 2017, 7, 84. [Google Scholar] [CrossRef]
- Mouanga, M.; Berçot, P. Comparison of corrosion behaviour of zinc in NaCl and in NaOH solutions; Part II: Electrochemical analyses. Corros. Sci. 2010, 52, 3993–4000. [Google Scholar] [CrossRef]
- Azevedo, M.S.; Allély, C.; Ogle, K.; Volovitch, P. Corrosion mechanisms of Zn(Mg,Al) coated steel: 2. The effect of Mg and Al alloying on the formation and properties of corrosion products in different electrolytes. Corros. Sci. 2015, 90, 482–490. [Google Scholar] [CrossRef]
- Kairy, S.K.; Birbilis, N. Clarifying the Role of Mg2Si and Si in Localized Corrosion of Aluminum Alloys by Quasi In Situ Transmission Electron Microscopy. Corrosion 2020, 76, 464–475. [Google Scholar] [CrossRef]
- Zhang, X.; Vu, T.-N.; Volovitch, P.; Leygraf, C.; Ogle, K.; Wallinder, I.O. The initial release of zinc and aluminum from non-treated Galvalume and the formation of corrosion products in chloride containing media. Appl. Surf. Sci. 2012, 258, 4351–4359. [Google Scholar] [CrossRef]
- Salinas, D.R.; García, S.G.; Bessone, J.B. Influence of alloying elements and microstructure on aluminium sacrificial anode performance: Case of Al–Zn. J. Appl. Electrochem. 1999, 29, 1063–1071. [Google Scholar] [CrossRef]
- Idusuyi, N.; Oluwole, O.O. Aluminium Anode Activation Research—A Review. 2012. Available online: https://www.researchgate.net/publication/256543719_Aluminium_Sacrificial_Anode_Activation_-_A_Review (accessed on 20 September 2024).
- LeBozec, N.; Thierry, D.; Persson, D.; Stoulil, J. Atmospheric Corrosion of Zinc-Aluminum Alloyed Coated Steel in Depleted Carbon Dioxide Environments. J. Electrochem. Soc. 2018, 165, C343–C353. [Google Scholar] [CrossRef]
- Ho, C.Y.; Wang, J.Y. Investigation of microstructure related to corrosion evolution, mechanical and electrochemical properties on doped Si of binary Zn–Al coating. Mater. Chem. Phys. 2023, 301, 127656. [Google Scholar] [CrossRef]
- Friel, J.J. Atmospheric Corrosion Products on Al, Zn, and AlZn Metallic Coatings. Corrosion 1986, 42, 422–426. [Google Scholar] [CrossRef]
- Shkirskiy, V.; Maltseva, A.; Ogle, K.; Volovitch, P. Corrosion mechanisms of Zn(Mg,Al) coated steel: The effect of HCO3− and NH4+ ions on the intrinsic reactivity of the coating. Electrochim. Acta. 2017, 153, 159–169. [Google Scholar] [CrossRef]
- Calderón, J.P.; Barragán, J.L.R.; Fierro, J.I.B.; Mejía, H.C.; González, C.D.A.; Vázquez, V.R.; Sánchez, K.P.; Torres-Mancera, M.T.; Retes-Mantilla, R.F.; Rodríguez-Díaz, R.A. Corrosion Behavior of Al Modified with Zn in Chloride Solution. Materials 2022, 15, 4229. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hreso, D. Morphology of 55% Al-Zn coating and the Al-Zn coating with Mg additions. In Proceedings of the 10th International Conference on Zinc and Zinc Alloy Coated Steel Sheet (Galvatech 2015), Toronto, ON, Canada, 31 May–4 June 2015; Association for Iron & Steel Technology: Warrendale, PA, USA, 2015; pp. 852–860. [Google Scholar]
- Xie, Y.; Artymowicz, D.M.; Lopes, P.P.; Aiello, A.; Wang, D.; Hart, J.L.; Anber, E.; Taheri, M.L.; Zhuang, H.; Newman, R.C.; et al. A percolation theory for designing corrosion-resistant alloys. Nat. Mater. 2021, 20, 789–793. [Google Scholar] [CrossRef]
- Jerauld, G.R.; Scriven, L.E.; Davis, H.T. Percolation and conduction on the 3D Voronoi and regular networks: A second case study in topological disorder. J. Phys. C Solid State Phys. 1984, 17, 3429–3439. [Google Scholar] [CrossRef]
- Becker, A.M.; Ziff, R.M. Percolation thresholds on two-dimensional Voronoi networks and Delaunay triangulations. Phys. Rev. E 2009, 80, 041101. [Google Scholar] [CrossRef]
- Prosek, T.; Nazarov, A.; Le Gac, A.; Thierry, D. Coil-coated Zn–Mg and Zn–Al–Mg: Effect of climatic parameters on the corrosion at cut edges. Prog. Org. Coat. 2015, 83, 26–35. [Google Scholar] [CrossRef]




| Sample | Al (wt.%) | Si (wt.%) | Mg (wt.%) | Zn | Thickness (µm) |
|---|---|---|---|---|---|
| AZ | 55% | 1.6% | – | Bal. | 22.43 ± 4.33 |
| AZM | 55% | 1–2% | 2–4% | Bal. | 24.88 ± 0.94 |
| L-AZM | 30–40% | 1–2% | 2–4% | Bal. | 22.17 ± 1.66 |
| AZ Standard | AZ New | AZM | L-AZM | |||||
|---|---|---|---|---|---|---|---|---|
| Phase | Surface | SD | Surface | SD | Surface | SD | Surface | SD |
| TOTAL | 100.00 | 0.00 | 100.00 | 0.00 | 100.00 | 0.00 | 100.00 | 0.00 |
| Al(Zn) | 82.37 | 4.87 | 44.88 (Al(lowZn)) | 5.96 | 68.21 | 7.04 | 32.49 | 3.83 |
| Binary ZnAl | 4.83 | 2.68 | 6.18 | 3.27 | 18.63 | 7.37 | 44.64 | 4.45 |
| Mg2Si | 0.00 | 0.00 | 0.00 | 0.00 | 2.49 | 2.22 | 0.35 | 0.58 |
| Si | 2.46 | 1.52 | 2.41 | 1.52 | 0.73 | 0.58 | 2.58 | 2.65 |
| Zn | 2.16 | 0.84 | 1.52 | 0.55 | 0.15 | 0.00 | 0.52 | 0.55 |
| Intermetallic | 1.96 | 0.00 | 2.90 | 0.00 | 1.53 | 0.45 | 0.86 | 0.00 |
| Zn2Mg | 0.04 | 0.00 | 0.03 | 0.00 | 3.56 | 1.67 | 11.91 | 2.28 |
| Ternary | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Binary ZnMg | 0.01 | 0.00 | 0.01 | 0.00 | 0.15 | 0.00 | 2.85 | 3.21 |
| Al(high Zn) | 38.84 | 3.63 | ||||||
| Not assigned | 6.16 | 2.86 | 3.22 | 1.00 | 4.55 | 1.10 | 3.80 | 1.14 |
| % Al(Zn) Dendrites Border Line in Contact with | ||||
|---|---|---|---|---|
| Sample | Binary ZnAl Phase | Zn2Mg Phase | Mg2Si Phase | Si Phase |
| AZ | 80.6 | 0.0 | 0.0 | 19.4 |
| AZM | 84.9 | 1.7 | 10.0 | 3.4 |
| L-AZM | 88.7 | 1.6 | 0.0 | 9.7 |
| Size of the Al(High Zn) Phase for AZ Coating (µm) | ||
|---|---|---|
| Zone1 | Max height | 20 |
| Max width | 30 | |
| Zone2 | Max height | 14 |
| Max width | 19 | |
| Zone3 | Max height | 21 |
| Max width | 25 | |
| Zone4 | Max height | 15 |
| Max width | 25 | |
| Zone5 | Max height | 15 |
| Max width | 29 | |
| Coating | |||
|---|---|---|---|
| Phase in Contact with Steel (%) | AZ | AZM | L-AZM |
| Dendrite’s zone with high Al | 5.84 ± 1.67 | 69.21 ± 16.01 | 19.03 ± 15.34 |
| Binary ZnAl | 0.38 ± 0.43 | 21.47 ± 14.16 | 43.2 ± 27.86 |
| Zn2Mg | 0 | 0 | 9.11 ± 12.86 |
| Mg2Si | 0 | 5.73 ± 6.62 | 12.43 ± 9.34 |
| Si | 19.63 ± 12.61 | 3.59 ± 5.08 | 16.22 ± 20.75 |
| Dendrite’s zone with low Al | 74.15 ± 12.96 | - | - |
| Phase | FeAlSi intermetallic | Si | Fe (steel) | Al | Zn | Zn(Al) (binary) | Zn2Mg | Mg2Si |
| E (V vs. SCE) | −0.59 [52] | −0.44 [53] | −0.6 | −0.94 [54] | −1.06 [55] | −1.04 [53] | −1.09 [53] | −1.35 [53] |
| Sample | Height of Sacrificial Phases (µm) | Width of Sacrificial Phases (µm) |
|---|---|---|
| AZ | 5.6 | 9.6 |
| AZM | 21 | >d |
| L-AZM | 26.6 | >d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adinolfi Colpaert Sartori, G.; Remy, B.; Machado Amorim, T.; Volovitch, P. Quantitative Microstructure of Multiphase Al-Zn-Si-(Mg) Coatings and Their Effects on Sacrificial Protection for Steel. Metals 2025, 15, 476. https://doi.org/10.3390/met15050476
Adinolfi Colpaert Sartori G, Remy B, Machado Amorim T, Volovitch P. Quantitative Microstructure of Multiphase Al-Zn-Si-(Mg) Coatings and Their Effects on Sacrificial Protection for Steel. Metals. 2025; 15(5):476. https://doi.org/10.3390/met15050476
Chicago/Turabian StyleAdinolfi Colpaert Sartori, Guilherme, Blandine Remy, Tiago Machado Amorim, and Polina Volovitch. 2025. "Quantitative Microstructure of Multiphase Al-Zn-Si-(Mg) Coatings and Their Effects on Sacrificial Protection for Steel" Metals 15, no. 5: 476. https://doi.org/10.3390/met15050476
APA StyleAdinolfi Colpaert Sartori, G., Remy, B., Machado Amorim, T., & Volovitch, P. (2025). Quantitative Microstructure of Multiphase Al-Zn-Si-(Mg) Coatings and Their Effects on Sacrificial Protection for Steel. Metals, 15(5), 476. https://doi.org/10.3390/met15050476







