Realization of a Novel FeSiAlCuSn Multicomponent Alloy and Characterization of Intermetallic Phases Formed at Different Temperatures During Cooling
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization Details
3. Results
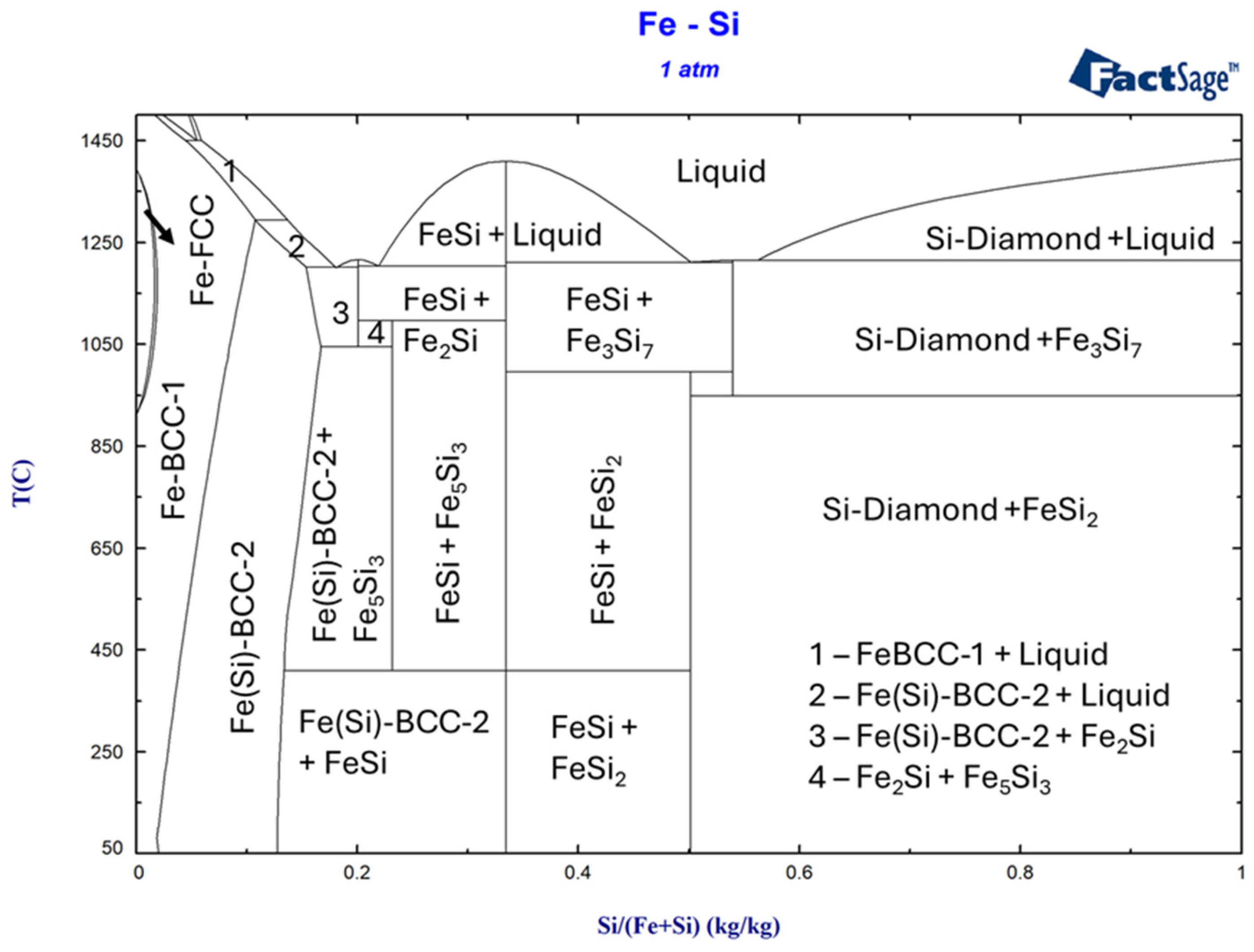
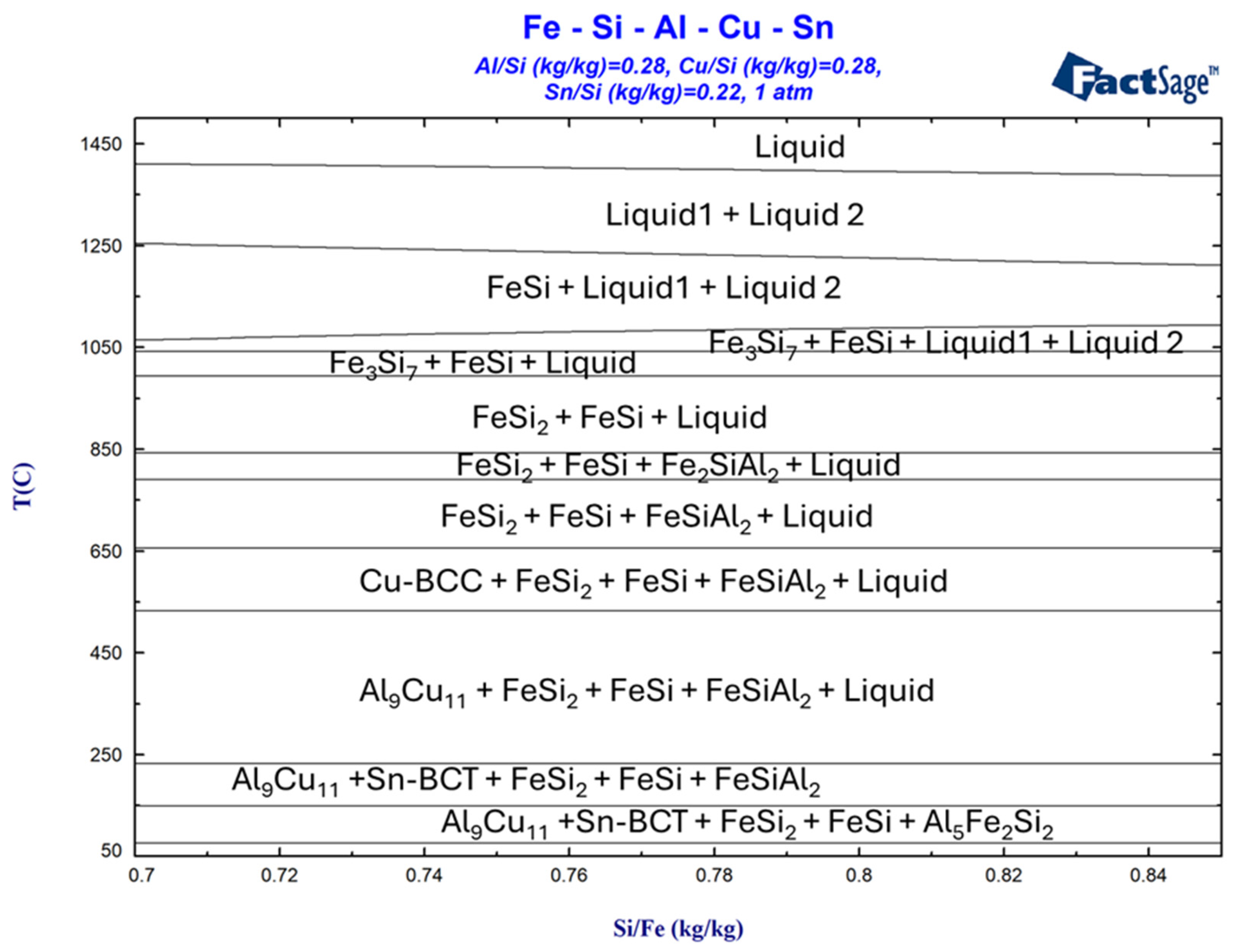
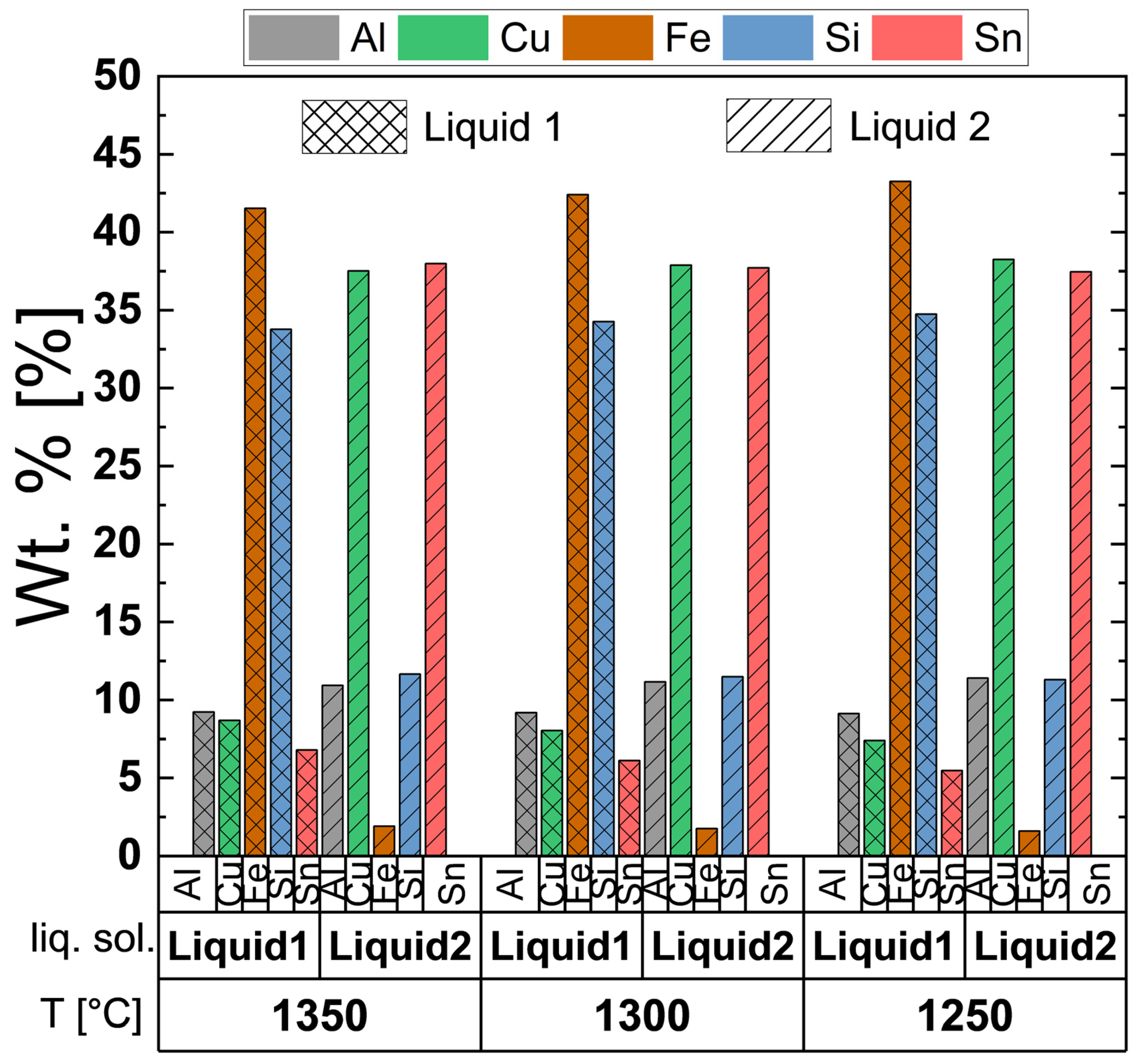
3.1. Simulation of Alloy Cooling Behavior Using FactSage
3.2. Analysis of the Microstructure of the Standard FeSi45 Sample
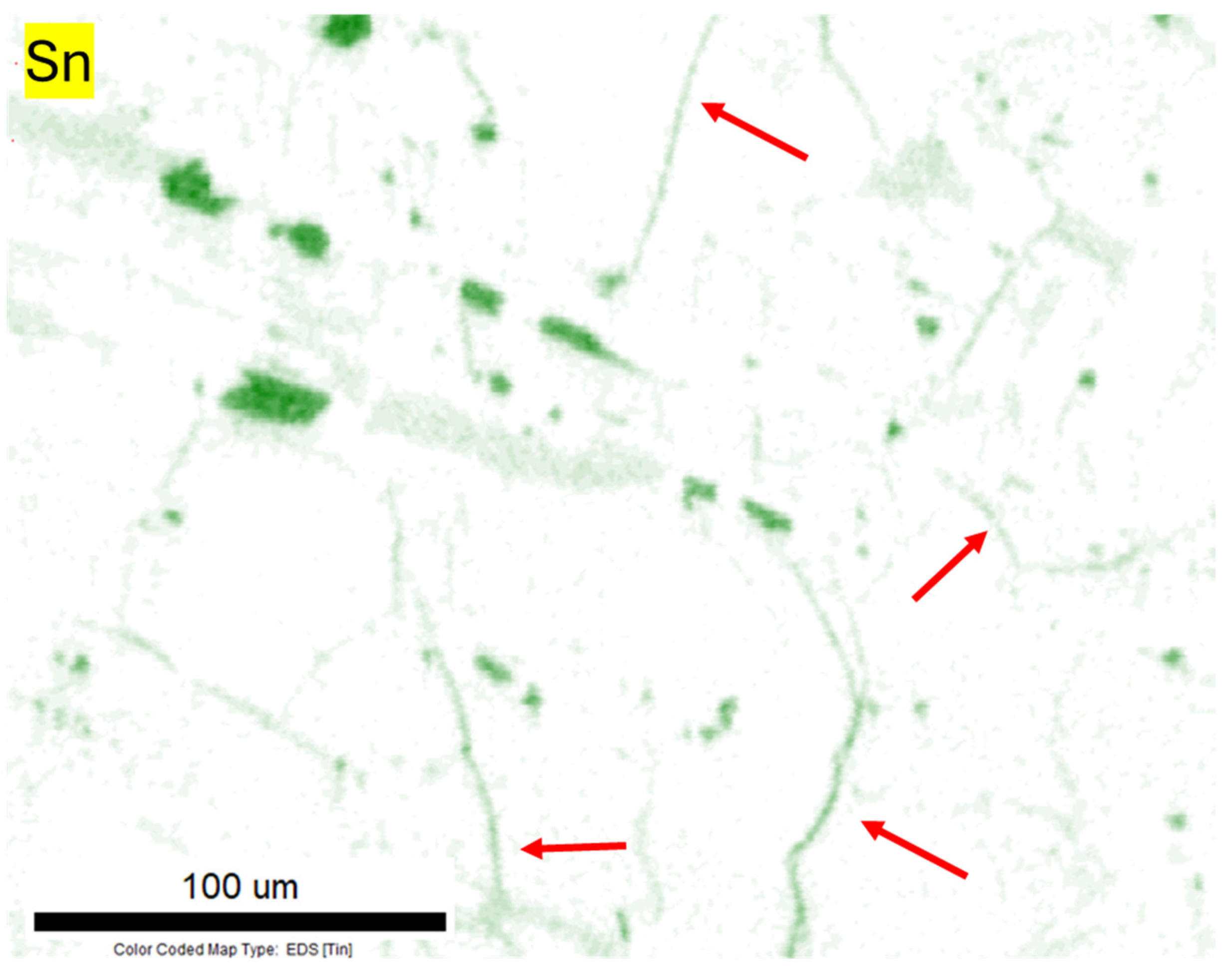
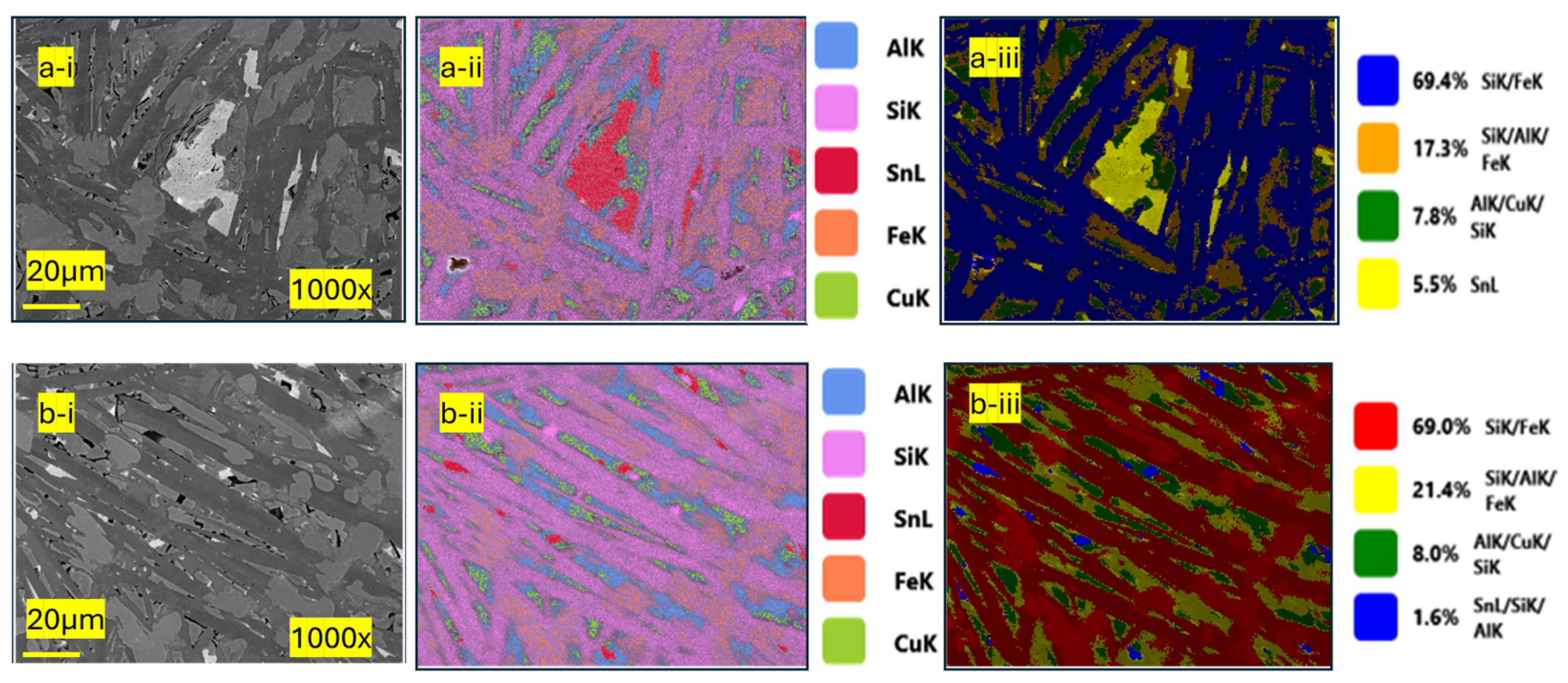
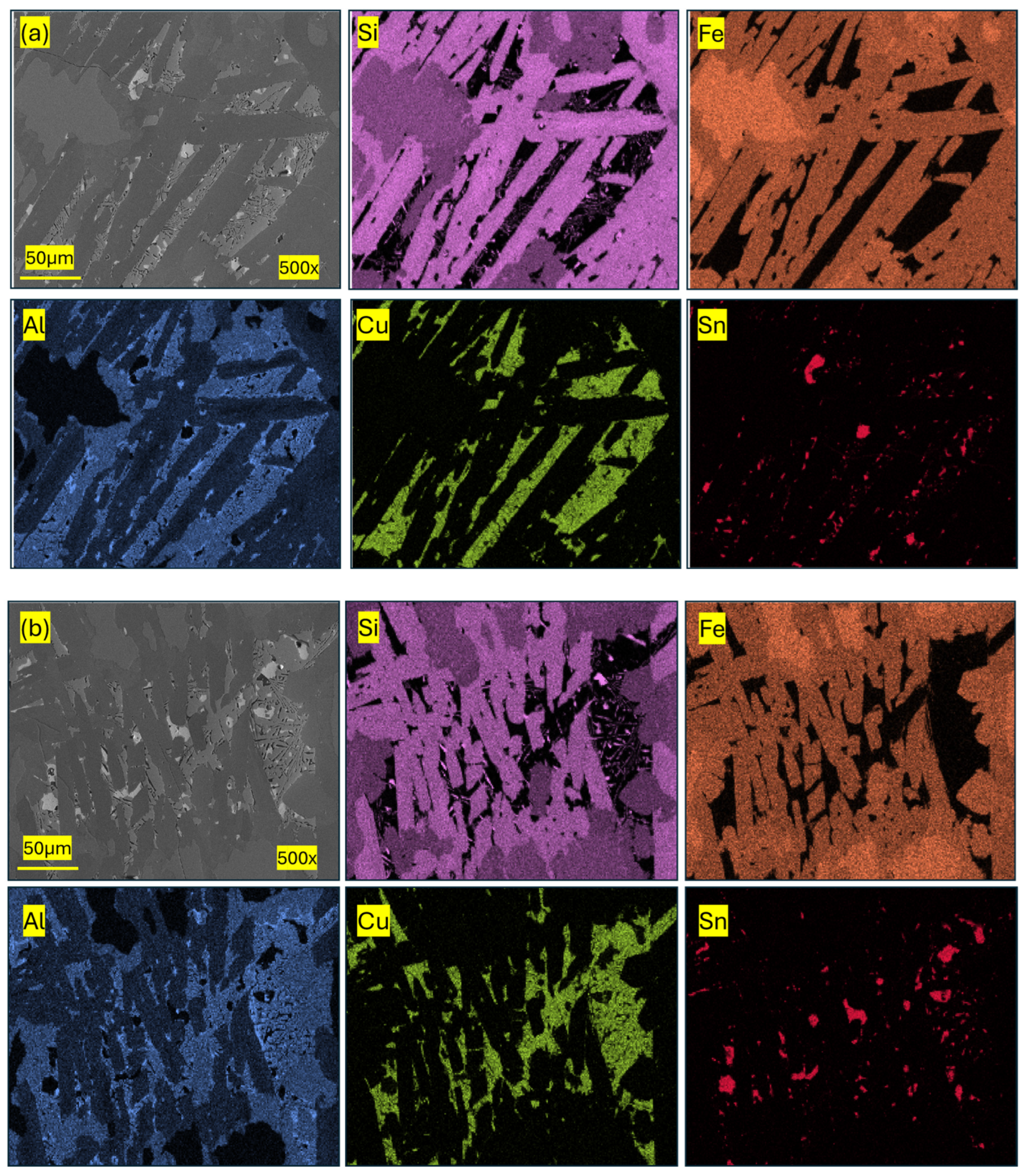
3.3. Analysis of the Microstructure of the Sample Cooled Under Ambient Conditions
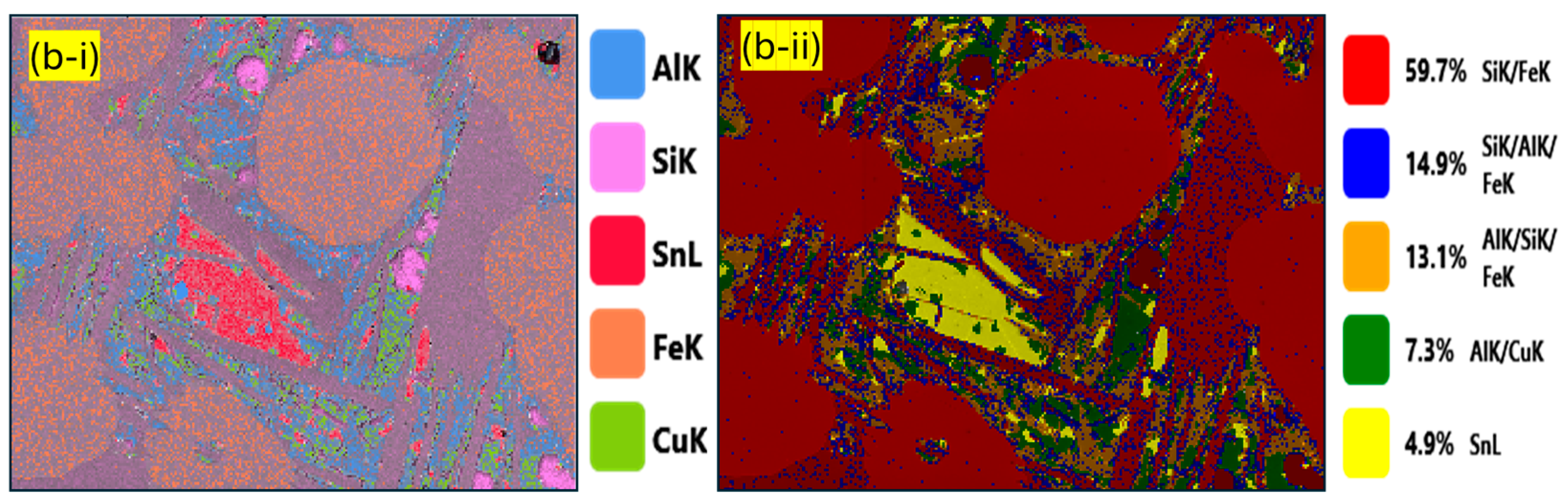
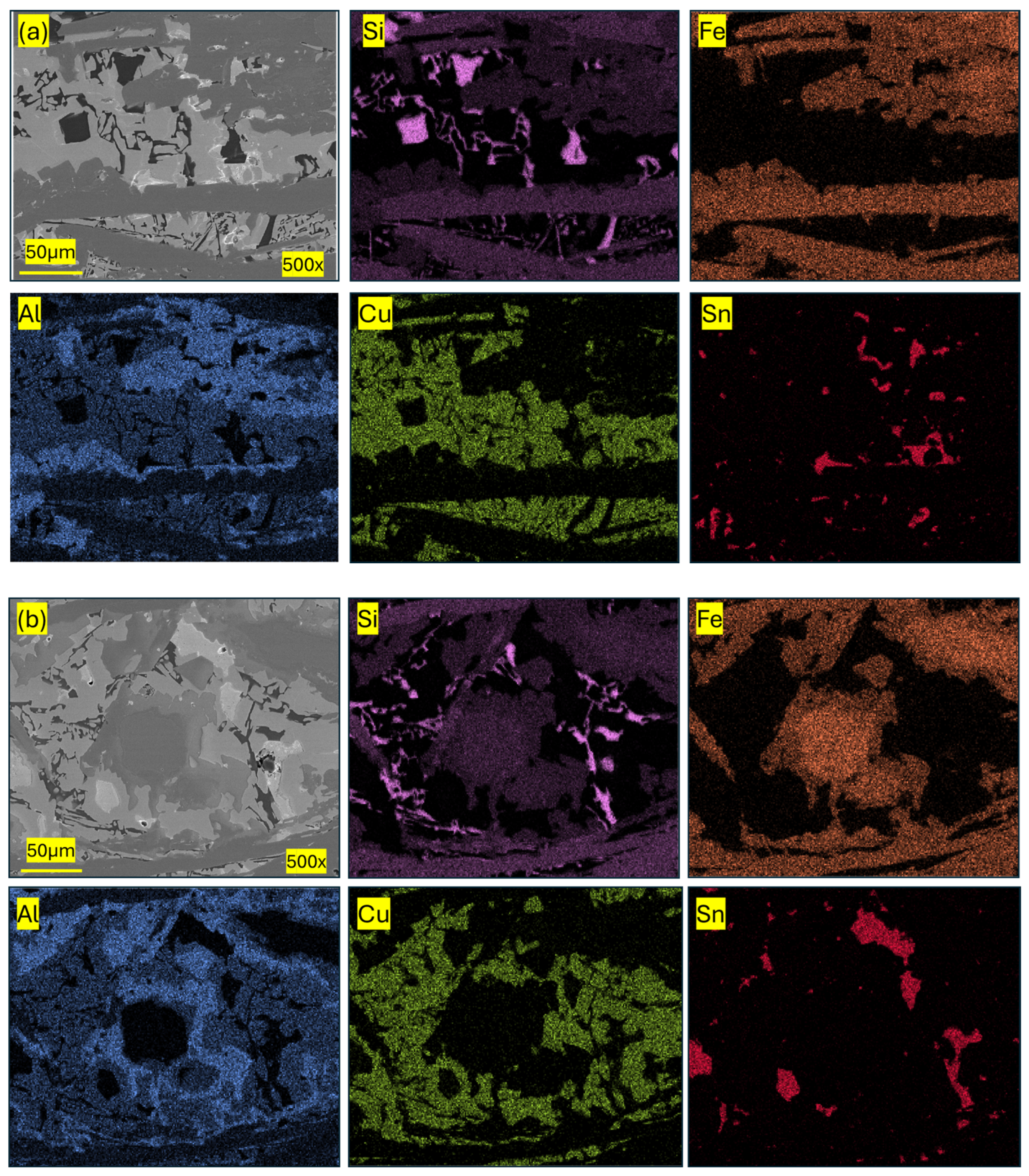
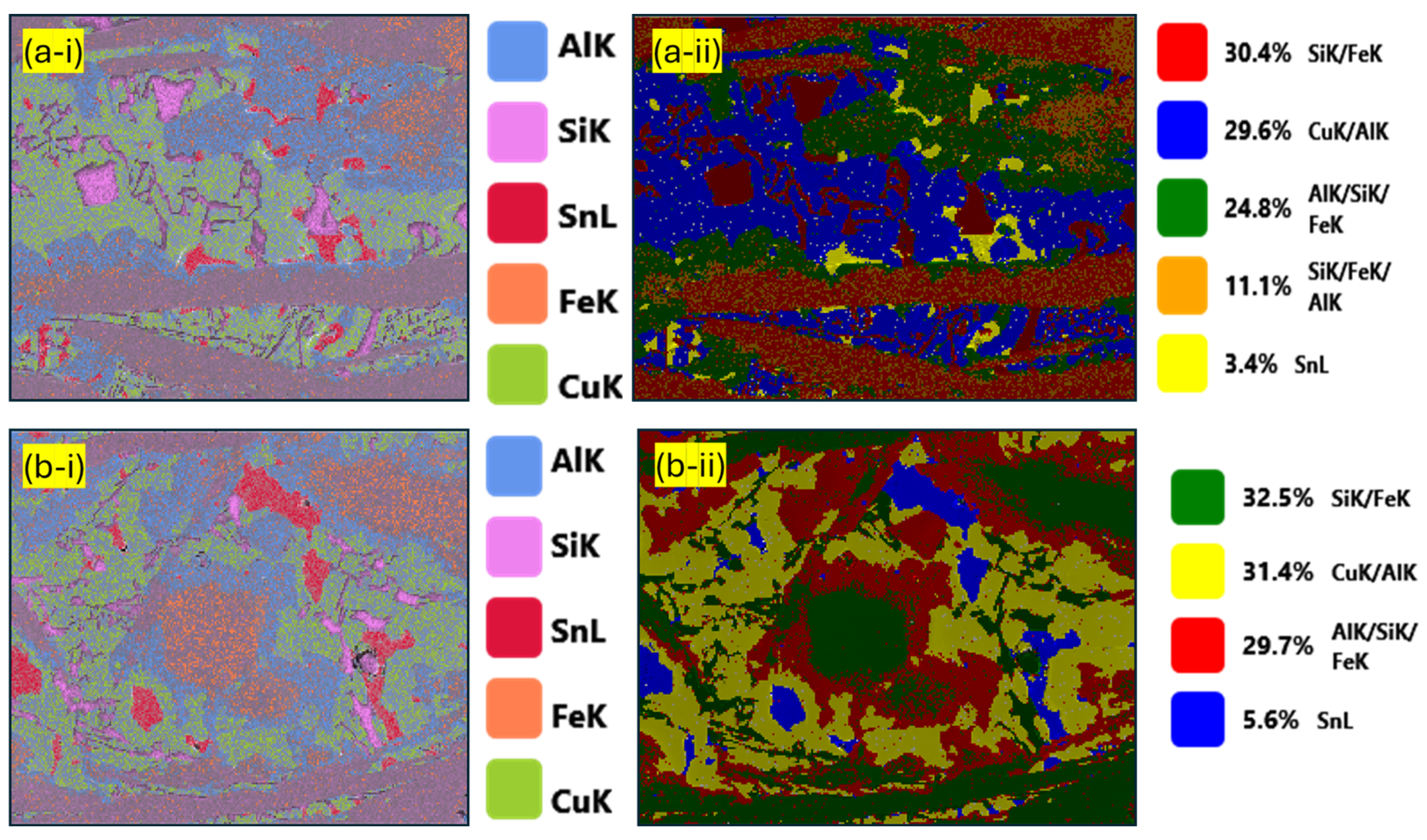
3.4. Analysis of Microstructure and Composition of Samples Annealed at 1000 °C
3.5. Analysis of Microstructure and Composition of Samples Annealed at 800 °C
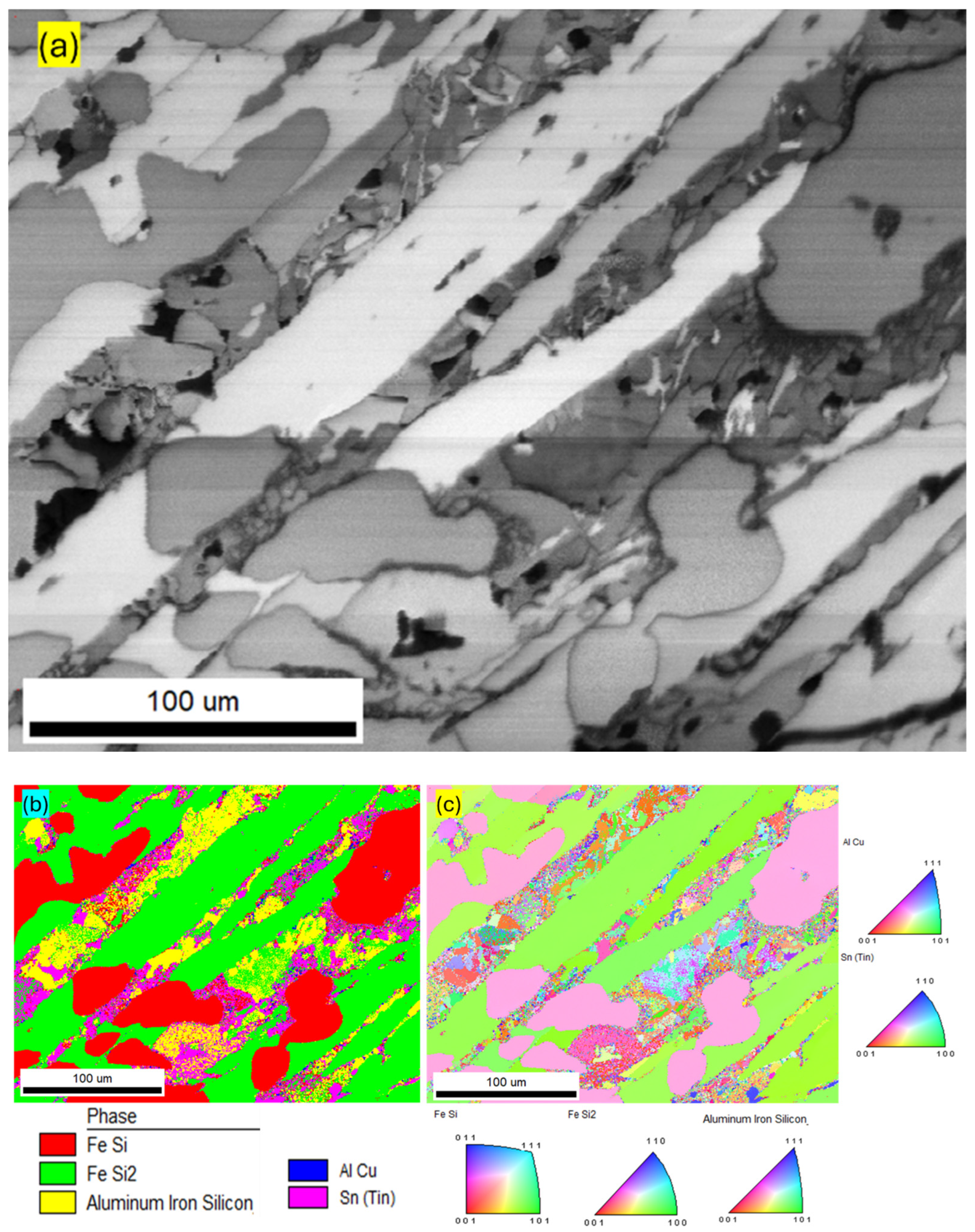
3.6. Analysis of Microstructure and Composition of Samples Annealed at 590 °C
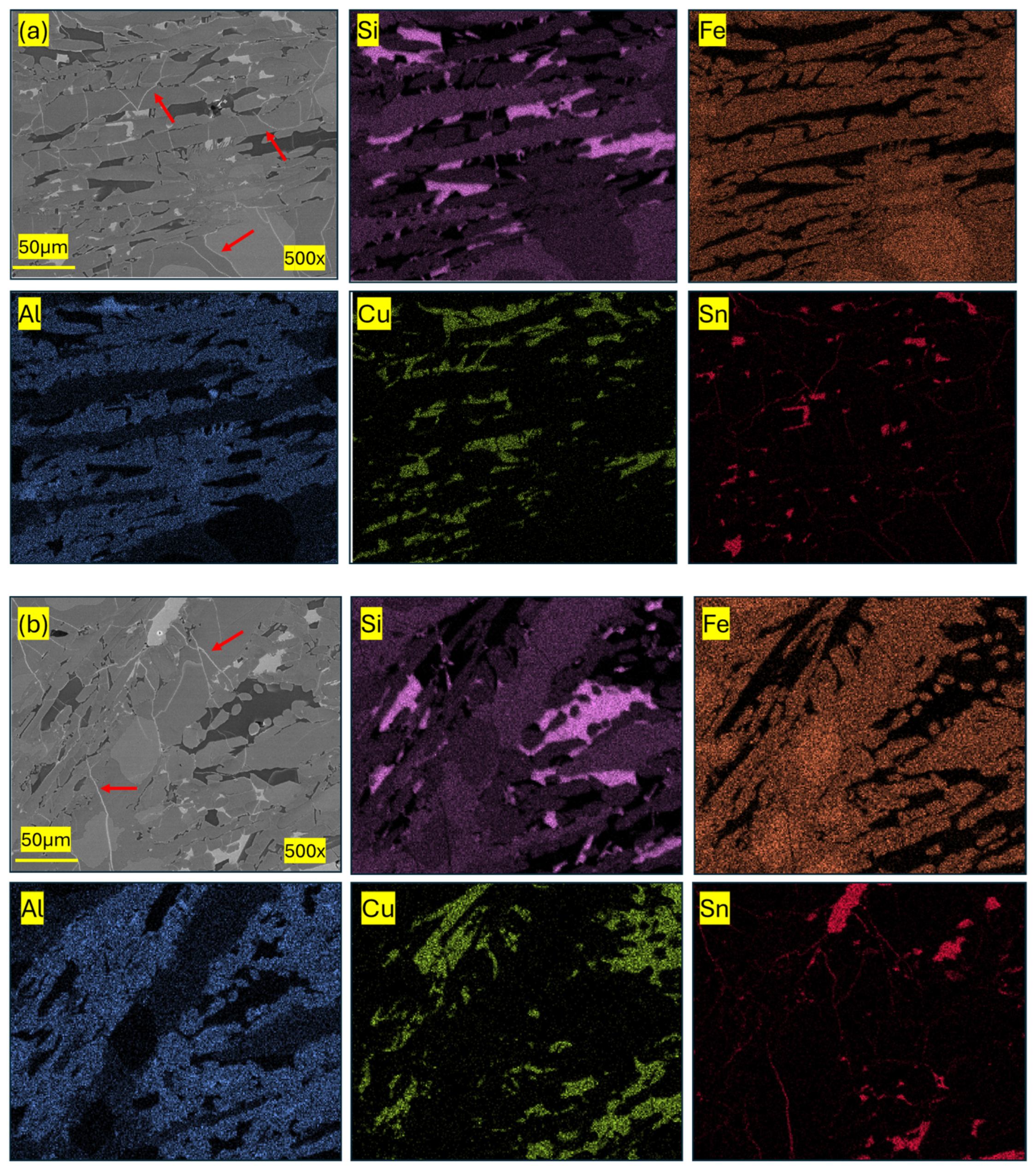
3.7. Analysis of Microstructure and Composition of Samples Annealed at 450 °C
3.8. Analysis of Microstructure and Composition of Samples Annealed at 200 °C
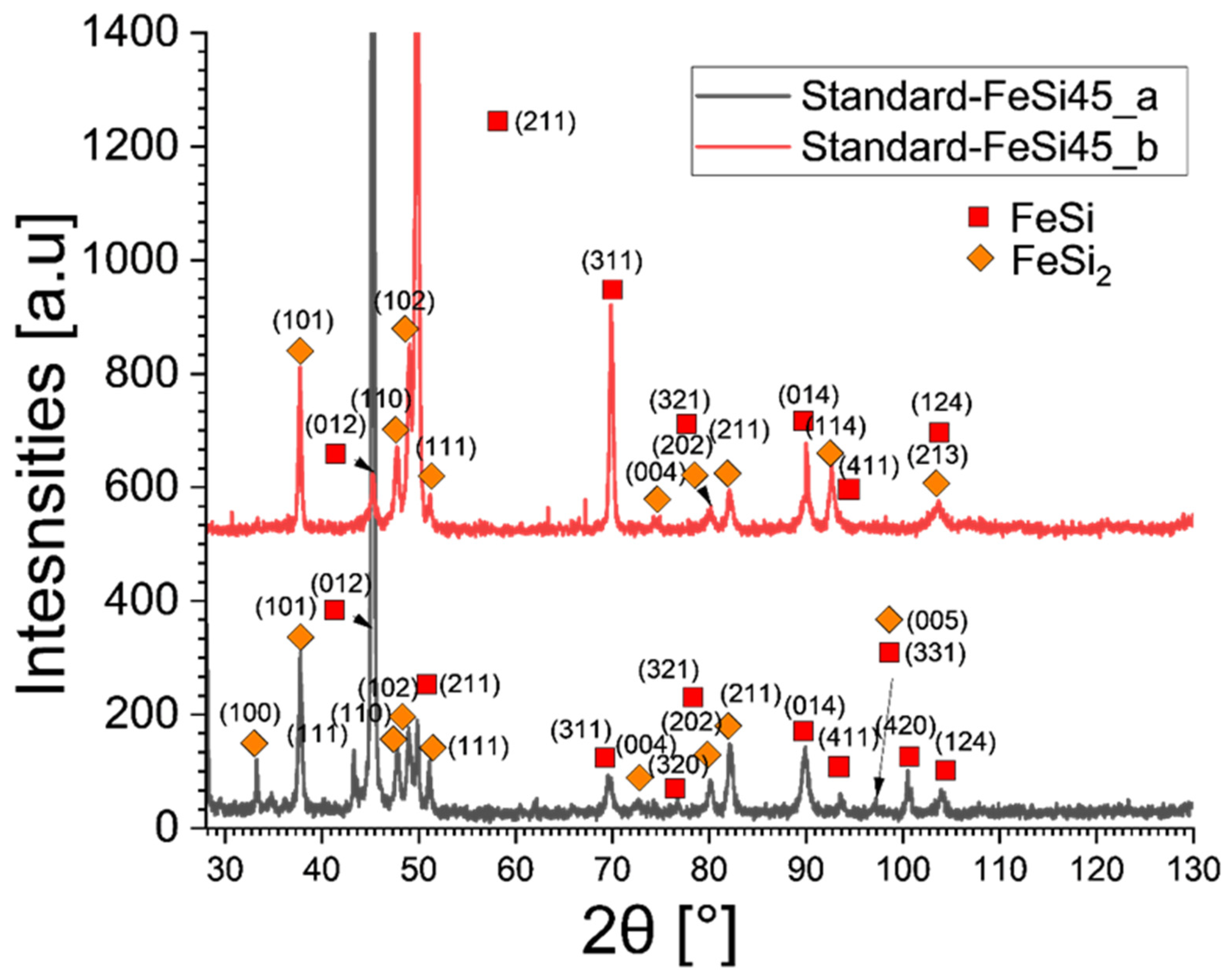
3.9. Analysis of Phases by XRD
3.10. Characterization of the Hardness of Samples Annealed at Different Temperatures
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Phases [Error] | FeSi | FeSi2 | Fe3Si | Fe2Si | FeAl | Fe3Al2Si3 |
|---|---|---|---|---|---|---|
| Annealing Temperature [°C] | ||||||
| 1000 | 52.77 (±2) | 2.9 (±0.3) | 23.1 (±1.5) | 5.41 (±0.4) | ||
| 800 | 11.01 (±1) | 48.2 (±2) | 17.7 (±0.3) | 8.34 (±1) | 1.6 (±0.3) | |
| 590 | 13.03 (±1) | 49.8 (±2) | 2.4 (±0.2) | 9.3 (±0.8) | ||
| 450 | 12.6 (±1) | 50.1 (±2) | 1.1 (±0.1) | 9.1 (±1.3) | ||
| 200 | 12.73 (±1) | 49.9 (±2) | 1.8 (±0.3) | 10.1 (±1.2) |
| Phases [Error] | Al2Cu | Al9Cu11 | Cu3Sn | Cu6Sn5 | Si | Sn |
|---|---|---|---|---|---|---|
| Annealing Temperature [°C] | ||||||
| 1000 | 7.08 (±0.6) | 3.21 (±0.3) | 5.41 (±0.5) | |||
| 800 | 6.87 (±0.6) | 1.09 (±0.1) | 5.22 (±0.5) | |||
| 590 | 11.09 (±1) | 5.12 (±0.4) | 5.35 (±0.5) | 4.77 (±0.5) | ||
| 450 | 11.4 (±1) | 6.8 (±0.5) | 4.63 (±0.5) | 4.25 (±0.5) | ||
| 200 | 9.35 (±1) | 5.14 (±0.4) | 2.1 (±0.1) | 4.2 (±0.5) | 4.68 (±0.5) |
Appendix B
References
- Hawezy, D. The influence of silicon content on physical properties of non-oriented silicon steel. Mater. Sci. Technol. 2017, 33, 1560–1569. [Google Scholar] [CrossRef]
- Selema, A.; Beretta, M.; Ibrahim, M.N.; Verwimp, J.; Rombouts, M.; Vleugels, J.; Kestens, L.A.I.; Sergeant, P. Material Engineering of 3D-Printed Silicon Steel Alloys for the Next Generation of Electrical Machines and Sustainable Electromobility. J. Magn. Magn. Mater. 2023, 584, 171106. [Google Scholar] [CrossRef]
- Haque, N.; Norgate, T. Estimation of greenhouse gas emissions from ferroalloy production using life cycle assessment with particular reference to Australia. J. Clean. Prod. 2013, 39, 220–230. [Google Scholar] [CrossRef]
- Sævarsdottir, G.; Kvande, H.; Magnusson, T. Greenhouse Gas Emissions from Silicon Production -Development of Carbon Footprint with Changing Energy Systems. SSRN Electron. J. 2021, 27–29. [Google Scholar] [CrossRef]
- Tangstad, M. Ferrosilicon and silicon technology. In Handbook of Ferroalloy; Elsevier: Amsterdam, The Netherlands, 2013; pp. 179–220. [Google Scholar] [CrossRef]
- Riva, L.; Surup, G.R.; Buø, T.V.; Nielsen, H.K. A study of densified biochar as carbon source in the silicon and ferrosilicon production. Energy 2019, 181, 985–996. [Google Scholar] [CrossRef]
- Blaesing, L.; Walnsch, A.; Hippmann, S.; Modrzynski, C.; Weidlich, C.; Pavón, S.; Bertau, M. Ferrosilicon Production from Silicon Wafer Breakage and Red Mud. ACS Sustain. Resour. Manag. 2024, 1, 404–416. [Google Scholar] [CrossRef]
- Wijk, O.; Brabie, V. The purity of ferrosilicon and its influence on inclusion cleanliness of steel. ISIJ Int. 1996, 36, S132–S135. [Google Scholar] [CrossRef]
- Wang, X.; Tian, X.; Chen, X.; Ren, L.; Geng, C. A review of end-of-life crystalline silicon solar photovoltaic panel recycling technology. Sol. Energy Mater. Sol. Cells 2022, 248, 111976. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, L. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef]
- Padhamnath, P.; Nalluri, S.; Kuśmierczyk, F.; Kopyściański, M.; Karbowniczek, J.; Leow, S.W.; Reindl, T. Electrohydraulic fragmentation processing enabling separation and recovery of all components in end-of-life silicon photovoltaic panels. Sol. Energy 2025, 289, 113329. [Google Scholar] [CrossRef]
- Su, P.; He, Y.; Feng, Y.; Wan, Q.; Li, T. Advancements in end-of-life crystalline silicon photovoltaic module recycling: Current state and future prospects. Sol. Energy Mater. Sol. Cells 2024, 277, 113109. [Google Scholar] [CrossRef]
- Yu, Y.; Bai, X.; Li, S.; Shi, J.; Wang, L.; Xi, F.; Ma, W.; Deng, R. Review of silicon recovery in the photovoltaic industry. Curr. Opin. Green Sustain. Chem. 2023, 44, 100870. [Google Scholar] [CrossRef]
- Heath, G.A.; Silverman, T.J.; Kempe, M.; Deceglie, M.; Ravikumar, D.; Remo, T.; Cui, H.; Sinha, P.; Libby, C.; Shaw, S.; et al. Research and development priorities for silicon photovoltaic module recycling to support a circular economy. Nat. Energy 2020, 5, 502–510. [Google Scholar] [CrossRef]
- Akhter, M.; Al Mansur, A.; Islam, M.I.; Lipu, M.S.H.; Karim, T.F.; Abdolrasol, M.G.M.; Alghamdi, T.A.H. Sustainable Strategies for Crystalline Solar Cell Recycling: A Review on Recycling Techniques, Companies, and Environmental Impact Analysis. Sustainability 2024, 16, 5785. [Google Scholar] [CrossRef]
- Palitzsch, W.; Loser, U. Integrated PV-recycling-more efficient, more effective. In Proceedings of the 2017 IEEE 44th Photovoltaic Specialist Conference (PVSC), Washington, DC, USA, 25–30 June 2017. [Google Scholar] [CrossRef]
- Draoua, A.D.; Martel, B.; Dubois, S.; Audoin, C.; Sérasset, M.; Fauveau, A.; Radhakrishnan, H.S.; Denafas, J.; Petreniene, L.; Severino, N. On the Fabrication of Solar Cells Based on Newly Produced Recycled Silicon Feedstocks From CABRISS-A Comparative Study Between Material Properties and Solar Cell Performances. In Proceedings of the EU PVSEC 2017-33rd European Photovoltaic Solar Energy Conference and Exhibition, Amsterdam, The Netherlands, 25–29 September 2017; pp. 483–487. [Google Scholar] [CrossRef]
- Jung, B.; Park, J.; Seo, D.; Park, N. Sustainable System for Raw-Metal Recovery from Crystalline Silicon Solar Panels: From Noble-Metal Extraction to Lead Removal. ACS Sustain. Chem. Eng. 2016, 4, 4079–4083. [Google Scholar] [CrossRef]
- Mao, D.; Yang, S.; Ma, L.; Ma, W.; Yu, Z.; Xi, F.; Yu, J. Overview of life cycle assessment of recycling end-of-life photovoltaic panels: A case study of crystalline silicon photovoltaic panels. J. Clean. Prod. 2024, 434, 140320. [Google Scholar] [CrossRef]
- Okoroigwe, F.C.; Okoroigwe, E.C.; Ajayi, O.O.; Agbo, S.N.; Chukwuma, J.N. Photovoltaic Modules Waste Management: Ethical Issues for Developing Nations. Energy Technol. 2020, 8, 2000543. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Huda, N.; Behnia, M. Environmental impacts and economic feasibility of end of life photovoltaic panels in Australia: A comprehensive assessment. J. Clean. Prod. 2020, 260, 120996. [Google Scholar] [CrossRef]
- Nain, P.; Kumar, A. Metal dissolution from end-of-life solar photovoltaics in real landfill leachate versus synthetic solutions: One-year study. Waste Manag. 2020, 114, 351–361. [Google Scholar] [CrossRef]
- Rajarao, R.; Farzana, R.; Sahajwalla, V. Transforming waste printed circuit boards and compact discs for the synthesis of valuable ferrosilicon alloy. J. Sustain. Metall. 2018, 4, 461–469. [Google Scholar] [CrossRef]
- Aryanto, D.; Sudiro, T. Preparation of ferrosilicon-aluminium coating using a mechanical alloying technique: Study of thermal annealing on their structural characteristics. Surf. Coat. Technol. 2018, 337, 35–43. [Google Scholar] [CrossRef]
- Tomé-Torquemada, S.; Glaser, B.; Hildal, K.; Sichen, D. Experimental Study on the Activities of Al and Ca in Ferrosilicon. Metall. Mater. Trans. B 2017, 48, 3251–3258. [Google Scholar] [CrossRef][Green Version]
- Baisanov, S.O.; Tolokonnikova, V.V.; Narikbayeva, G.I.; Korsukova, I.Y.; Vorobkalo, N.R. Development of Theoretical Basis for Low-Percentage Ferrotitanium Production Technology with Using Ferrosilicon Aluminium. High. Temp. 2022, 60, 775–780. [Google Scholar] [CrossRef]
- Dumay, C.; Chatillon, C.; Allibert, M. Mass spectrométrie determination of activity coefficients of dilute aluminium and calcium in liquid silicon and ferrosilicon alloys. J. De Chim. Phys. 1997, 94, 971–977. [Google Scholar] [CrossRef]
- Alaerts, L.; De Beeck, J.P.O.; Hoste, J. Simultaneous determination of silicon and aluminium in ferrosilicon by instrumental neutron activation analysis with the aid of an 227Ac-Be isotopic neutron source. Anal. Chim. Acta 1974, 70, 253–263. [Google Scholar] [CrossRef]
- Xu, J.; Wang, K.; Wang, Y.; Qu, Z.; Tu, X. Effects of ferrosilicon alloy, Si content of steel, and slag basicity on compositions of inclusions during ladle furnace refining of Al-killed steel. J. Iron Steel Res. Int. 2020, 27, 1011–1017. [Google Scholar] [CrossRef]
- Gui, Z.; Liang, W.; Zhang, Y. Enhancing ductility of the Al-Si coating on hot stamping steel by controlling the Fe-Al phase transformation during austenitization. Sci. China Technol. Sci. 2014, 57, 1785–1793. [Google Scholar] [CrossRef]
- Novák, P.; Nová, K.; Vanka, T.; Průša, F. High-temperature behaviour of new Fe-Al-Si alloy produced by powder metallurgy. Manuf. Technol. 2018, 18, 299–302. [Google Scholar] [CrossRef]
- Wolski, K.; Le Caer, G.; Delcroix, P.; Fillit, R.; Thevenot, F.; Le Coze, J. Influence of milling conditions on the FeAl intermetallic formation by mechanical alloying. Mater. Sci. Eng. A 1996, 207, 97–104. [Google Scholar] [CrossRef]
- Zhou, T.D.; Zhou, P.H.; Liang, D.F.; Deng, L.J. Structure and electromagnetic characteristics of flaky FeSiAl powders made by melt-quenching. J. Alloys. Compd. 2009, 484, 545–549. [Google Scholar] [CrossRef]
- Ni, J.L.; Duan, F.; Feng, S.J.; Hu, F.; Kan, X.C.; Liu, X.S. High performance of FeSiAl/hBN soft magnetic composites. J. Alloys Compd. 2022, 897, 163191. [Google Scholar] [CrossRef]
- Li, H.; Bai, G.; Zhao, R.; Yang, H.; Lu, Z.; Cheng, M.; Su, R.; Bandaru, S.; Zhang, Y.; Liu, X. High-performance FeSiAl soft magnetic composites achieved by confined solid-state reaction. Acta Mater. 2023, 255, 119102. [Google Scholar] [CrossRef]
- Zhou, L.; Yan, J.; Huang, J.; Wang, H.; Wang, X.; Wang, Z. Dielectric and microwave absorption properties of FeSiAl/Al2O3 composites containing FeSiAl particles of different sizes. Ceram. Int. 2021, 47, 7831–7836. [Google Scholar] [CrossRef]
- Morris, D.G.; Muñoz-Morris, M.A. A re-examination of the pinning mechanisms responsible for the stress anomaly in FeAl intermetallics. Intermetallics 2010, 18, 1279–1284. [Google Scholar] [CrossRef]
- Li, W.; Cai, H.; Kang, Y.; Ying, Y.; Yu, J.; Zheng, J.; Qiao, L.; Jiang, Y.; Che, S. High permeability and low loss bioinspired soft magnetic composites with nacre-like structure for high frequency applications. Acta Mater. 2019, 167, 267–274. [Google Scholar] [CrossRef]
- Shkolnik, V.S.; Zharmenov, A.A.; Tolymbekov, M.Z.; Baisanov, S.O.; Chekimbaev, A.F. Prospects of production complex aluminum silicon alloy. In Proceedings of the 13th International Ferroalloys Congress: Efficient Technologies in Ferroalloy Industry, Almaty, Kazakhstan, 9–12 June 2013; p. 311. [Google Scholar]
- Shevko, V.M.; Amanov, D.D.; Karataeva, G.E.; Aitkulov, D.K. Complex ferroalloy obtaining from silicon-and aluminum–containing silica clay. Kompleks. Ispolz. Miner. Syra Complex. Use Miner. Resour. 2016, 4, 39–45. [Google Scholar]
- Domain, C.; Becquart, C.S. Ab initio calculations of defects in Fe and dilute Fe-Cu alloys. Phys. Rev. B 2001, 65, 024103. [Google Scholar] [CrossRef]
- Kimura, Y.; Takaki, S. Phase transformation mechanism of Fe-Cu alloys. ISIJ Int. 1997, 37, 290–295. [Google Scholar] [CrossRef]
- Eckert, J.; Holzer, J.C.; Johnson, W.L. Thermal stability and grain growth behavior of mechanically alloyed nanocrystalline Fe-Cu alloys. J. Appl. Phys. 1993, 73, 131–141. [Google Scholar] [CrossRef]
- Jiang, J.Z.; Gente, C.; Bormann, R. Mechanical alloying in the Fe–Cu system. Mater. Sci. Eng. A 1998, 242, 268–277. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Liu, F.; Yang, G.C.; Xu, X.Q.; Zhou, Y.H. Rapid solidification of bulk undercooled hypoperitectic Fe–Cu alloy. J. Alloys Compd. 2007, 427, L1–L5. [Google Scholar] [CrossRef]
- Munitz, A. Liquid separation effects in Fe− Cu alloys solidified under different cooling rates. Metall. Trans. B 1987, 18, 565–575. [Google Scholar] [CrossRef]
- Chen, Q.; Jin, Z. The Fe-Cu system: A thermodynamic evaluation. Metall. Mater. Trans. A 1995, 26, 417–426. [Google Scholar] [CrossRef]
- Imai, N.; Tanaka, T.; Yuki, T.; Iida, T.; Morita, Z. Equilibrium distribution of Sn between solid and liquid phases in Fe-Sn and Fe-C-Sn alloys. Tetsu-to-Hagane 1991, 77, 224–230. [Google Scholar] [CrossRef][Green Version]
- Fayyazi, B.; Skokov, K.P.; Faske, T.; Opahle, I.; Duerrschnabel, M.; Helbig, T.; Soldatov, I.; Rohrmann, U.; Molina-Luna, L.; Gueth, K. Experimental and computational analysis of binary Fe-Sn ferromagnetic compounds. Acta Mater. 2019, 180, 126–140. [Google Scholar] [CrossRef]
- Dorofeev, G.A.; Elsukov, E.P. Thermodynamic modeling of mechanical alloying in the Fe–Sn system. Inorg. Mater. 2000, 36, 1228–1234. [Google Scholar] [CrossRef]
- Van Beek, J.A.; Stolk, S.A.; van Loo, F.J.J. Multiphase diffusion in the systems Fe-Sn and Si-Sn. Int. J. Mater. Res. 1982, 73, 439–444. [Google Scholar] [CrossRef]
- Yelsukov, E.P.; Konygin, G.N.; Voronina, E.V.; Korolyov, A.V.; Ulyanov, A.I.; Godovikov, S.K.; Zagainov, A.V. Magnetic behaviour of high Si (Sn) concentration nanocrystalline Fe–Si and Fe–Sn alloys obtained by mechanical grinding. J. Magn. Magn. Mater. 2000, 214, 258–268. [Google Scholar] [CrossRef]
- Arita, M.; Ohyama, M.; Goto, K.S. Someno, Measurements of Activity, Solubility, and Diffusivity in α and γ Fe-Sn Alloys between 1183 and 1680 K. Int. J. Mater. Res. 1981, 72, 244–250. [Google Scholar] [CrossRef]
- Giefers, H.; Nicol, M. High pressure X-ray diffraction study of all Fe–Sn intermetallic compounds and one Fe–Sn solid solution. J. Alloys Compd. 2006, 422, 132–144. [Google Scholar] [CrossRef]
- Padhamnath, P.; Ślęzak, M.; Karbowniczek, M. Disposing End of Life PV Modules–Reusing, Recycling and Upcycling. In Proceedings of the EU PVSEC 2023, EUPVSEC, Lisbon, Portugal, 18–22 September 2023; pp. 001–008. [Google Scholar] [CrossRef]
- Yeh, J.-W. Physical metallurgy of high-entropy alloys. JOM 2015, 67, 2254–2261. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.; Qi, H.; Zhang, W.; Chen, R.; Su, R.; Yu, B.; Qu, Y. Effect of non-metallic silicon content on the microstructure and corrosion behaviour of AlCoCrFeNi high-entropy alloys. Mater. Chem. Phys. 2024, 315, 128974. [Google Scholar] [CrossRef]
- Sohrabi, M.J.; Kalhor, A.; Mirzadeh, H.; Rodak, K.; Kim, H.S. Tailoring the strengthening mechanisms of high-entropy alloys toward excellent strength-ductility synergy by metalloid silicon alloying: A review. Prog. Mater. Sci. 2024, 101295. [Google Scholar] [CrossRef]
- Lee, H.; Sharma, A.; Ahn, B. Exploring strengthening mechanism of FeCoNiAl high-entropy alloy by non-metallic silicon addition produced via powder metallurgy. J. Alloys Compd. 2023, 947, 169545. [Google Scholar] [CrossRef]
- Nowell, M.M.; Wright, S.I. Phase differentiation via combined EBSD and XEDS. J. Microsc. 2004, 213, 296–305. [Google Scholar] [CrossRef]
- Bordín, S.F.; Limandri, S.; Ranalli, J.M.; Castellano, G. EBSD spatial resolution for detecting sigma phase in steels. Ultramicroscopy 2016, 171, 177–185. [Google Scholar] [CrossRef]
- Brodusch, N.; Gauvin, R. Phase differentiation based on x-ray energy spectrum correlation with an energy dispersive spectrometer (EDS). Ultramicroscopy 2022, 238, 113534. [Google Scholar] [CrossRef]
- Beniwal, D.; Shivam, V.; Palasyuk, O.; Kramer, M.J.; Phanikumar, G.; Ray, P.K. EDS-PhaSe: Phase segmentation and analysis from EDS elemental map images using markers of elemental segregation. Metallogr. Microstruct. Anal. 2023, 12, 924–933. [Google Scholar] [CrossRef]
- Parish, C.M. Cluster analysis of combined EDS and EBSD data to solve ambiguous phase identifications. Microsc. Microanal. 2022, 28, 371–382. [Google Scholar] [CrossRef]
- Ram, F.; Wright, S.; Singh, S.; De Graef, M. Error analysis of the crystal orientations obtained by the dictionary approach to EBSD indexing. Ultramicroscopy 2017, 181, 17–26. [Google Scholar] [CrossRef]
- Chen, Y.H.; Park, S.U.; Wei, D.; Newstadt, G.; Jackson, M.A.; Simmons, J.P.; De Graef, M.; Hero, A.O. A dictionary approach to electron backscatter diffraction indexing. Microsc. Microanal. 2015, 21, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Brewick, P.T.; Wright, S.I.; Rowenhorst, D.J. NLPAR: Non-local smoothing for enhanced EBSD pattern indexing. Ultramicroscopy 2019, 200, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Griesser, S.; Bernhard, C.; Dippenaar, R. Effect of nucleation undercooling on the kinetics and mechanism of the peritectic phase transition in steel. Acta Mater. 2014, 81, 111–120. [Google Scholar] [CrossRef]
- Herlach, D.M.; Gao, J.; Holland-Moritz, D.; Volkmann, T. Nucleation and phase-selection in undercooled melts. Mater. Sci. Eng. A 2004, 375, 9–15. [Google Scholar] [CrossRef]
- Perepezko, J.H.; Wilde, G. Melt undercooling and nucleation kinetics. Curr. Opin. Solid. State Mater. Sci. 2016, 20, 3–12. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. I General theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. II transformation-time relations for random distribution of nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Van der Zwaag, S. Kinetics of phase transformations in steels. In Phase Transformations in Steels; Elsevier: Amsterdam, The Netherlands, 2012; pp. 126–156. [Google Scholar]
- Smith, R.F.; Eggert, J.H.; Swift, D.C.; Wang, J.; Duffy, T.S.; Braun, D.G.; Rudd, R.E.; Reisman, D.B.; Davis, J.-P.; Knudson, M.D. Time-dependence of the alpha to epsilon phase transformation in iron. J. Appl. Phys. 2013, 114. [Google Scholar] [CrossRef]
- Madsen, I.C.; Scarlett, N.V.Y. Quantitative phase analysis. In Powder Diffraction: Theory and Practice; Royal Society of Chemistry: London, UK, 2008; pp. 298–331. [Google Scholar] [CrossRef]
- Gouttebroze, S.; Marthinsen, A.; Kroka, B.; Götz, A.; Ott, E. Modelling of major phases formation during solidification of Ferro-Silicon-Magnesium. IOP Conf. Ser. Mater. Sci. Eng. 2023, 128, 012052. [Google Scholar] [CrossRef]
- Degtyareva, O.; Degtyareva, V.F.; Porsch, F.; Holzapfel, W.B. Phase transitions under high pressure inbinary Sn alloys (with In, Hg and Ga). J. Phys. Condens. Matter. 2001, 14, 389. [Google Scholar] [CrossRef]
- Cheong, B.H.; Chang, K.-J. First-principles study of the structural properties of Sn under pressure. Phys. Rev. B 1991, 44, 4103. [Google Scholar] [CrossRef] [PubMed]
- Sim, K.; Lee, J. Phase stability of Ag–Sn alloy nanoparticles. J. Alloys Compd. 2014, 590, 140–146. [Google Scholar] [CrossRef]
- Milekhine, V.; Onsøien, M.I.; Solberg, J.K.; Skaland, T. Mechanical properties of FeSi (ε), FeSi2 (ζα) and Mg2Si. Intermetallics 2002, 10, 743–750. [Google Scholar] [CrossRef]
- Wu, J.; Chong, X.; Jiang, Y.; Feng, J. Stability, electronic structure, mechanical and thermodynamic properties of Fe-Si binary compounds. J. Alloys Compd. 2017, 693, 859–870. [Google Scholar] [CrossRef]
- Costa, B.F.O.; Le Caër, G.; de Campos, N.A. On the Role of Tin Solubility in the Precipitation of the Sigma-Phase in Fe–Cr–Sn Alloys. Phys. Status Solidi (A) 1997, 164, 687–697. [Google Scholar] [CrossRef]
- Hood, G.M. The diffusion of iron in aluminium. Philos. Mag. A J. Theor. Exp. Appl. Phys. 1970, 21, 305–328. [Google Scholar] [CrossRef]
- Salje, G.; Feller-Kniepmeier, M. The diffusion and solubility of copper in iron. J. Appl. Phys. 1977, 48, 1833–1839. [Google Scholar] [CrossRef]
- Novák, P.; Michalcová, A.; Marek, I.; Mudrová, M.; Saksl, K.; Bednarčík, J.; Zikmund, P.; Vojtěch, D. On the formation of intermetallics in Fe–Al system–An in situ XRD study. Intermetallics 2013, 32, 127–136. [Google Scholar] [CrossRef]
- Godlewska, E.; Szczepanik, S.; Mania, R.; Krawiarz, J.; Kozinski, S. FeAl materials from intermetallic powders. Intermetallics 2003, 11, 307–312. [Google Scholar] [CrossRef]
- Maitra, T.; Gupta, S.P. Intermetallic compound formation in Fe–Al–Si ternary system: Part II. Mater. Charact. 2002, 49, 293–311. [Google Scholar] [CrossRef]
- Ohnuma, I.; Abe, S.; Shimenouchi, S.; Omori, T.; Kainuma, R.; Ishida, K. Experimental and thermodynamic studies of the Fe–Si binary system. ISIJ Int. 2012, 52, 540–548. [Google Scholar] [CrossRef]
- Dézsi, I.; Fetzer, C.; Szűcs, I.; Dekoster, J.; Vantomme, A.; Caymax, M. Stable and metastable iron silicide phases on Si (1 0 0). Surf. Sci. 2005, 599, 122–127. [Google Scholar] [CrossRef]
- Ma, R.; Xie, Q.; Huang, J.; Yan, W.Y.; Guo, X.T. Theoretical study on the electronic structures and magnetism of Fe3Si intermetallic compound. J. Alloys Compd. 2013, 552, 324–328. [Google Scholar] [CrossRef]
- Novák, P.; Lamchaouri, A.; Nová, K.; Průša, F. Fe-Al-Si coatings for the Protection of Steel Against High-temperature Oxidation. Manuf. Technol. 2019, 19, 660–663. [Google Scholar] [CrossRef]
- Kang, S.; Han, K.; Kim, K.; Kang, Y.; Son, K.; Kim, D. Formation behavior of an intermetallic compound layer during the hot dip aluminizing of cast iron. ISIJ Int. 2012, 52, 1342–1347. [Google Scholar] [CrossRef]
- Zou, H.F.; Yang, H.J.; Zhang, Z.F. Morphologies, orientation relationships and evolution of Cu6Sn5 grains formed between molten Sn and Cu single crystals. Acta Mater. 2008, 56, 2649–2662. [Google Scholar] [CrossRef]
- Leineweber, A.; Wieser, C.; Hügel, W. Cu6Sn5 intermetallic: Reconciling composition and crystal structure. Scr. Mater. 2020, 183, 66–70. [Google Scholar] [CrossRef]
- Mu, D.K.; McDonald, S.D.; Read, J.; Huang, H.; Nogita, K. Critical properties of Cu6Sn5 in electronic devices: Recent progress and a review. Curr. Opin. Solid. State Mater. Sci. 2016, 20, 55–76. [Google Scholar] [CrossRef]
- Nutor, R.K.; Cao, Q.; Wang, X.; Zhang, D.; Fang, Y.; Zhang, Y.; Jiang, J.-Z. Phase selection, lattice distortions, and mechanical properties in high-entropy alloys. Adv. Eng. Mater. 2020, 22, 2000466. [Google Scholar] [CrossRef]
- Chang, C.-C.; Hsiao, Y.-T.; Chen, Y.-L.; Tsai, C.-Y.; Lee, Y.-J.; Ko, P.-H.; Chang, S.-Y. Lattice distortion or cocktail effect dominates the performance of Tantalum-based high-entropy nitride coatings. Appl. Surf. Sci. 2022, 577, 151894. [Google Scholar] [CrossRef]
- Michael, J.R. Phase identification using electron backscatter diffraction in the scanning electron microscope. In Electron Backscatter Diffraction in Materials Science; Springer: New York, NY, USA, 2000; pp. 75–89. [Google Scholar]
- El-Dasher, B.; Deal, A. Application of electron backscatter diffraction to phase identification. Electron Backscatter Diffr. Mater. Sci. 2009, 81–95. [Google Scholar]
- Münch, B.; Martin, L.H.J.; Leemann, A. Segmentation of elemental EDS maps by means of multiple clustering combined with phase identification. J. Microsc. 2015, 260, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Miler, M.; Mirtič, B. Accuracy and precision of EDS analysis for identification of metal-bearing minerals in polished and rough particle samples. Geologija 2013, 56, 5–17. [Google Scholar] [CrossRef]
- Chen, C.-L.; Thomson, R.C. The combined use of EBSD and EDX analyses for the identification of complex intermetallic phases in multicomponent Al–Si piston alloys. J. Alloys Compd. 2010, 490, 293–300. [Google Scholar] [CrossRef]
- Wu, T.H.; Wang, H. The Effect of Solidification Behavior of FeSi75 Alloy on Its Disintegration. In Proceedings of the of the Silicon for the Chemical & Solar Industry XVI 2022, Online, 14–16 June 2022. [Google Scholar]







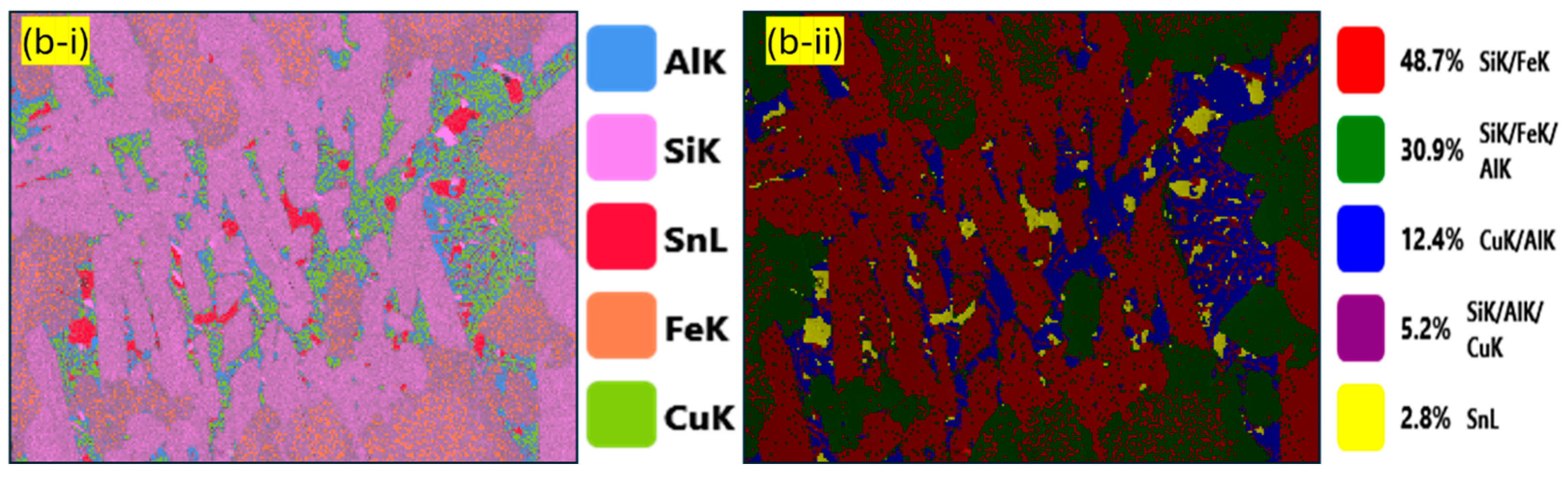
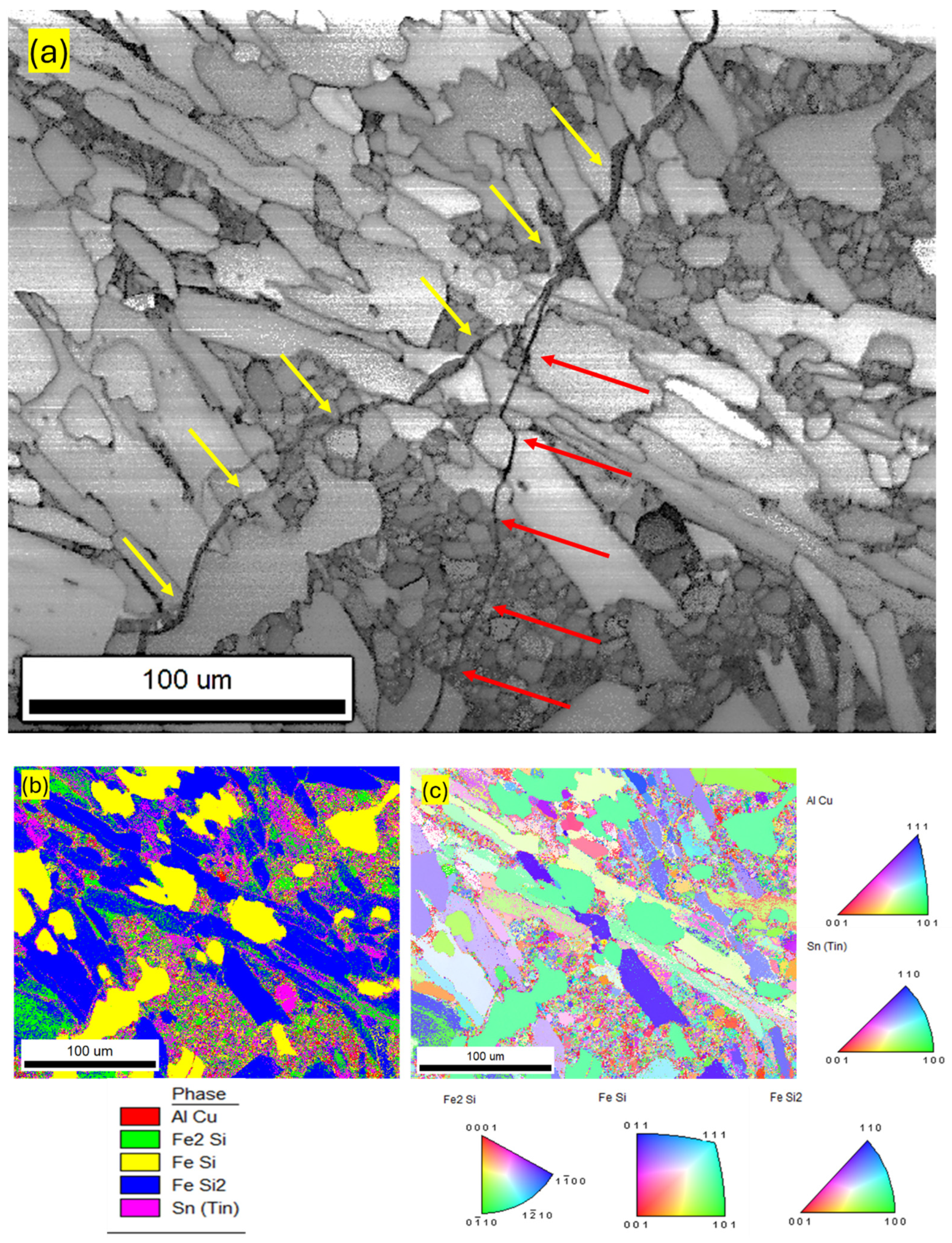
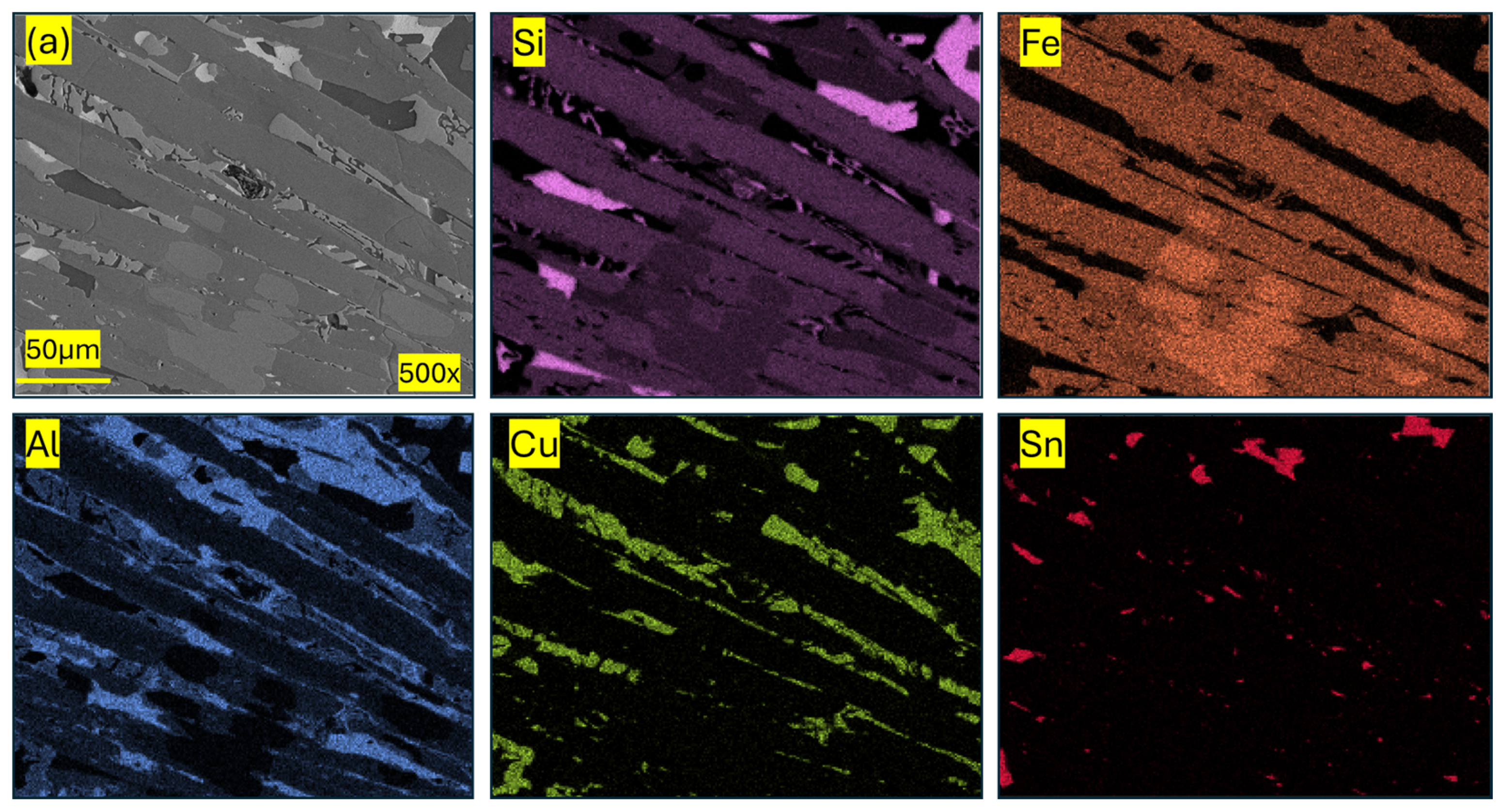
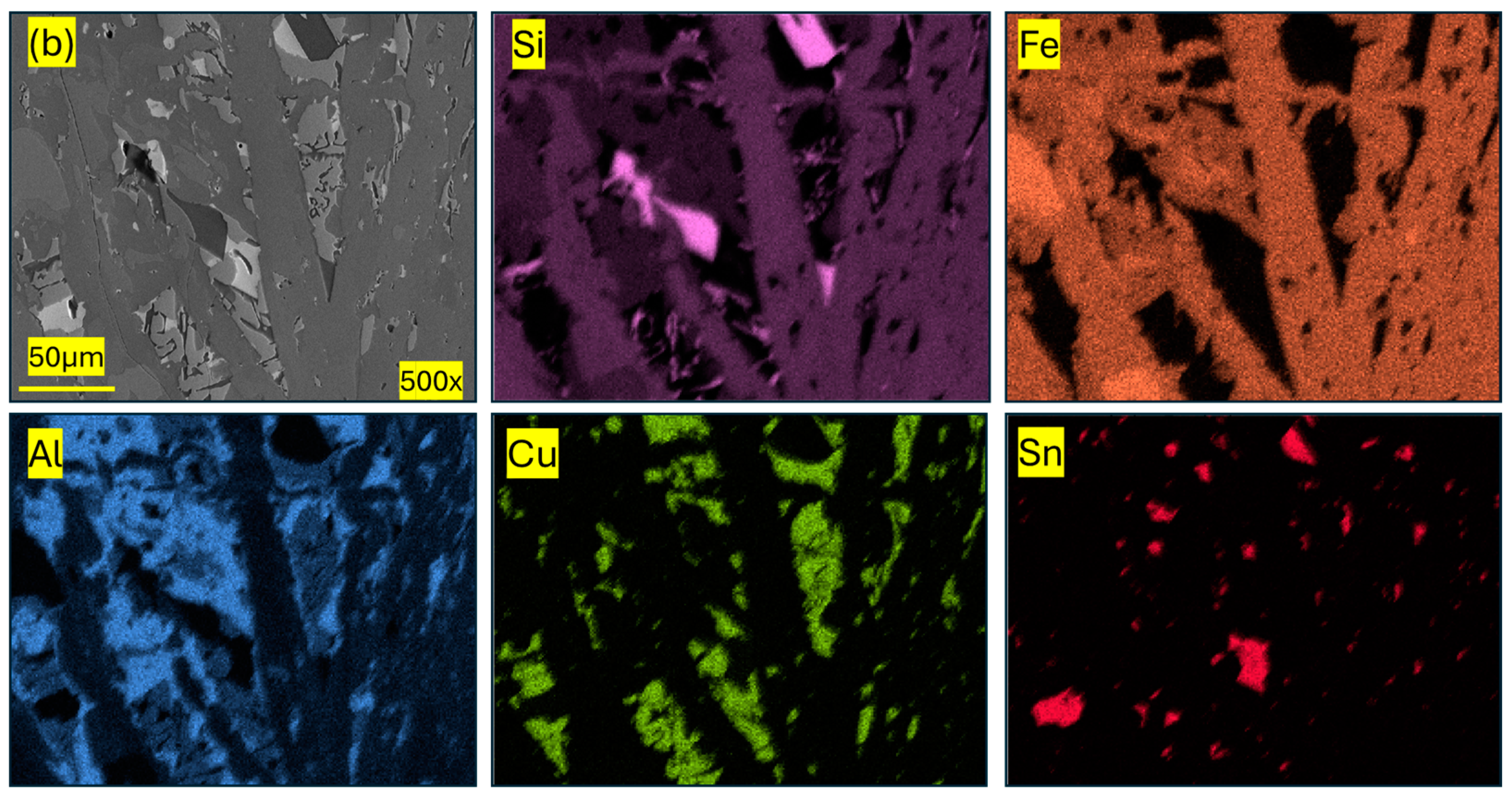





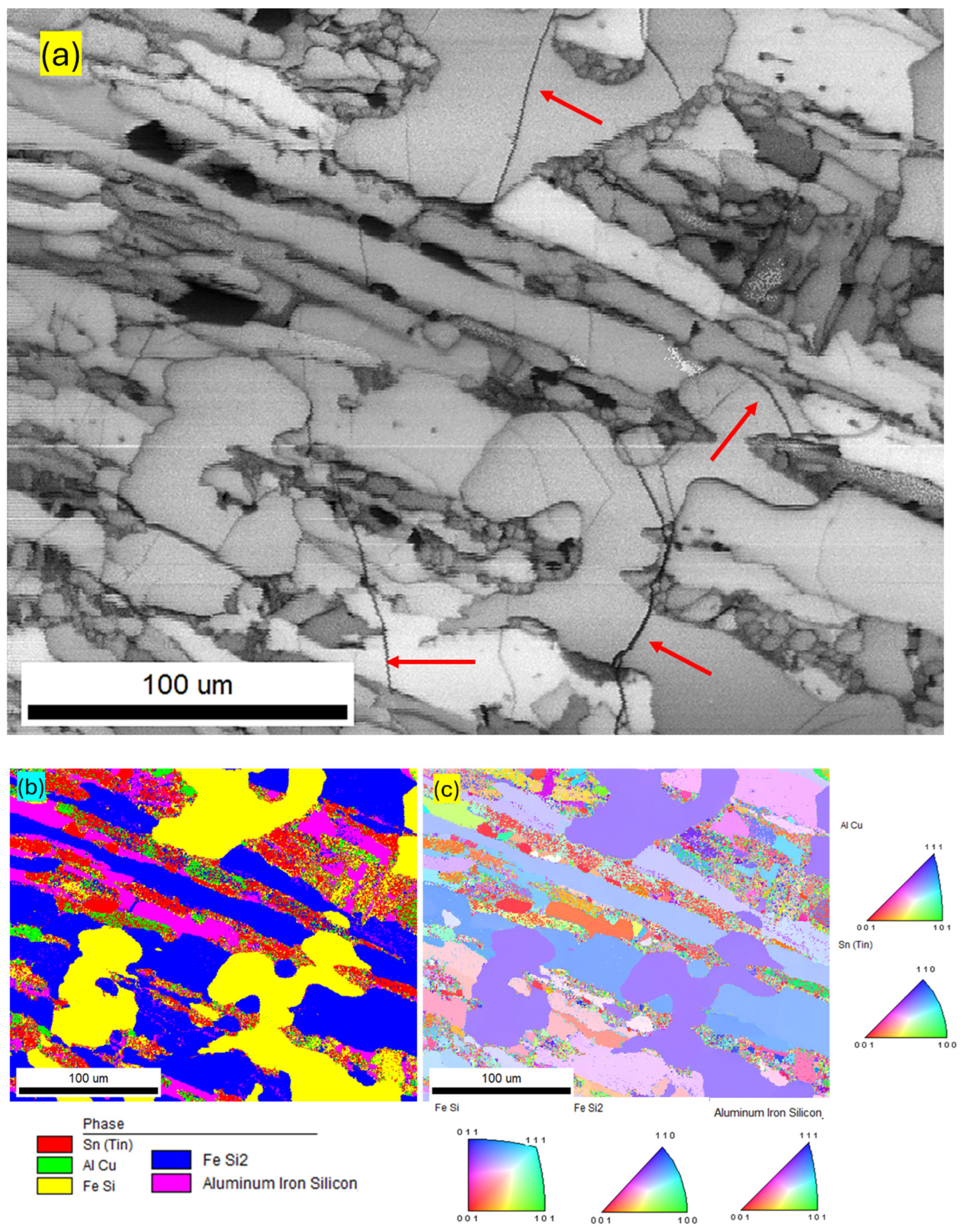

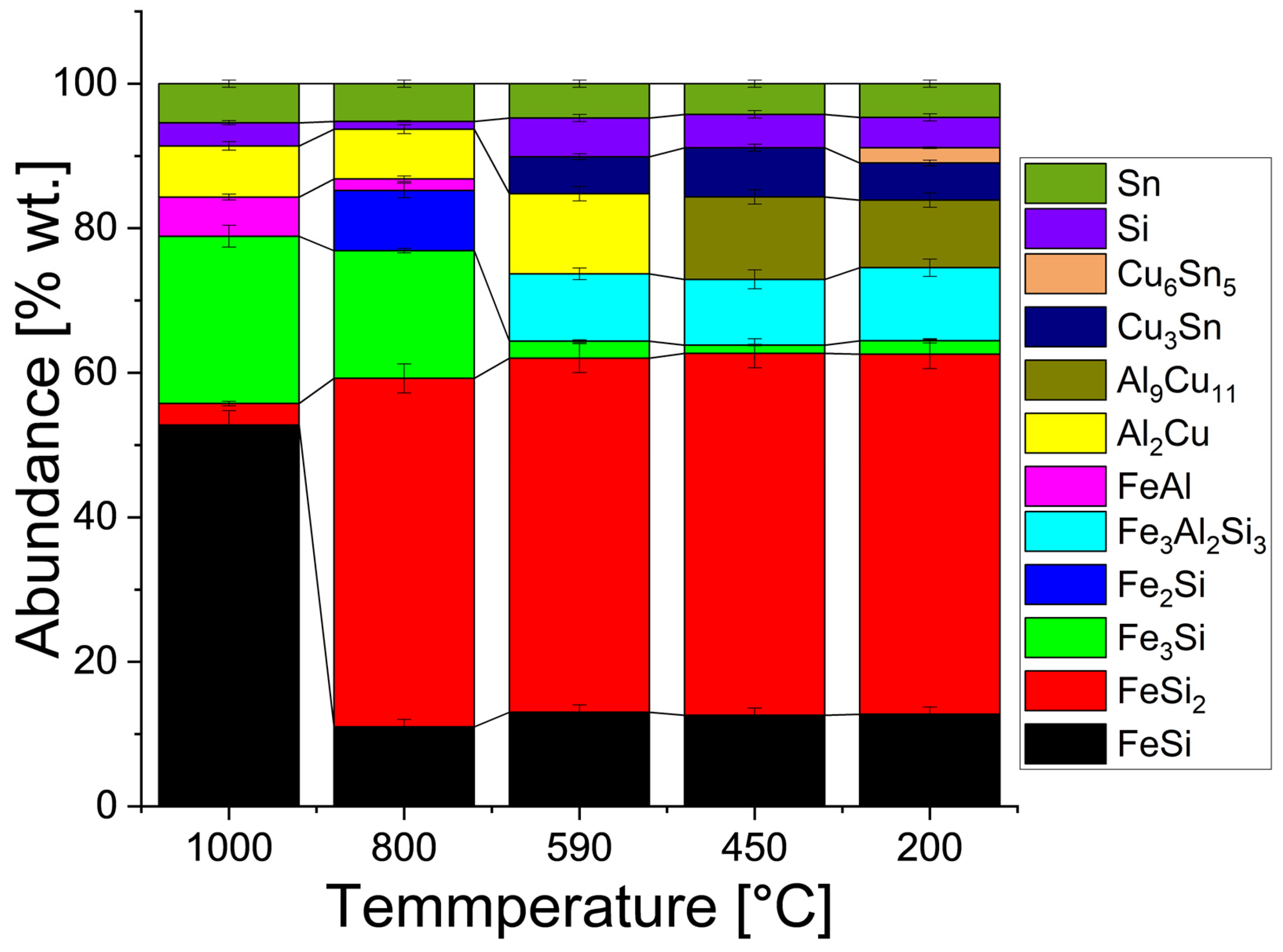

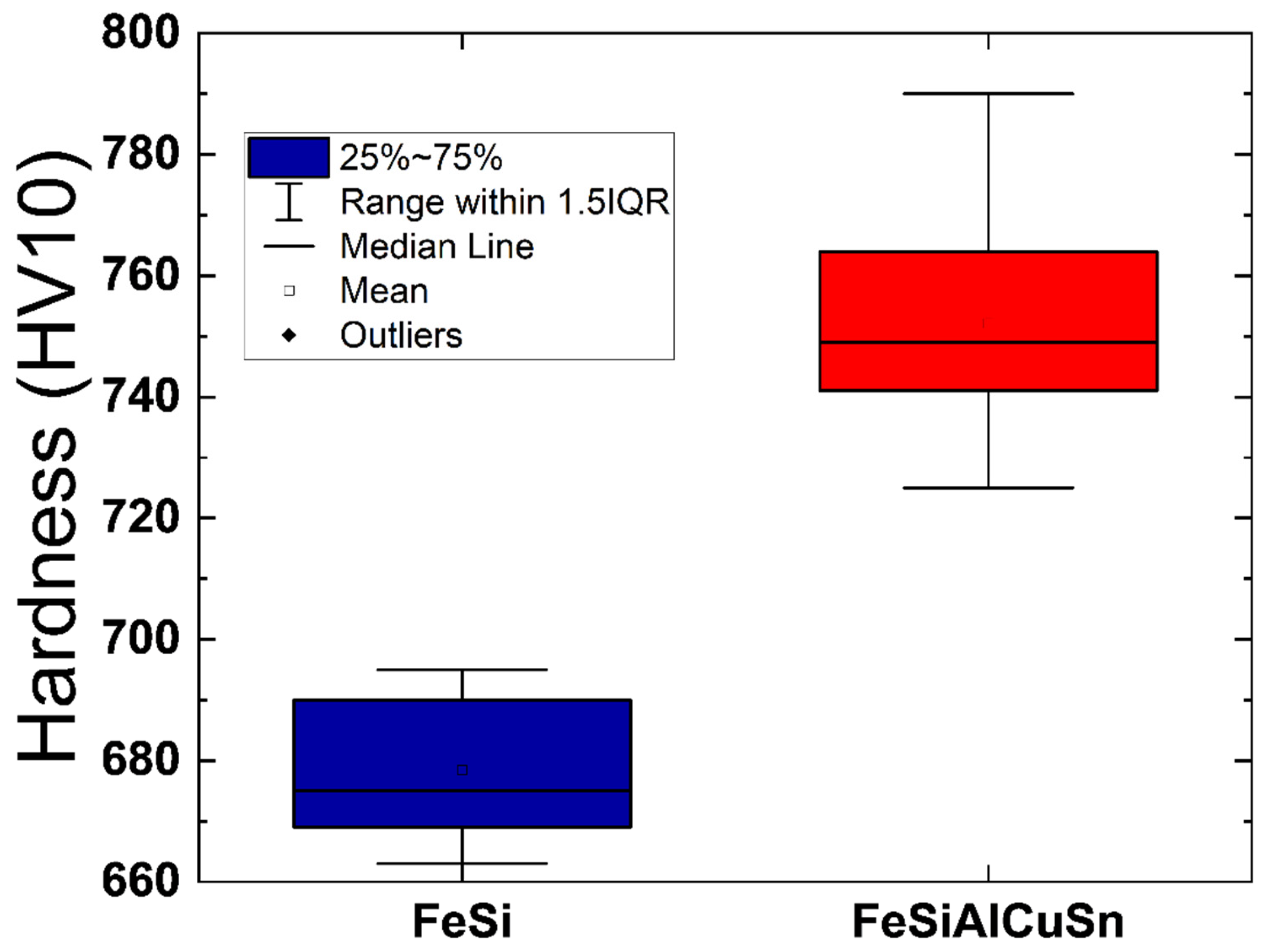
| Component (E) | Wt. [g] | E/Total [%] | E/Si [%] |
|---|---|---|---|
| Fe | 110 | 40.7 | 122.2 |
| Si | 90 | 33.3 | 1 |
| Al | 25 | 9.3 | 27.8 |
| Cu | 25 | 9.3 | 27.8 |
| Sn | 20 | 7.4 | 22.2 |
| Crucible Type | Height [mm] | Inner Diameter [mm] | Thickness [mm] | Weight as Received [g] | Weight After Baking [g] |
|---|---|---|---|---|---|
| Graphite | 100 | 55 | 25 | 1082 ± 3 | 871 ± 1.3 |
| Alumina | 80 | 48 | 2.5 | 120 ± 1.5 | 119 ± 0.2 |
| Sample-ID | Annealing Temp [°C] | Annealing Time [hours] |
|---|---|---|
| FeSi-1000 | 1000 | 24 |
| FeSi-800 | 800 | 36 |
| FeSi-590 | 590 | 48 |
| FeSi-450 | 450 | 60 |
| FeSi-200 | 200 | 72 |
| Phases | Mass [g] | Mass [%] |
|---|---|---|
| FeSi | 116.4 | 43.1 |
| Fe3(Si, Al)7 | 58.7 | 21.7 |
| Solution (liquid) (Al+Cu+Fe+Si+Sn) | 94.9 | 35.2 |
| Pseudo-Phase | Fe [% at.] | Si [% at.] | Al [% at.] | Cu [% at.] | Sn [% at.] |
|---|---|---|---|---|---|
| Si/Fe | 38.9 ± 11.6 | 48.3 ± 9.6 | 8.3 ± 5.4 | 3.5 ± 1.3 | 0.9 ± 0.1 |
| Al/Si/Fe | 25.5 ± 0.8 | 35.4 ± 7.6 | 30.7 ± 7.1 | 6.9 ± 0.8 | 1.9 ± 0.7 |
| Al/Cu | 3.6 ± 0.1 | 6.3 ± 0.1 | 36.5 ± 0.7 | 52.3 ± 1.1 | 1.2 ± 0.5 |
| Sn | 7.1 ± 2.1 | 6.7 ± 1.3 | 3.2 ± 1.2 | 4.9 ± 2.2 | 77.8 ± 1.8 |
| Phases | Mass [g] | Mass [%] |
|---|---|---|
| FeSi | 40.7 | 15.1 |
| FeSi2 | 137.2 | 50.8 |
| Fe2SiAl2 | 23.6 | 8.7 |
| Solution (liquid) (Al+Cu+Fe+Si+Sn) | 68.5 | 25.4 |
| Pseudo-Phase | Fe [% at.] | Si [% at.] | Al [% at.] | Cu [% at.] | Sn [% at.] |
|---|---|---|---|---|---|
| Si/Fe | 37.9 ± 7.6 | 53 ± 8.6 | 7.2 ± 0.1 | 1.6 ± 0.5 | 0.3 ± 0.2 |
| Si/Fe/Al | 44.6 ± 3.2 | 35.7 ± 1.6 | 16.3 ± 2.3 | 2.5 ± 0.7 | 0.9 ± 0.1 |
| Cu/Al | 2.7 ± 0.1 | 3.4 ± 0.6 | 34.7 ± 0.5 | 57.8 ± 1.4 | 1.4 ± 0.2 |
| Si/Al/Cu | 5 ± 0.9 | 27.3 ± 0.9 | 25.5 ± 0.8 | 40.2 ± 1.3 | 2 ± 0.7 |
| Sn | 4.4 ± 0.3 | 5.9 ± 0.7 | 7.2 ± 0.4 | 14.1 ± 1.7 | 68.3 ± 1.1 |
| Phases | Mass [g] | Mass [%] |
|---|---|---|
| FeSi | 36.9 | 13.7 |
| FeSi2 | 136.1 | 50.4 |
| FeSiAl2 | 43.3 | 16.1 |
| Al-Cu-Si-Sn (BCC) solid solution | 43.9 | 16.2 |
| Solution (liquid) (Al+Cu+Fe+Si+Sn) | 9.8 | 3.6 |
| Pseudo-Phase | Fe [% at.] | Si [% at.] | Al [% at.] | Cu [% at.] | Sn [% at.] |
|---|---|---|---|---|---|
| Si/Fe | 35.4 ± 7.2 | 55.1 ± 8.7 | 7.7 ± 1.2 | 1.5 ± 0.5 | 0.3 ± 0.2 |
| Si/Fe/Al | 23.8 ± 2.9 | 38.6 ± 5.6 | 27.4 ± 6.5 | 9 ± 3.1 | 1.6 ± 0.3 |
| Si/Al/Cu | 7.1 ± 2.1 | 17.5 ± 2.2 | 20.5 ± 1.5 | 53.2 ± 2.1 | 1.6 ± 0.7 |
| Sn/Si/Al/Cu | 8 ± 0.6 | 14.6 ± 3.7 | 13.9 ± 3.3 | 12.5 ± 1.3 | 48.7 ± 8.4 |
| Sn | 4.4 ± 0.3 | 5.9 ± 0.7 | 7.2 ± 0.4 | 14.1 ± 1.7 | 68.3 ± 1.1 |
| Phases | Mass [g] | Mass [%] |
|---|---|---|
| FeSi | 36.3 | 13.4 |
| FeSi2 | 138.4 | 51.3 |
| FeSiAl2 | 41.6 | 15.4 |
| Al9Cu11 | 33.4 | 12.4 |
| Solution (liquid) (primarily Sn) | 20.2 | 7.5 |
| Pseudo-Phase | Fe [% at.] | Si [% at.] | Al [% at.] | Cu [% at.] | Sn [% at.] |
|---|---|---|---|---|---|
| Si/Fe | 31.3 ± 7.8 | 56.9 ± 6.1 | 7.7 ± 0.2 | 3.7 ± 1.9 | 0.4 ± 0.1 |
| Si/Fe/Al | 25.8 ± 0.5 | 35.3 ± 8.3 | 35. ± 9.1 | 2.9 ± 1.1 | 0.6 ± 0.1 |
| Al/Cu | 3.9 ± 2.5 | 2.6 ± 0.1 | 36.5 ± 0.5 | 55.9 ± 2.4 | 1.2 ± 0.1 |
| Sn | 3.4 ± 0.5 | 1.9 ± 0.4 | 4.2 ± 0.9 | 4.3 ± 1.8 | 86.5 ± 0.4 |
| Phases | Mass [g] | Mass [%] |
|---|---|---|
| FeSi | 36.3 | 13.5 |
| FeSi2 | 138.3 | 51.3 |
| FeSiAl2 | 41.7 | 15.3 |
| Al9Cu11 | 33.7 | 12.5 |
| Sn-BCC | 20 | 7.4 |
| Pseudo-Phase | Fe [% at.] | Si [% at.] | Al [% at.] | Cu [% at.] | Sn [% at.] |
|---|---|---|---|---|---|
| Si/Fe | 28.6 ± 5.9 | 60.9 ± 6.9 | 5.9 ± 0.8 | 2.6 ± 0.7 | 0.3 ± 0.1 |
| Si/Fe/Al | 28.9 ± 2.8 | 56.4 ± 2.1 | 11.8 ± 1.5 | 1.9 ± 0.3 | 0.8 ± 0.1 |
| Al/Cu | 5.1 ± 1.8 | 6.7 ± 1.7 | 30.1 ± 8.2 | 56.6 ± 7.6 | 1.7 ± 0.9 |
| Sn | 2.1 ± 0.4 | 2.4 ± 0.5 | 6.1 ± 0.9 | 6.7 ± 0.9 | 82.6 ± 2.6 |
| Temperature | Phases | |||
|---|---|---|---|---|
| Simulation | EDS | EBSD | XRD | |
| 1000 | FeSi | Fe/Si | FeSi | FeSi |
| Fe3Si7 | Si/Fe/Al | FeSi2 | FeSi2 | |
| (FeSiAlCuSn) solution | Al/Cu | AlFeSi | Fe3Si | |
| Sn | AlCu | FeAl | ||
| Sn | AlCu | |||
| Si | ||||
| Sn | ||||
| 800 | FeSi | Si/Fe | FeSi | FeSi |
| FeSi2 | Si/Fe/Al | FeSi2 | FeSi2 | |
| Fe2SiAl2 | Cu/Al | Fe2Si | Fe3Si | |
| (FeSiAlCuSn) solution | Si/Al/Cu | AlCu | Fe2Si | |
| Sn | Sn | FeAl | ||
| AlCu | ||||
| Si | ||||
| Sn | ||||
| 590 | FeSi | Si/Fe | FeSi | FeSi |
| FeSi2 | Si/Fe/Al | FeSi2 | FeSi2 | |
| FeSiAl2 | Si/Al/Cu | AlFeSi | Fe3Si | |
| Al-Cu-Si-Sn (BCC) solid solution | Sn/Si/Al/Cu | Cu3Sn | Fe3Al2Si3 | |
| (FeSiAlCuSn) solution | Sn | AlCu | Al2Cu | |
| Sn | Cu3Sn | |||
| Si | ||||
| Sn | ||||
| 450 | FeSi | Si/Fe | FeSi | FeSi |
| FeSi2 | Si/Fe/Al | FeSi2 | FeSi2 | |
| FeSiAl2 | Al/Cu | AlFeSi | Fe3Si | |
| Al9Cu11 | Sn | AlCu | Fe3Al2Si3 | |
| Sn solution | Sn | Al9Cu11 | ||
| Cu3Sn | ||||
| Si | ||||
| Sn | ||||
| 200 | FeSi | Si/Fe | FeSi | FeSi |
| FeSi2 | Si/Fe/Al | FeSi2 | FeSi2 | |
| FeSiAl2 | Al/Cu | AlFeSi | Fe3Si | |
| Al9Cu11 | Sn | AlCu | Fe3Al2Si3 | |
| Sn-BCC | Sn | Al9Cu11 | ||
| Cu3Sn | ||||
| Cu6Sn5 | ||||
| Si | ||||
| Sn | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padhamnath, P.; Kuśmierczyk, F.; Kopyściański, M.; Gondek, Ł.; Migas, P.; Karbowniczek, M. Realization of a Novel FeSiAlCuSn Multicomponent Alloy and Characterization of Intermetallic Phases Formed at Different Temperatures During Cooling. Metals 2025, 15, 479. https://doi.org/10.3390/met15050479
Padhamnath P, Kuśmierczyk F, Kopyściański M, Gondek Ł, Migas P, Karbowniczek M. Realization of a Novel FeSiAlCuSn Multicomponent Alloy and Characterization of Intermetallic Phases Formed at Different Temperatures During Cooling. Metals. 2025; 15(5):479. https://doi.org/10.3390/met15050479
Chicago/Turabian StylePadhamnath, Pradeep, Filip Kuśmierczyk, Mateusz Kopyściański, Łukasz Gondek, Piotr Migas, and Mirosław Karbowniczek. 2025. "Realization of a Novel FeSiAlCuSn Multicomponent Alloy and Characterization of Intermetallic Phases Formed at Different Temperatures During Cooling" Metals 15, no. 5: 479. https://doi.org/10.3390/met15050479
APA StylePadhamnath, P., Kuśmierczyk, F., Kopyściański, M., Gondek, Ł., Migas, P., & Karbowniczek, M. (2025). Realization of a Novel FeSiAlCuSn Multicomponent Alloy and Characterization of Intermetallic Phases Formed at Different Temperatures During Cooling. Metals, 15(5), 479. https://doi.org/10.3390/met15050479








